Legacy Effect of Long-Term Elevated CO2 and Warming on Soil Properties Controls Soil Organic Matter Decomposition
Highlights
- Litter quality change does not affect SOM decomposition under elevated CO2 and warming.
- The legacy effect of elevated CO2 and warming on soil properties controls SOM decomposition.
- Elevated CO2 may promote SOC sequestration by suppressing SOM decomposition.
Abstract
1. Introduction
2. Materials and Methods
2.1. Soils and Plants Litter
2.2. Experimental Design
2.3. Plant and Soil Sample Analysis
2.4. Statistical Analysis
3. Results
3.1. Changes in Litter Quality under Elevated CO2 and Warming
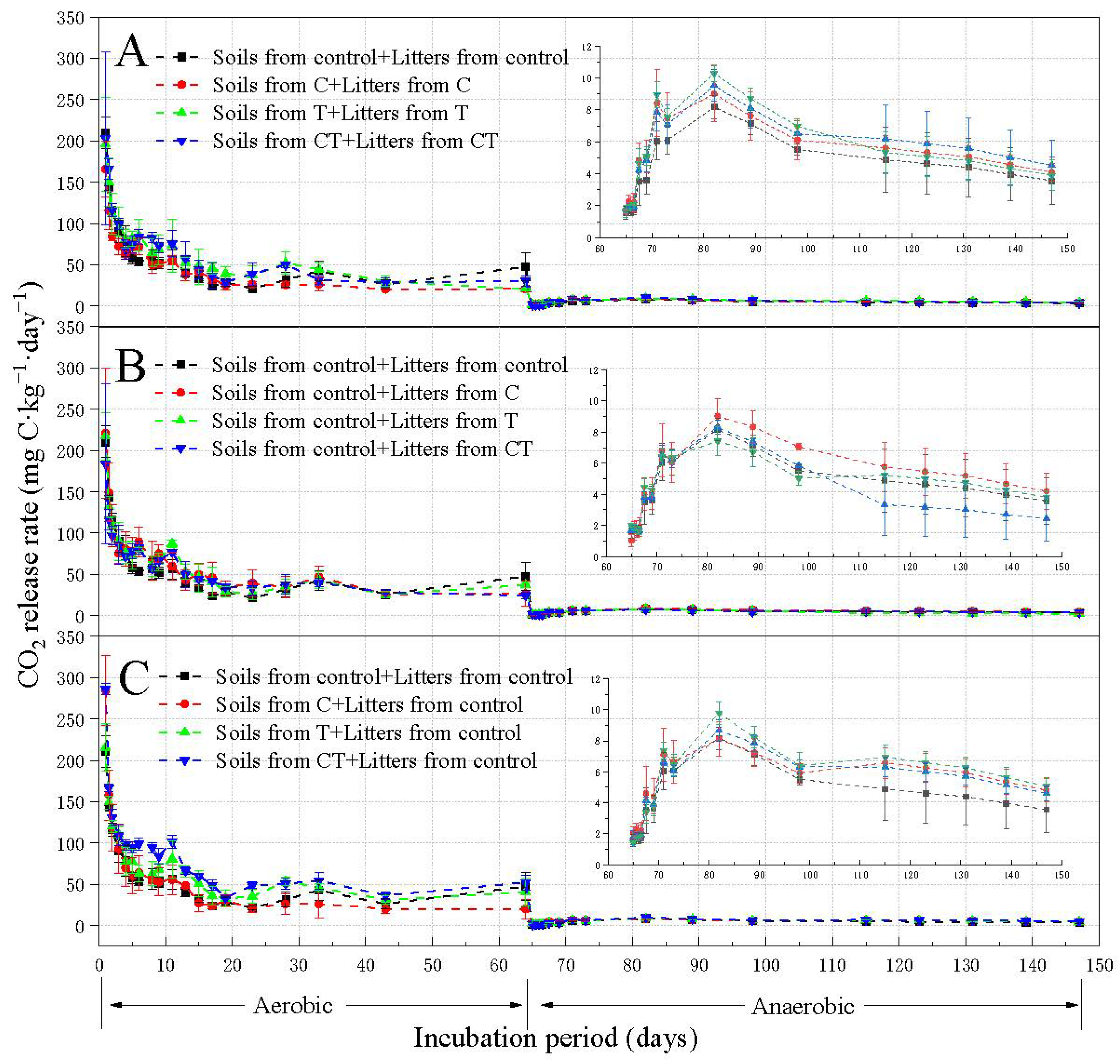
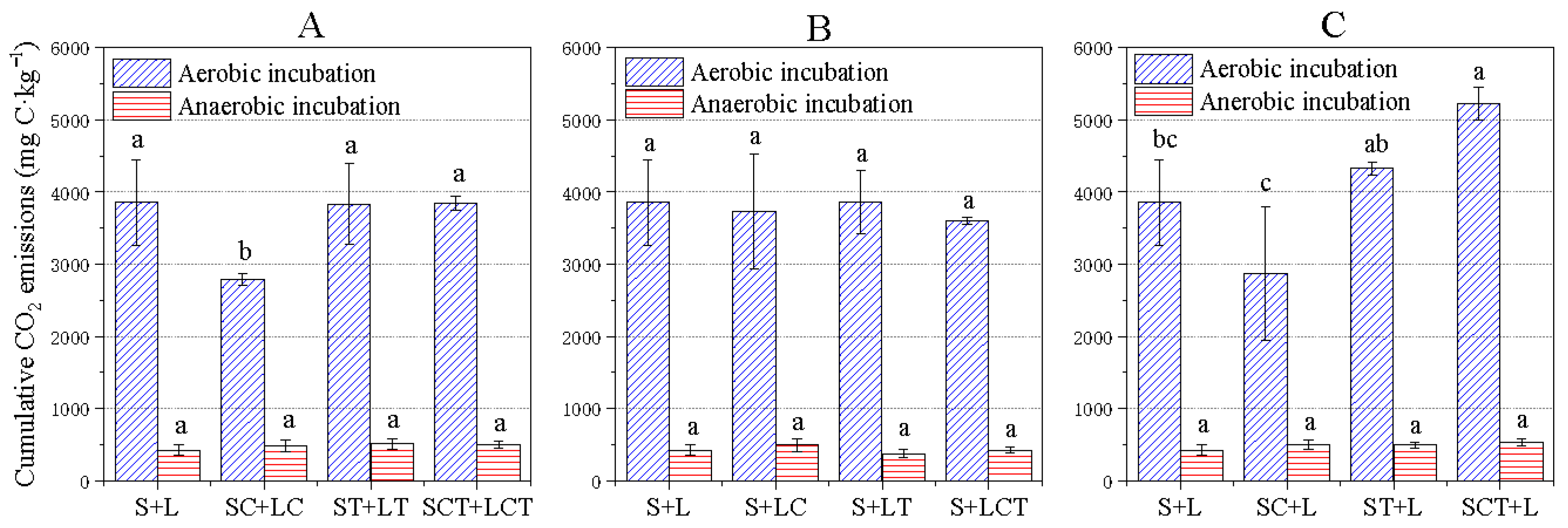
3.2. The Effect of Elevated CO2 and Warming on Soil Respiration (Experiment I)
3.3. The Effect of Litter Quality Change on Soil Respiration (Experiment II)
3.4. The Effect of Soil Property Change on Soil Respiration (Experiment III)
3.5. Correlation between Soil Respiration and Soil Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Climate Change 2014: Synthesis Repor; Pachauri, R.K., Mayer, L., Intergovernmental Panel on Climate Change, Eds.; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2015; ISBN 978-92-9169-143-2. [Google Scholar]
- Prentice, I.C.; Farquhar, G.D.; Fasham, M.J.R.; Goulden, M.L.; Heimann, M.; Jaramillo, V.J.; Kheshgi, H.S.; Le Quéré, C.; Scholes, R.J.; Wallace, D.W.R. The Carbon Cycle and Atmospheric Carbon Dioxide. In Climate Change 2001: The Scientific Basis; Cambridge University Press: Cambridge, UK, 2001; pp. 183–237. [Google Scholar]
- Climate Change 2007: The Physical Science Basis; Intergovernmental Panel on Climate Change, Ed.; Cambridge University Press: Cambridge, UK, 2007; ISBN 978-0-521-88009-1. [Google Scholar]
- Terrer, C.; Phillips, R.P.; Hungate, B.A.; Rosende, J.; Pett-Ridge, J.; Craig, M.E.; van Groenigen, K.J.; Keenan, T.F.; Sulman, B.N.; Stocker, B.D.; et al. A Trade-off between Plant and Soil Carbon Storage under Elevated CO2. Nature 2021, 591, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Hyvönen, R.; Ågren, G.I.; Linder, S.; Persson, T.; Cotrufo, M.F.; Ekblad, A.; Freeman, M.; Grelle, A.; Janssens, I.A.; Jarvis, P.G.; et al. The Likely Impact of Elevated CO2, Nitrogen Deposition, Increased Temperature and Management on Carbon Sequestration in Temperate and Boreal Forest Ecosystems: A Literature Review: Tansley Review. New Phytol. 2007, 173, 463–480. [Google Scholar] [CrossRef] [PubMed]
- Jastrow, J.D.; Michael Miller, R.; Matamala, R.; Norby, R.J.; Boutton, T.W.; Rice, C.W.; Owensby, C.E. Elevated Atmospheric Carbon Dioxide Increases Soil Carbon. Glob. Chang. Biol. 2005, 11, 2057–2064. [Google Scholar] [CrossRef]
- Liu, S.; Ji, C.; Wang, C.; Chen, J.; Jin, Y.; Zou, Z.; Li, S.; Niu, S.; Zou, J. Climatic role of terrestrial ecosystem under elevated CO2: A bottom-up greenhouse gases budget. Ecol. Lett. 2018, 21, 1108–1118. [Google Scholar] [CrossRef]
- Luo, Y.; Hui, D.; Zhang, D. Elevated CO2 stimulates net accumulations of carbon and nitrogen in land ecosystems: A meta-analysis. Ecology 2006, 87, 53–63. [Google Scholar] [CrossRef]
- Koyama, A.; Harlow, B.; Kuske, C.R.; Belnap, J.; Evans, R.D. Plant and microbial biomarkers suggest mechanisms of soil organic carbon accumulation in a mojave desert ecosystem under elevated CO2. Soil Biol. Biochem. 2018, 120, 48–57. [Google Scholar] [CrossRef]
- Keidel, L.; Lenhart, K.; Moser, G.; Müller, C. Depth-dependent response of soil aggregates and soil organic carbon content to long-term elevated CO2 in a temperate grassland soil. Soil Biol. Biochem. 2018, 123, 145–154. [Google Scholar] [CrossRef]
- Van Kessel, C.; Nitschelm, J.; Horwath, W.R.; Harris, D.; Walley, F.; Lüscher, A.; Hartwig, U. Carbon-13 input and turn-over in a pasture soil exposed to long-term elevated atmospheric CO2: Pasture soil C-cycling. Glob. Chang. Biol. 2000, 6, 123–135. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Horwath, W.R.; Dorodnikov, M.; Blagodatskaya, E. Review and synthesis of the effects of elevated atmospheric CO2 on soil processes: No changes in pools, but Increased Fluxes and Accelerated Cycles. Soil Biol. Biochem. 2019, 128, 66–78. [Google Scholar] [CrossRef]
- Chen, J.; Elsgaard, L.; Groenigen, K.J.; Olesen, J.E.; Liang, Z.; Jiang, Y.; Lærke, P.E.; Zhang, Y.; Luo, Y.; Hungate, B.A.; et al. Soil carbon loss with warming: New evidence from carbon-degrading enzymes. Glob. Chang. Biol. 2020, 26, 1944–1952. [Google Scholar] [CrossRef]
- Gao, W.; Yan, D. Warming suppresses microbial biomass but enhances N recycling. Soil Biol. Biochem. 2019, 131, 111–118. [Google Scholar] [CrossRef]
- Lu, M.; Zhou, X.; Yang, Q.; Li, H.; Luo, Y.; Fang, C.; Chen, J.; Yang, X.; Li, B. Responses of ecosystem carbon cycle to experimental warming: A meta-analysis. Ecology 2013, 94, 726–738. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yuan, W. Responses of microbial biomass carbon and nitrogen to experimental warming: A meta-analysis. Soil Biol. Biochem. 2017, 115, 265–274. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Shen, Z.X.; Fu, G. A meta-analysis of the effects of experimental warming on soil carbon and nitrogen dynamics on the Tibetan Plateau. Appl. Soil Ecol. 2015, 87, 32–38. [Google Scholar] [CrossRef]
- van de Geijn, S.C.; Veen, J.A. Implications of increased carbon dioxide levels for carbon input and turnover in soils. Vegetatio 1993, 104/105, 282–292. [Google Scholar] [CrossRef]
- Long, S.P.; Ainsworth, E.A.; Rogers, A.; Ort, D.R. Rising atmospheric carbon dioxide: Plants FACE the future. Annu. Rev. Plant Biol. 2004, 55, 591–628. [Google Scholar] [CrossRef]
- Sardans, J. The C:N:P stoichiometry of organisms and ecosystems in a changing world: A review and perspectives. Perspect. Plant Ecol. Evol. Syst. 2012, 14, 33–47. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Zhang, X.; Li, L.; Lam, S.K.; Pan, G. Changes in plant C, N and P ratios under elevated CO2 and canopy warming in a rice-winter wheat rotation system. Sci. Rep. 2019, 9, 5424. [Google Scholar] [CrossRef]
- Wang, J.; Li, L.; Lam, S.K.; Liu, X.; Pan, G. Responses of wheat and rice grain mineral quality to elevated carbon dioxide and canopy warming. Field Crops Res. 2020, 249, 107753. [Google Scholar] [CrossRef]
- He, X.; Wu, Y.; Cai, M.; Mu, C.; Luo, W.; Cheng, Y.; Zhu, W. The Effect of increased atmospheric temperature and CO2 concentration during crop growth on the chemical composition and in vitro rumen fermentation characteristics of wheat straw. J. Animal Sci. Biotechnol. 2015, 6, 46. [Google Scholar] [CrossRef]
- Elias, D.M.O.; Robinson, S.; Both, S.; Goodall, T.; Majalap-Lee, N.; Ostle, N.J.; McNamara, N.P. Soil microbial community and litter quality controls on decomposition across a tropical forest disturbance gradient. Front. For. Glob. Chang. 2020, 3, 81. [Google Scholar] [CrossRef]
- Fanin, N.; Hättenschwiler, S.; Barantal, S.; Schimann, H.; Fromin, N. Does variability in litter quality determine soil microbial respiration in an amazonian rainforest? Soil Biol. Biochem. 2011, 43, 1014–1022. [Google Scholar] [CrossRef]
- Butterly, C.R.; Phillips, L.A.; Wiltshire, J.L.; Franks, A.E.; Armstrong, R.D.; Chen, D.; Mele, P.M.; Tang, C. Long-term effects of elevated CO2 on carbon and nitrogen functional capacity of microbial communities in three contrasting soils. Soil Biol. Biochem. 2016, 97, 157–167. [Google Scholar] [CrossRef]
- He, Z.; Xiong, J.; Kent, A.D.; Deng, Y.; Xue, K.; Wang, G.; Wu, L.; Van Nostrand, J.D.; Zhou, J. Distinct responses of soil microbial communities to elevated CO2 and O3 in a soybean agro-ecosystem. ISME J. 2014, 8, 714–726. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, C.; Yang, J.; Liao, J.; Chen, H.Y.H.; Ruan, H. Elevated CO2 shifts soil microbial communities from K- to r-Strategists. Glob. Ecol. Biogeogr. 2021, 30, 961–972. [Google Scholar] [CrossRef]
- Carney, K.M.; Hungate, B.A.; Drake, B.G.; Megonigal, J.P. Altered soil microbial community at elevated CO2 leads to loss of soil carbon. Proc. Natl. Acad. Sci. USA 2007, 104, 4990–4995. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Zak, D.R.; Reich, P.B.; Ellsworth, D.S. Plant Species Richness, Elevated CO2, and atmospheric nitrogen deposition alter soil microbial community composition and function. Glob. Chang. Biol. 2007, 13, 980–989. [Google Scholar] [CrossRef]
- He, Z.; Xu, M.; Deng, Y.; Kang, S.; Kellogg, L.; Wu, L.; Van Nostrand, J.D.; Hobbie, S.E.; Reich, P.B.; Zhou, J. Metagenomic analysis reveals a marked divergence in the structure of belowground microbial communities at elevated CO2: Changes in the soil microbial community at elevated CO2. Ecol. Lett. 2010, 13, 564–575. [Google Scholar]
- Jin, J.; Wood, J.; Franks, A.; Armstrong, R.; Tang, C. Long-term CO2 enrichment alters the diversity and function of the microbial community in soils with high organic carbon. Soil Biol. Biochem. 2020, 144, 107780. [Google Scholar] [CrossRef]
- Lipson, D.A.; Wilson, R.F.; Oechel, W.C. Effects of elevated atmospheric CO2 on soil microbial biomass, activity, and diversity in a chaparral ecosystem. Appl. Environ. Microbiol. 2005, 71, 8573–8580. [Google Scholar] [CrossRef]
- Yang, S.; Zheng, Q.; Yuan, M.; Shi, Z.; Chiariello, N.R.; Docherty, K.M.; Dong, S.; Field, C.B.; Gu, Y.; Gutknecht, J.; et al. Long-term elevated CO2 shifts composition of soil microbial communities in a californian annual grassland, reducing growth and N utilization potentials. Sci. Total Environ. 2019, 652, 1474–1481. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Deng, Y.; He, Z.; Pendall, E.; Carrillo, Y.; Wang, S.; Jin, D.; Wu, L.; Wang, A.; Xu, Y.; et al. Stimulation of soil microbial functioning by elevated CO2 may surpass effects mediated by irrigation in a semiarid grassland. Geoderma 2021, 401, 115162. [Google Scholar] [CrossRef]
- Zhou, J.; Deng, Y.; Luo, F.; He, Z.; Yang, Y. Phylogenetic molecular ecological network of soil microbial communities in response to elevated CO2. mBio 2011, 2, e00122-11. [Google Scholar] [CrossRef] [PubMed]
- Drigo, B.; Pijl, A.S.; Duyts, H.; Kielak, A.M.; Gamper, H.A.; Houtekamer, M.J.; Boschker, H.T.S.; Bodelier, P.L.E.; Whiteley, A.S.; Veen, J.A.; et al. Shifting carbon flow from roots into associated microbial communities in response to elevated atmospheric CO2. Proc. Natl. Acad. Sci. USA 2010, 107, 10938–10942. [Google Scholar] [CrossRef] [PubMed]
- Cotton, T.E.A.; Fitter, A.H.; Miller, R.M.; Dumbrell, A.J.; Helgason, T. Fungi in the future: Interannual variation and effects of atmospheric change on arbuscular mycorrhizal fungal communities. New Phytol. 2015, 205, 1598–1607. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, N.; Yuan, M.; Xiao, J.; Qin, Y.; Deng, Y.; Tu, Q.; Xue, K.; Van Nostrand, J.D.; Wu, L.; et al. Warming enhances old organic carbon decomposition through altering functional microbial communities. ISME J. 2017, 11, 1825–1835. [Google Scholar] [CrossRef]
- Dai, Z.; Yu, M.; Chen, H.; Zhao, H.; Huang, Y.; Su, W.; Xia, F.; Chang, S.X.; Brookes, P.C.; Dahlgren, R.A.; et al. Elevated temperature shifts soil N cycling from microbial immobilization to enhanced mineralization, nitrification and denitrification across global terrestrial ecosystems. Glob. Chang. Biol. 2020, 26, 5267–5276. [Google Scholar] [CrossRef]
- Allison, S.D.; Treseder, K.K. Warming and Drying Suppress Microbial activity and carbon cycling in boreal forest soils: Warming suppresses microbial activity. Glob. Chang. Biol. 2008, 14, 2898–2909. [Google Scholar] [CrossRef]
- Frey, S.D.; Drijber, R.; Smith, H.; Melillo, J. Microbial biomass, functional capacity, and community structure after 12 years of soil warming. Soil Biol. 2008, 4, 2904–2907. [Google Scholar] [CrossRef]
- Guo, X.; Feng, J.; Shi, Z.; Zhou, X.; Yuan, M.; Tao, X.; Hale, L.; Yuan, T.; Wang, J.; Qin, Y.; et al. Climate warming leads to divergent succession of grassland microbial communities. Nature Clim. Chang. 2018, 8, 813–818. [Google Scholar] [CrossRef]
- Deslippe, J.R.; Hartmann, M.; Simard, S.W.; Mohn, W.W. Long-term warming alters the composition of arctic soil microbial communities. FEMS Microbiol Ecol. 2012, 82, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Sheik, C.S.; Beasley, W.H.; Elshahed, M.S.; Zhou, X.; Luo, Y.; Krumholz, L.R. Effect of warming and drought on grassland microbial communities. ISME J. 2011, 5, 1692–1700. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Deng, Y.; He, Z.; Van Nostrand, J.D.; Wang, S.; Jin, D.; Wang, A.; Wu, L.; Wang, D.; Tai, X.; et al. Elevated CO2 and warming altered grassland microbial communities in soil top-layers. Front. Microbiol. 2018, 9, 1790. [Google Scholar] [CrossRef] [PubMed]
- Lu, R. Analytical Methods for Soil Agricultural Chemistry; China Agricultural Science and Technology Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- FrostegArd, A.; BAAth, E. The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in Soil. Biol. Fertil. Soils 1996, 22, 59–65. [Google Scholar]
- Lieffering, M.; Kim, H.-Y.; Kobayashi, K.; Okada, M. The impact of elevated CO2 on the elemental concentrations of field-grown rice grains. Field Crops Res. 2004, 88, 279–286. [Google Scholar] [CrossRef]
- Hillstrom, M.; Meehan, T.D.; Kelly, K.; Lindroth, R.L. Soil carbon and nitrogen mineralization following deposition of insect frass and greenfall from forests under elevated CO2 and O3. Plant Soil. 2010, 336, 75–85. [Google Scholar] [CrossRef]
- Cornwell, W.K.; Cornelissen, J.H.C.; Amatangelo, K.; Dorrepaal, E.; Eviner, V.T.; Godoy, O.; Hobbie, S.E.; Hoorens, B.; Kurokawa, H.; Pérez-Harguindeguy, N.; et al. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol. Lett. 2008, 11, 1065–1071. [Google Scholar] [CrossRef]
- King, J.S.; Hanson, P.J.; Bernhardt, E.; DeAngelis, P.; Norby, R.J.; Pregitzer, K.S. A multiyear synthesis of soil respiration responses to elevated atmospheric CO2 from four forest FACE experiments: Elevated CO2 increases forest soil respiration. Glob. Chang. Biol. 2004, 10, 1027–1042. [Google Scholar] [CrossRef]
- Bader, M.K.-F.; Körner, C. No overall stimulation of soil respiration under mature deciduous forest trees after 7 years of CO2 enrichment: Forest soil respiration under elevated CO2. Glob. Chang. Biol. 2010, 16, 2830–2843. [Google Scholar] [CrossRef]
- Clark, K.L.; Skowronski, N.; Hom, J. Invasive insects impact forest carbon dynamics: Defoliation and forest carbon dynamics. Glob. Chang. Biol. 2010, 16, 88–101. [Google Scholar] [CrossRef]
- Keidel, L.; Kammann, C.; Grünhage, L.; Moser, G.; Müller, C. Positive feedback of elevated CO2 on soil respiration in late autumn and winter. Biogeosciences 2015, 12, 1257–1269. [Google Scholar] [CrossRef]
- Andrews, J.A.; Schlesinger, W.H. Soil CO2 dynamics, acidification, and chemical weathering in a temperate forest with experimental CO2 enrichment. Glob. Biogeochem. Cycles 2001, 15, 149–162. [Google Scholar] [CrossRef]
- Lagomarsino, A.; Lukac, M.; Godbold, D.L.; Marinari, S.; De Angelis, P. Drivers of increased soil respiration in a poplar coppice exposed to elevated CO2. Plant Soil. 2013, 362, 93–106. [Google Scholar] [CrossRef]
- Bernhardt, E.S.; Barber, J.J.; Pippen, J.S.; Taneva, L.; Andrews, J.A.; Schlesinger, W.H. Long-term effects of free air CO2 enrichment (FACE) on soil respiration. Biogeochemistry 2006, 77, 91–116. [Google Scholar] [CrossRef]
- Bradford, M.A. Thermal adaptation of decomposer communities in warming soils. Front. Microbiol. 2013, 4, 333. [Google Scholar] [CrossRef] [PubMed]

| Soils | Litters | Abbreviation | |
|---|---|---|---|
| Experiment I | Control | Control | S + L |
| C | C | SC + LC | |
| T | T | ST + LT | |
| CT | CT | SCT + LCT | |
| Experiment II | Control | Control | S + L |
| Control | C | S + LC | |
| Control | T | S + LT | |
| Control | CT | S + LCT | |
| Experiment III | Control | Control | S + L |
| C | Control | SC + L | |
| T | Control | ST + L | |
| CT | Control | SCT + L |
| Treatment | Rice Straw | Wheat Straw | ||||
|---|---|---|---|---|---|---|
| N (g·kg−1) | P (g·kg−1) | K (g·kg−1) | N (g·kg−1) | P (g·kg−1) | K (g·kg−1) | |
| Control | 10.59 ± 1.59 a | 1.06 ± 0.18 a | 16.70 ± 2.28 a | 9.28 ± 1.20 a | 1.11 ± 0.30 a | 15.87 ± 0.05 a |
| C | 8.84 ± 0.50 b | 0.90 ± 0.11 a | 14.90 ± 0.31 a | 5.60 ± 0.85 b | 0.67 ± 0.16 a | 11.56 ± 1.65 b |
| T | 11.42 ± 0.17 a | 0.97 ± 0.08 a | 16.69 ± 1.44 a | 6.94 ± 0.78 b | 0.89 ± 0.06 a | 7.47 ± 2.52 c |
| CT | 8.05 ± 0.71 b | 0.66 ± 0.03 b | 16.48 ± 0.54 a | 6.09 ± 0.65 b | 1.09 ± 0.29 a | 7.16 ± 1.98 c |
| Soil Characteristics | Soil Respiration (Experiment I) | Soil Respiration (Experiment III) |
|---|---|---|
| Soil organic carbon | 0.403 | 0.672 * |
| Dissolved organic carbon | 0.259 | 0.586 * |
| Microbial biomass carbon | −0.232 | −0.780 ** |
| Microbial metabolic quotient | 0.831 ** | 0.914 ** |
| Soil pH | 0.175 | −0.284 |
| Soil C/N | 0.676 * | 0.549 |
| Soil available K | 0.413 | 0.674 * |
| Soil available P | −0.601 * | −0.754 ** |
| Total PLFAs | 0.045 | −0.125 |
| Bacterial PLFAs | −0.062 | −0.199 |
| Fungal PLFAs | 0.135 | −0.037 |
| F/B ratio | 0.631 * | 0.429 |
| α-Glucosidase | 0.138 | 0.311 |
| β-Glucosidase | 0.236 | 0.664 * |
| N-acetyl-glucosaminidase | 0.738 ** | 0.426 |
| Cellobiohydrolase | −0.042 | 0.441 |
| β-Xylosidase | −0.163 | −0.016 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Sun, B.; Liu, C.; Drosos, M.; Zhang, X.; Liu, X.; Li, L.; Pan, G. Legacy Effect of Long-Term Elevated CO2 and Warming on Soil Properties Controls Soil Organic Matter Decomposition. Agriculture 2023, 13, 639. https://doi.org/10.3390/agriculture13030639
Li J, Sun B, Liu C, Drosos M, Zhang X, Liu X, Li L, Pan G. Legacy Effect of Long-Term Elevated CO2 and Warming on Soil Properties Controls Soil Organic Matter Decomposition. Agriculture. 2023; 13(3):639. https://doi.org/10.3390/agriculture13030639
Chicago/Turabian StyleLi, Jie, Baobao Sun, Cheng Liu, Marios Drosos, Xuhui Zhang, Xiaoyu Liu, Lianqing Li, and Genxing Pan. 2023. "Legacy Effect of Long-Term Elevated CO2 and Warming on Soil Properties Controls Soil Organic Matter Decomposition" Agriculture 13, no. 3: 639. https://doi.org/10.3390/agriculture13030639
APA StyleLi, J., Sun, B., Liu, C., Drosos, M., Zhang, X., Liu, X., Li, L., & Pan, G. (2023). Legacy Effect of Long-Term Elevated CO2 and Warming on Soil Properties Controls Soil Organic Matter Decomposition. Agriculture, 13(3), 639. https://doi.org/10.3390/agriculture13030639







