Condensed Tannins Attributes: Potential Solution to Fescue Toxicosis?
Abstract
:1. Introduction and Background
1.1. High-Valued Forage
1.2. Issues with Tall Fescue
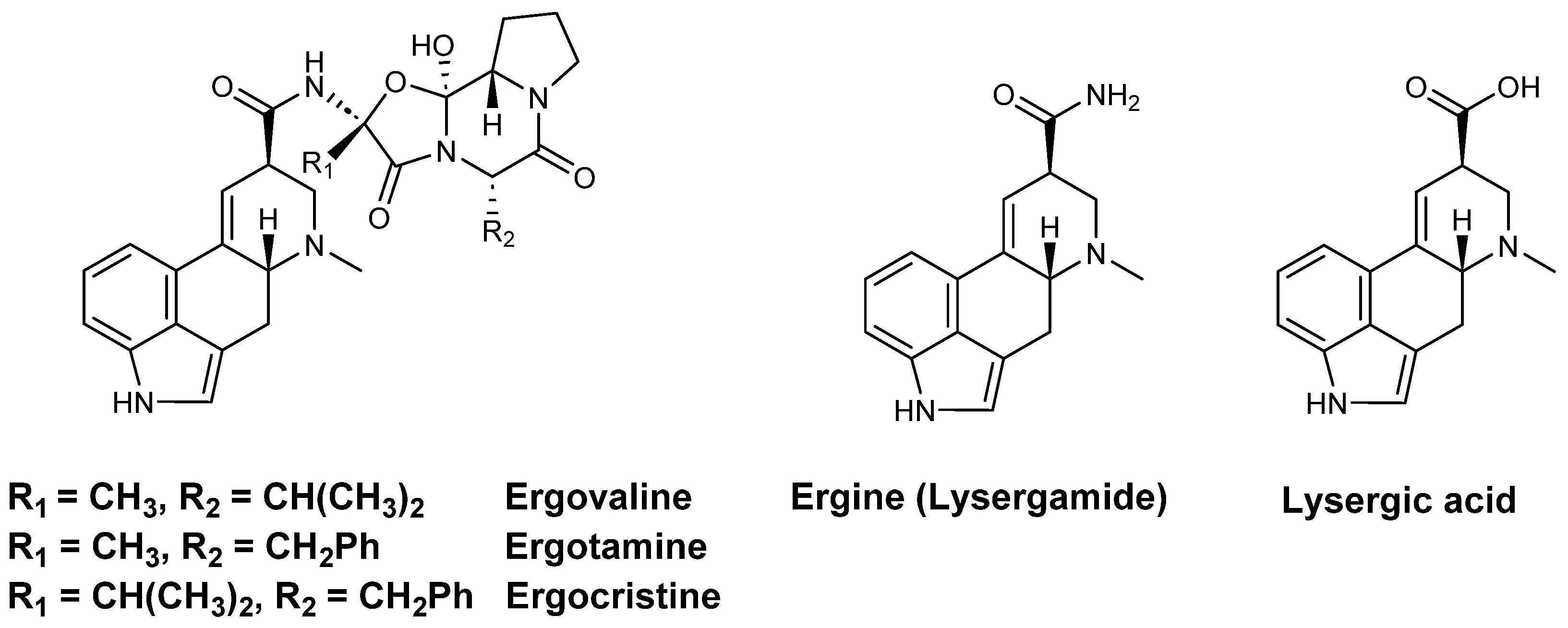
1.3. The Perpetrators
2. Effects of Fescue Toxicosis: Uptake by and Impacts on Ruminants
3. Current Tall Fescue Management Strategies to Mitigate Fescue Toxicosis
4. Tannin Biochemistry
5. Beneficial Effects of Condensed Tannins in Ruminant Production Systems
5.1. Increase in Nitrogen-Use Efficiency
5.2. Mitigation of Methane Production
5.3. Natural Anti-Parasitic Agents
6. Condensed Tannins: A Potential Solution for Fescue Toxicosis?
7. Common Condensed Tannin-Containing Forage Legumes in U.S. Pasture Systems
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, S.; Hall, J.B.; Johnson, G.D.; Peterson, P.R. Making the Most of Tall Fescue in Virginia; Virginia Cooperative Extension: Blacksburg, VA, USA, 2009; pp. 418–450. [Google Scholar]
- Cowan, J.R. Tall fescue. Adv. Agron. 1956, 8, 283–320. [Google Scholar]
- Stuedemann, J.A.; Hoveland, C.S. Fescue endophyte: History and impact on animal agriculture. J. Prod. Agric. 1988, 1, 39–44. [Google Scholar] [CrossRef]
- Hoveland, C.S. Importance and economic significance of the Acremonium endophytes to performance of animals and grass plant. Agric. Ecosyst. Environ. 1993, 44, 3–12. [Google Scholar] [CrossRef]
- Roberts, C.A.; Lacefield, G.D.; Ball, D.; Bates, G. Management to optimize grazing performance in the northern hemisphere. In Tall Fescue for the Tw Enty-First Century; Fribourg, H.A., Hannaway, D.B., West, C.P., Eds.; Wiley: New York, NY, USA, 2009; Volume 53, pp. 85–99. [Google Scholar]
- Williams, M.; Backman, P.; Crawford, M.; Schmidt, S.; King, C., Jr. Chemical control of the tall fescue endophyte and its relationship to cattle performance. N. Z. J. Exp. Agric. 1984, 12, 165–171. [Google Scholar] [CrossRef]
- Duell, R.W. Utilization of Fertilizer by Six Pasture Grasses. Agron. J. 1960, 52, 277–279. [Google Scholar] [CrossRef]
- Rayburn, E.; Blaser, R.; Wolf, D. Winter Tall Fescue Yield and Quality with Different Accumulation Periods and N Rates 1. Agron. J. 1979, 71, 959–963. [Google Scholar] [CrossRef]
- Prigge, E.C.; Bryan, W.B.; Goldman-Innis, E.S. Early-and late-season grazing of orchardgrass and fescue hayfields overseeded with red clover. Agron. J. 1999, 91, 690–696. [Google Scholar] [CrossRef]
- Dierking, R.; Young, C.; Kallenbach, R. Mediterranean and Continental Tall Fescue: I. Effects of Endophyte Status on Leaf Extension, Proline, Mono-and Disaccharides, Fructan, and Freezing Survivability. Crop Sci. 2012, 52, 451–459. [Google Scholar] [CrossRef]
- Taylor, T.; Templeton, W., Jr. Stockpiling Kentucky Bluegrass and Tall Fescue Forage for Winter Pasturage. Agron. J. 1976, 68, 235–239. [Google Scholar] [CrossRef]
- Porter, J. Analysis of endophyte toxins: Fescue and other grasses toxic to livestock. J. Anim. Sci. 1995, 73, 871–880. [Google Scholar] [CrossRef] [Green Version]
- Strickland, J.; Oliver, J.; Cross, D. Fescue toxicosis and its impact on animal agriculture. Vet. Hum. Toxicol. 1993, 35, 454–464. [Google Scholar] [PubMed]
- Bacon, C.W. Toxic endophyte-infected tall fescue and range grasses: Historic perspectives. J. Anim. Sci. 1995, 73, 861–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bush, L.; Boling, J.; Yates, S. Animal disorders. In Agronomy Monograph; Buckner, R.C., Bush, L.P., Eds.; ASA, CSSA, SSSA: Madison, WI, USA, 1979; Volume 20, pp. 247–292. [Google Scholar]
- Strickland, J.; Aiken, G.; Spiers, D.; Fletcher, L.; Oliver, J. Physiological basis of fescue toxicosis. In Tall Fescue for the Twenty-First Century; Fribourg, H., Hannaway, D., West, C., Eds.; Wiley: New York, NY, USA, 2009; Volume 53, pp. 203–227. [Google Scholar]
- Burke, J.; Rorie, R.; Piper, E.; Jackson, W. Reproductive responses to grazing endophyte-infected tall fescue by postpartum beef cows. Theriogenology 2001, 56, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Parish, J.A.; McCann, M.A.; Watson, R.H.; Paiva, N.N.; Hoveland, C.S.; Parks, A.H.; Upchurch, B.L.; Hill, N.S.; Bouton, J.H. Use of nonergot alkaloid-producing endophytes for alleviating tall fescue toxicosis in stocker cattle. J. Anim. Sci. 2003, 81, 2856–2868. [Google Scholar] [CrossRef]
- Nihsen, M.; Piper, E.; West, C.; Crawford, R., Jr.; Denard, T.; Johnson, Z.; Roberts, C.; Spiers, D.; Rosenkrans, C., Jr. Growth rate and physiology of steers grazing tall fescue inoculated with novel endophytes. J. Anim. Sci. 2004, 82, 878–883. [Google Scholar] [CrossRef]
- Kallenbach, R. BILL E. KUNKLE INTERDISCIPLINARY BEEF SYMPOSIUM: Coping with tall fescue toxicosis: Solutions and realities. J. Anim. Sci. 2015, 93, 5487–5495. [Google Scholar] [CrossRef]
- Bacon, C.; Porter, J.; Robbins, J.; Luttrell, E. Epichloe typhina from toxic tall fescue grasses. Appl. Environ. Microbiol. 1977, 34, 576–581. [Google Scholar] [CrossRef] [Green Version]
- Clay, K.; Holah, J. Fungal endophyte symbiosis and plant diversity in successional fields. Science 1999, 285, 1742–1744. [Google Scholar] [CrossRef]
- Bacon, C.W.; Siegel, M.R. Endophyte parasitism of tall fescue. J. Prod. Agric. 1988, 1, 45–55. [Google Scholar] [CrossRef]
- Glenn, A.E.; Bacon, C.W.; Price, R.; Hanlin, R.T. Molecular phylogeny of Acremonium and its taxonomic implications. Mycologia 1996, 88, 369–383. [Google Scholar] [CrossRef] [Green Version]
- Klotz, J.; Kirch, B.; Aiken, G.; Bush, L.; Strickland, J. Effects of selected combinations of tall fescue alkaloids on the vasoconstrictive capacity of fescue-naive bovine lateral saphenous veins. J. Anim. Sci. 2008, 86, 1021–1028. [Google Scholar] [CrossRef]
- Lyons, P.C.; Plattner, R.D.; Bacon, C.W. Occurrence of peptide and clavine ergot alkaloids in tall fescue grass. Science 1986, 232, 487–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yates, S.G.; Plattner, R.D.; Garner, G.B. Detection of ergopeptine alkaloids in endophyte-infected, toxic KY-31 tall fescue by mass spectrometry/mass spectrometry. J. Agric. Food Chem. 1985, 33, 719–722. [Google Scholar] [CrossRef]
- Bacon, C.W.; Lyons, P.C.; Porter, J.K.; Robbins, J.D. Ergot Toxicity from Endophyte-Infected Grasses: A Review. Agron. J. 1986, 78, 106–116. [Google Scholar] [CrossRef]
- Belesky, D.; Stuedemann, J.; Plattner, R.; Wilkinson, S. Ergopeptine alkaloids in grazed tall fescue. Agron. J. 1988, 80, 209–212. [Google Scholar] [CrossRef] [Green Version]
- Klotz, J.; Nicol, A. Ergovaline, an endophytic alkaloid. 1. Animal physiology and metabolism. Anim. Prod. Sci. 2016, 56, 1761–1774. [Google Scholar] [CrossRef] [Green Version]
- Schiff, P.L., Jr. Ergot and its alkaloids. Am. J. Pharm. Educ. 2006, 70, 98. [Google Scholar] [CrossRef]
- Strickland, J.R.; Looper, M.L.; Matthews, J.; Rosenkrans, C.F., Jr.; Flythe, M.; Brown, K. Board-invited review: St. Anthony’s Fire in livestock: Causes, mechanisms, and potential solutions. J. Anim. Sci. 2011, 89, 1603–1626. [Google Scholar] [CrossRef] [PubMed]
- Berde, B. Ergot compounds: A synopsis. In Ergot Compounds and Brain Function: Neuroendocrine and Neuropsychiatric Aspects; Goldstein, M., Lieberman, A., Calne, D.B., Thorner, M.O., Eds.; Raven Press: New York, NY, USA, 1980; pp. 4–23. [Google Scholar]
- Weber, H. The molecular architecture of ergopeptines: A basis for biological interaction. Adv. Biochem. Psychopharmacol. 1980, 23, 25–34. [Google Scholar]
- Klotz, J.; Bush, L.; Smith, D.; Shafer, W.; Smith, L.; Vevoda, A.; Craig, A.; Arrington, B.; Strickland, J. Assessment of vasoconstrictive potential of D-lysergic acid using an isolated bovine lateral saphenous vein bioassay. J. Anim. Sci. 2006, 84, 3167–3175. [Google Scholar] [CrossRef] [Green Version]
- Aiken, G.; Strickland, J.; Looper, M.; Bush, L.; Schrick, F. Hemodynamics are altered in the caudal artery of beef heifers fed different ergot alkaloid concentrations. J. Anim. Sci. 2009, 87, 2142–2150. [Google Scholar] [CrossRef] [PubMed]
- Thompson, F.; Stuedemann, J. Pathophysiology of fescue toxicosis. Agric. Ecosyst. Environ. 1993, 44, 263–281. [Google Scholar] [CrossRef]
- Oliver, J.W. Physiological manifestations of endophyte toxicosis in ruminant and laboratory species. In Neotyphodium/Grass Interactions; Bacon, C.W., Hill, N.S., Eds.; Plenum Publication: New York, NY, USA, 1997; pp. 311–346. [Google Scholar]
- Britt, J.L. The Impact of Ergot Alkaloid Consumption During Gestation on Maternal Performance and Fetoplacental Development. Ph.D. Dissertation, Clemson University, Clemson, SC, USA, 2019. [Google Scholar]
- McCollough, S.F.; Piper, E.L.; Moubarak, A.S.; Johnson, Z.B.; Petroski, R.J.; Flieger, M. Effect of tall fescue ergot alkaloids on peripheral blood flow and serum prolactin in steers. J. Anim. Sci. 1994, 72 (Suppl 1), 144. [Google Scholar]
- Bond, J.; Powell, J.; Weinland, B. Behavior of Steers Grazing Several Varieties of Tall Fescue During Summer Conditions. Agron. J. 1984, 76, 707–709. [Google Scholar] [CrossRef]
- Rhodes, M.; Paterson, J.; Kerley, M.; Garner, H.; Laughlin, M. Reduced blood flow to peripheral and core body tissues in sheep and cattle induced by endophyte-infected tall fescue. J. Anim. Sci. 1991, 69, 2033–2043. [Google Scholar] [CrossRef]
- Osborn, T.; Schmidt, S.; Marple, D.; Rahe, C.; Steenstra, J. Effect of consuming fungus-infected and fungus-free tall fescue and ergotamine tartrate on selected physiological variables of cattle in environmentally controlled conditions. J. Anim. Sci. 1992, 70, 2501–2509. [Google Scholar] [CrossRef]
- Zanzalari, K.; Heitmann, R.; McLaren, J.; Fribourg, H. Effects of endophyte-infected fescue and cimetidine on respiration rates, rectal temperatures and hepatic mixed function oxidase activity as measured by hepatic antipyrine metabolism in sheep. J. Anim. Sci. 1989, 67, 3370–3378. [Google Scholar] [CrossRef]
- Aldrich, C.; Paterson, J.; Tate, J.; Kerley, M. The effects of endophyte-infected tall fescue consumption on diet utilization and thermal regulation in cattle. J. Anim. Sci. 1993, 71, 164–170. [Google Scholar] [CrossRef]
- Bouton, J.H.; Latch, G.C.; Hill, N.S.; Hoveland, C.S.; McCann, M.A.; Watson, R.H.; Parish, J.A.; Hawkins, L.L.; Thompson, F.N. Reinfection of tall fescue cultivars with non-ergot alkaloid–producing endophytes. Agron. J. 2002, 94, 567–574. [Google Scholar]
- Hemken, R.; Boling, J.; Bull, L.; Hatton, R.; Buckner, R.; Bush, L. Interaction of environmental temperature and anti-quality factors on the severity of summer fescue toxicosis. J. Anim. Sci. 1981, 52, 710–714. [Google Scholar] [CrossRef] [Green Version]
- Hannah, S.; Paterson, J.; Williams, J.; Kerley, M.; Miner, J. Effects of increasing dietary levels of endophyte-infected tall fescue seed on diet digestibility and ruminal kinetics in sheep. J. Anim. Sci. 1990, 68, 1693–1701. [Google Scholar] [CrossRef] [PubMed]
- Bond, J.; Lynch, G.; Bolt, D.; Hawk, H.; Jackson, C., Jr.; Wall, R. Reproductive performance and lamb weight gains for ewes grazing fungus-infected tall fescue. Nutr. Rep. Int. 1988, 37, 1099–1115. [Google Scholar]
- Gay, N.; Boling, J.; Dew, R.; Miksch, D. Effects of endophyte-infected tall fescue on beef cow-calf performance. Appl. Agric. Res. 1988, 3, 182. [Google Scholar]
- Hemken, R.; Jackson, J., Jr.; Boling, J. Toxic factors in tall fescue. J. Anim. Sci. 1984, 58, 1011–1016. [Google Scholar] [CrossRef]
- Schmidt, S.; Osborn, T. Effects of endophyte-infected tall fescue on animal performance. Agric., Ecosyst. Environ. 1993, 44, 233–262. [Google Scholar] [CrossRef]
- McClanahan, L.; Aiken, G.; Dougherty, C. Influence of rough hair coats and steroid implants on the performance and physiology of steers grazing endophyte-infected tall fescue in the summer. Prof. Anim. Sci. 2008, 24, 269–276. [Google Scholar] [CrossRef]
- Aiken, G.; Klotz, J.; Looper, M.; Tabler, S.; Schrick, F. Disrupted hair follicle activity in cattle grazing endophyte-infected tall fescue in the summer insulates core body temperatures. Prof. Anim. Sci. 2011, 27, 336–343. [Google Scholar] [CrossRef]
- Hoveland, C.; Schmidt, S.; King, C., Jr.; Odom, J.; Clark, E.; McGuire, J.; Smith, L.; Grimes, H.; Holliman, J. Steer Performance and Association of Acremonium coenophialum Fungal Endophyte on Tall Fescue Pasture. Agron. J. 1983, 75, 821–824. [Google Scholar] [CrossRef]
- Oliver, J.W. Pathophysiologic response to endophyte toxins. In Neotyphodium in Cool-Season Grasses; Roberts, C.A., West, C.P., Spiers, D.E., Eds.; Blackwell Publishing: Ames, IA, USA, 2005; pp. 291–304. [Google Scholar]
- Howard, M.; Muntifering, R.; Bradley, N.; Mitchell Jr, G.; Lowry, S. Voluntary intake and ingestive behavior of steers grazing Johnstone or endophyte-infected Kentucky-31 tall fescue. J. Anim. Sci. 1992, 70, 1227–1237. [Google Scholar] [CrossRef] [Green Version]
- Stuedemann, J.; Wilkinson, S.; Belesky, D.; Devine, O.; Breedlove, D.; Thompson, F.; Hoveland, C.; Ciordia, H.; Townsend, W. Utilization and management of endophyte-infested tall fescue: Affects on steer performance and behavior. In Proceedings of the 41st Southern Pasture and Forage Crop Improvement Conference, Raleigh, NC, USA, 20–22 May 1985; pp. 17–20. [Google Scholar]
- Coffey, K.; Lomas, L.; Moyer, J. Grazing and subsequent feedlot performance by steers that grazed different types of fescue pasture. J. Prod. Agric. 1990, 3, 415–420. [Google Scholar] [CrossRef]
- Paterson, J.; Forwood, J.; Turner, K. University of Missouri report on fescue research activities. In Proceedings of the Tall Fescue Toxicosis Workshop, Memphis, TN, USA, 17–18 November 1987; pp. 17–18. [Google Scholar]
- Fike, J.H.; Pent, G.J. Strategies for Managing Endophyte Infected Tall Fescue--A Whole Farm Approach; Virginia Cooperative Extension: Washington, VA, USA, 2019; SPES-163P. [Google Scholar]
- Dougherty, C.; Lauriault, L.; Bradley, N.; Gay, N.; Cornelius, P. Induction of tall fescue toxicosis in heat-stressed cattle and its alleviation with thiamin. J. Anim. Sci. 1991, 69, 1008–1018. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.; Harmon, R.; Tabeidi, Z. Effect of dietary supplementation with vitamin E for lactating dairy cows fed tall fescue hay infected with endophyte. J. Dairy Sci. 1997, 80, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Aiken, G.; Piper, E.; Miesner, C. Influence of protein supplementation and implant status on alleviating fescue toxicosis. J. Anim. Sci. 2001, 79, 827–832. [Google Scholar] [CrossRef] [Green Version]
- Simeone, A.; Boissonneault, G.; Bush, L.; Mitchell, G. Comparison of two ammoniation procedures to reduce the toxicity of endophyte-infected tall fescue seed fed to rats. Drug Chem. Toxicol. 1998, 21, 79–95. [Google Scholar] [CrossRef] [PubMed]
- Filipov, N.; Thompson, F.; Hill, N.; Dawe, D.; Stuedemann, J.; Price, J.; Smith, C. Vaccination against ergot alkaloids and the effect of endophyte-infected fescue seed-based diets on rabbits. J. Anim. Sci. 1998, 76, 2456–2463. [Google Scholar] [CrossRef]
- Samford-Grigsby, M.; Larson, B.; Forcherio, J.; Lucas, D.; Paterson, J.; Kerley, M. Injection of a dopamine antagonist into Holstein steers to relieve symptoms of fescue toxicosis. J. Anim. Sci. 1997, 75, 1026–1031. [Google Scholar] [CrossRef] [Green Version]
- Chestnut, A.; Fribourg, H.; McLaren, J.; Keltner, D.; Reddick, B.; Carlisle, R.; Smith, M. Effects of Acremonium coenophialum infestation, bermudagrass, and nitrogen or clover on steers grazing tall fescue pastures. J. Prod. Agric. 1991, 4, 208–212. [Google Scholar] [CrossRef]
- Beck, P.; Haque, M.; Biermacher, J.; Hopkins, A.; Hubbell, D.; Hess, T. Impact of clover additions to toxic or nontoxic endophyte-infected tall fescue on animal performance and economics of stocker programs. Prof. Anim. Sci. 2012, 28, 433–442. [Google Scholar] [CrossRef] [Green Version]
- Lusby, K.; McMurphy, W.; Strasia, C.; Smith, S.; Muntz, S. Effects of fescue endophyte and interseeded clovers on subsequent finishing performance of steers. J. Prod. Agric. 1990, 3, 103–105. [Google Scholar] [CrossRef]
- Harlow, B.E.; Flythe, M.D.; Kagan, I.A.; Goodman, J.P.; Klotz, J.L.; Aiken, G.E. Isoflavone supplementation, via red clover hay, alters the rumen microbial community and promotes weight gain of steers grazing mixed grass pastures. PLoS ONE 2020, 15, e0229200. [Google Scholar] [CrossRef] [Green Version]
- Harlow, B.E.; Flythe, M.D.; Hamilton, T.A.; Ji, H.; Schrick, F.N.; Aiken, G.E. Effects of overseeding red clover in endophyte-infected tall fescue pastures on steer physiology and performance. App. Anim. Sci. 2021, 37, 748–757. [Google Scholar] [CrossRef]
- Harlow, B.E.; Flythe, M.D.; Goodman, J.P.; Ji, H.; Aiken, G.E. Isoflavone containing legumes mitigate ergot alkaloid-induced vasoconstriction in goats (Capra hircus). Animals 2022, 12, 750. [Google Scholar] [CrossRef] [PubMed]
- Beck, P.; Gunter, S.; Lusby, K.; West, C.; Watkins, K.; Hubbell, D., III. Animal performance and economic comparison of novel and toxic endophyte tall fescues to cool-season annuals. J. Anim. Sci. 2008, 86, 2043–2055. [Google Scholar] [CrossRef] [PubMed]
- Gunter, S.; Beck, P. Novel endophyte-infected tall fescue for growing beef cattle. J. Anim. Sci. 2004, 82, E75–E82. [Google Scholar] [PubMed]
- Marone, D.; Mastrangelo, A.M.; Borrelli, G.M.; Mores, A.; Laidò, G.; Russo, M.A.; Ficco, D.B.M. Specialized metabolites: Physiological and biochemical role in stress resistance, strategies to improve their accumulation, and new applications in crop breeding and management. Plant Physiol. Biochem. 2022, 172, 48–55. [Google Scholar] [CrossRef]
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef] [PubMed]
- Hagerman, A.E.; Butler, L.G. The specificity of proanthocyanidin-protein interactions. J. Biol. Chem. 1981, 256, 4494–4497. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Amelot, M.E.; Oliveros-Bastidas, A.; Calcagno-Pisarelli, M.P. Phenolics and condensed tannins of high altitude Pteridium arachnoideum in relation to sunlight exposure, elevation, and rain regime. Biochem. Syst. Ecol. 2007, 35, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Hassanpour, S.; MaheriSis, N.; Eshratkhah, B. Plants and secondary metabolites (Tannins): A Review. Int. J. For. Soil Erosion 2011, 1, 47–53. [Google Scholar]
- Hagerman, A.E.; Robbins, C.T.; Weerasuriya, Y.; Wilson, T.C.; McArthur, C. Tannin chemistry in relation to digestion. J. Range Manag. 1992, 45, 57–62. [Google Scholar] [CrossRef]
- Min, B.; Hart, S. Tannins for suppression of internal parasites. J. Anim. Sci. 2003, 81, E102–E109. [Google Scholar]
- Mangan, J. Nutritional effects of tannins in animal feeds. Nutr. Res. Rev. 1988, 1, 209–231. [Google Scholar] [CrossRef] [PubMed]
- Stewart, E.K.; Villalba, J.J.; Rood, K.A. Environmental and Animal Benefits When Beef Cattle Consume Condensed and Hydrolysable Tannins; Utah State University Extension: Logan, UT, USA, 2018; p. 1858. [Google Scholar]
- Amarowicz, R.; Janiak, M. Hydrolysable Tannins. In Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 337–343. [Google Scholar]
- Reed, J.D. Nutritional toxicology of tannins and related polyphenols in forage legumes. J. Anim. Sci. 1995, 73, 1516–1528. [Google Scholar] [CrossRef] [PubMed]
- Aboagye, I.A.; Beauchemin, K.A. Potential of molecular weight and structure of tannins to reduce methane emissions from ruminants: A review. Animals 2019, 9, 856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barry, T.; McNabb, W. The implications of condensed tannins on the nutritive value of temperate forages fed to ruminants. Br. J. Nutr. 1999, 81, 263–272. [Google Scholar] [CrossRef] [Green Version]
- Degen, A.A.; Becker, K.; Makkar, H.P.; Borowy, N. Acacia saligna as a fodder tree for desert livestock and the interaction of its tannins with fibre fractions. J. Sci. Food Agric. 1995, 68, 65–71. [Google Scholar] [CrossRef]
- Barry, T. The role of condensed tannins in the nutritional value of Lotus pedunculatus for sheep:* 3. Rates of body and wool growth. Br. J. Nutr. 1985, 54, 211–217. [Google Scholar] [CrossRef] [Green Version]
- Zeller, W.E. Activity, purification, and analysis of condensed tannins: Current state of affairs and future endeavors. Crop Sci. 2019, 59, 886–904. [Google Scholar] [CrossRef]
- Chalupa, W. Rumen bypass and protection of proteins and amino acids. J. Dairy Sci. 1975, 58, 1198–1218. [Google Scholar] [CrossRef]
- Hartinger, T.; Gresner, N.; Südekum, K.-H. Does intra-ruminal nitrogen recycling waste valuable resources? A review of major players and their manipulation. J. Anim. Sci. Biotechnol. 2018, 9, 33. [Google Scholar] [CrossRef] [Green Version]
- Powell, J.; Aguerre, M.; Wattiaux, M. Tannin extracts abate ammonia emissions from simulated dairy barn floors. J. Environ. Qual. 2011, 40, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Samanta, P.; Horn, H.; Saravia, F. Impact of Livestock Farming on Nitrogen Pollution and the Corresponding Energy Demand for Zero Liquid Discharge. Water 2022, 14, 1278. [Google Scholar] [CrossRef]
- Jones, W.T.; Mangan, J.L. Complexes of the condensed tannins of sainfoin (Onobrychis viciifolia Scop.) with fraction 1 leaf protein and with submaxillary mucoprotein, and their reversal by polyethylene glycol and pH. J. Sci. Food Agric. 1977, 28, 126–136. [Google Scholar] [CrossRef]
- Min, B.; McNabb, W.; Barry, T.; Peters, J. Solubilization and degradation of ribulose-1, 5-bisphosphate carboxylase/oxygenase (EC 4.1. 1.39; Rubisco) protein from white clover (Trifolium repens) and Lotus corniculatus by rumen microorganisms and the effect of condensed tannins on these processes. J. Agric. Sci. 2000, 134, 305–317. [Google Scholar] [CrossRef]
- Patra, A.K.; Saxena, J. Exploitation of dietary tannins to improve rumen metabolism and ruminant nutrition. J. Sci. Food Agric. 2011, 91, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Robbins, C.; Hanley, T.; Hagerman, A.; Hjeljord, O.; Baker, D.; Schwartz, C.; Mautz, W. Role of tannins in defending plants against ruminants: Reduction in protein availability. Ecology 1987, 68, 98–107. [Google Scholar] [CrossRef]
- Waghorn, G. Beneficial and detrimental effects of dietary condensed tannins for sustainable sheep and goat production—Progress and challenges. Anim. Feed Sci. Technol. 2008, 147, 116–139. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, X.; Cao, Z.; Wang, Y.; Yang, H.; Azarfar, A.; Li, S. Effect of different tannin sources on nutrient intake, digestibility, performance, nitrogen utilization, and blood parameters in dairy cows. Animals 2019, 9, 507. [Google Scholar] [CrossRef] [Green Version]
- Perez-Maldonado, R.; Norton, B. The effects of condensed tannins from Desmodium intortum and Calliandra calothyrsus on protein and carbohydrate digestion in sheep and goats. Br. J. Nutr. 1996, 76, 515–533. [Google Scholar] [CrossRef] [Green Version]
- Place, S.E.; Mitloehner, F. Invited review: Contemporary environmental issues: A review of the dairy industry’s role in climate change and air quality and the potential of mitigation through improved production efficiency. J. Dairy Sci. 2010, 93, 3407–3416. [Google Scholar] [CrossRef]
- Waghorn, G.; Tavendale, M.; Woodfield, D. Methanogenesis from forages fed to sheep. Proc. N. Z. Grassl. Assoc. 2002, 64, 167–171. [Google Scholar] [CrossRef]
- Hess, H.; Valencia, F.; Monsalve, L.; Lascano, C. Effects of tannins in Calliandra calothyrsus and supplemental molasses on ruminal fermentation. J. Anim. Feed Sci. 2004, 13, 95–98. [Google Scholar] [CrossRef]
- Min, B.R.; Solaiman, S.; Waldrip, H.M.; Parker, D.; Todd, R.W.; Brauer, D. Dietary mitigation of enteric methane emissions from ruminants: A review of plant tannin mitigation options. Anim. Nutr. 2020, 6, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Gerber, P.J.; Steinfeld, H.; Henderson, B.; Mottet, A.; Opio, C.; Dijkman, J.; Falcucci, A.; Tempio, G. Tackling Climate Change through Livestock: A Global Assessment of Emissions and Mitigation Opportunities; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2013. [Google Scholar]
- Johnson, K.A.; Johnson, D.E. Methane emissions from cattle. J. Anim. Sci. 1995, 73, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- McAllister, T.; Newbold, C. Redirecting rumen fermentation to reduce methanogenesis. Aust. J. Anim. Agric. 2008, 48, 7–13. [Google Scholar] [CrossRef]
- Janssen, P.H. Influence of hydrogen on rumen methane formation and fermentation balances through microbial growth kinetics and fermentation thermodynamics. Anim. Feed Sci. Technol. 2010, 160, 1–22. [Google Scholar] [CrossRef]
- Tedeschi, L.O.; Callaway, T.R.; Muir, J.P.; Anderson, R.C. Potential environmental benefits of feed additives and other strategies for ruminant production. Rev. Bras. De Zootec. 2011, 40, 291–309. [Google Scholar]
- Huang, X.; Liang, J.; Tan, H.; Yahya, R.; Ho, Y. Effects of Leucaena condensed tannins of differing molecular weights on in vitro CH4 production. Anim. Feed Sci. Technol. 2011, 166, 373–376. [Google Scholar] [CrossRef]
- Pellikaan, W.F.; Stringano, E.; Leenaars, J.; Bongers, D.J.; van Laar-van Schuppen, S.; Plant, J.; Mueller-Harvey, I. Evaluating effects of tannins on extent and rate of in vitro gas and CH4 production using an automated pressure evaluation system (APES). Anim. Feed Sci. Technol. 2011, 166, 377–390. [Google Scholar] [CrossRef]
- Animut, G.; Puchala, R.; Goetsch, A.; Patra, A.; Sahlu, T.; Varel, V.; Wells, J. Methane emission by goats consuming different sources of condensed tannins. Anim. Feed Sci. Technol. 2008, 144, 228–241. [Google Scholar] [CrossRef]
- Kongvongxay, S.; Preston, T.; Leng, R.; Khang, D.N. Effect of a tannin-rich foliage (Mimosa pigra) on feed intake, digestibility, N retention and methane production in goats fed a basal diet of Muntingia calabura. Livest. Res. Rural Dev. 2011, 23. [Google Scholar]
- Williams, C.; Eun, J.-S.; MacAdam, J.W.; Young, A.; Fellner, V.; Min, B. Effects of forage legumes containing condensed tannins on methane and ammonia production in continuous cultures of mixed ruminal microorganisms. Anim. Feed Sci. Technol. 2011, 166, 364–372. [Google Scholar] [CrossRef]
- Tavendale, M.H.; Meagher, L.P.; Pacheco, D.; Walker, N.; Attwood, G.T.; Sivakumaran, S. Methane production from in vitro rumen incubations with Lotus pedunculatus and Medicago sativa, and effects of extractable condensed tannin fractions on methanogenesis. Anim. Feed Sci. Technol. 2005, 123, 403–419. [Google Scholar] [CrossRef]
- Dschaak, C.; Williams, C.; Holt, M.; Eun, J.-S.; Young, A.; Min, B. Effects of supplementing condensed tannin extract on intake, digestion, ruminal fermentation, and milk production of lactating dairy cows. J. Dairy Sci. 2011, 94, 2508–2519. [Google Scholar] [CrossRef] [Green Version]
- Becker, P.M.; van Wikselaar, P.G.; Franssen, M.C.; de Vos, R.C.; Hall, R.D.; Beekwilder, J. Evidence for a hydrogen-sink mechanism of (+) catechin-mediated emission reduction of the ruminant greenhouse gas methane. Metabolomics 2014, 10, 179–189. [Google Scholar] [CrossRef]
- Pathak, A.; Dutta, N.; Pattanaik, A.; Chaturvedi, V.; Sharma, K. Effect of condensed tannins from Ficus infectoria and Psidium guajava leaf meal mixture on nutrient metabolism, methane emission and performance of lambs. Australas. J. Anim. Sci. 2017, 30, 1702. [Google Scholar] [CrossRef] [Green Version]
- Lisonbee, L.D.; Villalba, J.J.; Provenza, F.D.; Hall, J.O. Tannins and self-medication: Implications for sustainable parasite control in herbivores. Behav. Process. 2009, 82, 184–189. [Google Scholar] [CrossRef]
- Hoste, H.; Torres-Acosta, J.; Sandoval-Castro, C.A.; Mueller-Harvey, I.; Sotiraki, S.; Louvandini, H.; Thamsborg, S.M.; Terrill, T.H. Tannin containing legumes as a model for nutraceuticals against digestive parasites in livestock. Vet. Parasitol. 2015, 212, 5–17. [Google Scholar] [CrossRef]
- Hoste, H.; Meza-OCampos, G.; Marchand, S.; Sotiraki, S.; Sarasti, K.; Blomstrand, B.M.; Williams, A.R.; Thamsborg, S.M.; Athanasiadou, S.; Enemark, H.L. Use of agro-industrial by-products containing tannins for the integrated control of gastrointestinal nematodes in ruminants. Parasite 2022, 29, 10. [Google Scholar] [CrossRef]
- Karonen, M.; Ahern, J.R.; Legroux, L.; Suvanto, J.; Engström, M.T.; Sinkkonen, J.; Salminen, J.-P.; Hoste, H. Ellagitannins inhibit the exsheathment of Haemonchus contortus and Trichostrongylus colubriformis larvae: The efficiency increases together with the molecular size. J. Agric. Food Chem. 2020, 68, 4176–4186. [Google Scholar] [CrossRef] [Green Version]
- Kahiya, C.; Mukaratirwa, S.; Thamsborg, S. Effects of Acacia nilotica and Acacia karoo diets on Haemonchus contortus infection in goats. Vet. Parasitol. 2003, 115, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Hoste, H.; Torres-Acosta, J.; Paolini, V.; Aguilar-Caballero, A.; Etter, E.; Lefrileux, Y.; Chartier, C.; Broqua, C. Interactions between nutrition and gastrointestinal infections with parasitic nematodes in goats. Small Rumin. Res. 2005, 60, 141–151. [Google Scholar] [CrossRef]
- Hur, S.N.; Molan, A.L.; Cha, J.O. Effects of feeding condensed tannin-containing plants on natural coccidian infection in goats. Australas. J. Anim. Sci. 2005, 18, 1262–1266. [Google Scholar] [CrossRef]
- Max, R.; Kimambo, A.; Kassuku, A.; Mtenga, L.; Buttery, P. Effect of tanniniferous browse meal on nematode faecal egg counts and internal parasite burdens in sheep and goats. S. Afr. J. Anim. Sci. 2007, 37, 97–106. [Google Scholar] [CrossRef]
- Min, B.R.; Pomroy, W.E.; Hart, S.P.; Sahlu, T. The effect of short-term consumption of a forage containing condensed tannins on gastro-intestinal nematode parasite infections in grazing wether goats. Small Rumin. Res. 2004, 51, 279–283. [Google Scholar] [CrossRef]
- Shaik, S.; Terrill, T.; Miller, J.; Kouakou, B.; Kannan, G.; Kaplan, R.; Burke, J.; Mosjidis, J. Sericea lespedeza hay as a natural deworming agent against gastrointestinal nematode infection in goats. Vet. Parasitol. 2006, 139, 150–157. [Google Scholar] [CrossRef]
- Desrues, O.; Mueller-Harvey, I.; Pellikaan, W.F.; Enemark, H.L.; Thamsborg, S.M. Condensed tannins in the gastrointestinal tract of cattle after sainfoin (Onobrychis viciifolia) intake and their possible relationship with anthelmintic effects. J. Agric. Food Chem. 2017, 65, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Min, B.; Hernandez, K.; Pinchak, W.; Anderson, R.; Miller, J.; Valencia, E. Effects of plant tannin extracts supplementation on animal performance and gastrointestinal parasites infestation in steers grazing winter wheat. Open J. Anim. Sci. 2015, 5, 343. [Google Scholar] [CrossRef] [Green Version]
- Novobilský, A.; Mueller-Harvey, I.; Thamsborg, S.M. Condensed tannins act against cattle nematodes. Vet. Parasitol. 2011, 182, 213–220. [Google Scholar] [CrossRef]
- Novobilský, A.; Stringano, E.; Carbonero, C.H.; Smith, L.; Enemark, H.L.; Mueller-Harvey, I.; Thamsborg, S. In vitro effects of extracts and purified tannins of sainfoin (Onobrychis viciifolia) against two cattle nematodes. Vet. Parasitol. 2013, 196, 532–537. [Google Scholar] [CrossRef]
- Collas, C.; Sallé, G.; Dumont, B.; Cabaret, J.; Cortet, J.; Martin-Rosset, W.; Wimel, L.; Fleurance, G. Are sainfoin or protein supplements alternatives to control small strongyle infection in horses? Animal 2018, 12, 359–365. [Google Scholar] [CrossRef]
- Grimm, P.; Laroche, N.; Julliand, S.; Sorci, G. Inclusion of Sainfoin in the Diet Might Alter Strongyle Infection in Naturally Infected Horses. Animals 2022, 12, 955. [Google Scholar] [CrossRef] [PubMed]
- Malsa, J.; Courtot, É.; Boisseau, M.; Dumont, B.; Gombault, P.; Kuzmina, T.A.; Basiaga, M.; Lluch, J.; Annonay, G.; Dhorne-Pollet, S. Effect of sainfoin (Onobrychis viciifolia) on cyathostomin eggs excretion, larval development, larval community structure and efficacy of ivermectin treatment in horses. Parasitology 2022, 149, 1439–1449. [Google Scholar] [CrossRef] [PubMed]
- Payne, S.; Kotze, A.; Durmic, Z.; Vercoe, P. Australian plants show anthelmintic activity toward equine cyathostomins in vitro. Vet. Parasitol. 2013, 196, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Min, B.; Hart, S.; Miller, D.; Tomita, G.; Loetz, E.; Sahlu, T. The effect of grazing forage containing condensed tannins on gastro-intestinal parasite infection and milk composition in Angora does. Vet. Parasitol. 2005, 130, 105–113. [Google Scholar] [CrossRef]
- Malinow, M.; McLaughlin, P.; Stafford, C.; Livingston, A.L.; Kohler, G.O.; Cheeke, P.R. Comparative effects of alfalfa saponins and alfalfa fiber on cholesterol absorption in rats. Am. J. Clin. Nutr. 1979, 32, 1810–1812. [Google Scholar] [CrossRef]
- Okuda, T.; Mori, K.; Shiota, M. Effects of the interaction of tannins with coexisting substances. III. Formation and solubilization of precipitates with alkaloids. J. Pharma. Soc. Jpn. 1982, 102, 854–858. [Google Scholar] [CrossRef] [Green Version]
- Catanese, F.; Distel, R.A.; Villalba, J.J. Effects of supplementing endophyte-infected tall fescue with sainfoin and polyethylene glycol on the physiology and ingestive behavior of sheep. J. Anim. Sci. 2014, 92, 744–757. [Google Scholar] [CrossRef]
- Villalba, J.J.; Spackman, C.; Goff, B.; Klotz, J.; Griggs, T.; MacAdam, J.W. Interaction between a tannin-containing legume and endophyte-infected tall fescue seed on lambs’ feeding behavior and physiology. J. Anim. Sci. 2016, 94, 845–857. [Google Scholar] [CrossRef] [Green Version]
- Lyman, T.; Provenza, F.; Villalba, J.; Wiedmeier, R. Cattle preferences differ when endophyte-infected tall fescue, birdsfoot trefoil, and alfalfa are grazed in different sequences. J. Anim. Sci. 2011, 89, 1131–1137. [Google Scholar] [CrossRef]
- Lyman, T.D.; Provenza, F.D.; Villalba, J.J. Sheep foraging behavior in response to interactions among alkaloids, tannins and saponins. J. Sci. Food Agric. 2008, 88, 824–831. [Google Scholar] [CrossRef]
- Owens, J.; Provenza, F.D.; Wiedmeier, R.D.; Villalba, J.J. Supplementing endophyte-infected tall fescue or reed canarygrass with alfalfa or birdsfoot trefoil increases forage intake and digestibility by sheep. J. Sci. Food Agric. 2012, 92, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Maughan, B.; Provenza, F.; Tansawat, R.; Maughan, C.; Martini, S.; Ward, R.; Clemensen, A.; Song, X.; Cornforth, D.; Villalba, J. Importance of grass-legume choices on cattle grazing behavior, performance, and meat characteristics. J. Anim. Sci. 2014, 92, 2309–2324. [Google Scholar] [CrossRef] [PubMed]
- Grote, A.; Nieman, C.; Morgan, A.; Coffey, K.; Philipp, D.; Kegley, E.; Edwards, J. Using supplemental condensed tannin to mitigate tall fescue toxicosis in non-pregnant, non-lactating ewes consuming tall fescue silage. Anim. Feed Sci. Technol. 2023, 295, 115516. [Google Scholar] [CrossRef]
- Haslam, E. Vegetable tannins–Lessons of a phytochemical lifetime. Phytochemistry 2007, 68, 2713–2721. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, H.; Nakatsubo, F.; Murakami, K. Quantitative determination of tannin and protein in the precipitates by high-performance liquid chromatography. Phytochemistry 1995, 40, 1503–1505. [Google Scholar] [CrossRef]
- de Freitas, V.; Carvalho, E.; Mateus, N. Study of carbohydrate influence on protein–tannin aggregation by nephelometry. Food Chem. 2003, 81, 503–509. [Google Scholar] [CrossRef]
- Fierer, N.; Schimel, J.P.; Cates, R.G.; Zou, J. Influence of balsam poplar tannin fractions on carbon and nitrogen dynamics in Alaskan taiga floodplain soils. Soil Biol. Biochem. 2001, 33, 1827–1839. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Butler, L.G. Protein precipitation method for the quantitative determination of tannins. J. Agric. Food Chem. 1978, 26, 809–812. [Google Scholar] [CrossRef]
- Eckert, H.; Kiechel, J.R.; Rosenthaler, J.; Schmidt, R.; Schreier, E. Biopharmaceutical aspects. In Ergot Alkaloids and Related Compounds; Berde, B., Schild, H.O., Eds.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1978; pp. 1–28. [Google Scholar]
- Westendorf, M.; Mitchell, G.; Tucker, R. Influence of rumen fermentation on response to endophyte—Infected tall fescue seed measured by a rat bioassay. Drug Chem. Toxicol. 1992, 15, 351–364. [Google Scholar] [CrossRef]
- Hill, N.; Thompson, F.; Stuedemann, J.; Rottinghaus, G.; Ju, H.; Dawe, D.; Hiatt, E., III. Ergot alkaloid transport across ruminant gastric tissues. J. Anim. Sci. 2001, 79, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.B.; Rychlik, J.L. Factors that alter rumen microbial ecology. Science 2001, 292, 1119–1122. [Google Scholar] [CrossRef] [PubMed]
- Naumann, H.D.; Tedeschi, L.O.; Zeller, W.E.; Huntley, N.F. The role of condensed tannins in ruminant animal production: Advances, limitations and future directions. Rev. Bras. De Zootec. 2017, 46, 929–949. [Google Scholar] [CrossRef] [Green Version]
- Mueller-Harvey, I. Unravelling the conundrum of tannins in animal nutrition and health. J. Sci. Food Agric. 2006, 86, 2010–2037. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, M. Tannins: Their adverse role in ruminant nutrition. J. Agric. Food Chem. 1984, 32, 447–453. [Google Scholar] [CrossRef]
- Welsh, S.; Atwood, N.; Goodrich, S.; Higgins, L. A Utah Flora; Brigham Young University: Provo, UT, USA, 2003. [Google Scholar]
- Wang, Y.; McAllister, T.A.; Acharya, S. Condensed tannins in sainfoin: Composition, concentration, and effects on nutritive and feeding value of sainfoin forage. Crop Sci. 2015, 55, 13–22. [Google Scholar] [CrossRef]
- Lees, G.; Suttill, N.; Gruber, M. Condensed tannins in sainfoin. 1. A histological and cytological survey of plant tissues. Canad. J. Bot. 1993, 71, 1147–1152. [Google Scholar] [CrossRef]
- Karnezos, T.; Matches, A.; Brown, C. Spring lamb production on alfalfa, sainfoin, and wheatgrass pastures. Agron. J. 1994, 86, 497–502. [Google Scholar] [CrossRef]
- Lagrange, S.; Beauchemin, K.A.; MacAdam, J.; Villalba, J.J. Grazing diverse combinations of tanniferous and non-tanniferous legumes: Implications for beef cattle performance and environmental impact. Sci. Total Environ. 2020, 746, 140788. [Google Scholar] [CrossRef]
- Sengul, S. Performance of some forage grasses or legumes and their mixtures under dry land conditions. Eur. J. Agron. 2003, 19, 401–409. [Google Scholar] [CrossRef]
- Marten, G.; Ehle, F.; Ristau, E. Performance and photosensitization of cattle related to forage quality of four legumes. Crop Sci. 1987, 27, 138–145. [Google Scholar] [CrossRef]
- Grabber, J.; Coblentz, W.; Riday, H.; Griggs, T.; Min, D.; MacAdam, J.; Cassida, K. Protein and Dry-Matter Degradability of European-and Mediterranean-Derived Birdsfoot Trefoil Cultivars Grown in the Colder Continental USA. Crop Sci. 2015, 55, 1356–1364. [Google Scholar] [CrossRef]
- Sheaffer, C.C.; Wyse, D.L.; Ehlke, N.J. Palatability and nutritive value of native legumes. Nat. Plants J. 2009, 10, 224–231. [Google Scholar] [CrossRef]
- Huang, Q.; Peng, K.; Iwaasa, A.; Schellenberg, M.; McAllister, T.; Wang, Y. PSXI-26 Effects of condensed tannins in purple prairie and white prairie clover on ruminal fermentation and methane production in vitro. J. Anim. Sci. 2018, 96, 422. [Google Scholar] [CrossRef]
- Jin, L.; Wang, Y.; Iwaasa, A.; Li, Y.; Xu, Z.; Schellenberg, M.; Liu, X.; McAllister, T.; Stanford, K. Purple prairie clover (Dalea purpurea Vent) reduces fecal shedding of Escherichia coli in pastured cattle. J. Food Prot. 2015, 78, 1434–1441. [Google Scholar] [CrossRef]
- Liu, X.-L.; Hao, Y.-Q.; Jin, L.; Xu, Z.-J.; McAllister, T.A.; Wang, Y. Anti-Escherichia coli O157: H7 properties of purple prairie clover and sainfoin condensed tannins. Molecules 2013, 18, 2183–2199. [Google Scholar] [CrossRef]
- Pieters, A. Lespedeza; US Department of Agriculture: Washington, DC, USA, 1933.
- Helm, C.A.; Etheridge, W.C. Lespedeza sericea. In The Newest Legume for Missouri; University of Missouri College of Agriculture Agricultural Experiment Station Bulletin 331; University of Missouri: Columbia, MO, USA, 1933. [Google Scholar]
- Mechineni, A.; Kommuru, D.; Gujja, S.; Mosjidis, J.; Miller, J.; Burke, J.; Ramsay, A.; Mueller-Harvey, I.; Kannan, G.; Lee, J. Effect of fall-grazed sericea lespedeza (Lespedeza cuneata) on gastrointestinal nematode infections of growing goats. Vet. Parasitol. 2014, 204, 221–228. [Google Scholar] [CrossRef]
- Cope, W.; Burns, J. Relationship Between Tannin Levels and Nutritive Value of Sericea. Crop Sci. 1971, 11, 231–233. [Google Scholar] [CrossRef]
- Terrill, T.; Windham, W.; Evans, J.; Hoveland, C. Condensed tannin concentraton in sericea lespedeza as influenced by preservation method. Crop Sci. 1990, 30, 219–224. [Google Scholar] [CrossRef]
- Mosjidis, J. Registration of ‘AU Grazer’ Sericea Lespedeza. Crop Sci. 2001, 41, 262. [Google Scholar] [CrossRef]
- Burke, J.; Miller, J.; Terrill, T.; Mosjidis, J. The effects of supplemental sericea lespedeza pellets in lambs and kids on growth rate. Livest. Sci. 2014, 159, 29–36. [Google Scholar] [CrossRef]
- Lange, K.; Olcott, D.; Miller, J.; Mosjidis, J.; Terrill, T.; Burke, J.; Kearney, M. Effect of sericea lespedeza (Lespedeza cuneata) fed as hay, on natural and experimental Haemonchus contortus infections in lambs. Vet. Parasitol. 2006, 141, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Solaiman, S.; Thomas, J.; Dupre, Y.; Min, B.; Gurung, N.; Terrill, T.; Haenlein, G. Effect of feeding sericea lespedeza (Lespedeza cuneata) on growth performance, blood metabolites, and carcass characteristics of Kiko crossbred male kids. Small Rumin. Res. 2010, 93, 149–156. [Google Scholar] [CrossRef]
- Kommuru, D.; Whitley, N.C.; Miller, J.; Mosjidis, J.; Burke, J.; Gujja, S.; Mechineni, A.; Terrill, T. Effect of sericea lespedeza leaf meal pellets on adult female Haemonchus contortus in goats. Vet. Parasitol. 2015, 207, 170–175. [Google Scholar] [CrossRef]
- Du Toit, C.J.L.; Van Niekerk, W.A.; Meissner, H.; Erasmus, L.J.; Coertze, R.J. Methane emissions from sheep fed Eragrostis curvula hay substituted with Lespedeza cuneata. Anim. Prod. Sci. 2020, 60, 1777–1784. [Google Scholar] [CrossRef]
- Puchala, R.; Min, B.; Goetsch, A.; Sahlu, T. The effect of a condensed tannin-containing forage on methane emission by goats. J. Anim. Sci. 2005, 83, 182–186. [Google Scholar] [CrossRef]
- Pech-Cervantes, A.A.; Terrill, T.H.; Ogunade, I.M.; Estrada-Reyes, Z.M. Meta-analysis of the effects of dietary inclusion of sericea lespedeza (Lespedeza cuneata) forage on performance, digestibility, and rumen fermentation of small ruminants. Livest. Sci. 2021, 253, 104707. [Google Scholar] [CrossRef]
- Lagrange, S.P.; MacAdam, J.W.; Villalba, J.J. The use of temperate tannin containing forage legumes to improve sustainability in forage–livestock production. Agronomy 2021, 11, 2264. [Google Scholar] [CrossRef]
- Piluzza, G.; Sulas, L.; Bullitta, S. Tannins in forage plants and their role in animal husbandry and environmental sustainability: A review. Grass Forage Sci. 2014, 69, 32–48. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poudel, S.; Zeller, W.E.; Fike, J.; Pent, G. Condensed Tannins Attributes: Potential Solution to Fescue Toxicosis? Agriculture 2023, 13, 672. https://doi.org/10.3390/agriculture13030672
Poudel S, Zeller WE, Fike J, Pent G. Condensed Tannins Attributes: Potential Solution to Fescue Toxicosis? Agriculture. 2023; 13(3):672. https://doi.org/10.3390/agriculture13030672
Chicago/Turabian StylePoudel, Sanjok, Wayne E. Zeller, John Fike, and Gabriel Pent. 2023. "Condensed Tannins Attributes: Potential Solution to Fescue Toxicosis?" Agriculture 13, no. 3: 672. https://doi.org/10.3390/agriculture13030672





