Abstract
Saffron is a rare and valuable crop that is only cultivated in specific regions with suitable topographical conditions. To improve saffron cultivation, it is crucial to monitor and precisely control the crop’s agronomic variables over at least one growth cycle to create a fully automated environment. To this end, agronomic variables in the Punjab region of India were analyzed and set points were calculated using third-order polynomial equations through the application of image processing techniques. The relationship between canopy cover, growth percentage, and agronomic variables was also investigated for optimal yield and quality. The addition of adulterants, such as turmeric and artificial colorants, to saffron is a major concern due to the potential for quality compromise and fraud by supply chain vendors. Hence, there is a need for devising an easy, reliable, and user-friendly mechanism to help in the detection of adulterants added to the saffron stigmas. This paper proposes an automated IoT-based saffron cultivation environment using sensors for determining set points of agronomical variables. In addition, a sensor-based chamber has been proposed to provide quality and adulteration checks of saffron and to eliminate product counterfeiting. The AquaCrop simulator was employed to evaluate the proposed framework’s performance. The results of the simulation show improved biomass, yield, and harvest index compared with the existing solutions in precision agriculture. Given the high value and demand for saffron, ensuring its purity and quality is essential to sustain its cultivation and the economic viability of the market.
Keywords:
IoT; saffron; artificial cultivation; hydroponics; saffron adulteration; saffron quality; AquaCrop 1. Introduction
Saffron, botanical name Crocus Sativa, is a precious spice known as one of the most important and scantily cultivated agricultural products. One kg of saffron is derived from about 150,000 flowers, resulting in a high cost. Due to numerous medicinal qualities and pharmacological applications, it is one of the highest-valued spices. The saffron flower comprises only three stigmas that are beneficial in many applications, including usage as a food additive due to its rich aroma, bright color, and bitter taste. Although efforts are continuously being made to increase productivity and to increase areas under cultivation, several constraints are limiting these efforts [1]. Automated environments using Internet of Things (IoT) sensors can help in cultivating saffron in an artificial environment. Challenges in artificial saffron cultivation include the identification of optimal values of agronomical variables significant for growth. Agronomical variables are defined as the important parameters that impact the growth of saffron, such as temperature, humidity, and corm size [2]. The cultivation of saffron is a tedious process, requiring conscious human efforts to pluck stigmas with care so that plant remains unharmed [3,4]. While drying the stigmas for storage, care must be taken to maintain low temperatures for drying (below 55 °C) so that the benefits of the stigmas remain intact [5,6]. Another reason for compromised quality is the absence of proper storage spaces near the harvesting spaces, which are designed as per scientific norms, providing dry and dark ambiance so that the crop is protected from rots.
Due to the immense economic potential of saffron, adulteration is common these days. Various similar substances such as beet, turmeric, pomegranate fibers, artificial colorant, inexpensive plants, and additives are added, resulting in a low-cost product with high profit margins [4,5,6]. Substances such as glycerin, honey, etc., are used for immersion to increase saffron mass. All the enlisted adulteration methods result in a change of chemical composition and compromise in benefits associated with saffron.
Iran is the largest saffron-producing nation in the world, with 220 tons per year, followed by Spain, Greece, Italy, Turkey, and India. About 90% of the total production of saffron comes from Iran. The total area under saffron production has reduced from 5.707 thousand hectares in 1997 to 3.674 thousand hectares. This has resulted in a decline of annual production and productivity from 15.95 tons and productivity of 2.8 kg/ha to an annual production of 9.6 tons and 2.61 kg/ha of productivity [7,8,9,10]. This trending decline in the saffron production due to factors such as counterfeiting and adulteration has made its impact on the Indian subcontinent as well. The absence of a standardized quality control mechanism and certification makes the situation worse, leading to the loss of Kashmiri saffron value. To make the Indian-produced saffron a recognized brand that is economically potent, quality analysis should be provided as per ISO standards. The production of saffron worldwide is estimated at 300 tons per year and the cost per kg is estimated at USD 5000/kg. The market for saffron worldwide is growing at CAGR of 5.8% and is estimated to reach USD 1 billion. There is a demand and supply gap in the saffron market due to the decline in the production of saffron [11].
Problem Formulation
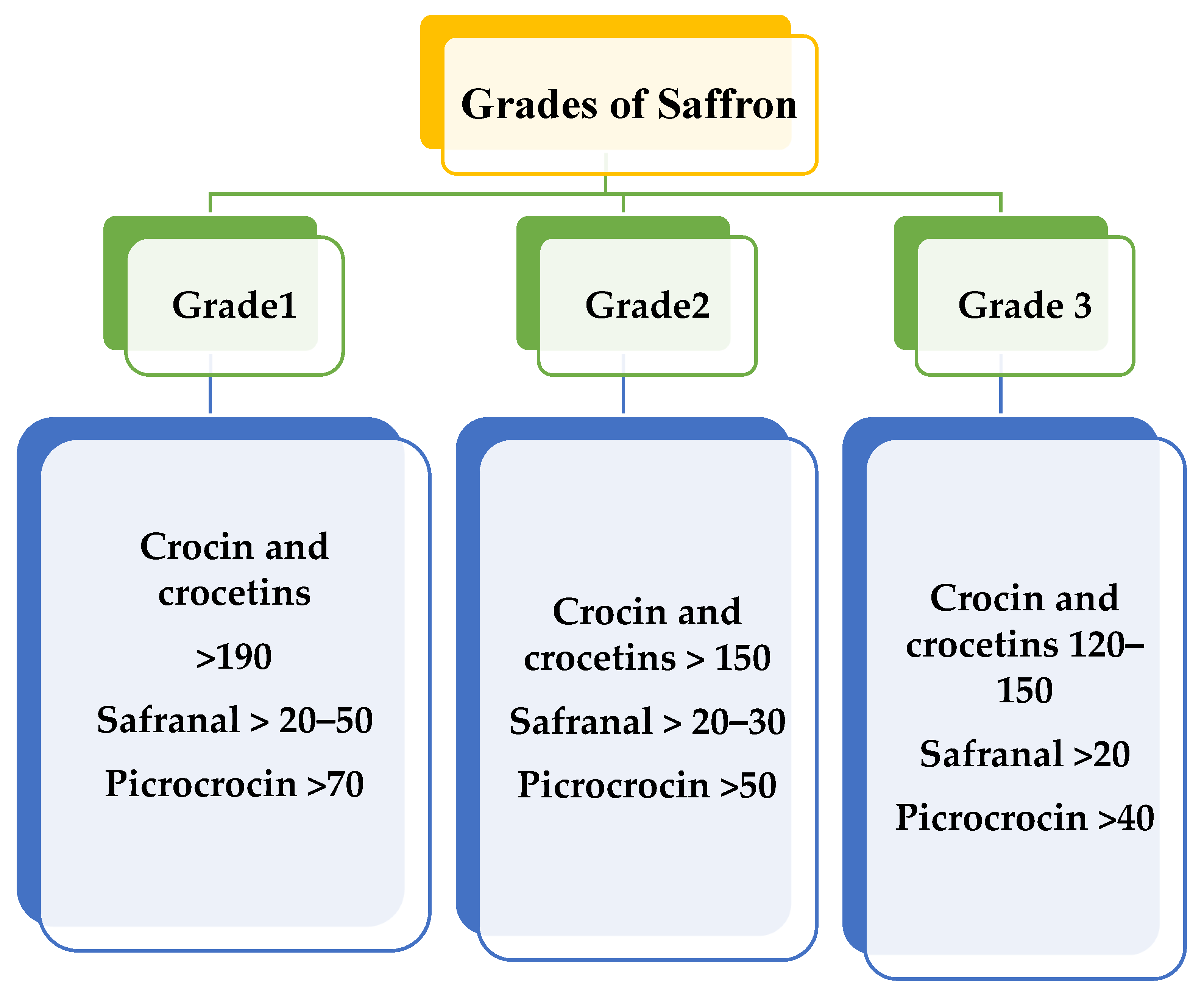
Various factors that decide the quality of the saffron crop and that are responsible for its grading include moisture content, coloring intensity depending on the pigment crocin, aroma strength depending on safranal, and flavor depending on picrocrocin. As per the presence of the above pigments, saffron is graded mainly into three classes, Grade 1, Grade 2, and Grade 3 in order of their quality, as shown in Figure 1. The grading of saffron is influenced by factors such as taste, presence of mycotoxins, color, smell, moisture content, and presence of other floral parts. Grade 3 is the lowest grade of saffron, followed by Grade 2, with Grade 1 being the purest. Grading is governed by ISO 3632, based on 440-nm light absorbency. High absorbency indicates a high concentration of crocin and safranal. As per the standards, Iranian saffron is classified as Grade 1. Indian saffron, also known as Mongra, falls into Grades 2 and 3 due to the intact styles of the flower, which is yellow or white [10,11,12]. The issues with the saffron supply chain involve adulteration and replacement with a similar stigma of plants readily available in the market.
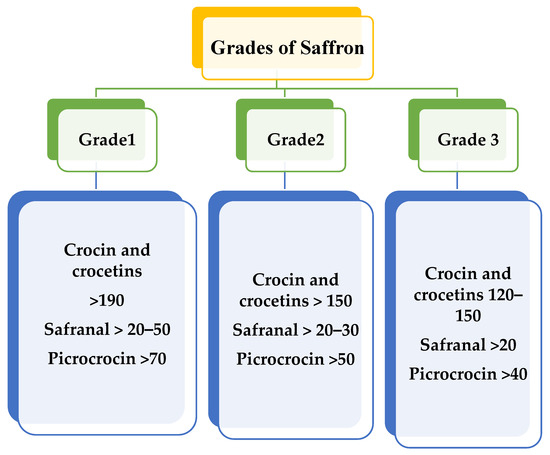
Figure 1.
Grades of saffron based on quality traits.
Therefore, authenticity is a great concern for saffron. There is a requirement to obviate the above-mentioned problems by providing a cost-effective, portable, and reliable solution that can be operated without much complexity. This paper focuses on solving the issues and devising a mechanism to solve issues related to product counterfeiting.
The existing artificial cultivation systems have challenges such as complexity, scalability, and lack of optimization. Other than this, there is no proper analysis of the set points required for the optimal yield in an automated artificial environment. Various substances such as turmeric, artificial colorant, inexpensive plants, and additives are added and sold at the same price, which is a great concern for the authenticity of saffron [11]. Though different techniques are used for quality evaluation, none of them are reliable or less time-consuming. The developed solution is cost-effective, reliable, and can be operated by any person without much complexity.
This paper addresses the stated problems by providing a framework used to monitor and control all the important agronomical variables of saffron for optimized saffron yield and detection of adulteration in saffron. The optimization of the model is performed by using regression analysis and machine-learning techniques that show the variance of growth percentage and canopy cover concerning changes in temperature, humidity, and corm size. The designed framework for quality analysis is portable and easy to operate, thus enabling a user to perform various tests at a time to detect the quality of the saffron. Moreover, adulteration in the saffron is analyzed, which is displayed using a LED, enabling the user to test the quality of the saffron easily. This manuscript provides a framework for performing various tests at a time to detect adulteration in saffron. It enables a user to perform the test with cost-efficiency and reliability. The paper provides the following contributions:
- Designing an automated IoT-based saffron cultivation environment using sensors by determination of set points for agronomical variable control.
- Designing a four-layer mechanism for the identification of adulterants added to the saffron.
- Detailed study of the impact of agronomical variables on growth percentage and its optimization.
2. The Literature Survey
Saffron also known as “Red Gold”, is a rare and expensive spice out of all the 85 known spices in the world. Cultivation and harvesting of saffron are carried out traditionally by hand, which is a time-consuming and laborious process [8]. The color and aroma of saffron that are important for quality analysis are determined by different techniques such as gas chromatography, UV/Vis spectrometry, High Performance Liquid Chromatography (HPLC), and mass spectrometry. The other existing solutions include chromatography and spectroscopy, which are expensive, difficult to be performed at home, and are also time-consuming. There is a lack of analytical methods that are easy, less time-consuming, and reliable to grade saffron [12]. Different researchers have focused on the use of using different analytic methods for saffron cultivation. Different regression techniques have been used by them such as variance and Tukey tests [13,14,15,16]. All the papers suffer from one or more limitations as stated. Researchers have studied the quality of saffron using different techniques. The detailed studies related to saffron quality analysis and techniques used by researchers have been listed in Table 1. However, not all the metrics have been considered by them.

Table 1.
Various saffron quality analyses and adulteration check techniques.
In [11], Luca Giupponi et al. studied the quality traits of Italian saffron based on various ISO standards. The data consisted of 484 samples collected over 4 years. Various variables affecting the quality such as aroma strength, coloring strength, and flavor strength were studied for each sample. Analysis of pigments responsible for the above stated qualities such as safranal, crocin, and picrocrocin, respectively, was also performed.
Poursorkh et al. [12] used a Thin Layer Chromatography (TLC) and image analysis method for the study of quality and to identify adulteration in saffron. TLC is widely used to perform analysis of carotenoids in food. These methods provide successful results in the case of clustering and classification based on smaller sample sizes; however, Linear Discriminant Analysis (LDA) cannot be used as a potential solution for large sample sizes.
Mohammad and Hossein et al. [13] presented a genetic study and method for barcoding Iran’s saffron. Different techniques used included: Inter Simple Sequence Repeat (ISSR) and Chloroplast DNA (trnH-psbA). High Resolution Melting (HRM) analysis along with real-time PCR assay were also used in this study. Analysis of Molecular Variance (AMOVA) among saffron produced in different regions produced significant genetic differences among the studied saffron populations. To study the data obtained and the identification of suitable molecular markers, Data-centered Correspondence Analysis (DCA) was used. cp-DNA sequences were used to identify genotype groupings of saffron and the separation of Iranian saffron from the world based on generated barcode sequences.
In [14], the authors have proposed a setup to evaluate the quality traits of saffron using Principal Component Analysis (PCA). The apparatus was designed to detect both saffron adulteration and pure saffron separation from adulterants. The saffron adulteration was detected satisfactorily. However, the system could only identify adulterants when their percentage exceeded 10%. Pure saffron was mixed with safflower and beetroot dye extracts in 10 to 50% proportions and analyzed 15 times by an electronic nose. However, this setup requires an oxygen cylinder and a data acquisition card, which makes it heavy and complex to be operated by the farmer.
In [15], an integrated system using e-nose and Computer Vision System (CVS) was designed for detecting saffron adulteration. The color and aroma characteristics of approximately ten samples containing adulterants such as artificially colored styles of saffron and safflower were examined. For analysis, Support Vector Machines (SVMs) and Hierarchical Cluster Analysis (HCA) were used. Dataset analysis revealed the presence of adulterants and a success rate of 89% was achieved. However, the system is based on complex methods of PCA and Hierarchical Cluster Analysis (HCA), which are not farmer-friendly. Furthermore, the identification of eleven parameters for analysis is a lengthy and cumbersome process.
In [16], different traditional and sensor-based techniques aimed at detecting saffron adulteration are discussed. The authors used spectroscopic and chromatographic techniques in addition to sensor and molecular techniques for adulterant determination. Quality compromise in saffron due to chemical adulterants leads to health complications. The authors thoroughly studied various techniques used for identifying chemical and natural adulterants. The simplest methods to perform adulteration using water for immersion to check quality have also been proposed. On immersion, the original saffron strand changes to a cone-like shape and releases color slowly, unlike dyed saffron, which releases color instantly. The authors also suggested the use of color sensors and software packages for the detection and analysis of impurities.
The detailed analysis of different studies carried out by researchers is summarized in Table 1. The features and the shortcomings of different techniques used by researchers in Precision Agriculture (PA) for evaluating the quality of saffron are summed up in Table 1.
The analysis from the study of related research papers discussing different artificial cultivation techniques and metrics considered for saffron cultivation are presented in Table 2.

Table 2.
Different artificial techniques for saffron cultivation.
3. Proposed System Methodology
The proposed solution includes two steps; saffron cultivation analysis in artificial and natural medium and quality analysis of saffron to check adulterants added and percentage purity.
3.1. Artificial Saffron Cultivation
To study the effect of optimized agronomical variables on saffron cultivation, parallel cultivation was conducted in both natural and artificial environments. For the artificial environment, an automated IoT-based environment was created for the hydroponic cultivation of plants. High-quality corms were used for sowing in both environments. The corms were planted at the end of September and are currently under observation. The data from temperature and humidity sensors are constantly analyzed to find the best-optimized environment for maximum yield and quality [31,32,33,34]. The setup for artificial soilless cultivation and naturally growing corms is shown in Figure 2.

Figure 2.
(A) Saffron corms dried after treatment with fungicidal solution;(B) hardware components for artificial environment; (C) artificial saffron cultivation set up;(D) saffron corms sown in natural medium; (E) sprouting of saffron corms;(F) flowering of corms.
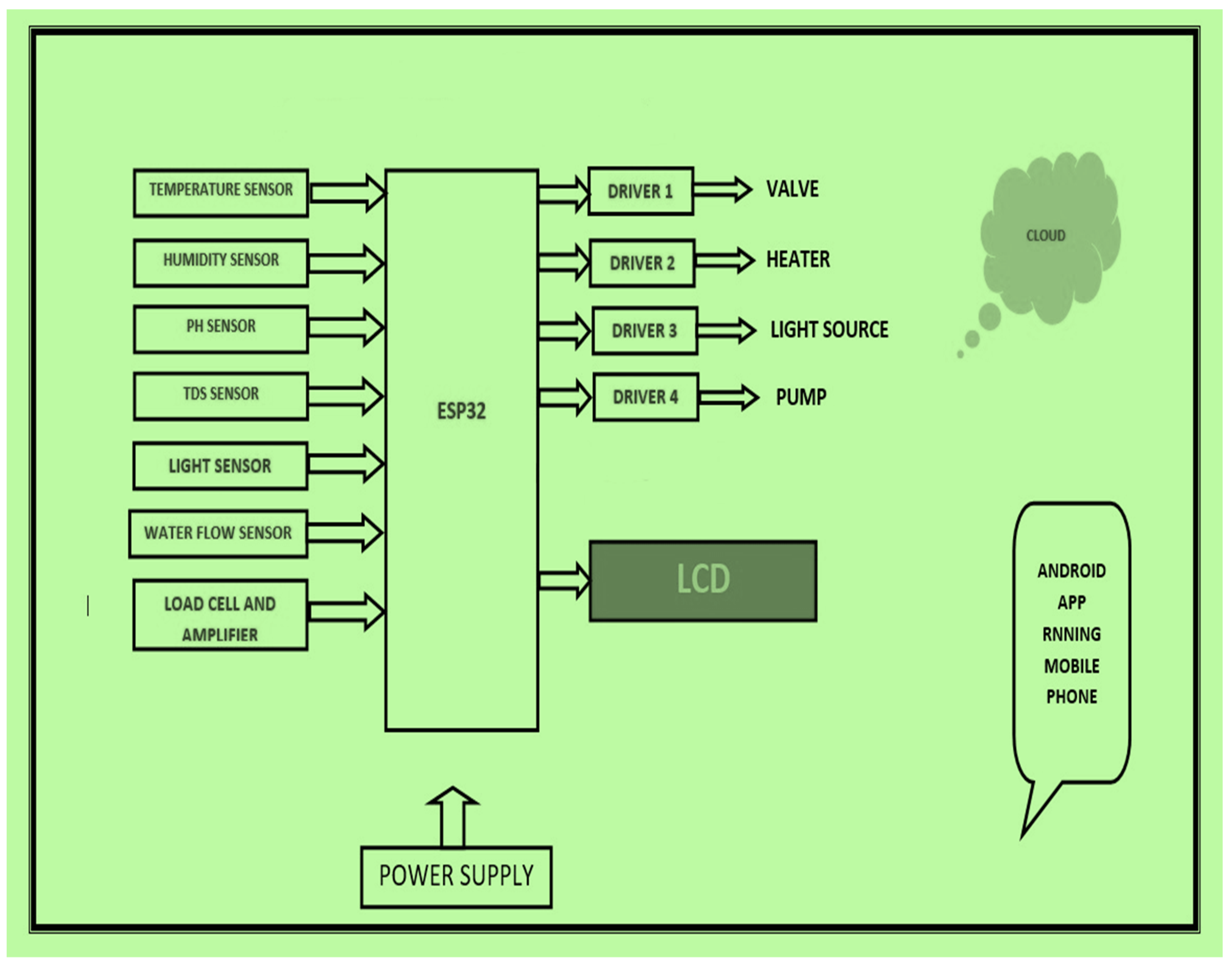
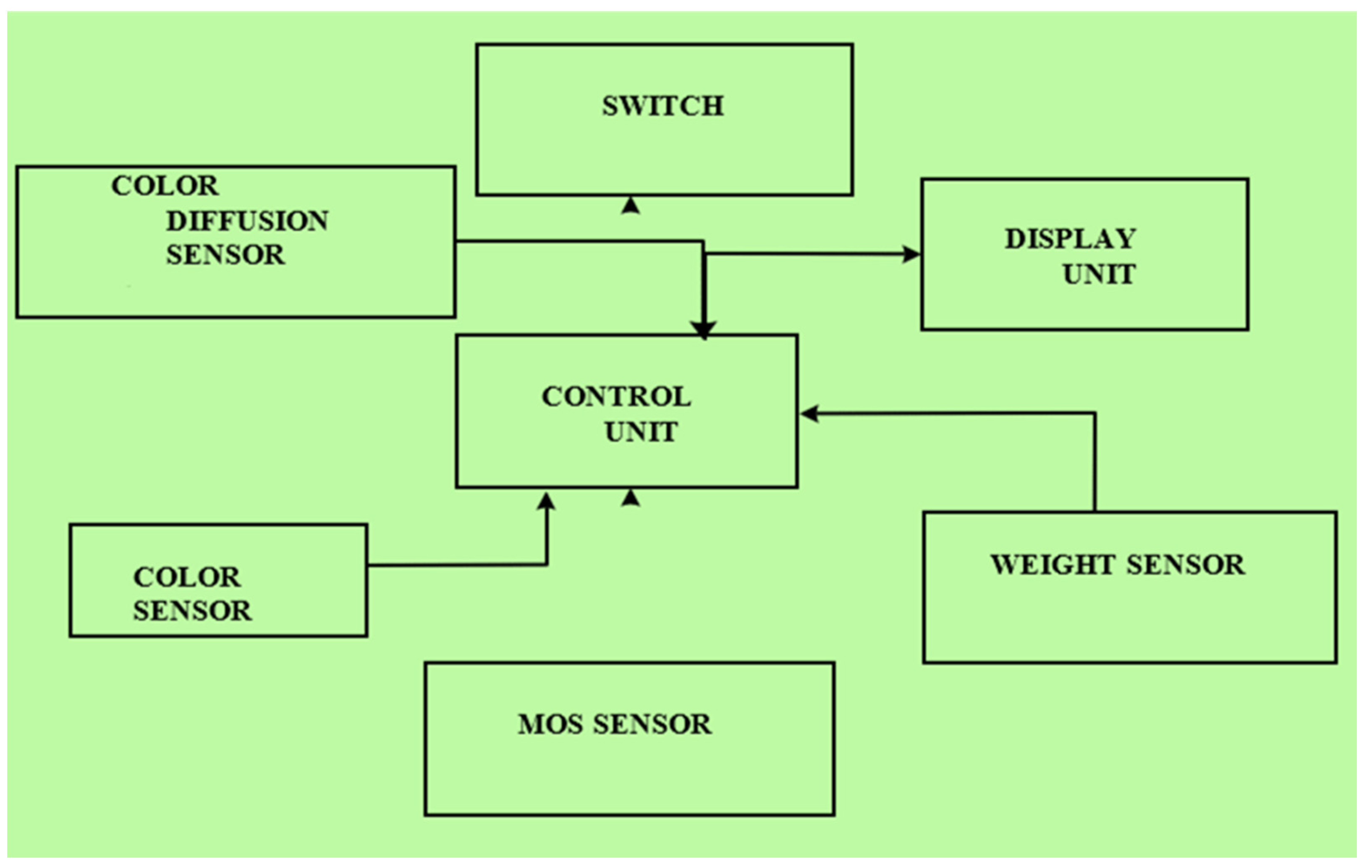
These sensors monitor and control parameters such as temperature, humidity, weight, turbidity, pH, water flow, and relay. The block diagram of the artificial system for saffron cultivation is given in Figure 3. It consists of an array of sensors discussed in detail in Table 3. The sensors are connected to the ESP 32 chip, which supports inbuilt networking on a highly scalable level in both Bluetooth and Wi-Fi mode. The different IoT sensors and components used are listed in Table 3.
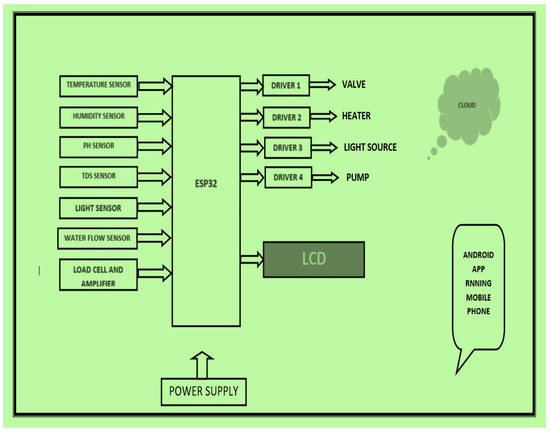
Figure 3.
Block diagram of artificial saffron cultivation set up.

Table 3.
Hardware components of optimized cultivation system.
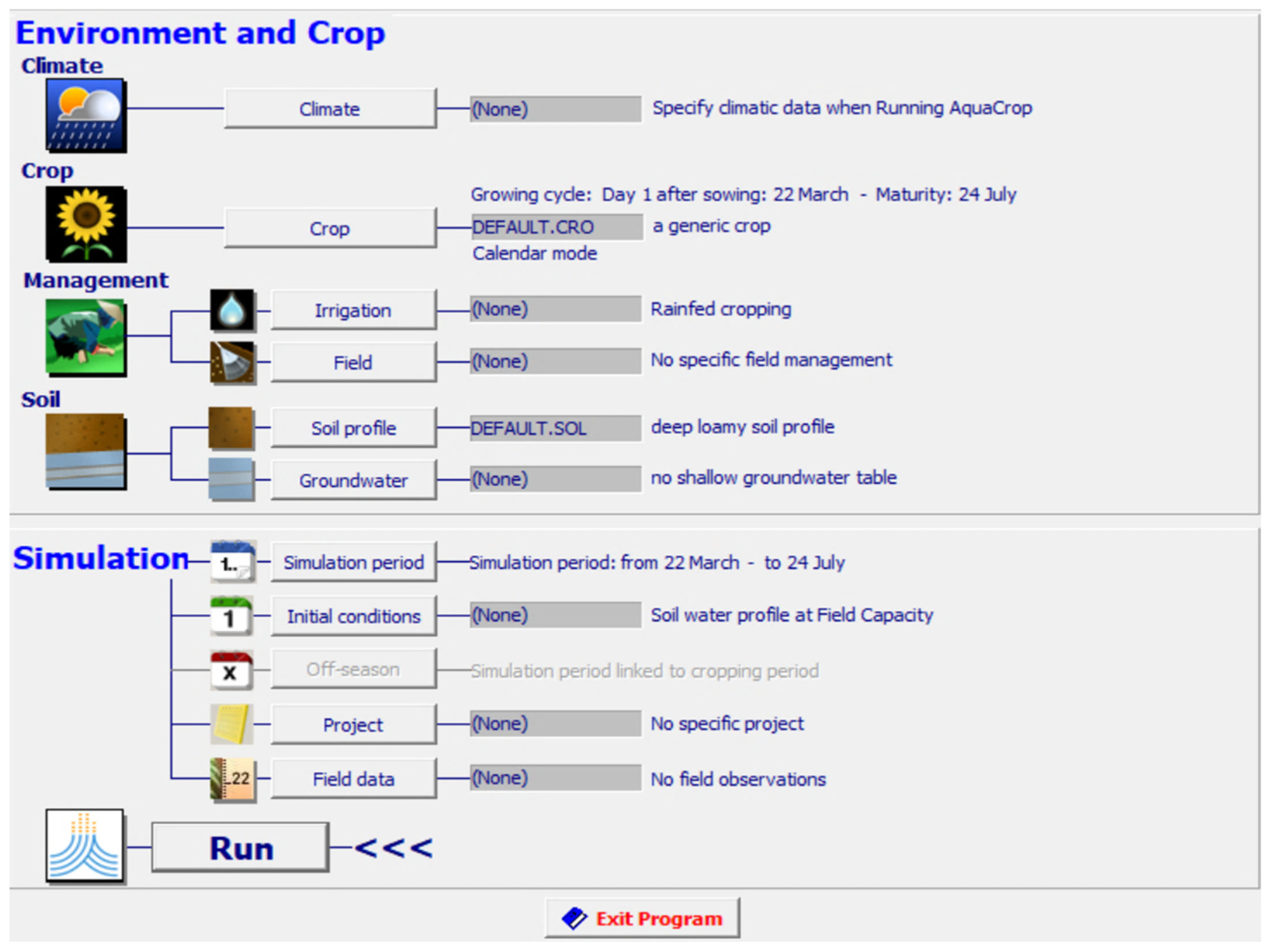
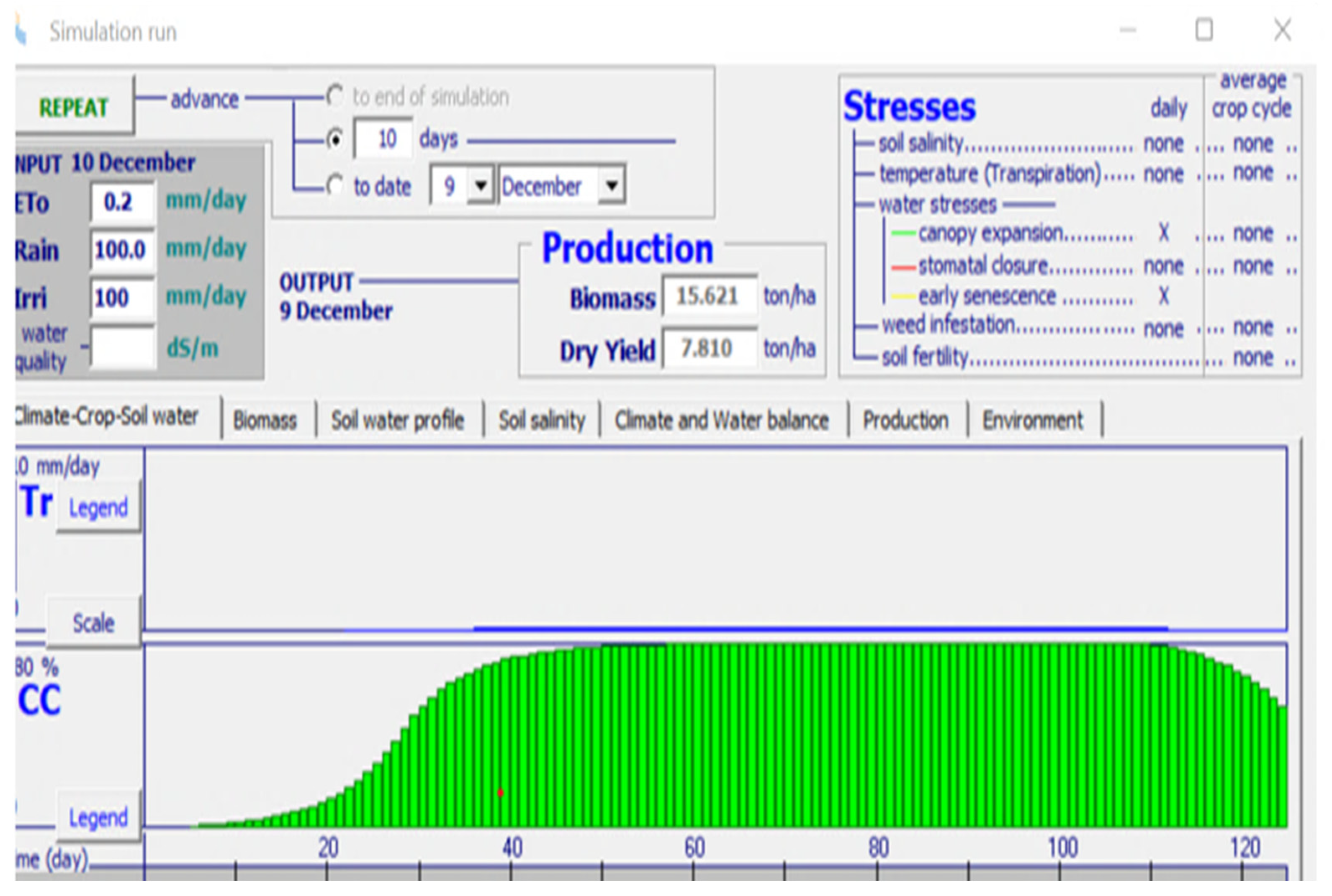
The data from the sensors are analyzed using an AquaCrop simulator. It is a crop growth model which was developed by the land and water division of the Food and Agriculture Organization (FAO) in 2009. AquaCrop is used to simulate the impact of water on the different parameters of growth such as yield and biomass of crops. It has been parameterized for the majority of field crops. It simulates the response of yield to different water conditions and impact on herbaceous crops. It is of great importance in studying conditions where water is a limiting factor in crop production. AquaCrop is used to compare attainable and actual yields. The screenshot of input parameters considered in the AquaCrop simulator for artificial saffron cultivation is shown in Figure 4.
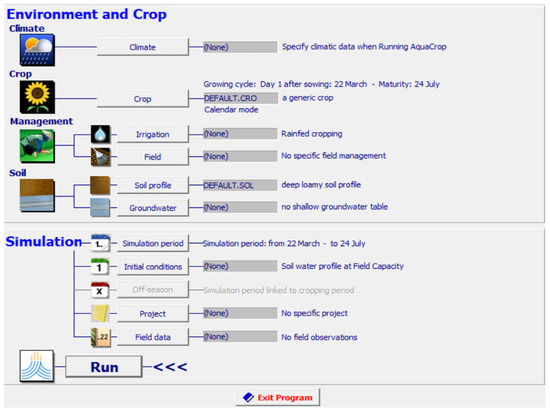
Figure 4.
Screen shot of input parameters for simulation in AquaCrop.
The climate file includes a yearly analysis of temperature and humidity in the region of Punjab, India where the optimized framework was installed for study. Crop File includes details related to the saffron crop. Other parameters such as irrigation schedule, soil texture, and moisture are also specified before running the simulations.
3.2. Proposed Framework for Quality Analysis
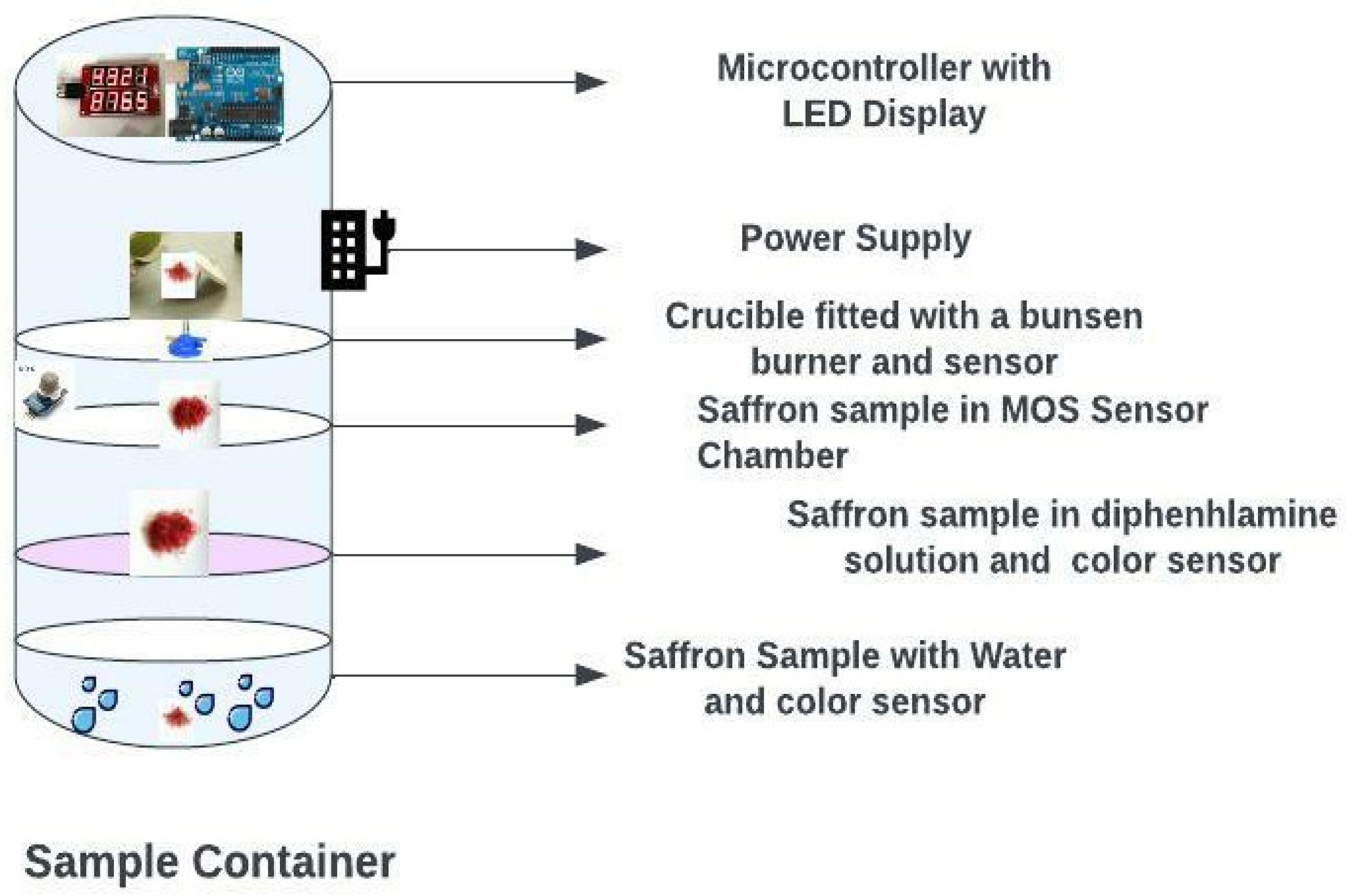
The framework for the saffron quality check comprises a body, four compartments, and a control unit, as shown in Figure 5.
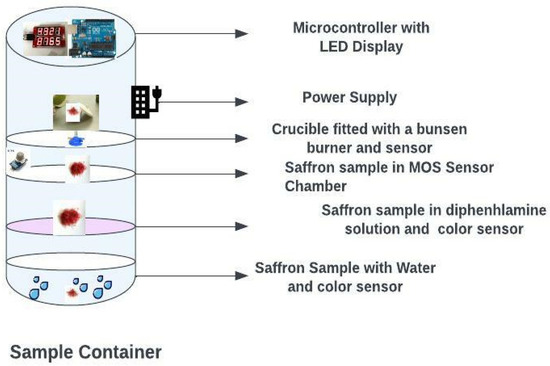
Figure 5.
Smart saffron quality check chamber.
All the compartments receive samples of saffron to perform different tests for quality determination. A rim is provided at the top of each compartment to hold the other compartments in place. The first compartment is designed to store water and a color diffusion sensor [35]. The color diffusion sensor is configured to detect the color of water upon receiving one or more strands of saffron in the water. In the first compartment, when the first sample (i.e., one or more strands of saffron) is dipped in water, the color diffusion sensor detects the color of the water upon receiving the first sample [36]. For example, real saffron strands change the color of the water into a yellowish-golden hue and the color release is slow and steady without losing its deep reddish color. Additionally, when the real saffron strands are taken out of the water, the original deep-red color remains the same. However, if the saffron is adulterated, complete or partial discoloration of the saffron strands occurs, and the saffron loses its original deep red color, such as dyed corn silk. The second compartment is attached to the body and configured to store a diphenylamine solution and a color sensor. The color sensor is configured to detect the color of the diphenylamine solution upon receiving one or more strands of the saffron in the diphenylamine solution. When the saffron is adulterated, the diphenylamine solution turns blue, indicating the presence of adulterants rich in nitrates. The third compartment attached to the body has a Metal Oxide Sensor (MOS) coupled to detect one or more gases in one or more strands of the saffron. If the oxygen concentration is detected in the third sample by the MOS, that indicates the presence of impurities in the saffron [37,38,39].
The fourth compartment attached to the body has a burner mounted to burn the received one or more strands of saffron, coupled with a weight sensor to determine the weight of the ash of the burnt one or more strands of the saffron. The fourth sample of the saffron can be positioned in the crucible and burned until turned into ash. Further, the weight of the ash can be detected by an attached weight sensor. If the saffron is real, the ash content is less than eight percent. The control unit consisting of multiple processors is attached to the body and operatively coupled to the color diffusion sensor, the color sensor, the MOS, and the weight sensor to receive collected information, which is analyzed the outcome is shown on a display unit that is coupled to the control unit [40,41]. The control unit operatively coupled to each of the compartments is configured to determine the quality of the saffron and correspondingly displays the quality on the display unit, which is configured to receive the color diffusion speed of one or more strands ofsaffron from the color diffusion sensor.
It is configured to receive the color of the diphenylamine solution to detect the amount of nitrate in the saffron [42]. To analyze the received sample of saffron, one or more gases are used to detect one or more impurities in the saffron. A crucible is positioned at the Bunsen burner to accommodate one or more strands of the saffron [43]. The control unit is configured to receive the weight of ash from the weight sensor and to analyze the percentage of ash concerning the amount of one or more strands of the saffron received in the fourth compartment. The control unit receives information from all the sensors and the collected information is analyzed by the control unit; the outcome is shown on a display unit.
The display unit is made by a combination of Organic Light-Emitting Diodes (OLED), Light-Emitting Diodes (LED), and Liquid Crystal Display (LCD) [44,45]. The display unit includes a touch panel screen to receive input signals from the user.
3.3. Implementation of Proposed Framework
A charging port electrically coupled to a power supply unit is attached to the body. The charging port comprises a charger that is electrically plugged for charging and a power supply unit with a power source. Upon actuation of a switch, electricity is supplied to the color diffusion sensor, the color sensor, the MOS sensor, the weight sensor, the control unit, the display unit, and the switch for the apparatus to start [46]. The apparatus is communicatively coupled with a mobile computing device through wireless communication such as Wireless LAN (WLAN) or Wireless Fidelity (Wi-Fi)). Mobil computing devices such as smart phones, tablets, personal digital assistants, and portable media devices can be used for operating the designed apparatus [47]. During a communication session with the mobile computing device, the apparatus provides a set of machine-readable instructions that are interpreted by the smart phone using the web client or the application [48,49,50]. In this way, the user can check the outcome and test analysis from the apparatus, also on the smart phone. The block diagram of the proposed quality check framework can be seen in Figure 6.
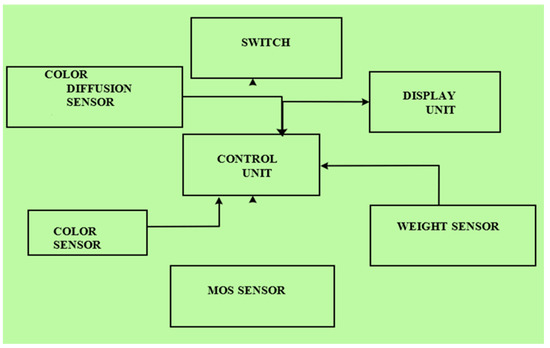
Figure 6.
Block diagram of the quality check chamber.
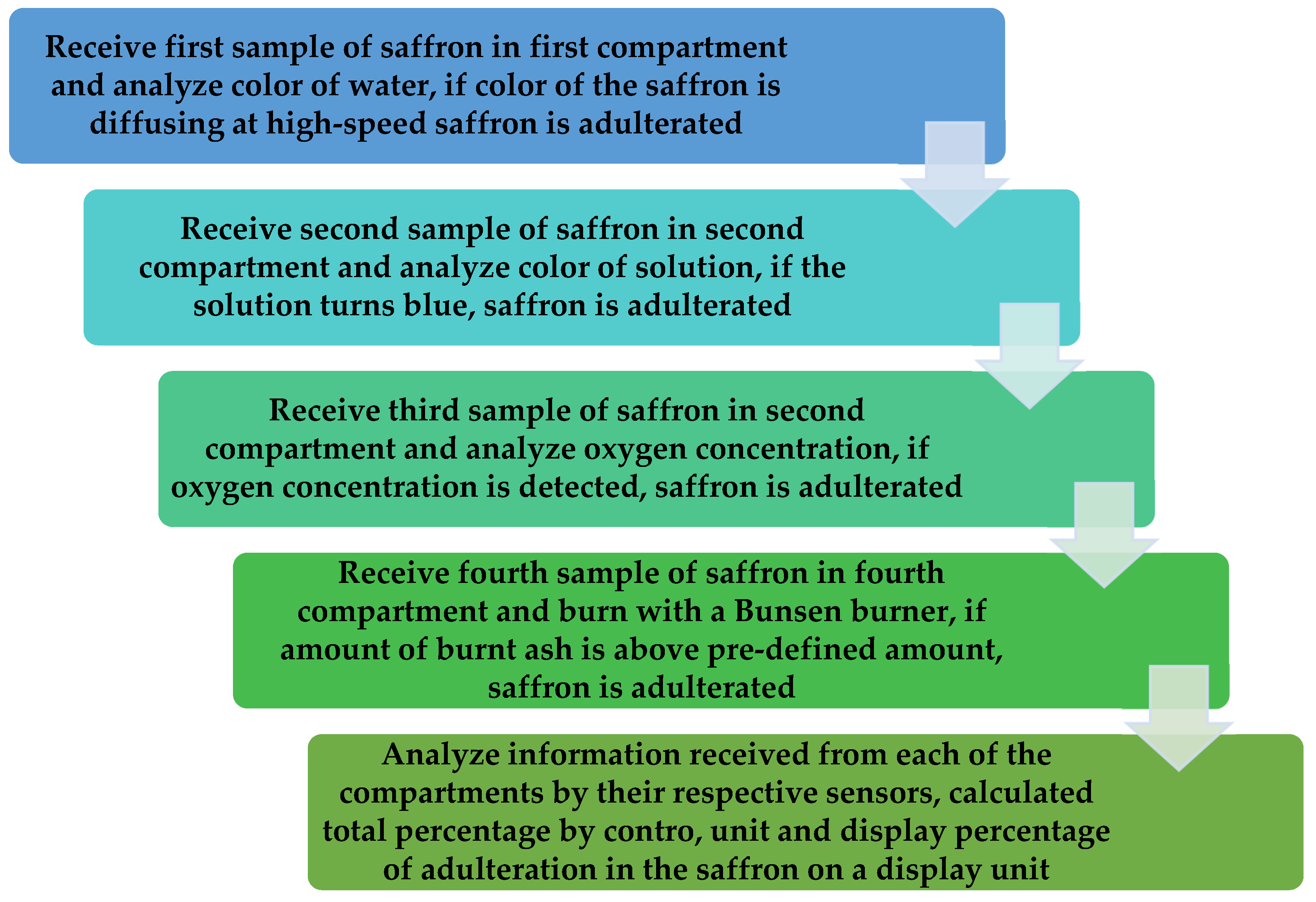
A detailed flowchart to illustrate the functioning of the proposed apparatus is shown in Figure 7. In Step 1, the apparatus receives the first sample of saffron in the first compartment and analyze the color of the water; if the color of the saffron is diffusing at a high-speed, the saffron is adulterated [51,52,53,54].
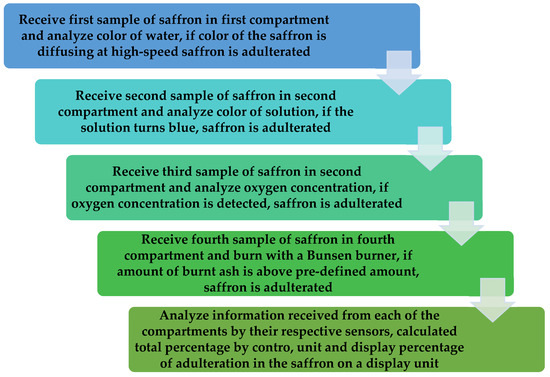
Figure 7.
Flowchart of the quality check chamber.
In Step 2, the apparatus receives a second sample of saffron in the second compartment and analyzes the color of the solution; if the solution turns blue, the saffron is adulterated. In Step 3, the apparatus receives a third sample of saffron in a third compartment and analyzes the oxygen concentration; if the oxygen concentration is detected, the saffron is adulterated. In Step 4, the apparatus receives a fourth sample of saffron in the fourth compartment and burns it with a Bunsen burner; if the amount of burnt ash is above a pre-defined amount, the saffron is adulterated. In Step 5, information is collected in the first compartment, the second compartment, the third compartment, and the fourth compartment from their respective sensors, i.e., color diffusion sensors, color sensors, MOS sensor, and the weight sensor transmitted to a control unit to analyze the receive information and correspondingly display a percentage of adulteration in the saffron.
4. Experimental Results
In previous research works, the control of agronomical variables for optimized yield and quality has not been considered. In this paper, agronomical variables such as temperature and humidity have been used for evaluating the performance of the designed artificial cultivation model. The image-based dataset, shown in Figure 8, collected from traditionally grown saffron, was collected throughout one growth cycle. The data from these images were processed using the AquaCrop simulator to indicate the percentage increase in growth and yield.

Figure 8.
Canopy cover development in different stages of saffron growth. (A) Images shot by camera; (B) images processed by software.
Based on the development of canopy cover (the total ground area covered by native vegetation as viewed vertically) over a growing cycle in percentage, the temperature and humidity set points for optimized yield were obtained. The set points are provided by fitting third-order polynomial equations and using image processing techniques. The set points for the optimal cultivation of saffron are determined depending on the stage of growth, by plotting temperature and humidity values collected over a period of time against the optimal values needed obtained from sensors.
This can be given in the form of Equations (1) and (2). The data used for analysis were from plants growing in the natural environment. The values of humidity and temperature obtained from equations were plotted against percentage growth, i.e., the increase in canopy cover per month. Therefore, in this model, different images were used from a natural cultivation medium and evaluated and calibrated using image processing techniques.
−9.009 × 10−6 x3 + 0.001044x2 + 0.02955x + 0.7957
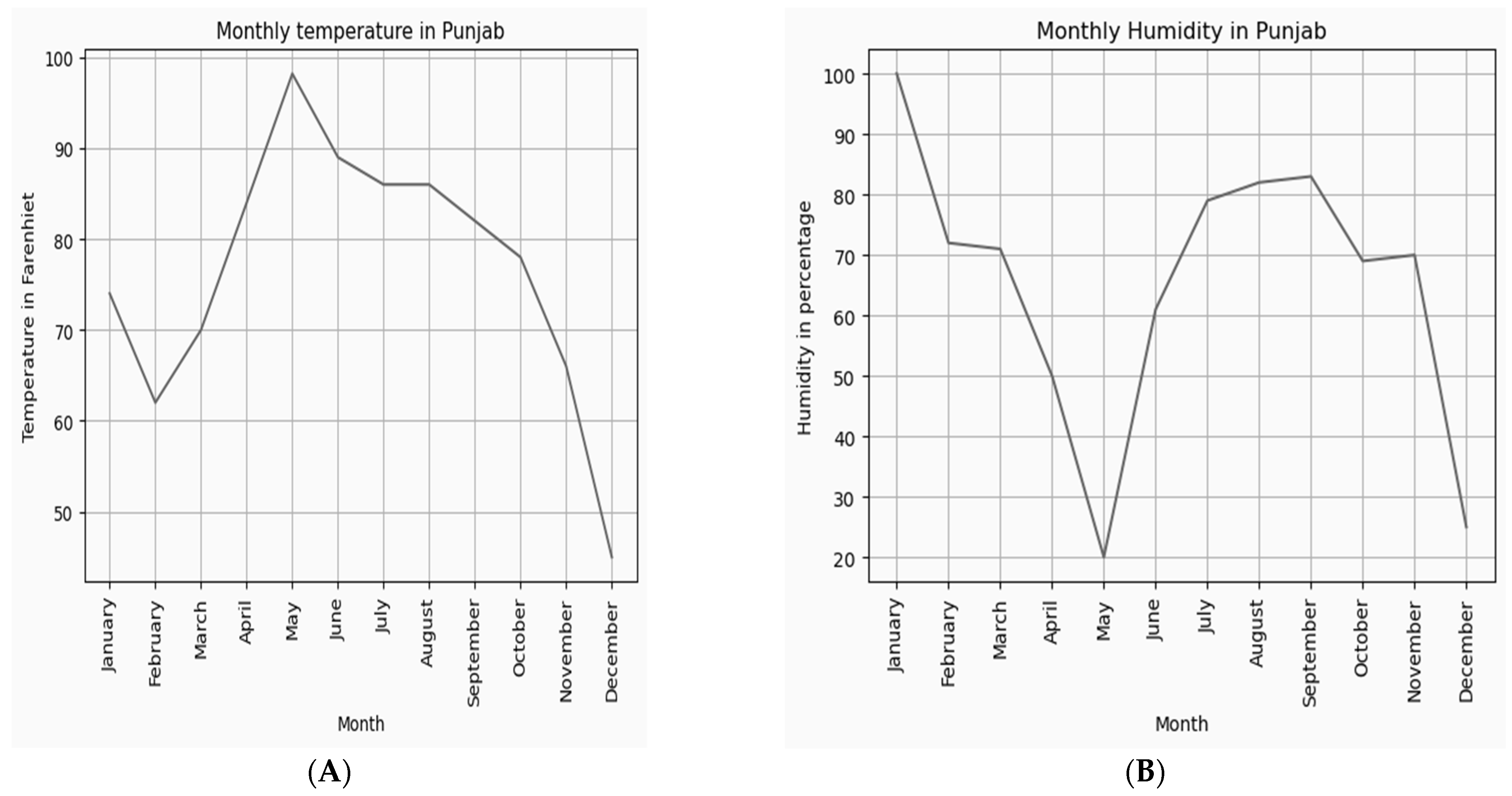
The dataset of temperature and humidity values of the cultivated regions was analyzed annually and plotted, as shown in Figure 9A,B. Optimal growth of saffron requires an average temperature and humidity in the range of (68–86°F) and (60−80%), respectively. The dataset shows that June-November are the best months for natural saffron cultivation.
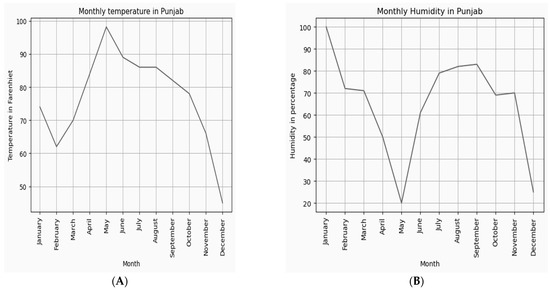
Figure 9.
(A) Average temperature and (B) average humidity values throughout the year.
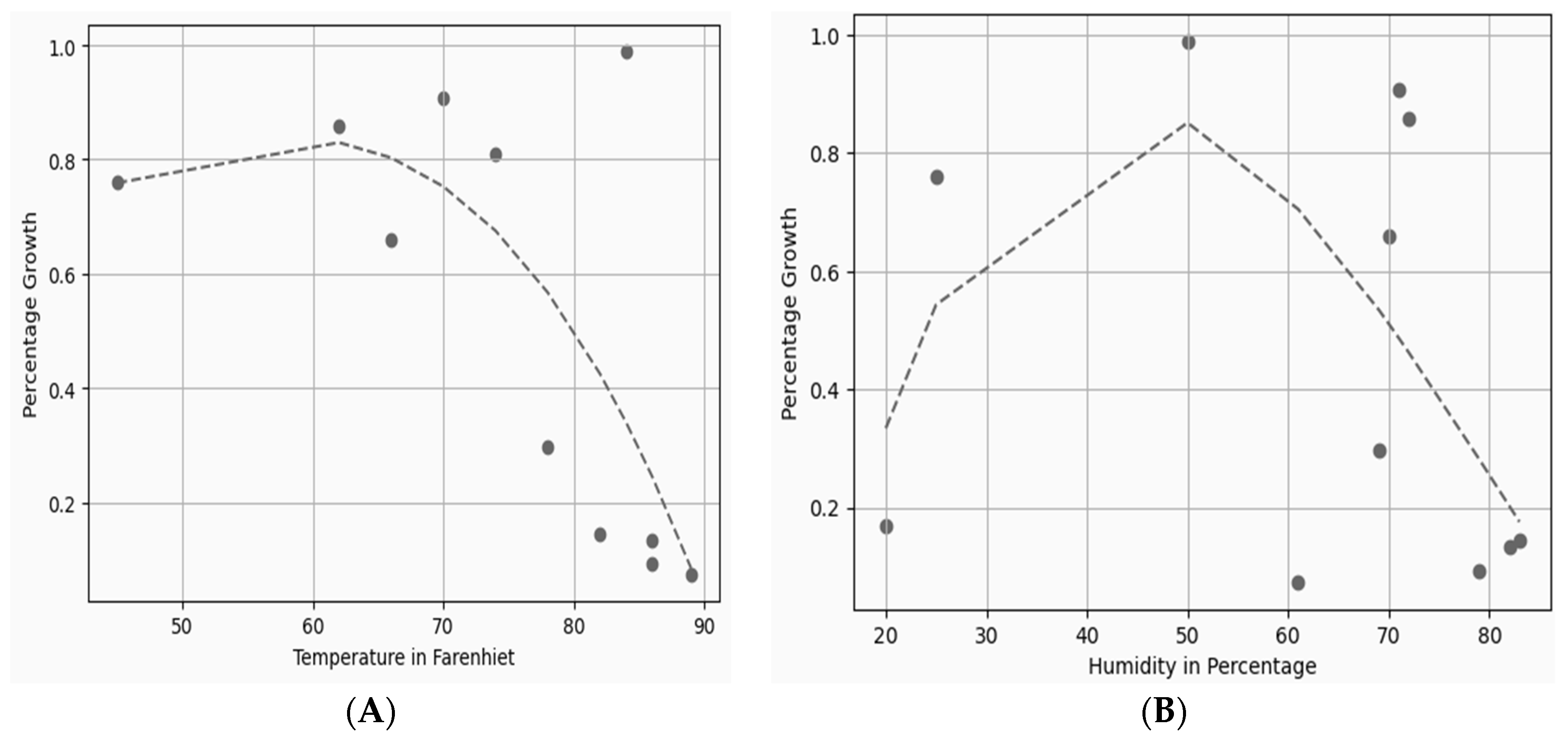
However, if temperature and humidity are monitored along with other growth parameters, it can be cultivated anytime year round in an automated environment. The relationship between percentage growth, temperature, and humidity can be seen in Figure 10A,B. By using curve-fitting Equations (1) and (2), the obtained values plotted in Figure 10A,B indicate much less difference between the predicted and actual measured values in an artificial cultivation environment.
6.652 × 10−6 x3 − 0.001617x2 + 0.1045x − 1.162
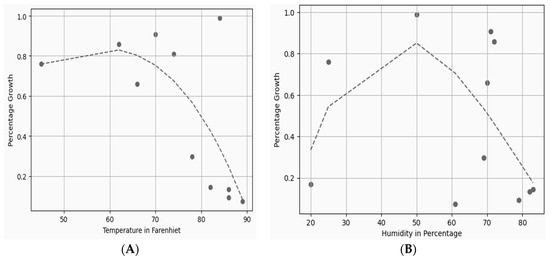
Figure 10.
(A) Temperature and percentage growth plot; (B) humidity and percentage growth plot.
5. Discussion
Cultivation of saffron in a hydroponic medium has been carried out by only a few researchers. AquaCrop is a simulator based on water productivity that is used to evaluate the performance of hydroponic systems. The performance of such systems is measured by using different metrics such as canopy cover, biomass, yield, and harvest index. Canopy cover (the total ground area covered by native vegetation as viewed vertically) is measured over a growing cycle by percentage. Biomass is the measurement of total mass of living tissue obtained after cultivation measured by mass per unit area. The weight of the stigma of flowers divided by the total biomass is the Harvest Index (HI). Crop Yield (Y) is obtained by using the product of biomass and HI − (y = B* HI). Water productivity is the value of biomass produced per meter of water lost in evapotranspiration. After providing the value for input parameters, different simulation results are run, which indicate the initial canopy cover to be very high, as shown in Figure 11.
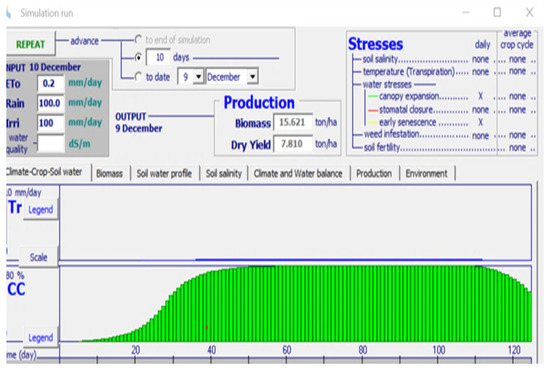
Figure 11.
Canopy cover, biomass, and yield in the AquaCrop model for saffron.
Based on results obtained and comparison with other similar models developed earlier, it was seen that the framework proposed is better in different performance metrics such as initial and maximum canopy cover, flowering, yield formation, and harvest time, as shown in Table 4.

Table 4.
Comparison of models based on performance metrics.
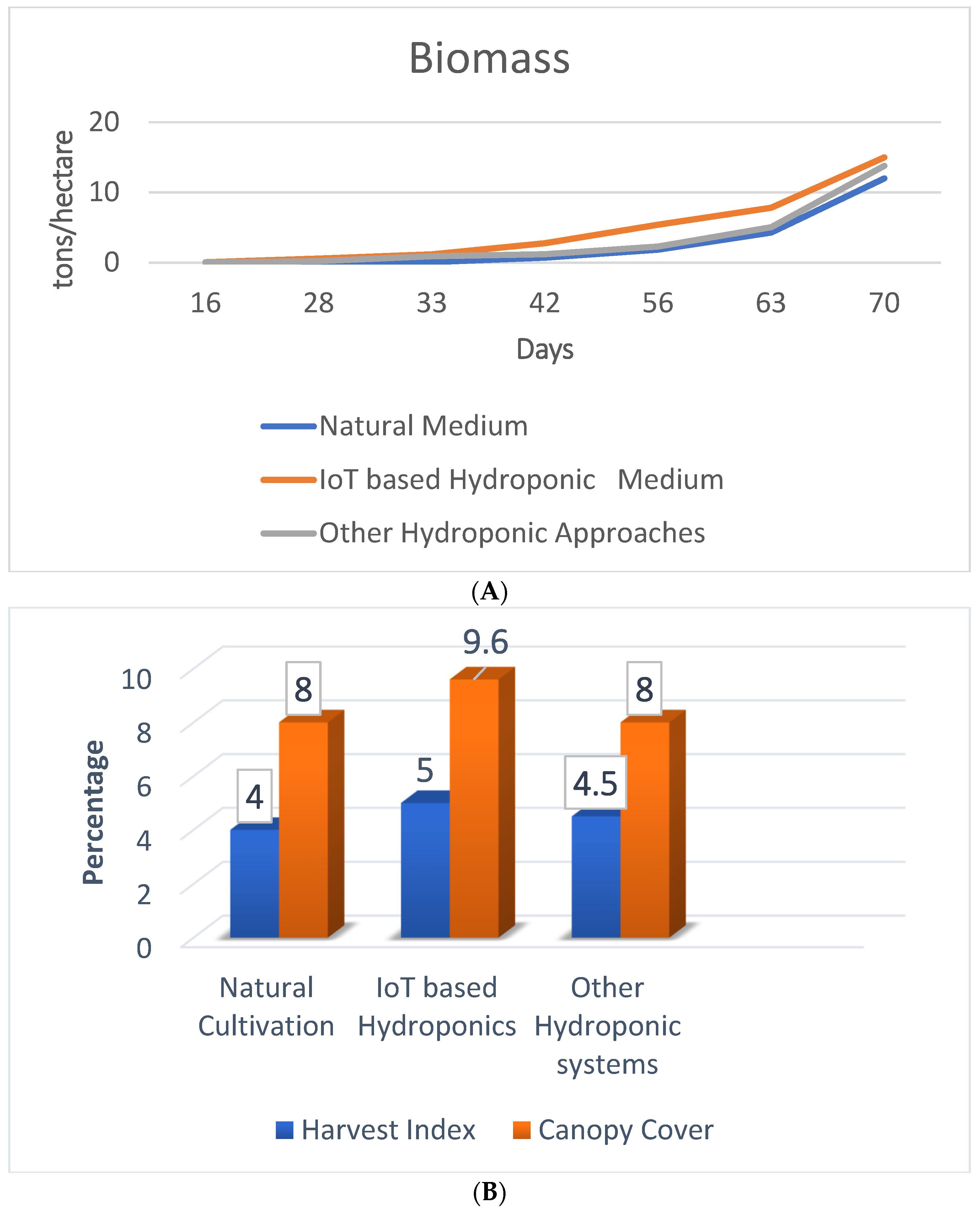
The performance of the IoT-based hydroponic model is best in terms of biomass production, canopy cover, and harvest index, as shown in Figure 12A,B. The higher values of biomass, harvest index, and canopy cover indicate better performance of the proposed framework.
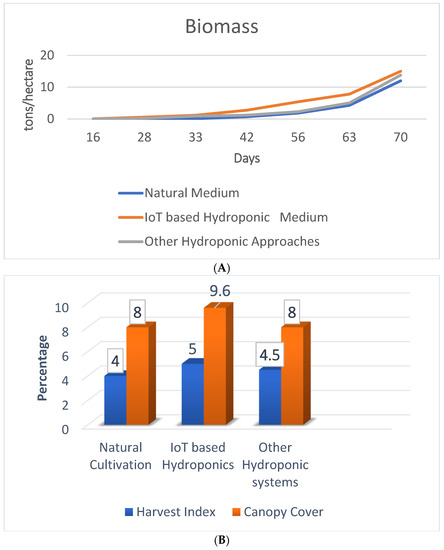
Figure 12.
(A) Comparison of biomass in different saffron cultivation models. (B) Comparison of harvest index and canopy cover for different saffron cultivation models.
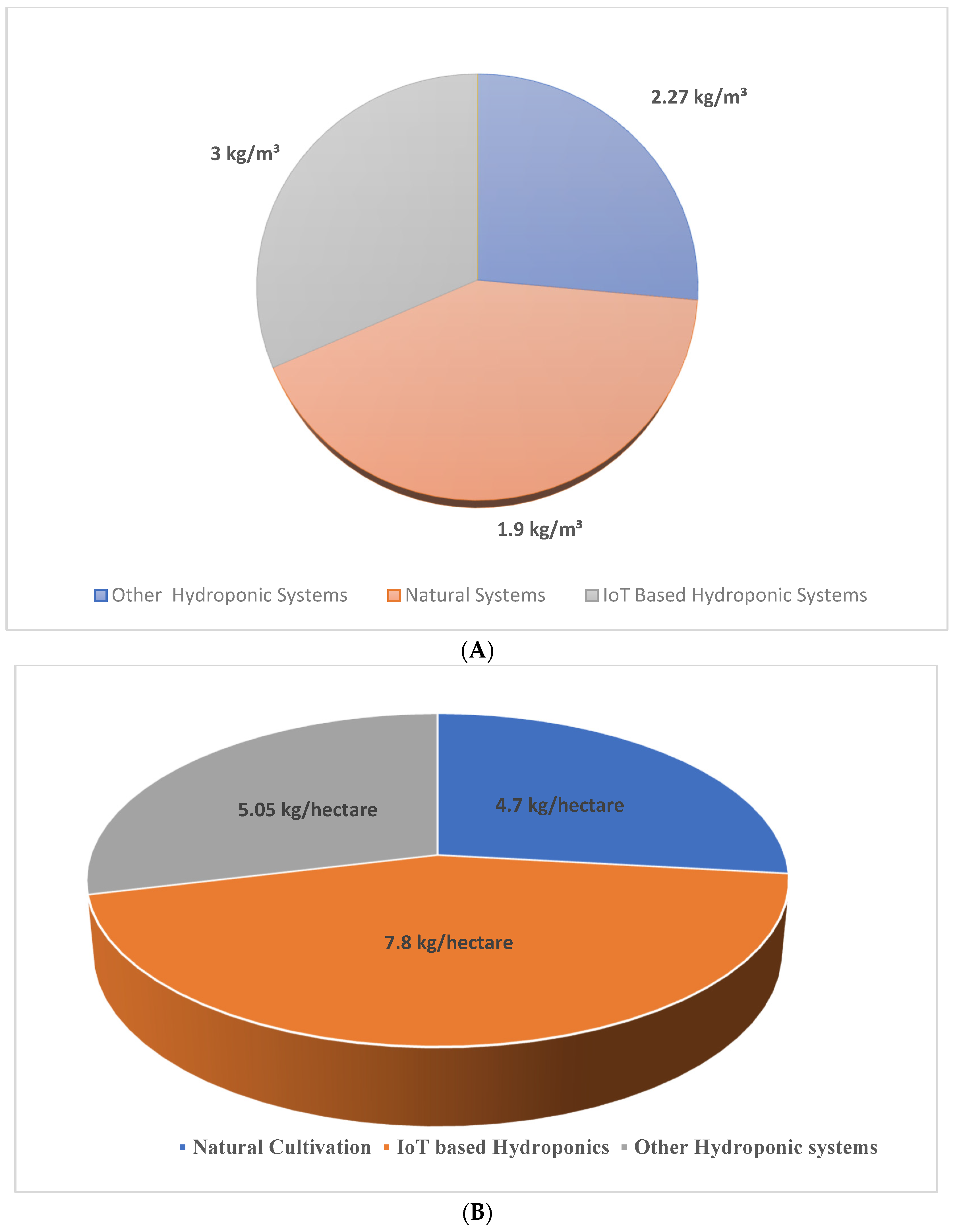
Water productivity is the efficiency of the model to produce biomass with proper utilization of water. In the designed framework, the water productivity value obtained is 3kg/m3 higher than other models and naturally cultivated saffron, as shown in Figure 13 A,B. The yield obtained in the designed framework is 7.8 kg/hectare, which is higher than all other existing models.
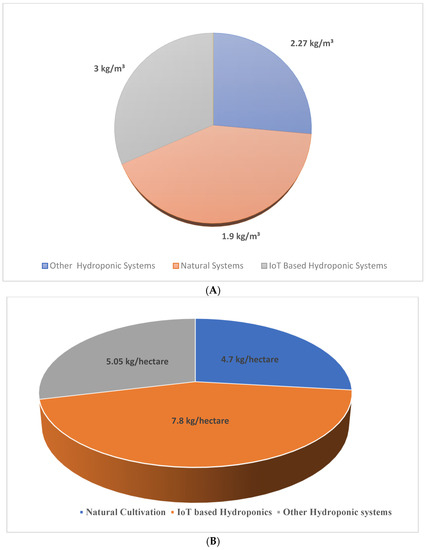
Figure 13.
(A) Comparison of water productivity in different saffron cultivation models.(B) Comparison of yield in different saffron cultivation models.
6. Conclusions and Future Scope
The world population is on a trending increase and soilless cultivation is taking over from traditional soil-based cultivation due to urbanization. In the present study, a framework for artificial saffron cultivation in an automated environment is proposed and evaluated, along with a quality analysis of saffron. For this purpose, saffron was grown in both artificial and natural media and agronomic variables such as temperature and humidity were studied in both modes to obtain optimized values required for obtaining high yield and quality. The study for the determination of set points of temperature and humidity in the growth region was conducted over one year. It was observed that, by using sensors and IoT components for growth, the temperature and humidity values can be analyzed and controlled, resulting in better growth percentage and yield required to boost the economy of the country. The results of the designed framework were compared by using the AquaCrop model with an error of less than 10% compared with other models, which showed errors higher than 11%. The values for yield, biomass, harvest index, and water productivity were also higher than traditional methods and other existing models. The yield obtained for the designed framework was about 20% more compared with the naturally growing saffron and other hydroponic models. It can be clearly seen that the water productivity of the proposed framework was 40% more than natural cultivation, which makes it sustainable and useful even in areas having water scarcity.
The designed framework also provides a mechanism to check the adulteration and to eliminate product counterfeiting of saffron (which has immense health benefits if present in good quality). The framework consists of a total of four chambers, with the bottom chamber filled with water and a color diffusion sensor. The sample placed in this chamber is tested by inserting saffron in water and detecting the color diffusion speed of the orangish-red color. The original or unadulterated saffron sample takes time to release its color compared with the adulterated saffron. The chamber above this is equipped with a diphenylamine solution to detect the presence of nitrates, if any. The blue color of the solution indicates the presence of nitrates. The chamber above this contains a MOS sensor to detect oxygen concentration, which tells us about the presence of impurities, if any. The topmost chamber is fitted with a crucible and a Bunsen burner to burn the sample and to analyze the ashes. Original saffron leaves have an ash content of less than 8 percent, which can be detected using sensors. The data from sensors in all the chambers are passed to the microprocessor, which analyzes the data against stored values; the quality percentage is displayed on the LED display fitted at the top of the chamber. The saffron sample is distributed in different chambers to check for adulteration and is evaluated for 2–3min to detect changes by different sensors. Depending on the quality of the sample and the nature of the adulterants added, the sample experiences a change in color, ash percentage left, and the nature detecting the color, ash percentage left, and dark red-brown color in case of the presence of nitrate in different chambers. The results from different sensor readings are analyzed by the connected microprocessor and the already programmed data are used to compare results and to display the output on the LED display attached to the top of the container. The proposed solution is reliable, portable, and cost-efficient and provides a comprehensive system to detect and prevent product counterfeiting in the saffron industry.
The future scope of the paper will focus on developing a prototype of the proposed model to test the performance, efficiency, and quality analysis in the real world. The future work will also focus on the improvement of the recognition rate of the model by collecting datasets and analyzing them.
Author Contributions
Conceptualization, K.K., D.G., J.R. and K.G., funding acquisition, J.K. and J.R.; investigation, K.K., D.G., J.R., K.G., J.K., K.H. and K.M; methodology, K.K., D.G., J.R., K.G. and J.K.; resources, K.K., D.G., J.R. and K.G.; visualization, K.K., D.G., J.R., K.G., J.K., K.H. and K.M.; writing—review and editing, K.K., D.G., J.R., K.G., J.K., K.H. and K.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Technology Development Program of MSS (No. S3033853).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Acknowledgments
The authors thank the anonymous reviewers who helped to improve the quality of the paper. Authors extend their appreciation to King Khalid University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ganaie, D.B.; Singh, Y. Saffron in Jammu & Kashmir. Int. J. Res. Geogr. 2019, 5, 1–12. [Google Scholar]
- Kour, K.; Gupta, D.; Gupta, K.; Juneja, S.; Kaur, M.; Alharbi, A.H.; Lee, H.-N. Controlling Agronomic Variables of Saffron Crop Using IoT for Sustainable Agriculture. Sustainability 2022, 14, 5607. [Google Scholar] [CrossRef]
- Ruiz Pulpón, Á.R.; Cañizares Ruiz, M.D.C.; Martínez Sánchez-Mateos, H.S. Regional Identity and Intangible Heritage Related to Saffron Cultivation in Castilla-La Mancha (Spain). Heritage 2023, 6, 2453–2472. [Google Scholar] [CrossRef]
- Kour, K.; Gupta, D.; Gupta, K. IoT and Fog Enabled Model for Saffron Cultivation in Precision Farming. In Proceedings of the 2021 3rd International Conference on Advances in Computing, Communication Control and Networking (ICAC3N), Greater Noida, India, 17–18 December 2021; pp. 614–619. [Google Scholar]
- Cid-Pérez, T.S.; Nevárez-Moorillón, G.V.; Ochoa-Velasco, C.E.; Navarro-Cruz, A.R.; Hernández-Carranza, P.; Avila-Sosa, R. The Relation between Drying Conditions and the Development of Volatile Compounds in Saffron (Crocus sativus). Molecules 2021, 26, 6954. [Google Scholar] [CrossRef] [PubMed]
- Cardone, L.; Castronuovo, D.; Perniola, M.; Cicco, N.; Candido, V. Saffron (Crocus sativus L.), the king of spices: An overview. Sci. Hortic. 2020, 272, 109560. [Google Scholar] [CrossRef]
- Caser, M.; Demasi, S.; Victorino, Í.M.M.; Donno, D.; Faccio, A.; Lumini, E.; Bianciotto, V.; Scariot, V. Arbuscular Mycorrhizal Fungi Modulate the Crop Performance and Metabolic Profile of Saffron in Soilless Cultivation. Agronomy 2019, 9, 232. [Google Scholar] [CrossRef]
- Mzabri, I.; Addi, M.; Berrichi, A. Traditional and Modern Uses of Saffron (Crocus sativus). Cosmetics 2019, 6, 63. [Google Scholar] [CrossRef]
- Avila-Sosa, R.; Nevárez-Moorillón, G.V.; Ochoa-Velasco, C.E.; Navarro-Cruz, A.R.; Hernández-Carranza, P.; Cid-Pérez, T.S. Detection of Saffron’s Main Bioactive Compounds and Their Relationship with Commercial Quality. Foods 2022, 11, 3245. [Google Scholar] [CrossRef]
- Li, S.; Xing, B.; Lin, D.; Yi, H.; Shao, Q. Rapid detection of saffron (Crocus sativus L.) Adulterated with lotus stamens and corn stigmas by near-infrared spectroscopy and chemometrics. Ind. Crop. Prod. 2020, 152, 112539. [Google Scholar] [CrossRef]
- Giupponi, L.; Ceciliani, G.; Leoni, V.; Panseri, S.; Pavlovic, R.; Lingua, G.; Di Filippo, A.; Giorgi, A. Quality traits of saffron produced in Italy: Geographical area effect and good practices. J. Appl. Bot. Food Qual. 2019, 92, 336–342. [Google Scholar]
- Sereshti, H.; Poursorkh, Z.; Aliakbarzadeh, G.; Zarre, S. Quality control of saffron and evaluation of potential adulteration by means of thin layer chromatography-image analysis and chemometrics methods. Food Control. 2018, 90, 48–57. [Google Scholar] [CrossRef]
- Anabat, M.M.; Riahi, H.; Sheidai, M.; Koohdar, F. Population genetic study and barcoding in Iran saffron (Crocus sativus L.). Ind. Crop. Prod. 2020, 143, 111915. [Google Scholar] [CrossRef]
- Heidarbeigi, K.; Mohtasebi, S.S.; Foroughirad, A.; Ghasemi-Varnamkhasti, M.; Rafiee, S.; Rezaei, K. Detection of Adulteration in Saffron Samples Using Electronic Nose. Int. J. Food Prop. 2015, 18, 1391–1401. [Google Scholar] [CrossRef]
- Kiani, S.; Minaei, S.; Ghasemi-Varnamkhasti, M. Integration of computer vision and electronic nose as non-destructive systems for saffron adulteration detection. Comput. Electron. Agric. 2017, 141, 46–53. [Google Scholar] [CrossRef]
- Kumari, L.; Jaiswal, P.; Tripathy, S.S. Various techniques useful for determination of adulterants in valuable saffron: A review. Trends Food Sci. Technol. 2021, 111, 301–321. [Google Scholar] [CrossRef]
- Aghaei, Z.; Jafari, S.M.; Dehnad, D.; Ghorbani, M.; Hemmati, K. Refractance-window as an innovative approach for the drying of saffron petals and stigma. J. Food Process. Eng. 2018, 41, e12863. [Google Scholar] [CrossRef]
- Najeeb, S.; Rather, A.G.; Sheikh, F.A.; Ahanger, M.A.; Teli, N.A. Baby corn (Zea mays L.): A means of crop diversification under temperate conditions of Kashmir. Maize Genet. Coop. Newsl. 2011, 85, 1–5. [Google Scholar]
- Shokati, B.; Feizizadeh, B. Sensitivity and uncertainty analysis of agro-ecological modeling for saffron plant cultivation using GIS spatial decision-making methods. J. Environ. Plan. Manag. 2019, 62, 517–533. [Google Scholar] [CrossRef]
- Villa, C.; Costa, J.; Meira, L.; Oliveira, M.B.P.; Mafra, I. Exploiting DNA mini-barcodes as molecular markers to authenticate saffron (Crocus sativus L.). Food Control 2016, 65, 21–31. [Google Scholar] [CrossRef]
- Placidi, P.; Morbidelli, R.; Fortunati, D.; Papini, N.; Gobbi, F.; Scorzoni, A. Monitoring Soil and Ambient Parameters in the IoT Precision Agriculture Scenario: An Original Modeling Approach Dedicated to Low-Cost Soil Water Content Sensors. Sensors 2021, 21, 5110. [Google Scholar] [CrossRef] [PubMed]
- Khilare, V.; Tiknaik, A.; Chandraprakash, K.; Ughade, B.; Korhale, G.; Nalage, D.; Ahmed, N.; Khedkar, C.; Khedkar, G. Multiple tests on saffron find new adulterant materials and reveal that Ist grade saffron is rare in the market. Food Chem. 2019, 272, 635–642. [Google Scholar] [CrossRef]
- Moratalla-López, N.; Parizad, S.; Habibi, M.K.; Winter, S.; Kalantari, S.; Bera, S.; Lorenzo, C.; García-Rodríguez, M.V.; Dizadji, A.; Alonso, G.L. Impact of two different dehydration methods on saffron quality, concerning the prevalence of Saffron latent virus (SaLV) in Iran. Food Chem. 2021, 337, 127786. [Google Scholar] [CrossRef] [PubMed]
- Amanpour, A.; Kelebek, H.; Selli, S. GLC/HPLC Methods for Saffron (L.). In Bioactive Molecules in Food; Springer: Cham, Switzerland, 2019; pp. 1987–2035. [Google Scholar]
- Dewir, Y.H.; Alsadon, A.; Ibrahim, A.; El-Mahrouk, M. Effects of Growing Substrate, Mode of Nutrient Supply, and Saffron Corm Size on Flowering, Growth, Photosynthetic Competence, and Cormlet Formation in Hydroponics. Horttechnology 2022, 32, 234–240. [Google Scholar] [CrossRef]
- Schroeder, F.G.; Lozoya, D.R.; Ruser, P. Hydroponic forcing of saffron (Crocus sativus L.). In Proceedings of the XXX International Horticultural Congress IHC2018: II International Symposium on Soilless Culture and VIII International Symposium on Seed, Transplant and Stand Establishment of Horticultural Crops, Istanbul, Turkey, 23 March 2020; pp. 281–288. [Google Scholar] [CrossRef]
- Khajeh-Hosseini, M.; Fallahpour, F. Emerging innovation in saffron production. In Saffron; Woodhead Publishing: Cambridge, UK, 2020; pp. 205–216. [Google Scholar] [CrossRef]
- Askari-Khorasgani, O.; Pessarakli, M. Shifting saffron (Crocus sativus L.) culture from traditional farmland to controlled environment (greenhouse) condition to avoid the negative impact of climate changes and increase its productivity. J. Plant Nutr. 2019, 42, 2642–2665. [Google Scholar] [CrossRef]
- Perez-Vidal, C.; Gracia, L. Computer based production of Saffron (Crocus sativus L.): From mechanical design to electronic control. Comput. Electron. Agric. 2020, 169, 105198. [Google Scholar] [CrossRef]
- Salas, M.D.C.; Montero, J.L.; Diaz, J.G.; Berti, F.; Quintero, M.F.; Guzmán, M.; Orsini, F. Defining optimal strength of the nutrient solution for soilless cultivation of saffron in the Mediterranean. Agronomy 2020, 10, 1311. [Google Scholar] [CrossRef]
- García, L.; Parra, L.; Jimenez, J.M.; Lloret, J.; Lorenz, P. IoT-Based Smart Irrigation Systems: An Overview on the Recent Trends on Sensors and IoT Systems for Irrigation in Precision Agriculture. Sensors 2020, 20, 1042. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.S.; Riaz, S.; Abid, A.; Umer, T.; Zikria, Y.B. Role of IoT Technology in Agriculture: A Systematic Literature Review. Electronics 2020, 9, 319. [Google Scholar] [CrossRef]
- Chawade, A.; van Ham, J.; Blomquist, H.; Bagge, O.; Alexandersson, E.; Ortiz, R. High-Throughput Field-Phenotyping Tools for Plant Breeding and Precision Agriculture. Agronomy 2019, 9, 258. [Google Scholar] [CrossRef]
- Thakur, D.; Kumar, Y.; Kumar, A.; Singh, P.K. Applicability of Wireless Sensor Networks in Precision Agriculture: A Review. Wirel. Pers. Commun. 2019, 107, 471–512. [Google Scholar] [CrossRef]
- Bauer, A.; Bostrom, A.G.; Ball, J.; Applegate, C.; Cheng, T.; Laycock, S.; Rojas, S.M.; Kirwan, J.; Zhou, J. Combining computer vision and deep learning to enable ultra-scale aerial phenotyping and precision agriculture: A case study of lettuce production. Hortic. Res. 2019, 6, 70. [Google Scholar] [CrossRef]
- Roy, S.K.; De, D. Genetic Algorithm based Internet of Precision Agricultural Things (IopaT) for Agriculture 4.0. Internet Things 2022, 18, 100201. [Google Scholar] [CrossRef]
- Zheng, Y.-Y.; Kong, J.-L.; Jin, X.-B.; Wang, X.-Y.; Su, T.-L.; Zuo, M. CropDeep: The Crop Vision Dataset for Deep-Learning-Based Classification and Detection in Precision Agriculture. Sensors 2019, 19, 1058. [Google Scholar] [CrossRef] [PubMed]
- Ferrández-Pastor, F.J.; García-Chamizo, J.M.; Nieto-Hidalgo, M.; Mora-Martínez, J. Precision Agriculture Design Method Using a Distributed Computing Architecture on Internet of Things Context. Sensors 2018, 18, 1731. [Google Scholar] [CrossRef]
- Shafi, U.; Mumtaz, R.; García-Nieto, J.; Hassan, S.A.; Zaidi, S.A.R.; Iqbal, N. Precision Agriculture Techniques and Practices: From Considerations to Applications. Sensors 2019, 19, 3796. [Google Scholar] [CrossRef] [PubMed]
- Guntukula, R. Assessing the impact of climate change on Indian agriculture: Evidence from major crop yields. J. Public Aff. 2020, 20, e2040. [Google Scholar] [CrossRef]
- Khalili, M.R.; Asadi, M.E.; Torkashvand, A.M.; Pazira, E. Regression Analysis for Yield Comparison of Saffron as Affected by Physicochemical Properties of the Soil, Case Study in Northeast of Iran. Agric. Res. 2020, 9, 568–576. [Google Scholar] [CrossRef]
- Thakur, A.K.; Singh, R.; Gehlot, A.; Kaviti, A.K.; Aseer, R.; Suraparajud, S.K.; Natarajand, S.K.; Sikarwar, V.S. Ad-vancements in solar technologies for sustainable development of agricultural sector in India: A comprehensive review on challenges and opportunities. Environ. Sci. Pollut. Res. 2022, 29, 43607–43634. [Google Scholar] [CrossRef]
- Aqeel-Ur-Rehman; Abbasi, A.Z.; Islam, N.; Shaikh, Z.A. A review of wireless sensors and networks’ applications in agriculture. Comput. Stand. Interfaces 2014, 36, 263–270. [Google Scholar] [CrossRef]
- Kour, K.; Gupta, D.; Gupta, K.; Dhiman, G.; Juneja, S.; Viriyasitavat, W.; Mohafez, H.; Islam, M.A. Smart-Hydroponic-Based Framework for Saffron Cultivation: A Precision Smart Agriculture Perspective. Sustainability 2022, 14, 1120. [Google Scholar] [CrossRef]
- Sabzian, M.; Rahimikhoob, A.; Mashal, M.; Aliniaeifard, S.; Dehghani, T. Comparison of water productivity and crop performance in hydroponic and soil cultivation using AquaCrop software* A case study of lettuce cultivation in Pakdasht, Iran. Irrig. Drain. 2021, 70, 1261–1272. [Google Scholar] [CrossRef]
- Mirsafi, Z.-S.; Sepaskhah, A.R.; Ahmadi, S.H.; Kamgar-Haghighi, A.A. Assessment of AquaCrop model for simulating growth and yield of saffron (Crocus sativus L.). Sci. Hortic. 2016, 211, 343–351. [Google Scholar] [CrossRef]
- Kour, K.; Gupta, D.; Gupta, K.; Anand, D.; Elkamchouchi, D.H.; Pérez-Oleaga, C.M.; Ibrahim, M.; Goyal, N. Moni-toring Ambient Parameters in the IoT Precision Agriculture Scenario: An Approach to Sensor Selection and Hydroponic Saffron Cultivation. Sensors 2022, 22, 8905. [Google Scholar] [CrossRef] [PubMed]
- Juneja, S.; Dhiman, G.; Kautish, S.; Viriyasitavat, W.; Yadav, K. A perspective roadmap for IoMT-based early detection and care of the neural disorder, dementia. J. Healthc. Eng. 2021, 2021, 6712424. [Google Scholar] [CrossRef] [PubMed]
- Anand, R.; Sindhwani, N. Cognitive Internet of Things, Its Applications, and Its Challenges: A Survey. In Harnessing the Internet of Things (IoT) for a Hyper-Connected Smart World; Apple Academic Press: Washington, DC, USA, 2022; pp. 91–113. [Google Scholar]
- Sharma, S.; Gupta, K.; Gupta, D.; Juneja, S.; Turabieh, H.; Sharma, S. SWOT: A Hybrid Hardware-Based Approach for Robust Fault-Tolerant Framework in a Smart Day Care. Secur. Commun. Netw. 2022, 2022, 2939469. [Google Scholar] [CrossRef]
- Upadhyay, H.K.; Muhammad, G.; Nauman, A.; Awad, N.A. Analysis of IoT-Related Ergonomics-Based Healthcare Issues Using Analytic Hierarchy Process Methodology. Sensors 2022, 22, 8232. [Google Scholar] [CrossRef]
- Shao, C.; Yang, Y.; Juneja, S.; Gseetharam, T. IoT data visualization for business intelligence in corporate finance. Inf. Process. Manag. 2022, 59, 102736. [Google Scholar] [CrossRef]
- Kaur, A.; Singh, G.; Kukreja, V.; Sharma, S.; Singh, S.; Yoon, B. Adaptation of IoT with Blockchain in Food Supply Chain Management: An Analysis-Based Review in Development, Benefits and Potential Applications. Sensors 2022, 22, 8174. [Google Scholar] [CrossRef]
- Kumar, D.; Kukreja, V. Deep learning in wheat diseases classification: A systematic review. Multimed. Tools Appl. 2022, 81, 10143–10187. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).