Effect of Osmopriming with Melatonin on Germination, Vigor and Health of Daucus carota L. Seeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Seed Germination Test
2.4. Seed Vigor Test
2.5. Seed Health Test
3. Results
Seed Germination Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Xue, Z.; Wang, R.; Ning, H.; Li, W.; Bai, Y.; Li, Y. Evaluation and management of fungal infected carrot seeds. Sci. Rep. 2020, 10, 10808. [Google Scholar] [CrossRef]
- Richardson, M.J. An Annotated List of Seed-Borne Diseases; International Seed Testing Association: Zurich, Switzerland, 1990. [Google Scholar]
- Que, F.; Ho, X.L.; Wang, G.L.; Xu, Z.S.; Tan, G.F.; Li, T.; Wang, Y.H.; Khadr, A.; Xiong, A.S. Advances in research on the carrot, an important root vegetable in the Apiaceae family. Hort. Res. 2019, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- Moliszewska, E.; Smiatek, V. Toxic properties of Alternaria radicina culture filtrates against carrot seeds and seedlings. Ecol. Chem. Eng. A 2009, 16, 1515–1523. [Google Scholar]
- Chen, H.; Singh, H.; Bhardwaj, N.; Bhardwaj, S.K.; Khatri, M.; Kim, K.-H.; Peng, W. An exploration on the toxicity mechanisms of phytotoxins and their potential utilities. Crit. Rev. Environ. Sci. Technol. 2020, 52, 395–435. [Google Scholar] [CrossRef]
- Gugino, B.K.; Carroll, J.; Chen, J.; Ludwig, J.; Abawi, G. Carrot Leaf Blight Diseases and Their Management in New York; Cornell University Press: New York, NY, USA, 2004. [Google Scholar]
- Farrar, J.J.; Pryor, B.A.; Davis, R.M. Alternaria diseases of carrot. Plant Dis. 2007, 88, 776–784. [Google Scholar] [CrossRef]
- Gugino, B.; Carroll, J.; Widmer, T.; Chen, P.; Abawi, G. Field evaluation of carrot cultivars for susceptibility to fungal leaf blight diseases in New York. Crop Prot. 2007, 26, 709–714. [Google Scholar] [CrossRef]
- Courtial, J.; Hamama, L.; Hélesbeux, J.-J.; Lecomte, M.; Renaud, Y.; Guichard, E.; Voisine, L.; Yovanopoulos, C.; Hamon, B.; Ogé, L.L.; et al. Aldaulactone—An original phytotoxic secondary metabolite involved in the aggressiveness of Alternaria dauci on carrot. Front. Plant Sci. 2018, 9, 502. [Google Scholar] [CrossRef]
- Lecomte, M.; Hamama, L.; Voisine, L.; Gatto, J.; Hélesbeux, J.-J.; Séraphin, D.; Peña-Rodriguez, L.M.; Richomme, P.; Boedo, C.; Yovanopoulos, C.; et al. Partial resistance of carrot to Alternaria dauci correlates with in vitro cultured carrot cell resistance to fungal exudates. PLoS ONE 2014, 9, e101008. [Google Scholar] [CrossRef]
- Meena, M.; Zehra, A.; Dubey, M.K.; Aamir, M.; Gupta, V.K.; Upadhyay, R.S. Comparative evaluation of biochemical changes in tomato (Lycopersicon esculentum Mill.) infected by Alternaria alternata and its toxic metabolites (TeA, AOH, and AME). Front. Plant Sci. 2016, 7, 1408. [Google Scholar] [CrossRef]
- Barash, I.; Mor, H.; Netzer, D.; Kashman, Y. Production of zinniol by Alternaria dauci and its phytotoxic effect on carrot. Physiol. Plant Pathol. 1981, 19, 7–16. [Google Scholar] [CrossRef]
- Bradford, K.J. Manipulation of seed water relations via osmotic priming to improve germination under stress conditions. Hort. Sci. 1986, 21, 1105–1112. [Google Scholar] [CrossRef]
- Aazami, M.A.; Zahedi, S.M. Germination of carrot (Daucus carota L.) seeds in response to osmotic priming. Thai J. Agri. Sci. 2018, 51, 188–194. [Google Scholar]
- Amooaghaie, R. The effect of hydro and osmopriming on alfalfa seed germination and antioxidant defenses under salt stress. Afr. J. Biotechnol. 2011, 10, 6269–6275. [Google Scholar] [CrossRef]
- Miladinov, Z.; Balešević-Tubić, S.; Đukić, V.; Ilić, A.; Čobanović, L.; Dozet, G.; Merkulov-Popadić, L. Effect of priming on soybean seed germination parameters. Acta Agric. Serb. 2018, 23, 15–26. [Google Scholar] [CrossRef]
- Aazami, M.A.; Mohammadi, S. Determination of the best temperature and dry condition in seeds-carrot primed. Pak. J. Biol. Sci. 2008, 11, 1502–1505. [Google Scholar] [CrossRef]
- Draganic, I.; Lekic, S. Seed priming with antioxidants improves sunflower seed germination and seedling growth under unfavorable germination conditions. Turk. J. Agric. For. 2012, 36, 421–428. [Google Scholar] [CrossRef]
- Dellan, E.; Lagunovschi-Luchian, V. Germination and vigour of primed Daucus carota L. seeds under saline stress conditions. Rom. Biotechnol. Lett. 2015, 20, 10833–10840. [Google Scholar]
- Tylkowska, K.; Biniek, A. Fungi and germination of carrot and parsley seeds under osmopriming and fungicide treatment. Phytopatol. Pol. 1996, 12, 51–61. [Google Scholar]
- Nascimento, W.M.; West, S.H. Microorganism growth during muskmelon seed priming. Seed Sci. Technol. 1998, 26, 531–534. [Google Scholar]
- Tylkowska, K.; van den Bulk, R.W. Effects of osmo- and hydropraming on fungal infestation levels and germination of carrot (Daucus carota L.) seeds contaminated with Alternaria spp. Seed Sci. Technol. 2001, 29, 365–375. [Google Scholar]
- Zhao, X.; Li, Y.; Dorna, H. Effect of priming and fungicide treatment on germination of China aster (Callistephus chinensis L.) seeds. Seed Sci. Technol. 2004, 32, 417–424. [Google Scholar] [CrossRef]
- Dorna, H.; Tylkowska, K.; Zhao, X. Effects of osmopriming and Rovral on health and germination of China aster seeds. Phytopatol. Pol. 2001, 21, 35–44. [Google Scholar]
- Kolár, J.; Machácková, I. Melatonin in higher plants: Occurrence and possible functions. J. Pineal Res. 2005, 39, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: Plant growth regulator and/or biostimulator during stress? Trends Plant Sci. 2014, 19, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Functions of melatonin in plants: A review. J. Pineal Res. 2015, 59, 133–150. [Google Scholar] [CrossRef]
- Hernández-Ruiz, J.; Arnao, M.B. Phytomelatonin, an interesting tool for agricultural crops. Focus Sci. 2016, 2, 1–7. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Huang, Y.; Bie, Z.; Ahmed, W.; Reiter, R.J.; Niu, M.; Hameed, S. Melatonin: Current status and future perspectives in plant science. Front. Plant Sci. 2016, 6, 1230. [Google Scholar] [CrossRef]
- Li, C.; Wang, P.; Wei, Z.; Liang, D.; Liu, C.; Yin, L.; Jia, D.; Fu, M.; Ma, F. The mitigation effects of exogenous melatonin on salinity-induced stress in Malus hupehensis. J. Pineal Res. 2012, 53, 298–306. [Google Scholar] [CrossRef]
- Li, C.; Zhao, Q.; Gao, T.; Wang, H.; Zhang, Z.; Liang, B.; Wei, Z.; Liu, C.; Ma, F. The mitigation effects of exogenous melatonin on replant disease in apple. J. Pineal Res. 2018, 65, e12523. [Google Scholar] [CrossRef]
- Szafrańska, K.; Glińska, S.; Jamas, K.M. Ameliorative effect of melatonin on meristematic cells of chilled and re-warmed Vigna radiata roots. Biol. Plant. 2013, 57, 91–96. [Google Scholar] [CrossRef]
- Shi, H.; Tan, D.X.; Reiter, R.J.; Ye, T.; Yang, F.; Chan, Z. Melatonin induces class A1 heat shock factors (HSFA1s) and their possible involvement of thermotolerance in Arabidopsis. J. Pineal Res. 2015, 58, 335–342. [Google Scholar] [CrossRef]
- Ye, J.; Wang, S.; Deng, X.; Yin, L.; Xiong, B.; Wang, X. Melatonin increased maize (Zea mays) seedling drought tolerance by alleviating drought-induced photosynthetic inhibition and oxidative damage. Acta Physiol. Plant. 2016, 38, 48. [Google Scholar] [CrossRef]
- Kobylińska, A.; Borek, S.; Posmyk, M. Melatonin redirects carbohydrates metabolism during sugar starvation in plant cells. J. Pineal Res. 2018, 64, e12466. [Google Scholar] [CrossRef]
- Tan, D.X.; Chen, L.D.; Poeggeler, B.; Manchester, L.C.; Reiter, R.J. Melatonin: A potent, endogenous hydroxyl radical scavenger. Endocr. J. 1993, 1, 57–60. [Google Scholar]
- Galano, A.; Tan, D.X.; Reiter, R.J. Melatonin as a natural ally against oxidative stress: A physicochemical examination. J. Pineal Res. 2011, 51, 1–16. [Google Scholar] [CrossRef]
- Kołodziejczyk, I.; Kaźmierczak, A.; Posmyk, M.M. Melatonin application modifies antioxidant defense and induces endoreplication in maize seeds exposed to chilling stress. Int. J. Mol. Sci. 2021, 22, 8628. [Google Scholar] [CrossRef]
- Manchester, L.C.; Tan, D.X.; Reiter, R.J.; Park, W.; Monis, K.; Qi, W. High levels of melatonin in the seeds of edible plants: Possible function in germ tissue protection. Life Sci. 2000, 67, 3023–3029. [Google Scholar] [CrossRef]
- Murch, S.J.; Alan, A.R.; Cao, J.; Saxena, P.K. Melatonin and serotonin in flowers and fruits of Datura metel L. J. Pineal Res. 2009, 47, 277–283. [Google Scholar] [CrossRef]
- Xiao, S.; Liu, L.; Wang, H.; Li, D.; Bai, Z.; Zhang, Y.; Sun, H.; Zhang, K.; Li, C. Exogenous melatonin accelerates seed germination in cotton (Gossypium hirsutum L.). PLoS ONE 2019, 14, e0216575. [Google Scholar] [CrossRef]
- Bałabusta, M.; Szafrańska, K.; Posmyk, M.M. Exogenous melatonin improves antioxidant defense in cucumber seeds (Cucumis sativus L.) germinated under chilling stress. Front. Plant Sci. 2016, 7, 575. [Google Scholar]
- Michel, B.E.; Kaufmann, M.R. The osmotic potential of polyethylene glycol 6000. Plant Physiolol. 1973, 51, 914–916. [Google Scholar] [CrossRef] [PubMed]
- ISTA International Rules for Seed Testing; International Seed Testing Association: Zurich, Switzerland, 2020.
- Mallone, J.P.; Muskett, A.E. Seed Borne-Fungi. Description of 77 Fungus Species; The International Seed Testing Association: Zurych, Switzerland, 1964. [Google Scholar]
- Watanabe, T. Pictorial Atlas of Soil and Seed Fungi Morphologies of Cultured Fungi and Key to Species; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Mathur, S.B.; Kongsdal, O. Common Laboratory Seed Health Testing Methods for Detecting Fungi; The International Seed Testing Association: Basserdorf, Switzerland, 2003. [Google Scholar]
- Jalink, H.; van der Schoor, R. SeedCalculator, Version 2.1. License number: 100200122. Plant Research International: Wageningen, The Netherlands, 1999.
- Hattori, A.; Migitaka, H.; Masayaki, I.; Itoh, M.; Yamamoto, K.; Ohtani-Kaneko, R.; Hara, M.; Suzuki, T.; Reiter, R.J. Identification of melatonin in plant seed its effects on plasma melatonin level sand binding to melatonin receptors in vertebrates. Biochem. Mol. Biol. Int. 1995, 35, 627–634. [Google Scholar] [PubMed]
- Murch, S.J.; Erland, L.A.E. A systematic review of melatonin in plants: An example of evolution of literature. Front. Plant Sci. 2021, 12, 683047. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.X.; Lv, B.S.; Li, X.W.; Wang, M.M.; Ma, H.Y.; Yang, H.Y.; Yang, R.F.; Piao, Z.Z.; Wang, Z.H.; Lou, J.H.; et al. Priming of rice (Oryza sativa L.) seedlings with abscisic acid enhance seedling survival, plant growth, and grain yield in saline-alkaline paddy fields. Field Crops Res. 2017, 203, 86–93. [Google Scholar] [CrossRef]
- Cao, Q.; Li, G.; Cui, Z.; Yang, F.; Jiang, X.; Diallo, L.; Kong, F. Seed priming with melatonin improves the seed germination of waxy maize under chilling stress via promoting the antioxidant system and starch metabolism. Sci. Rep. 2019, 9, 15044. [Google Scholar] [CrossRef]
- Posmyk, M.M.; Kuran, H.; Marciniak, K.; Janas, K.M. Presowing seed treatment with melatonin protects red cabbage seedlings against toxic copper ion concentrations. J. Pineal Res. 2008, 45, 24–31. [Google Scholar] [CrossRef]
- Posmyk, M.; Bałabusta, M.; Wieczorek, M.; Śliwińska, E.; Janas, K.M. Melatonin applied to cucumber (Cucumis sativus L.) seeds improves germination during chilling stress. J. Pineal Res. 2009, 46, 214–223. [Google Scholar] [CrossRef]
- Tiryaki, I.; Keles, H. Reversal of the inhibitory effect of light and high temperature on germination of Phacelia tanacetifolia seeds by melatonin. J. Pineal Res. 2012, 52, 332–339. [Google Scholar] [CrossRef]
- Akbari, A.G.; Heshmati, S.; Soltani, E.; Dehaghi, M.A. Influence of seed priming on seed yield, oil content and fatty acid composition of safflower (Carthamus tinctorius L.) grown under water deficit. Int. J. Plant Prod. 2020, 14, 245–258. [Google Scholar] [CrossRef]
- Jiang, X.W.; Li, H.Q.; Song, X.Y. Seed priming with melatonin effects on seed germination and seedling growth in maize under salinity stress. Pak. J. Bot. 2016, 48, 1345–1352. [Google Scholar]
- Rosińska, A.; Dorna, H.; Szopińska, D. Effects of osmopriming on germination, vigour and health of Silybum marianum (L.) Gaertn. seeds. Acta Sci. Pol. Hortorum Cultus 2017, 16, 13–25. [Google Scholar] [CrossRef]
- Rhaman, M.S.; Rauf, F.; Tania, S.S.; Khatun, S. Seed priming methods: Application in field crops and future perspectives. Asian J. Res. Crop Sci. 2020, 5, 8–19. [Google Scholar] [CrossRef]
- Iranshahy, M.; Etemad, L.; Shakeri, A.; Badibostan, H.; Karimi, G. Protective activity of melatonin against mycotoxins-induced toxicity: A review. Toxicol. Environ. Chem. 2020, 101, 435–450. [Google Scholar] [CrossRef]
- Hernández-Ruiz, J.; Giraldo-Acosta, M.; El Mihyaoui, A.; Cano, A.; Arnao, M.B. Melatonin as a possible natural anti-viral compound in plant biocontrol. Plants 2023, 12, 781. [Google Scholar] [CrossRef]
- Janas, K.M.; Ciupińska, E.; Posmyk, M.M. Melatonin applied by hydropriming as a biostimulator improving sweet corn (Zea mays L.) seedling growth in abiotic stress condition. In Progress in Environmental Science and Technology; Li, S., Wang, Y., Ca, F., Huang, P., Zhang, Y., Eds.; Science Press: Princeton Junction, NJ, USA, 2009; Volume 2, pp. 383–388. [Google Scholar]
- Posmyk, M.M.; Janas, K.M. Melatonin in plants. Acta Physiol. Plant. 2009, 31, 1–11. [Google Scholar] [CrossRef]
- Altaf, M.A.; Shahid, R.; Ren, M.X.; Mora-Poblete, F.; Arnao, M.B.; Naz, S.; Anwar, M.; Altaf, M.M.; Shahid, S.; Shakoor, A.; et al. Phytomelatonin: An overview of the importance and mediating functions of melatonin against environmental stresses. Physiol. Plant. 2021, 172, 820–846. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin as a Chemical Substance or as Phytomelatonin Rich-Extracts for Use as Plant Protector and/or Biostimulant in Accordance with EC Legislation. Agronomy 2019, 9, 570. [Google Scholar] [CrossRef]
- Chen, Q.; Qi, W.B.; Reiter, R.J.; Wei, W.; Wang, B.M. Exogenously applied melatonin stimulates root growth and raises endogenous indoleacetic acid in roots of etiolated seedlings of Brassica juncea. J. Plant Physiol. 2009, 166, 324–328. [Google Scholar] [CrossRef]
- Bai, Y.; Xiao, S.; Zhang, Z.; Zhang, Y.; Sun, H.; Zhang, K.; Wang, X.; Bai, Z.; Li, C.; Liu, L. Melatonin improves the germination rate of cotton seeds under drought stress by opening pores in the seed coat. PeerJ 2020, 8, e9450. [Google Scholar] [CrossRef]
- Chipenete, G.H.N.; dos Santos Dias, D.C.F.; Pinheiro, D.T.; da Silva, L.J.; Pazzin, D.; da Silva, A.L. Carrot seeds vigor on plant performance and crop yield. Rev. Verde 2021, 16, 48–59. [Google Scholar] [CrossRef]
- Guo, Y.; Li, D.; Liu, L.; Sun, H.; Zhu, L.; Zhang, K.; Zhao, H.; Zhang, Y.; Li, A.; Bai, Z.; et al. Seed priming with melatonin promotes seed germination and seedling growth of Triticale hexaploide L. under PEG-6000 induced drought stress. Front. Plant Sci. 2022, 13, 932912. [Google Scholar] [CrossRef] [PubMed]
- Hanci, F. The effect of L-tryptophan and melatonin on seed germination of some cool season vegetable species under salinity stress. Düzce Üniversitesi Bilim Ve Teknol. Derg. 2019, 7, 1879–1891. [Google Scholar] [CrossRef]
- Simlat, M.; Ptak, A.; Skrzypek, E.; Warchoł, M.; Morańska, E.; Piórkowska, E. Melatonin significantly influence seed germination and seedling growth of Stevia rebaudiana Bertoni. PeerJ 2018, 6, e5009. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. The physiological function of melatonin in plants. Plant Signal. Behav. 2006, 1, 89–95. [Google Scholar] [CrossRef]
- Yin, L.; Wang, P.; Li, M.; Ke, X.; Li, C.; Liang, D.; Wu, S.; Ma, X.; Li, C.; Zou, Y.; et al. Exogenous melatonin improves Malus resistance to Marssonina apple blotch. J. Pineal Res. 2013, 54, 426–434. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, S.; Zhang, J.; Reiter, R.J.; Wang, Y.; Qiu, D.; Luo, X.; Rehman Khalid, A.; Wang, H.; Feng, L.; et al. Synergistic anti-oomycete effect of melatonin with a biofungicide against oomycetic black shank disease. J. Pineal Res. 2018, 65, e12492. [Google Scholar] [CrossRef]
- Lee, H.; Byeon, Y.; Back, K. Melatonin as a signal molecule triggering defense responses against pathogen attack in Arabidopsis and tobacco. J. Pineal Res. 2014, 57, 262–268. [Google Scholar] [CrossRef]
- Liu, C.; Chen, L.; Zhao, R.; Li, R.; Zhang, S.; Yu, W.; Sheng, J.; Shen, L. Melatonin induces disease resistance to Botrytis cinerea in tomato fruit by activating jasmonic acid signaling pathway. J. Agric. Food Chem. 2019, 67, 6116–6124. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, L.; Su, T.; Jiang, Y.; Hu, L.; Ma, F. Melatonin regulates carbohydrate metabolism and defenses against Pseudomonas syringae pv. tomato DC3000 infection in Arabidopsis thaliana. J. Pineal Res. 2015, 59, 109–119. [Google Scholar] [CrossRef]


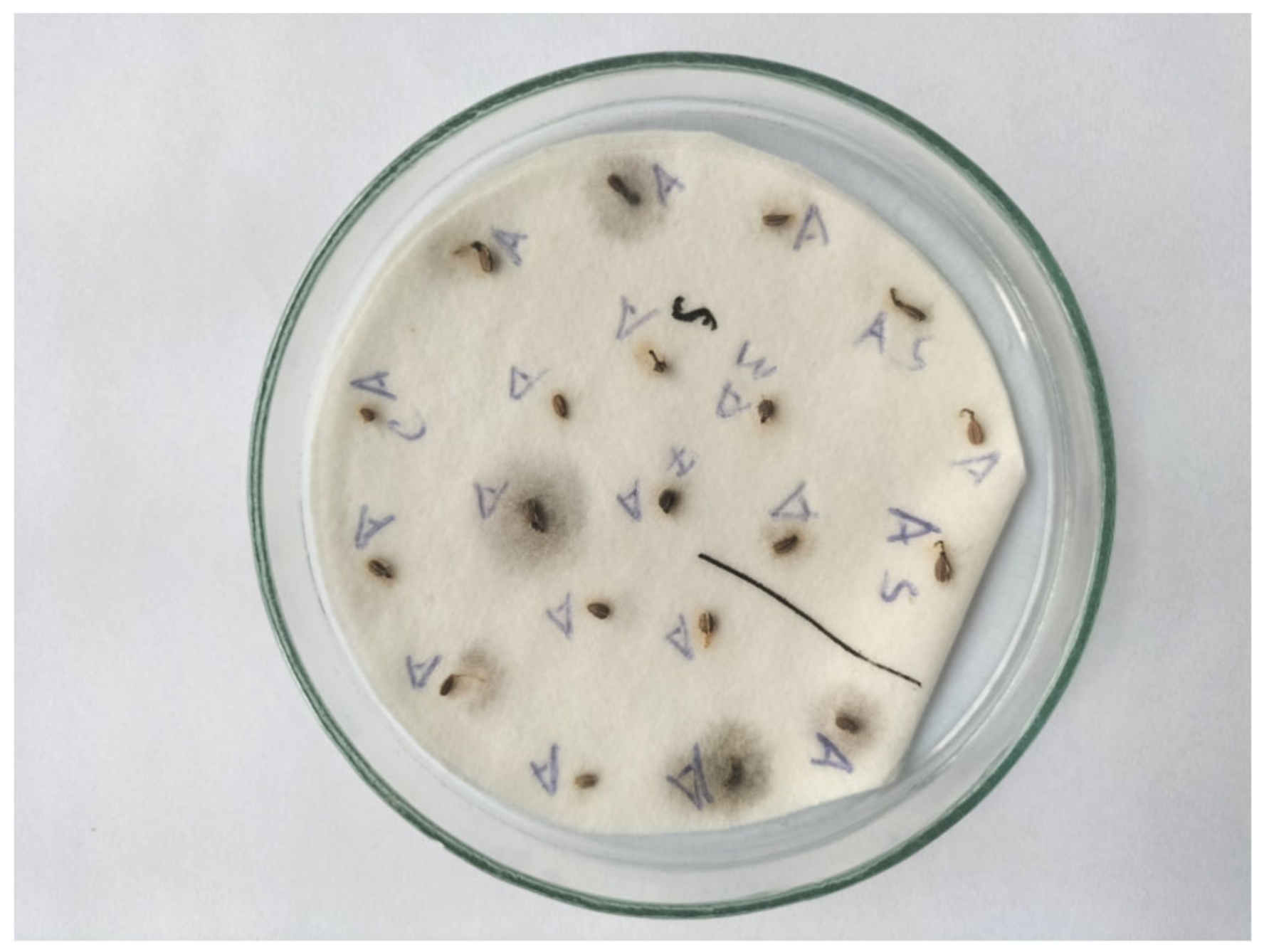


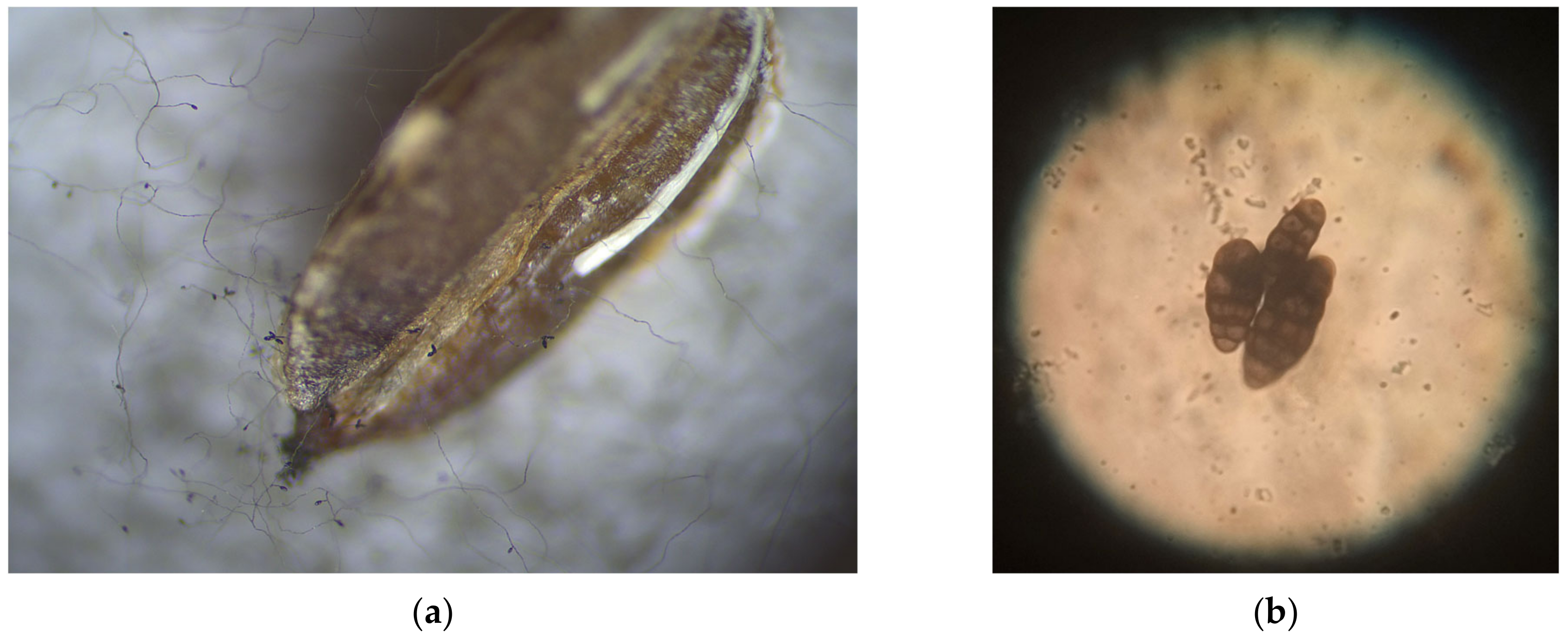
| Seed Treatment | Germination Capacity | Abnormal Diseased Seedlings | Abnormal Deformed Seedlings | Fresh Seeds | Dead Seeds | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| At First Count | At Final Count | |||||||||||
| Sample I | ||||||||||||
| U * | 79.0 | a | 79.7 | a | 5.7 | b | 0.7 | a | 2.0 | ab | 12.0 | c |
| F | 92.0 | c | 94.0 | c | 1.0 | a | 0 | a | 0 | a | 5.0 | a–c |
| OS25 | 86.3 | b | 87.7 | b | 2.3 | a | 0 | a | 2.3 | b | 7.7 | bc |
| OS50 | 91.7 | bc | 92.3 | bc | 1.3 | a | 0 | a | 1.3 | ab | 5.0 | a–c |
| OS100 | 89.3 | bc | 94.3 | c | 1.0 | a | 0 | a | 2.0 | ab | 2.7 | a |
| OS200 | 89.7 | bc | 91.7 | bc | 1.0 | a | 0 | a | 4.3 | b | 3.0 | ab |
| Sample II | ||||||||||||
| U | 46.3 | a | 46.3 | a | 31.0 | d | 0 | a | 5.7 | a | 17.0 | b |
| F | 87.7 | cd | 93.7 | d | 0.7 | a | 1.0 | a | 2.7 | a | 2.0 | a |
| OS25 | 82.3 | bc | 83.0 | b | 11.3 | c | 0.3 | a | 1.3 | a | 4.0 | a |
| OS50 | 89.0 | d | 90.0 | cd | 5.0 | b | 0.7 | a | 2.0 | a | 2.3 | a |
| OS100 | 81.7 | b | 85.3 | bc | 6.3 | b | 0 | a | 6.0 | a | 2.3 | a |
| OS200 | 83.0 | b-d | 86.0 | bc | 9.0 | bc | 0 | a | 1.7 | a | 3.3 | a |
| Seed Treatment | T1 ** | T25 | MGT | U75-25 | ||||
|---|---|---|---|---|---|---|---|---|
| Sample I | ||||||||
| U * | 1.4 | c | 1.93 | b | 2.44 | b | 0.71 | a |
| F | 1.11 | b | 1.95 | b | 2.52 | b | 0.99 | a |
| OS25 | 0.45 | a | 1.33 | a | 1.90 | a | 1.06 | a |
| OS50 | 0.48 | a | 1.40 | a | 1.97 | a | 1.06 | a |
| OS100 | 1.31 | bc | 1.94 | b | 2.53 | b | 0.88 | a |
| OS200 | 1.07 | b | 1.90 | b | 2.46 | b | 0.97 | a |
| Sample II | ||||||||
| U * | 1.94 | d | 2.59 | d | 3.13 | d | 0.83 | a |
| F | 2.00 | d | 2.68 | d | 3.34 | e | 0.95 | a |
| OS25 | 0.99 | a | 1.75 | a | 2.23 | a | 0.85 | a |
| OS50 | 1.49 | b | 2.07 | b | 2.50 | b | 0.70 | a |
| OS100 | 1.77 | cd | 2.31 | c | 2.81 | c | 0.72 | a |
| OS200 | 1.68 | c | 2.33 | c | 2.85 | c | 0.81 | a |
| Seed Treatment | Alternaria alternata | Cladosporium spp. | Fusarium spp. | Melanospora simplex | Stemphylium botryosum | Non-Sporulating Hyphae | Seeds Free of Fungi | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| U * | 80.5 | d | 50.5 | c | 36.5 | e | 50.0 | b | 18.5 | ab | 11.0 | a | 1.0 | a |
| F | 16.5 | a | 6.0 | a | 4.0 | a | 0.5 | a | 10.0 | a | 10.5 | a | 59.0 | b |
| OS25 | 51.0 | b | 37.0 | b | 20.5 | cd | 46.5 | b | 24.5 | b | 30.5 | c | 2.5 | a |
| OS50 | 75.0 | cd | 40.5 | b | 22.0 | d | 42.0 | b | 27.5 | b | 50.0 | d | 1.5 | a |
| OS100 | 65.0 | bc | 34.0 | b | 12.0 | b | 41.5 | b | 27.5 | b | 41.5 | d | 2.5 | a |
| OS200 | 61.5 | bc | 41.5 | bc | 13.0 | bc | 39.0 | b | 24.0 | b | 19.0 | b | 0 | a |
| Seed Treatment | Alternaria alternata | Alternaria radicina | Cladosporium spp. | Fusarium spp. | Melanospora simplex | Stemphylium botryosum | Non-Sporulating Hyphae | Seeds Free of Fungi | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| U * | 97.0 | d | 25.0 | b | 29.5 | b | 8.5 | b | 28.0 | b | 12.0 | bc | 4.0 | a | 0 | a |
| F | 27.5 | a | 3.0 | a | 3.5 | a | 1.5 | a | 0 | a | 6.0 | a | 7.0 | ab | 58.5 | b |
| OS25 | 84.0 | c | 13.5 | a | 31.0 | b | 2.5 | a | 33.5 | bc | 18.0 | c | 18.0 | c | 0 | a |
| OS50 | 69.5 | b | 9.0 | a | 30.0 | b | 1.5 | a | 38.0 | c | 17.5 | c | 44.5 | d | 0.5 | a |
| OS100 | 70.0 | b | 4.5 | a | 30.0 | b | 3.0 | a | 32.0 | bc | 13.5 | bc | 11.0 | bc | 0 | a |
| OS200 | 80.0 | c | 8.0 | a | 33.5 | b | 4.0 | ab | 39.5 | c | 8.5 | ab | 9.5 | a-c | 0 | a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosińska, A.; Andrzejak, R.; Kakkerla, V. Effect of Osmopriming with Melatonin on Germination, Vigor and Health of Daucus carota L. Seeds. Agriculture 2023, 13, 749. https://doi.org/10.3390/agriculture13040749
Rosińska A, Andrzejak R, Kakkerla V. Effect of Osmopriming with Melatonin on Germination, Vigor and Health of Daucus carota L. Seeds. Agriculture. 2023; 13(4):749. https://doi.org/10.3390/agriculture13040749
Chicago/Turabian StyleRosińska, Agnieszka, Roman Andrzejak, and Vignan Kakkerla. 2023. "Effect of Osmopriming with Melatonin on Germination, Vigor and Health of Daucus carota L. Seeds" Agriculture 13, no. 4: 749. https://doi.org/10.3390/agriculture13040749
APA StyleRosińska, A., Andrzejak, R., & Kakkerla, V. (2023). Effect of Osmopriming with Melatonin on Germination, Vigor and Health of Daucus carota L. Seeds. Agriculture, 13(4), 749. https://doi.org/10.3390/agriculture13040749






