Dissecting Physiological and Agronomic Diversity in Safflower Populations Using Proximal Phenotyping
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Field Experiments
2.2. Agronomic Data Acquisition
2.3. Aerial Data Acquisition
2.4. Aerial Data Processing
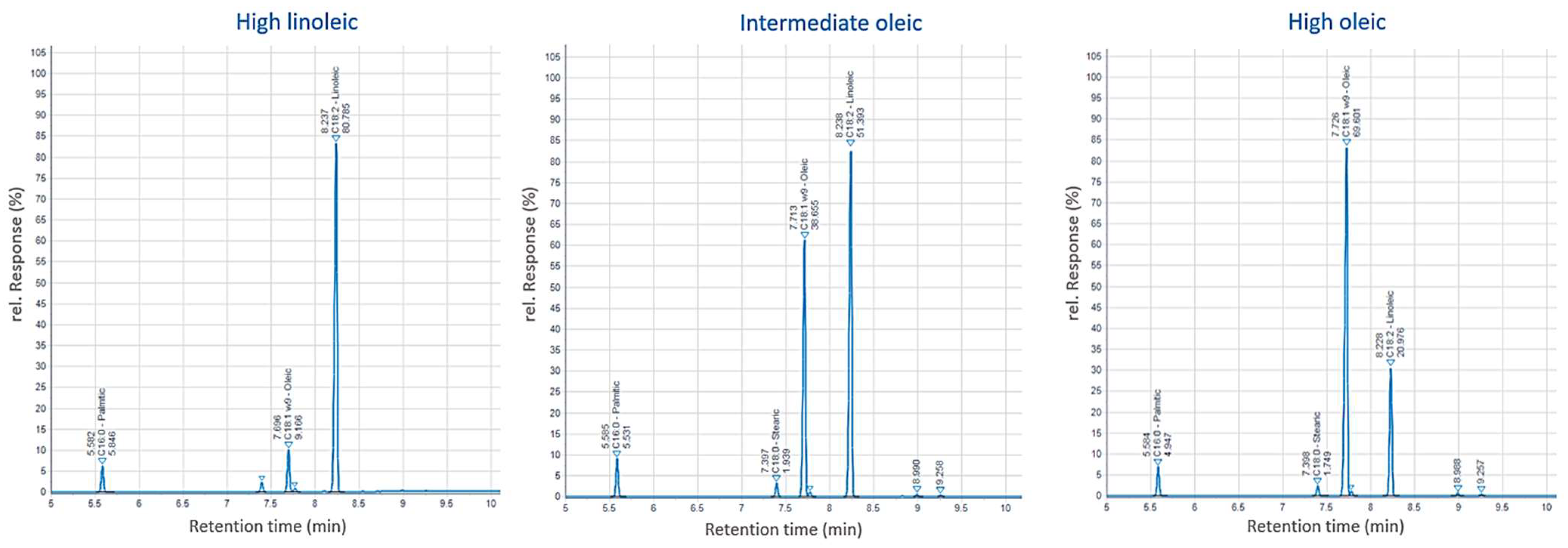
2.5. Grain Quality Analysis
2.6. Statistical Analysis
3. Results
3.1. Weather Variations
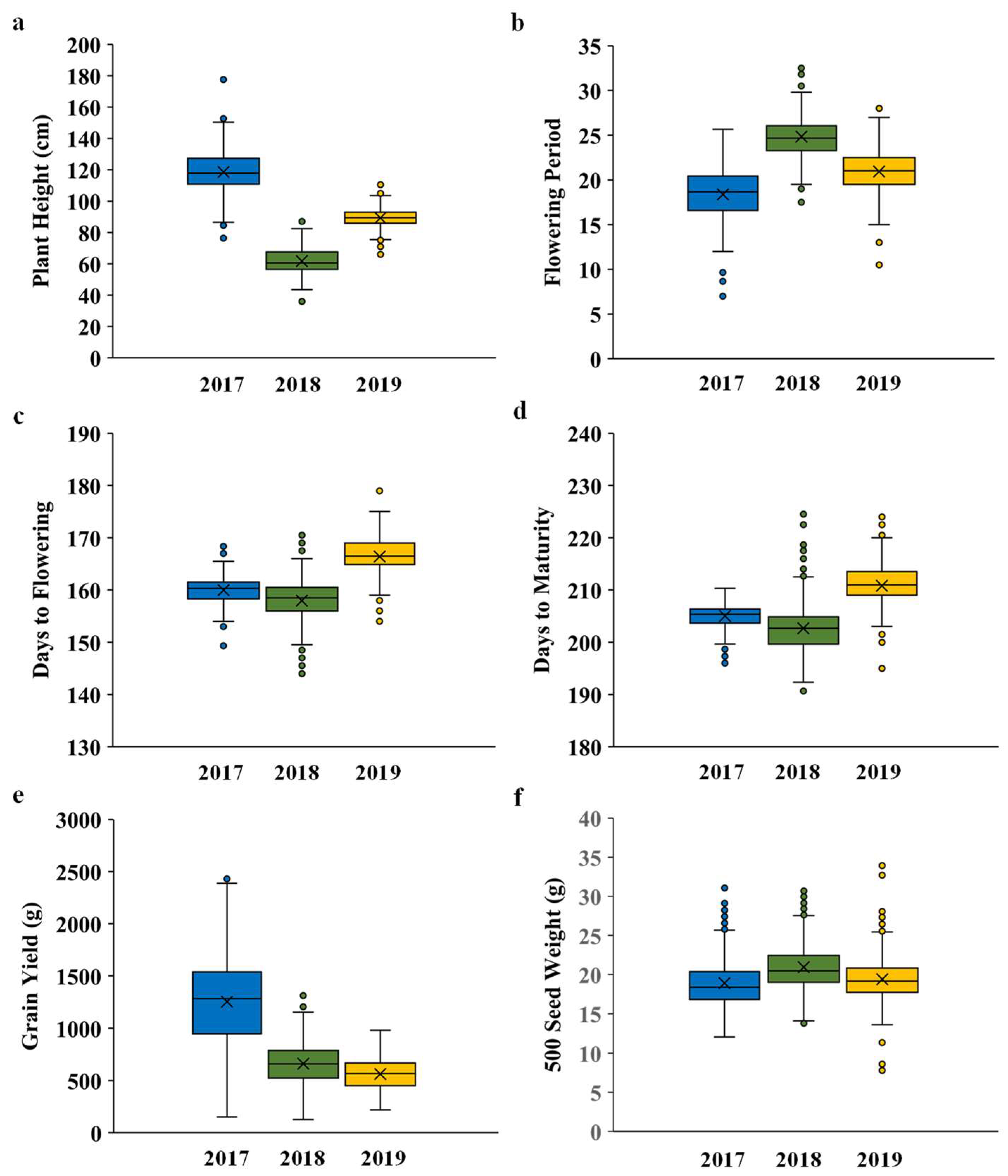
3.2. Plant Growth and Phenology
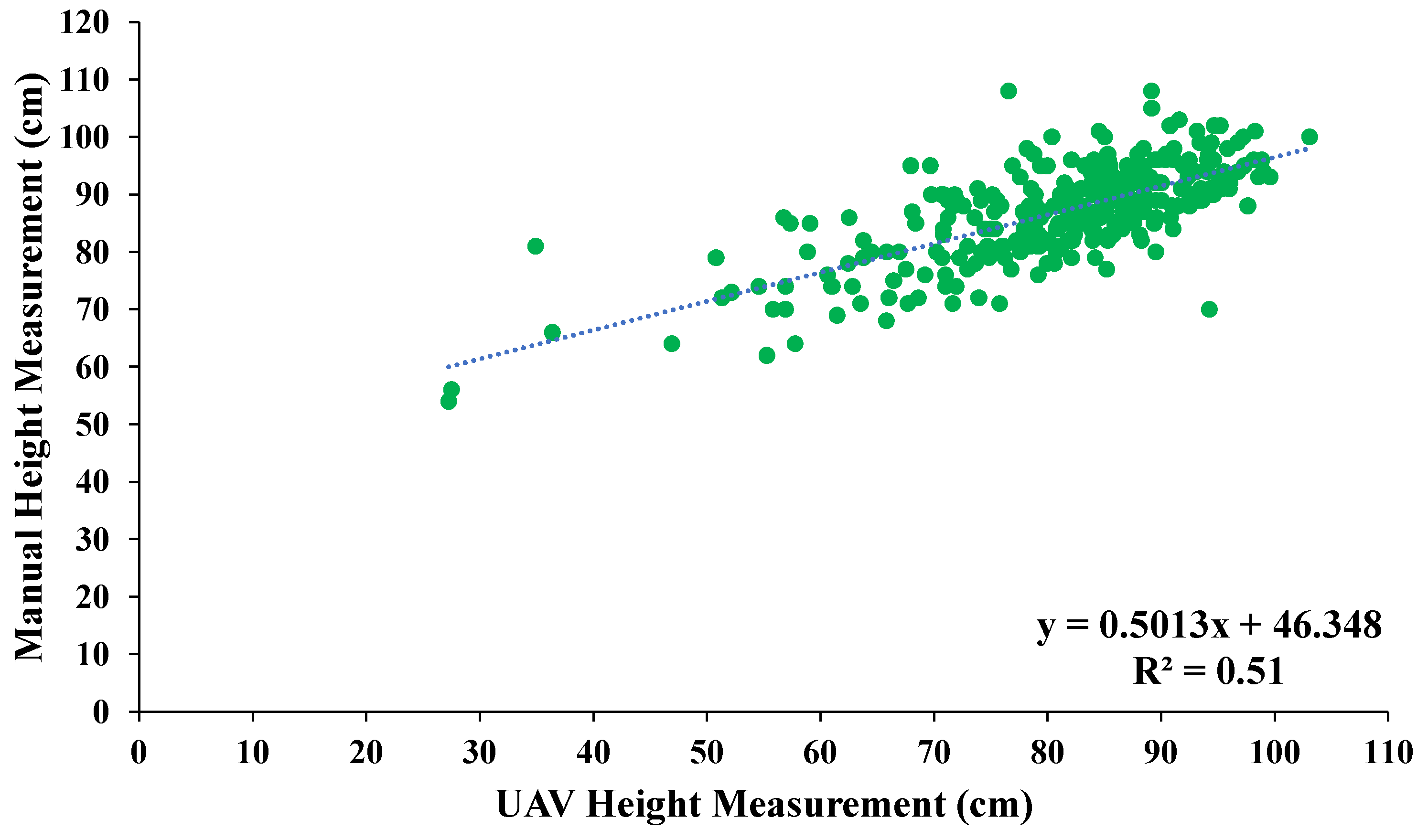
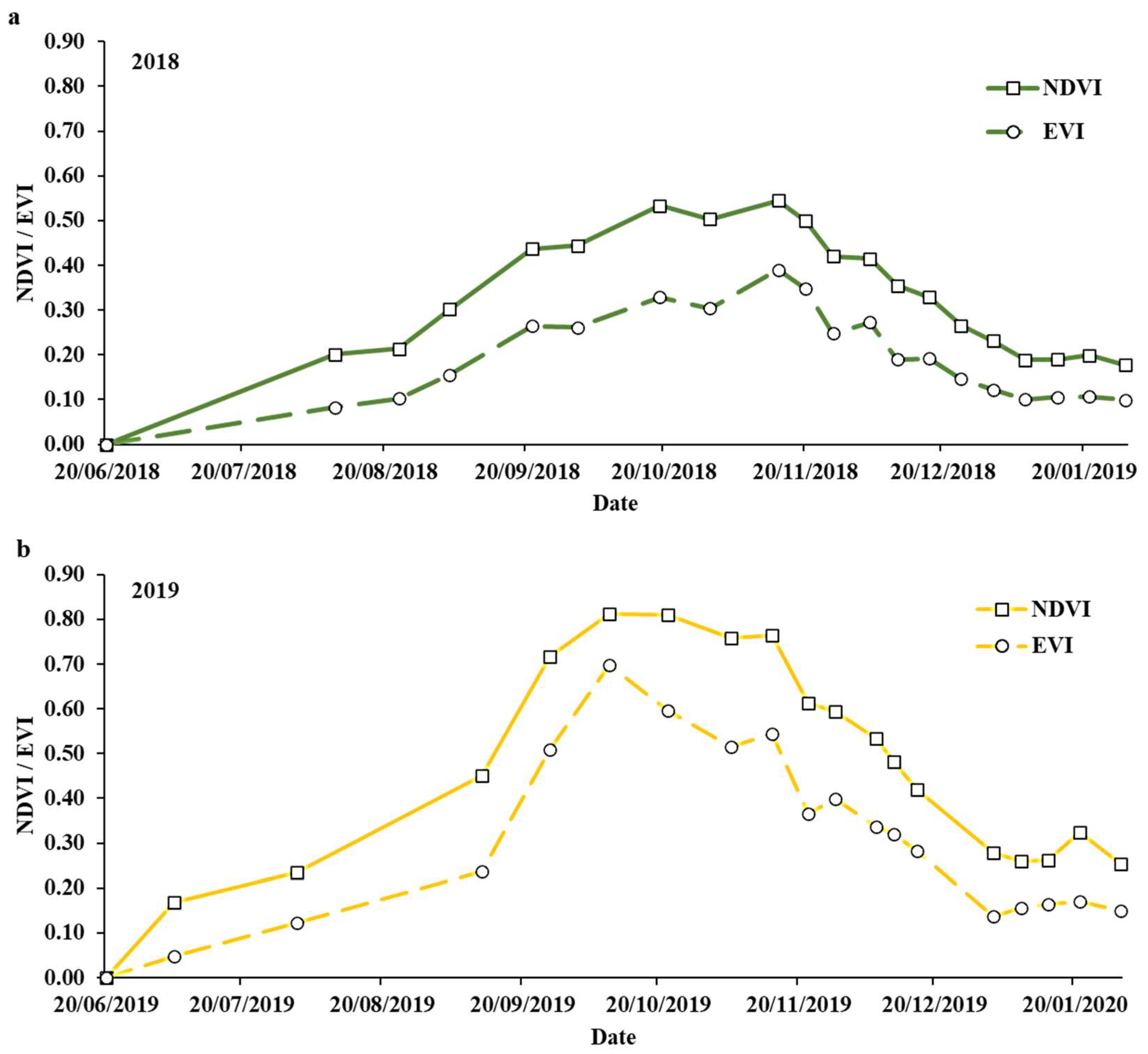
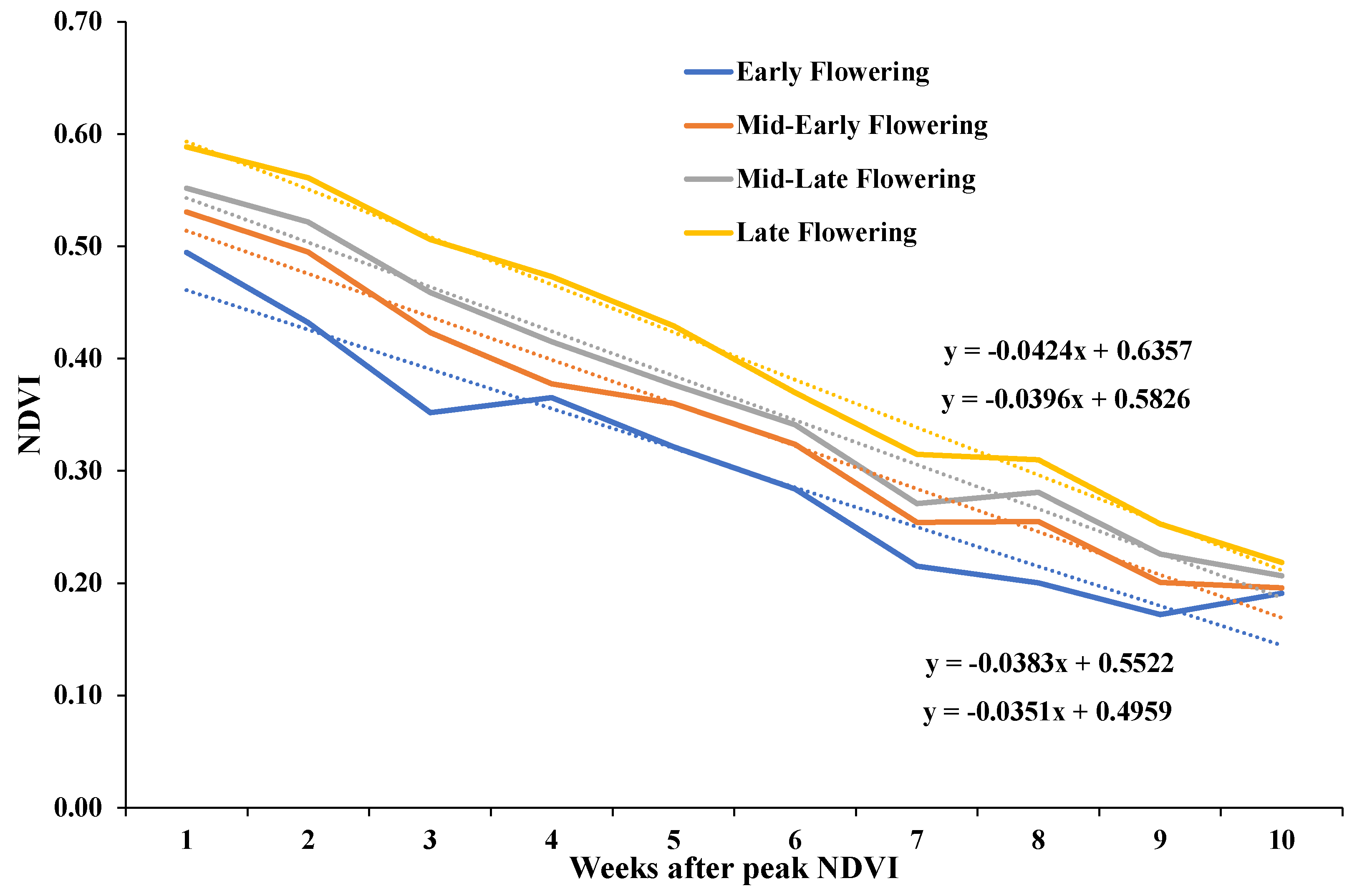
3.3. Aerially Derived Traits
3.4. Oil Quality Traits
4. Discussion
4.1. Seasonal Effects
4.2. High-Performing Genotypes
4.3. UAV-Derived Traits
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dajue, L.; Mündel, H.-H. Safflower. Carthamus tinctorius L.; Institute of Plant Genetics and Crop Plant Research, Gatersleben/International Plant Genetic Resources Institute: Rome, Italy, 1996; Volume 7. [Google Scholar]
- Emongor, V. Safflower (Carthamus tinctorius L.) the underutilized and neglected crop: A review. Asian J. Plant Sci. 2010, 9, 299–306. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.; Nimbkar, N. Safflower. In Breeding Oilseed Crops for Sustainable Production; Gupta, S.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 149–167. [Google Scholar]
- Hussain, M.I.; Lyra, D.-A.; Farooq, M.; Nikoloudakis, N.; Khalid, N. Salt and drought stresses in safflower: A review. Agron. Sustain. Dev. 2016, 36, 4. [Google Scholar] [CrossRef] [Green Version]
- Nuttall, J.G.; Davies, S.L.; Armstrong, R.A.; Peoples, M.B. Testing the primer-plant concept: Wheat yields can be increased on alkaline sodic soils when an effective primer phase is used. Aust. J. Agric. Res. 2008, 59, 331–338. [Google Scholar] [CrossRef]
- Knights, S.; Wachsmann, N.; Norton, R. Safflower (Carthamus tinctorius L.) production and research in southern Australia. In Proceedings of the 5th International Safflower Conference, Williston, North Dakota, Sidney, MT, USA, 23–27 July 2001; pp. 213–217. [Google Scholar]
- Jochinke, D.; Wachsmann, N.; Potter, T.; Norton, R. Growing safflower in Australia: Part 1—History, experiences and current constraints on production. In Proceedings of the 7th International Safflower Conference, Wagga Wagga, Australia, 3–6 November 2008. [Google Scholar]
- Wachsmann, N.; Knights, S.; Norton, R. The potential role of safflower (Carthamus tinctorius L.) in Australia’s southern farming systems. In Proceedings of the 10th Australian Agronomy Conference, Hobart, Tasmania, 28 January–1 February 2001. [Google Scholar]
- La Bella, S.; Tuttolomondo, T.; Lazzeri, L.; Matteo, R.; Leto, C.; Licata, M. An agronomic evaluation of new Safflower (Carthamus tinctorius L.) germplasm for seed and oil yields under Mediterranean climate conditions. Agronomy 2019, 9, 468. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Martinez, J.; del Rio, M.; de Haro, A. Survey of safflower (Carthamus tinctorius L.) germplasm for variants in fatty acid composition and other seed characters. Euphytica 1993, 69, 115–122. [Google Scholar] [CrossRef]
- Gecgel, U.; Demirci, M.; Esendal, E.; Tasan, M. Fatty acid composition of the oil from developing seeds of different varieties of Safflower (Carthamus tinctorius L.). J. Am. Oil Chem. Soc. 2006, 84, 47–54. [Google Scholar] [CrossRef]
- Tultabaev, M.; Chomanov, U.; Tultabaeva, T.; Shoman, A.; Dodaev, K.; Azimov, U.; Zhumanova, U. Identifying patterns in the fatty-acid composition of safflower depending on agroclimatic conditions. East.-Eur. J. Enterp. Technol. 2022, 2, 116. [Google Scholar] [CrossRef]
- Wouters, J.; Grzywacz, C.M.; Claro, A. Markers for identification of faded Safflower (Carthamus tinctorius L.) colorants by HPLC-PDA-MS—Ancient fibres, pigments, paints and cosmetics derived from antique recipes. Stud. Conserv. 2010, 55, 186–203. [Google Scholar] [CrossRef]
- Zemour, K.; Labdelli, A.; Adda, A.; Dellal, A.; Talou, T.; Merah, O. Phenol content and antioxidant and antiaging activity of safflower seed oil (Carthamus Tinctorius L.). Cosmetics 2019, 6, 55. [Google Scholar] [CrossRef] [Green Version]
- Asgarpanah, J.; Kazemivash, N. Phytochemistry, pharmacology and medicinal properties of Carthamus tinctorius L. Chin. J. Integr. Med. 2013, 19, 153–159. [Google Scholar] [CrossRef]
- Carvalho, I.S.; Miranda, I.; Pereira, H. Evaluation of oil composition of some crops suitable for human nutrition. Ind. Crop Prod. 2006, 24, 75–78. [Google Scholar] [CrossRef]
- de Oliveira, C.V.K.; Santos, R.F.; Siqueira, J.A.C.; Bariccatti, R.A.; Lenz, N.B.G.; Cruz, G.S.; Tokura, L.K.; Klajn, F.F. Chemical characterization of oil and biodiesel from four safflower genotypes. Ind. Crop Prod. 2018, 123, 192–196. [Google Scholar] [CrossRef]
- Meka, P.K.; Tripathi, V.; Singh, R.P. Synthesis of biodiesel fuel from Safflower oil using various reaction parameters. J. Oleo Sci. 2007, 56, 9–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khemchandani, B.; Jaiswal, A.; Sayanna, E.; Forsyth, M. Mixture of safflower oil and synthetic ester as a base stock for biodegradable lubricants. Lubr. Sci. 2014, 26, 67–80. [Google Scholar] [CrossRef]
- Anjani, K.; Yadav, P. High yielding-high oleic non-genetically modified Indian safflower cultivars. Ind. Crop Prod. 2017, 104, 7–12. [Google Scholar] [CrossRef]
- Dhruw, P.; Chandrakar, P.; Shrivastava, R. Assessment of genetic variability, heritability and genetic advance for seed yield and its contributing traits in elite germplasm accessions of safflower (Carthamus tinctorius L.). J. Pharma Innov. 2022, 11, 311–313. [Google Scholar]
- Feizi, M.; Hajabbasi, M.A.; Mostafazadeh-Fard, B. Saline irrigation water management strategies for better yield of safflower (Carthamus tinctorius L.) in an arid region. Aust. J. Crop Sci. 2010, 4, 408–414. [Google Scholar]
- Javed, S.; Bukhari, S.A.; Ashraf, M.Y.; Mahmood, S.; Iftikhar, T. Effects of salinity on growth, biochemical parameters and fatty acid composition in Safflower (Carthamus tinctorius L.). Pak. J. Bot. 2014, 46, 1153–1158. [Google Scholar]
- Yeilaghi, H.; Arzani, A.; Ghaderian, M. Evaluating the contribution of ionic and agronomic components toward salinity tolerance in safflower. Agron. J. 2015, 107, 2205–2212. [Google Scholar] [CrossRef]
- Beyyavas, V.; Haliloglu, H.; Copur, O.; Yilmaz, A. Determination of seed yield and yield components of some safflower (Carthamus tinctorius L.) cultivars, lines and populations under the semi-arid conditions. Afr. J. Biotechnol. 2011, 10, 527–534. [Google Scholar] [CrossRef]
- Bijanzadeh, E.; Moosavi, S.M.; Bahadori, F. Quantifying water stress of safflower (Carthamus tinctorius L.) cultivars by crop water stress index under different irrigation regimes. Heliyon 2022, 8, e09010. [Google Scholar] [CrossRef] [PubMed]
- Bortolheiro, F.P.A.P.; Silva, M.A. Physiological response and productivity of safflower lines under water deficit and rehydration. An. Acad. Bras. Cienc. 2017, 89, 3051–3066. [Google Scholar] [CrossRef] [Green Version]
- Koutroubas, S.D.; Damalas, C.A.; Fotiadis, S. Safflower assimilate remobilization, yield, and oil content in response to nitrogen availability, sowing time, and genotype. Field Crop. Res. 2021, 274, 108313. [Google Scholar] [CrossRef]
- Shahrokhnia, M.H.; Sepaskhah, A.R. Physiologic and agronomic traits in safflower under various irrigation strategies, planting methods and nitrogen fertilization. Ind. Crop Prod. 2017, 95, 126–139. [Google Scholar] [CrossRef]
- Stavropoulos, P.; Roussis, I.; Kakabouki, I.; Mavroeidis, A.; Zotos, A.; Triantafyllidis, V.; Kosma, C.; Beslemes, D.; Tigka, E.; Papadopoulos, G. Influence of urea fertilization with and without inhibitors on growth and yield of safflower (Carthamus tinctorius L.) under different tillage practices. Bull. Univ. Agric. Sci. 2022, 79, 90–94. [Google Scholar]
- Yolci, M.S.; Tunçtürk, R. The effect of inorganic fertilizer and biofertilizer applications on some quality and biochemical properties of safflower (Carthamus tinctorius L.). YYU J. Agric. Sci. 2022, 32, 740–753. [Google Scholar] [CrossRef]
- Chehade, L.A.; Angelini, L.G.; Tavarini, S. Genotype and seasonal variation affect yield and oil quality of safflower (Carthamus tinctorius L.) under Mediterranean conditions. Agronomy 2022, 12, 122. [Google Scholar] [CrossRef]
- Yeilaghi, H.; Arzani, A.; Ghaderian, M.; Fotovat, R.; Feizi, M.; Pourdad, S.S. Effect of salinity on seed oil content and fatty acid composition of safflower (Carthamus tinctorius L.) genotypes. Food Chem. 2012, 130, 618–625. [Google Scholar] [CrossRef]
- Arslan, B.; Culpan, E. Identification of suitable safflower genotypes for the development of new cultivars with high seed yield, oil content and oil quality. Azarian J. Agric. 2018, 5, 133–141. [Google Scholar]
- Bidgoli, A.M.; Akbari, G.A.; Mirhadi, M.J.; Zand, E.; Soufizadeh, S. Path analysis of the relationships between seed yield and some morphological and phenological traits in safflower (Carthamus tinctorius L.). Euphytica 2006, 148, 261–268. [Google Scholar] [CrossRef]
- El Bey, N.; Kurt, O. Correlation and path coefficient analysis for seed yield and yield contributing components, phenological and some morphological traits in Safflower (Carthamus tinctorius L.). Int. J. Phytol. Res. 2023, 3, 1–6. [Google Scholar]
- Malve, A.S.; Rathod, A.H.; Shinde, S.L.; Zate, D.K. Correlation analysis between seed yield and yield contributing traits over the locations in genotypes of safflower (Carthamus tinctorius L.). J. Pharma Innov. 2022, 11, 3407–3412. [Google Scholar]
- Kumar, S.; Ambreen, H.; Variath, M.T.; Rao, A.R.; Agarwal, M.; Kumar, A.; Goel, S.; Jagannath, A. Utilization of molecular, phenotypic, and geographical diversity to develop compact composite core collection in the oilseed crop, Safflower (Carthamus tinctorius L.) through maximization strategy. Front. Plant Sci. 2016, 7, 1554. [Google Scholar] [CrossRef] [Green Version]
- Omidi, A.H.; Khazaei, H.; Hongbo, S. Variation for some important agronomic traits in 100 spring Safflower (Carthamus tinctorius L.) genotypes. Am.-Eurasian J. Agric. Environ. Sci. 2009, 5, 791–795. [Google Scholar]
- Nabloussi, A.; El Fechtali, M.; Lyagoubi, S.; Knights, S.; Potter, T. Agronomic and technological evaluation of a world safflower collection in Moroccan conditions. In Proceedings of the 7th International Safflower Conference, Wagga Wagga, Australia, 3–6 November 2008. [Google Scholar]
- Pavithra, K.; Patil, R.S.; Harijan, Y.; Nishanth, G. Correlation and path analysis studies in safflower (Carthamus tinctorius L.) germplasm. Res. J. Agric. Sci. 2016, 7, 428–432. [Google Scholar]
- Devi, Y.L.; Shrivastava, R.; Parihar, R. Inheritance pattern of important qualitative traits in safflower (Carthamus tinctorious L.). Int. J. Plant Soil Sci. 2022, 34, 1154–1165. [Google Scholar] [CrossRef]
- Kose, A.; Onder, O.; Bilir, O.; Kosar, F. Application of multivariate statistical analysis for breeding strategies of sping Safflower (Carthamus tinctorius L.). Turk. J. Field Crops 2018, 23, 12–19. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Y.; Petkowski, J.; Kant, S.; Hayden, M.J.; Daetwyler, H.D. Genomic prediction and genomic heritability of grain yield and its related traits in a safflower genebank collection. Plant Genome 2021, 14, e20064. [Google Scholar] [CrossRef]
- Zhao, H.; Savin, K.W.; Li, Y.; Breen, E.J.; Maharjan, P.; Tibbits, J.F.; Kant, S.; Hayden, M.J.; Daetwyler, H.D. Genome-wide association studies dissect the G × E interaction for agronomic traits in a worldwide collection of safflowers (Carthamus tinctorius L.). Mol. Breed. 2022, 42, 24. [Google Scholar] [CrossRef]
- Al-Tamimi, N.; Langan, P.; Bernád, V.; Walsh, J.; Mangina, E.; Negrão, S. Capturing crop adaptation to abiotic stress using image-based technologies. Open Biol. 2022, 12, 210353. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Q.; Huang, D. A review of imaging techniques for plant phenotyping. Sensors 2014, 14, 20078–20111. [Google Scholar] [CrossRef] [PubMed]
- Homolova, L.; Malenovský, Z.; Clevers, J.G.; García-Santos, G.; Schaepman, M.E. Review of optical-based remote sensing for plant trait mapping. Ecol. Complex. 2013, 15, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Koh, J.C.O.; Hayden, M.; Daetwyler, H.; Kant, S. Estimation of crop plant density at early mixed growth stages using UAV imagery. Plant Methods 2019, 15, 64. [Google Scholar] [CrossRef] [Green Version]
- Papadopoulos, G.; Mavroeidis, A.; Roussis, I.; Kakabouki, I.; Stavropoulos, P.; Bilalis, D. Evaluation of tillage & fertilization in Carthamus tinctorius L. using remote sensing. Smart Agric. Technol. 2023, 4, 100158. [Google Scholar] [CrossRef]
- Joshi, S.; Thoday-Kennedy, E.; Daetwyler, H.D.; Hayden, M.; Spangenberg, G.; Kant, S. High-throughput phenotyping to dissect genotypic differences in safflower for drought tolerance. PLoS ONE 2021, 16, e0254908. [Google Scholar] [CrossRef] [PubMed]
- Thoday-Kennedy, E.; Joshi, S.; Daetwyler, H.D.; Hayden, M.; Hudson, D.; Spangenberg, G.; Kant, S. Digital phenotyping to delineate salinity response in safflower genotypes. Front. Plant Sci. 2021, 12, 662498. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, B.P.; Spangenberg, G.; Kant, S. Fusion of spectral and structural information from aerial images for improved biomass estimation. Remote Sens. 2020, 12, 3164. [Google Scholar] [CrossRef]
- Rudolphi, S.; Becker, H.C.; Schierholt, A.; von Witzke-Ehbrecht, S. Improved estimation of oil, linoleic and oleic acid and seed hull fractions in Safflower by NIRS. J. Am. Oil Chem. Soc. 2012, 89, 363–369. [Google Scholar] [CrossRef] [Green Version]
- Arshad, A.; Qamar, H.; Siti-Sundari, R.; Zhang, Y.; Zubair, M.; Raza, M.A.; Habib-ur-Rehman, M.; Zhang, L. Phenotypic plasticity of spineless safflower (Carthamus tinctorius L.) cultivars in response to exogenous application of salicylic acid under rainfed climate conditions. Pak. J. Agric. Res. 2020, 33, 729–743. [Google Scholar] [CrossRef]
- Moghaddam, M.J.; Torbaghan, M.E.; Mirzaee, A. Analysis of genotype × environment interaction for seed yield in spineless safflower (Carthamus tinctorius L.) genotypes. Crop Breed. J. 2014, 4, 47–56. [Google Scholar] [CrossRef]
- Bhattarai, B.; Singh, S.; Angadi, S.V.; Begna, S.; Saini, R.; Auld, D. Spring safflower water use patterns in response to preseason and in-season irrigation applications. Agric. Water Manag. 2020, 228, 105876. [Google Scholar] [CrossRef]
- Merrill, S.; Tanaka, D.; Krupinsky, J.; Ries, R. Safflower root growth and water use in comparison with other crops. In Proceedings of the 5th International Safflower Conference, Williston, North Dakota, Sidney, MT, USA, 23–27 July 2001; Safflower: A multipurpose species with unexploited potential and world adaptability. pp. 227–231. [Google Scholar]
- Krupinsky, J.; Tanaka, D.; Merrill, S.; Liebig, M.; Hanson, J. Crop sequence effects of 10 crops in the northern Great Plains. Agric. Syst. 2006, 88, 227–254. [Google Scholar] [CrossRef]
- Tanaka, D.; Krupinsky, J.; Merrill, S.; Ries, R. Safflower production as influenced by previous crops. In Proceedings of the 5th International Safflower Conference, Williston, North Dakota, Sidney, MT, USA, 23–27 July 2001; pp. 259–262. [Google Scholar]
- Bassil, E.; Kaffka, S.; Hutmacher, R. Response of safflower (Carthamus tinctorius L.) to residual soil N following cotton (Gossypium spp.) in rotation in the San Joaquin Valley of California. J. Agric. Sci. 2002, 138, 395–402. [Google Scholar] [CrossRef]
- Wachsmann, N.; Knights, S.; Norton, R. Phenotypic variation of a selection of safflower (Carthamus tinctorius L.) lines and their potential in southern Australia. In Proceedings of the 5th International Safflower Conference, Williston, North Dakota, Sidney, MT, USA, 23–27 July 2001; Safflower: A multipurpose species with unexploited potential and world adaptability. 2001; pp. 263–267. [Google Scholar]
- Sadighfard, S.; Eslam, B.P.; Amirnia, R.; Zanghani, E. Evaluation of photosynthetic roles of different canopy strata and capitulum on seed yield and its components of spring Safflower (Carthamus tinctorius L.). Tirkish JAF Sci. Technol. 2022, 10, 1382–1389. [Google Scholar] [CrossRef]
- Esendal, A.; Istanbulluoglu, B.; Arslan, B.; Paşa, C. Effect of water stress on growth components of winter safflower (Carthamus tinctorius L.). In Proceedings of the 7th International safflower conference, Wagga Wagga, Australia, 3–6 November 2008; pp. 1–3. [Google Scholar]
- Berzsenyi, Z.; Győrffy, B.; Lap, D. Effect of crop rotation and fertilisation on maize and wheat yields and yield stability in a long-term experiment. Eur. J. Agron. 2000, 13, 225–244. [Google Scholar] [CrossRef]
- Armstrong, R.; Perris, R.; Munn, M.; Dunsford, K.; Robertson, F.; Hollaway, G.; O’Leary, G. Effects of long-term rotation and tillage practice on grain yield and protein of wheat and soil fertility on a Vertosol in a medium-rainfall temperate environment. Crop Pasture Sci. 2019, 70, 1–15. [Google Scholar] [CrossRef]
- Çamaş, N.; Çirak, C.; Esendal, E. Seed yield, oil content and fatty acids composition of safflower (Carthamus tinctorius L.) grown in northern Turkey conditions. J. Fac. Agric. OMU 2007, 22, 98–104. [Google Scholar] [CrossRef]
- Wachsmann, N.; Jochinke, D.; Potter, T.; Norton, R. Growing safflower in Australia: Part 2—Agronomic research and suggestions to increase yields and production. In Proceedings of the 7th International Safflower Conference, Wagga Wagga, Australia, 3–6 November 2008. [Google Scholar]
- Koutroubas, S.D.; Papakosta, D.K.; Doitsinis, A. Cultivar and seasonal effects on the contribution of pre-anthesis assimilates to safflower yield. Field Crop. Res. 2004, 90, 263–274. [Google Scholar] [CrossRef]
- Singh, S.; Nimbkar, N. Safflower (Carthamus tinctorius L.). In Genetic Resources, Chromosome Engineering, and Crop Improvement: Oil Crops; Singh, R.J., Ed.; CRC Press: New York, NY, USA, 2007; Volume 4, pp. 168–194. [Google Scholar]
- Saxena, M.; Singh, J.; Deshpande, S.; Choudhari, R. Two decades of safflower in Madhya Pradesh from 1984–2004. In Proceedings of the 7th International Safflower Conference, Wagga Wagga, Australia, 3–6 November 2008. [Google Scholar]
- Knights, S. Raising the Bar with Better Safflower Agronomy; Knights, S., Wachsmann, N., McCaffery, D., Cribb, M., Potter, T., Eds.; Grains Research & Development Corporation: Canberra, Australia, 2010. [Google Scholar]
- Tunçtürk, M.; Çiftçi, V. Relationships among traits using correlation and path coefficient analysis in safflower (Carthamus tinctorius L.) sown different fertilization levels and row spacing. Asian J. Plant Sci. 2004, 3, 683–686. [Google Scholar] [CrossRef]
- Malve, A.; Rathod, A.; Shinde, S.; Ghuge, S. Stability analysis in genotypes of safflower (Carthamus tinctorius L.). J. Pharma Innov. 2022, 11, 2246–2250. [Google Scholar]
- Thalji, T. Effect of planting date on yield performance and some agronomical traits in safflower (Carthamus Tincotorius L.) under rainfed and irrigation conditions in Jordan. Adv. Environ. Biol. 2015, 9, 367–370. [Google Scholar]
- Hassan, M.A.; Yang, M.; Rasheed, A.; Yang, G.; Reynolds, M.; Xia, X.; Xiao, Y.; He, Z. A rapid monitoring of NDVI across the wheat growth cycle for grain yield prediction using a multi-spectral UAV platform. Plant Sci. 2019, 282, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Cen, H.; Zhu, J.; Zhang, J.; Zhu, Y.; Sun, D.; Du, X.; Zhai, L.; Weng, H.; Li, Y. Grain yield prediction of rice using multi-temporal UAV-based RGB and multispectral images and model transfer—A case study of small farmlands in the South of China. Agric. For. Meterol. 2020, 291, 108096. [Google Scholar] [CrossRef]
- Zhao, H.; Pandey, B.R.; Khansefid, M.; Khahrood, H.V.; Sudheesh, S.; Joshi, S.; Kant, S.; Kaur, S.; Rosewarne, G.M. Combining NDVI and bacterial blight score to predict grain yield in field pea. Front. Plant Sci. 2022, 13, 923381. [Google Scholar] [CrossRef]
- Tefera, A.T.; Banerjee, B.P.; Pandey, B.R.; James, L.; Puri, R.R.; Cooray, O.; Marsh, J.; Richards, M.; Kant, S.; Fitzgerald, G.J.; et al. Estimating early season growth and biomass of field pea for selection of divergent ideotypes using proximal sensing. Field Crop. Res. 2022, 277, 108407. [Google Scholar] [CrossRef]
- Koh, J.C.O.; Spangenberg, G.; Kant, S. Automated machine learning for high-throughput image-based plant phenotyping. Remote Sens. 2021, 13, 858. [Google Scholar] [CrossRef]
- Gallmann, J.; Schüpbach, B.; Jacot, K.; Albrecht, M.; Winizki, J.; Kirchgessner, N.; Aasen, H. Flower mapping in grasslands with drones and deep learning. Front. Plant Sci. 2022, 12, e774965. [Google Scholar] [CrossRef]
- Wan, L.; Li, Y.; Cen, H.; Zhu, J.; Yin, W.; Wu, W.; Zhu, H.; Sun, D.; Zhou, W.; He, Y. Combining UAV-based vegetation indices and image classification to estimate flower number in oilseed rape. Remote Sens. 2018, 10, 1484. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Vail, S.; Duddu, H.S.; Parkin, I.A.; Guo, X.; Johnson, E.N.; Shirtliffe, S.J. Phenotyping flowering in Canola (Brassica napus L.) and estimating seed yield using an unmanned aerial vehicle-based imagery. Front. Plant Sci. 2021, 12, 686332. [Google Scholar] [CrossRef]
- Ichiura, S.; Yoshihiro, H.; Sato, K.; Onodera, R.; Katahira, M. Safflower production management ECOSYSTEM with AI harvester. In Proceedings of the 2020 ASABE Annual International Virtual Meeting, St. Joseph, MI, USA, 13–15 July 2020; p. 1. [Google Scholar]
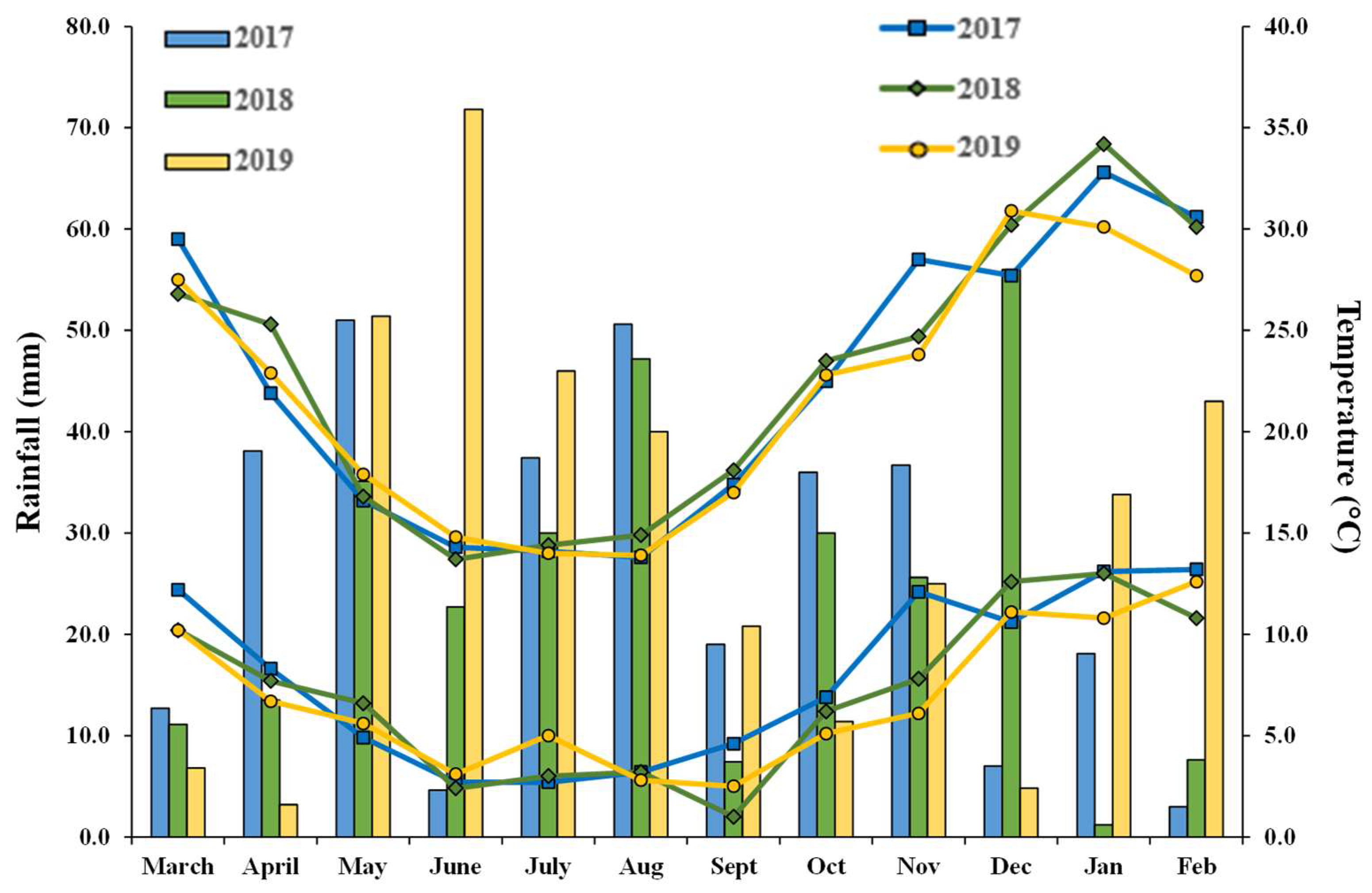
| Temperature (°C) | ||||
| Minimum | Average | Maximum | ||
| 2017 | ||||
| Mean | 11.8 | 21.2 | 29.7 | |
| Range | 0.2–22.1 | 10.0–32.4 | 18.8–44.4 | |
| 2018 | ||||
| Mean | 11.6 | 21.2 | 30.6 | |
| Range | 2.8–24.1 | 11.3–33.5 | 16.4–43.7 | |
| 2019 | ||||
| Mean | 10.0 | 20.1 | 29.7 | |
| Range | 1.8–23.0 | 11.3–32.4 | 16.3–46.7 | |
| Rainfall (mm) | ||||
| Pre-Sowing | Growth Period | |||
| 2017 | 144.5 | 203.5 | ||
| 2018 | 102.5 | 198.4 | ||
| 2019 | 113.2 | 202.0 | ||
| Genotype | DTFS | DTFE | DTM | FP | Plant Height (cm) | Seed Yield (g) | Capitulum Morphology | |
|---|---|---|---|---|---|---|---|---|
| 2017 | AVS-SAFF-6 | 159 | 179 | 206 | 20 | 136.67 | 2430.43 | Spined |
| AVS-SAFF-11 | 161 | 182 | 208 | 21 | 123.93 | 2107.49 | Spineless | |
| AVS-SAFF-53 | 157 | 177 | 204 | 20 | 124.47 | 1987.62 | Spined | |
| AVS-SAFF-116 | 162 | 181 | 208 | 19 | 118.67 | 1952.60 | Spined | |
| AVS-SAFF-117 | 158 | 181 | 207 | 22 | 149.87 | 1980.21 | Spineless | |
| AVS-SAFF-118 | 160 | 180 | 207 | 20 | 131.47 | 1947.35 | Spined | |
| AVS-SAFF-142 | 157 | 175 | 205 | 18 | 133.00 | 1967.18 | Spineless | |
| AVS-SAFF-229 | 157 | 176 | 204 | 18 | 135.40 | 2017.80 | Spined | |
| AVS-SAFF-232 | 157 | 175 | 203 | 19 | 146.33 | 2209.51 | Spineless | |
| AVS-SAFF-263 | 162 | 180 | 207 | 18 | 121.40 | 2144.79 | Spined | |
| AVS-SAFF-265 | 155 | 175 | 204 | 20 | 123.53 | 1934.13 | Spineless | |
| AVS-SAFF-269 | 161 | 178 | 204 | 17 | 111.73 | 2476.32 | Spined | |
| AVS-SAFF-299 | 161 | 180 | 205 | 19 | 138.33 | 2389.06 | Spineless | |
| AVS-SAFF-312 | 161 | 177 | 206 | 16 | 124.33 | 2012.51 | Spined | |
| AVS-SAFF-321 | 160 | 181 | 208 | 21 | 128.13 | 2028.79 | Spined | |
| AVS-SAFF-325 | 161 | 181 | 206 | 20 | 132.40 | 2055.95 | Spined | |
| AVS-SAFF-335 | 160 | 180 | 206 | 20 | 118.20 | 2046.02 | Spined | |
| AVS-SAFF-357 | 161 | 182 | 208 | 22 | 135.67 | 1933.29 | Spined | |
| AVS-SAFF-364 | 159 | 180 | 206 | 20 | 128.00 | 1990.00 | Spined | |
| AVS-SAFF-365 | 168 | 185 | 208 | 17 | 144.27 | 1979.89 | Spineless | |
| 2018 | AVS-SAFF-33 | 156 | 182 | 201 | 26 | 66.50 | 1048.57 | Spineless |
| AVS-SAFF-50 | 158 | 181 | 200 | 24 | 69.50 | 1151.53 | Spined | |
| AVS-SAFF-73 | 156 | 182 | 200 | 26 | 65.00 | 1065.76 | Spined | |
| AVS-SAFF-118 | 161 | 185 | 204 | 24 | 67.00 | 1044.44 | Spined | |
| AVS-SAFF-129 | 161 | 185 | 203 | 24 | 75.50 | 1151.74 | Spineless | |
| AVS-SAFF-144 | 165 | 187 | 209 | 23 | 74.50 | 1100.34 | Spineless | |
| AVS-SAFF-150 | 160 | 184 | 202 | 24 | 72.50 | 1204.25 | Spineless | |
| AVS-SAFF-157 | 162 | 191 | 223 | 30 | 57.50 | 1078.83 | Spined | |
| AVS-SAFF-162 | 159 | 183 | 206 | 24 | 65.50 | 1096.71 | Spined | |
| AVS-SAFF-218 | 151 | 180 | 199 | 29 | 56.50 | 1063.27 | Spined | |
| AVS-SAFF-230 | 159 | 184 | 201 | 25 | 68.50 | 1038.84 | Spined | |
| AVS-SAFF-238 | 166 | 194 | 215 | 29 | 74.00 | 1062.23 | Spineless | |
| AVS-SAFF-265 | 158 | 183 | 198 | 25 | 77.50 | 1041.90 | Spineless | |
| AVS-SAFF-295 | 156 | 181 | 199 | 25 | 57.50 | 1090.99 | Spined | |
| AVS-SAFF-296 | 161 | 185 | 204 | 24 | 82.50 | 1312.66 | Spineless | |
| AVS-SAFF-298 | 160 | 186 | 203 | 26 | 79.50 | 1073.10 | Spined | |
| AVS-SAFF-335 | 159 | 185 | 203 | 26 | 62.00 | 1090.40 | Spined | |
| AVS-SAFF-350 | 162 | 194 | 216 | 33 | 71.00 | 1055.54 | Spined | |
| AVS-SAFF-365 | 171 | 196 | 215 | 25 | 78.50 | 1145.36 | Spineless | |
| AVS-SAFF-368 | 161 | 186 | 205 | 26 | 81.50 | 1050.44 | Spined |
| DTFS | DTFE | DTM | FP | Height | Yield | NDVI Slope | NDVI y–int | |
|---|---|---|---|---|---|---|---|---|
| DTFS | ||||||||
| DTFE | 0.843 *** | |||||||
| DTM | 0.839 *** | 0.830 *** | ||||||
| FP | –0.558 *** | –0.023 | –0.280 *** | |||||
| Height | 0.800 *** | 0.617 *** | 0.736 *** | –0.535 *** | ||||
| Yield | –0.197 *** | –0.049 | –0.083 * | 0.290 *** | –0.087 * | |||
| NDVI Slope | –0.310 *** | –0.311 *** | –0.254 *** | 0.100 ** | –0.358 *** | –0.318 *** | ||
| NDVI y–int | 0.308 *** | 0.357 *** | 0.263 *** | –0.026 | 0.281 *** | 0.356 *** | –0.944 *** |
| Seed Yield (g) | Oil Characteristics | |||||||
|---|---|---|---|---|---|---|---|---|
| n | Oil Content (%) | Oleic (%) | Linoleic (%) | |||||
| Mean | Range | Mean | Range | Mean | Range | |||
| 2017 | ||||||||
| High linoleic | 1271.73 | 331 | 30.43 | 24.47–41.07 | 12.88 | 7.57 – 21.75 | 77.73 | 68.20–83.10 |
| Intermediate oleic | 886.20 | 11 | 30.69 | 24.10–37.13 | 38.05 | 26.33–51.75 | 52.22 | 39.65–64.20 |
| High oleic | 1036.84 | 8 | 37.41 | 35.30–40.67 | 67.00 | 59.93–74.50 | 24.13 | 17.00–32.67 |
| 2018 | ||||||||
| High linoleic | 672.33 | 331 | 29.44 | 23.10–37.60 | 14.71 | 11.00–23.30 | 75.16 | 67.40–78.85 |
| Intermediate oleic | 526.88 | 11 | 30.13 | 24.15–34.15 | 41.30 | 30.00–54.85 | 49.15 | 35.55–60.85 |
| High oleic | 457.71 | 8 | 35.38 | 34.60–36.55 | 59.98 | 56.65–70.20 | 30.94 | 20.60–34.20 |
| 2019 | ||||||||
| High linoleic | 564.92 | 331 | 27.2 | 20.60–30.75 | 16.1 | 10.90–23.60 | 73.0 | 64.60–78.65 |
| Intermediate oleic | 564.13 | 13 | 28.77 | 23.05–33.70 | 40.41 | 26.75–54.25 | 49.07 | 35.50–60.80 |
| High oleic | 435.73 | 6 | 31.14 | 29.65–33.50 | 59.19 | 55.60–64.80 | 30.84 | 25.25–34.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thoday-Kennedy, E.; Banerjee, B.; Panozzo, J.; Maharjan, P.; Hudson, D.; Spangenberg, G.; Hayden, M.; Kant, S. Dissecting Physiological and Agronomic Diversity in Safflower Populations Using Proximal Phenotyping. Agriculture 2023, 13, 620. https://doi.org/10.3390/agriculture13030620
Thoday-Kennedy E, Banerjee B, Panozzo J, Maharjan P, Hudson D, Spangenberg G, Hayden M, Kant S. Dissecting Physiological and Agronomic Diversity in Safflower Populations Using Proximal Phenotyping. Agriculture. 2023; 13(3):620. https://doi.org/10.3390/agriculture13030620
Chicago/Turabian StyleThoday-Kennedy, Emily, Bikram Banerjee, Joe Panozzo, Pankaj Maharjan, David Hudson, German Spangenberg, Matthew Hayden, and Surya Kant. 2023. "Dissecting Physiological and Agronomic Diversity in Safflower Populations Using Proximal Phenotyping" Agriculture 13, no. 3: 620. https://doi.org/10.3390/agriculture13030620







