From Endophyte Community Analysis to Field Application: Control of Apple Canker (Neonectria ditissima) with Epicoccum nigrum B14-1
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of Endophytic Epicoccum Strains
2.2. In Vitro Antagonism Assays against N. ditissima
2.2.1. Antagonism in Co-Culture Assay
2.2.2. Antagonism of Soluble Metabolites
2.2.3. Antagonism of Volatile Metabolites
2.3. Field Antagonism Assays
2.3.1. Inoculum Preparation
2.3.2. Leaf Scar Protection
2.3.3. Pruning Wound Protection
2.3.4. Field Data Analysis
2.4. Endophytic Colonisation of B14-1
2.4.1. Quantification of E. nigrum DNA in Plant Tissues
2.4.2. Colonisation Data Analysis
2.5. Investigating Potential Adverse Effects of B14-1
2.5.1. Effects of B14-1 on Apple Tree Growth
2.5.2. Effects of B14-1 on Apple Fruit
2.6. General Statistical and Visualisation Tools
3. Results
3.1. Isolation and Identification of Epicoccum Strains
3.2. In Vitro Antagonism
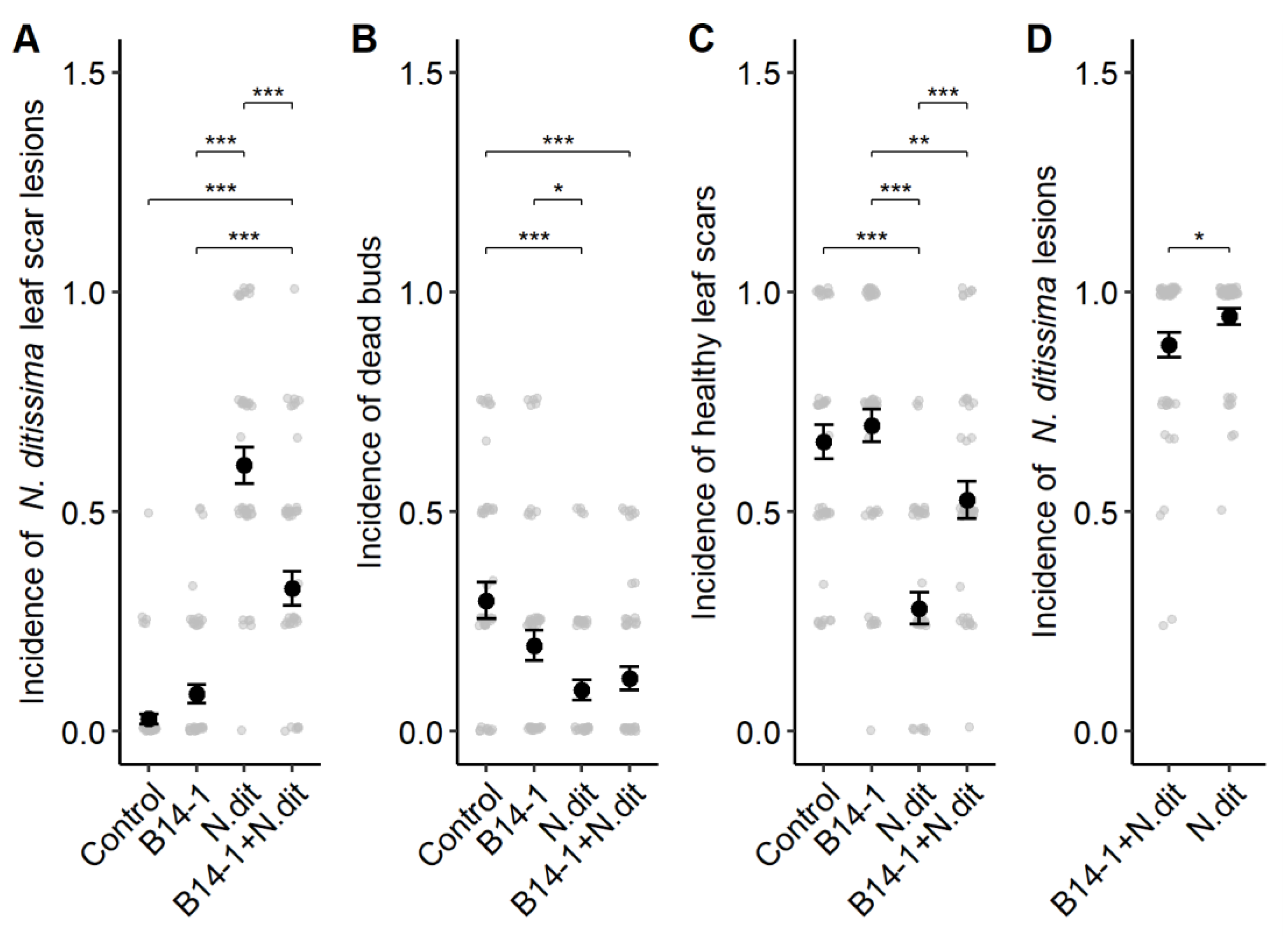
3.3. Antagonism in Field Conditions
3.4. Colonisation of Apple Tissues
3.5. Potential Adverse Effects of B14-1 on Apple
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Creemers, P. Nectria Canker. In Compendium of Apple and Pear Diseases and Pests, 2nd ed.; Sutton, T.B., Aldwinckle, H.S., Agnello, A.M., Walgenbach, J.F., Eds.; The American Phytopathological Society: St. Paul, MN, USA, 2014; pp. 49–51. ISBN 978-0-89054-433-4. [Google Scholar]
- Xu, X.M.; Butt, D.J.; Ridout, M.S. The Effects of Inoculum Dose, Duration of Wet Period, Temperature and Wound Age on Infection by Nectria Galligena of Pruning Wounds on Apple. Eur. J. Plant Pathol. 1998, 104, 511–519. [Google Scholar] [CrossRef]
- Dubin, H.J. Factors Affecting Apple Leaf Scar Infection by Nectria Galligena Conidia. Phytopathology 1974, 64, 1201. [Google Scholar] [CrossRef]
- Amponsah, N.T.; Walter, M.; Beresford, R.M.; Scheper, R.W.A. Seasonal Wound Presence and Susceptibility to Neonectria Ditissima Infection in New Zealand Apple Trees. N. Z. Plant Prot. 2015, 68, 250–256. [Google Scholar] [CrossRef]
- Weber, R.W.S.; Børve, J. Infection Biology as the Basis of Integrated Control of Apple Canker (Neonectria Ditissima) in Northern Europe. CABI Agric. Biosci. 2021, 2, 5. [Google Scholar] [CrossRef]
- Saville, R.; Olivieri, L. Fungal Diseases of Fruit: Apple Cankers in Europe. In Integrated Management of Diseases and Insect Pests of Tree Fruit; Xu, X., Fountain, M., Eds.; Burleigh Dodds Science Publishing: Cambridge, UK, 2019; pp. 59–84. [Google Scholar]
- Wenneker, M.; Goedhart, P.W.; van der Steeg, P.; van de Weg, W.E.; Schouten, H.J. Methods for the Quantification of Resistance of Apple Genotypes to European Fruit Tree Canker Caused by Neonectria Ditissima. Plant Dis. 2017, 101, 2012–2019. [Google Scholar] [CrossRef]
- Walter, M.; Roy, S.; Fisher, B.M.; Mackle, L.; Amponsah, N.T.; Curnow, T.; Campbell, R.E.; Braun, P.; Reineke, A.; Scheper, R.W.A. How Many Conidia Are Required for Wound Infection of Apple Plants by Neonectria Ditissima? N. Z. Plant Prot. 2016, 69, 238–245. [Google Scholar] [CrossRef]
- Børve, J.; Dalen, M.; Stensvand, A. Development of Neonectria Ditissima Infections Initiated at Grafting of Apple Trees. Eur. J. Plant Pathol. 2019, 155, 1225–1239. [Google Scholar] [CrossRef]
- Gómez-Cortecero, A.; Saville, R.J.; Scheper, R.W.A.A.; Bowen, J.K.; Agripino De Medeiros, H.; Kingsnorth, J.; Xu, X.; Harrison, R.J. Variation in Host and Pathogen in the Neonectria/Malus Interaction; toward an Understanding of the Genetic Basis of Resistance to European Canker. Front. Plant Sci. 2016, 7, 1365. [Google Scholar] [CrossRef] [PubMed]
- Ghasemkhani, M.; Sehic, J.; Ahmadi-Afzadi, M.; Nybom, H.; Garkava-Gustavsson, L. Screening for Partial Resistance to Fruit Tree Canker in Apple Cultivars. Acta Hortic. 2015, 1099, 687–690. [Google Scholar] [CrossRef]
- Smith, J.T.; Walter, M.; Campbell, R.E.; Turner, L. Can Phosphorous Acid Be Used to Control Neonectria Ditissima in New Zealand Grown Apples? N. Z. Plant Prot. 2019, 72, 117–122. [Google Scholar] [CrossRef]
- Walter, M.; Campbell, R.E.; Amponsah, N.T.; Turner, L.; Rainham, D.; Kerer, U.; Butler, R.C. Can Biological Products Control Neonectria Ditissima Picking Wound and Leaf Scar Infections in Apples? N. Z. Plant Prot. 2017, 70, 63–72. [Google Scholar] [CrossRef]
- Elena, G.; Groenenboom-de Haas, B.H.; Houwers, I.; de Lange, E.; Schnabel, S.K.; Köhl, J. Systematic Stepwise Screening of New Microbial Antagonists for Biological Control of European Canker. Biol. Control 2022, 174, 105009. [Google Scholar] [CrossRef]
- Easton, H.S. Grasses and Neotyphodium Endophytes: Co-Adaptation and Adaptive Breeding. Euphytica 2006, 154, 295–306. [Google Scholar] [CrossRef]
- Card, S.; Johnson, L.; Teasdale, S.; Caradus, J. Deciphering Endophyte Behaviour: The Link between Endophyte Biology and Efficacious Biological Control Agents. FEMS Microbiol. Ecol. 2016, 92, fiw114. [Google Scholar] [CrossRef]
- Arnold, A.E.; Mejía, L.C.; Kyllo, D.; Rojas, E.I.; Maynard, Z.; Robbins, N.; Herre, E.A. Fungal Endophytes Limit Pathogen Damage in a Tropical Tree. Proc. Natl. Acad. Sci. USA 2003, 100, 15649–15654. [Google Scholar] [CrossRef]
- Halecker, S.; Wennrich, J.P.; Rodrigo, S.; Andrée, N.; Rabsch, L.; Baschien, C.; Steinert, M.; Stadler, M.; Surup, F.; Schulz, B. Fungal Endophytes for Biocontrol of Ash Dieback: The Antagonistic Potential of Hypoxylon Rubiginosum. Fungal Ecol. 2020, 45, 100918. [Google Scholar] [CrossRef]
- Busby, P.E.; Ridout, M.; Newcombe, G. Fungal Endophytes: Modifiers of Plant Disease. Plant Mol. Biol. 2016, 90, 645–655. [Google Scholar] [CrossRef]
- Gao, F.; Dai, C.; Liu, X. Mechanisms of Fungal Endophytes in Plant Protection against Pathogens. Afr. J. Microbiol. Res. 2010, 4, 1346–1351. [Google Scholar]
- Chitnis, V.R.; Suryanarayanan, T.S.; Nataraja, K.N.; Prasad, S.R.; Oelmüller, R.; Shaanker, R.U. Fungal Endophyte-Mediated Crop Improvement: The Way Ahead. Front. Plant Sci. 2020, 11, 561007. [Google Scholar] [CrossRef]
- Olivieri, L.; Saville, R.J.; Gange, A.C.; Xu, X. Apple Endophyte Community in Relation to Location, Scion and Rootstock Genotypes and Susceptibility to European Canker. FEMS Microbiol. Ecol. 2021, 97, fiab131. [Google Scholar] [CrossRef]
- Liu, J.; Ridgway, H.J.; Jones, E.E. Apple Endophyte Community Is Shaped by Tissue Type, Cultivar and Site and Has Members with Biocontrol Potential against Neonectria Ditissima. J. Appl. Microbiol. 2020, 128, 1735–1753. [Google Scholar] [CrossRef]
- Liu, J.; Abdelfattah, A.; Norelli, J.; Burchard, E.; Schena, L.; Droby, S.; Wisniewski, M. Apple Endophytic Microbiota of Different Rootstock/Scion Combinations Suggests a Genotype-Specific Influence. Microbiome 2018, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Schulz, B.; Boyle, C. The Endophytic Continuum. Mycol. Res. 2005, 109, 661–686. [Google Scholar] [CrossRef] [PubMed]
- Fávaro, L.C.D.L.; Sebastianes, F.L.d.S.; Araújo, W.L. Epicoccum Nigrum P16, a Sugarcane Endophyte, Produces Antifungal Compounds and Induces Root Growth. PLoS ONE 2012, 7, e36826. [Google Scholar] [CrossRef]
- De Cal, A.; Larena, I.; Liñán, M.; Torres, R.; Lamarca, N.; Usall, J.; Domenichini, P.; Bellini, A.; de Eribe, X.O.; Melgarejo, P. Population Dynamics of Epicoccum Nigrum, a Biocontrol Agent against Brown Rot in Stone Fruit. J. Appl. Microbiol. 2009, 106, 592–605. [Google Scholar] [CrossRef]
- Musetti, R.; Grisan, S.; Polizzotto, R.; Martini, M.; Paduano, C.; Osler, R. Interactions between “Candidatus Phytoplasma Mali” and the Apple Endophyte Epicoccum Nigrum in Catharanthus Roseus Plants. J. Appl. Microbiol. 2011, 110, 746–756. [Google Scholar] [CrossRef]
- Hashem, M.; Ali, E. Epicoccum Nigrum as Biocontrol Agent of Pythium Damping-off and Root-Rot of Cotton Seedlings. Arch. Phytopathol. Plant Prot. 2004, 37, 283–297. [Google Scholar] [CrossRef]
- Bian, J.Y.; Fang, Y.L.; Song, Q.; Sun, M.L.; Yang, J.Y.; Ju, Y.W.; Li, D.W.; Huang, L. The Fungal Endophyte Epicoccum Dendrobii as a Potential Biocontrol Agent against Colletotrichum Gloeosporioides. Phytopathology 2021, 111, 293–303. [Google Scholar] [CrossRef]
- Schulz, B.; Wanke, U.; Draeger, S.; Aust, H.J. Endophytes from Herbaceous Plants and Shrubs: Effectiveness of Surface Sterilization Methods. Mycol. Res. 1993, 97, 1447–1450. [Google Scholar] [CrossRef]
- Ellis, D.; Davis, S.; Alexiou, H.; Handke, R.; Bartley, R. Descriptions of Medical Fungi, 2nd ed.; University of Adelaide: Adelaide, Australia, 2007. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols; Elsevier: Amsterdam, The Netherlands, 1990; pp. 315–322. [Google Scholar]
- Bensch, K.; Groenewald, J.Z.; Dijksterhuis, J.; Starink-Willemse, M.; Andersen, B.; Summerell, B.A.; Shin, H.D.; Dugan, F.M.; Schroers, H.J.; Braun, U.; et al. Species and Ecological Diversity within the Cladosporium Cladosporioides Complex (Davidiellaceae, Capnodiales). Stud. Mycol. 2010, 67, 94. [Google Scholar] [CrossRef]
- Carbone, I.; Kohn, L.M. A Method for Designing Primer Sets for Speciation Studies in Filamentous Ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 48. [Google Scholar] [CrossRef]
- Larena, I.; De Cal, A.; Melgarejo, P. Solid Substrate Production of Epicoccum Nigrum Conidia for Biological Control of Brown Rot on Stone Fruits. Int. J. Food Microbiol. 2004, 94, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, L.; Saville, R.J.; Gange, A.C.; Xu, X. Limited Asymptomatic Colonization of Apple Tree Shoots by Neonectria Ditissima Following Infection of Leaf Scars and Pruning Wounds. Plant Pathol. 2021, 70, 1838–1849. [Google Scholar] [CrossRef]
- Mollie, E.B.; Kristensen, K.; Koen, J.; Magnusson, A.; Casper, W.B.; Nielsen, A.; Hans, J.S.; Mächler, M.; Benjamin, M.B. glmmTMB Balances Speed and Flexibility Among Packages for Zero-inflated Generalized Linear Mixed Modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models, R Package Version 0.4.4; 2021. Available online: https://CRAN.R-project.org/package=DHARMa (accessed on 5 May 2021).
- Lenth, R.V. Emmeans: Estimated Marginal Means, Aka Least-Squares Means, Version 1.7.0; 2021. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 15 April 2021).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Kassambara, A. Ggpubr: “ggplot2” Based Publication Ready Plots, R Package version 0.4.0; 2020. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 10 October 2021).
- Del Frari, G.; Cabral, A.; Nascimento, T.; Boavida Ferreira, R.; Oliveira, H. Epicoccum Layuense a Potential Biological Control Agent of Esca-Associated Fungi in Grapevine. PLoS ONE 2019, 14, e0213273. [Google Scholar] [CrossRef]
- Kosawang, C.; Amby, D.B.; Bussaban, B.; McKinney, L.V.; Xu, J.; Kjær, E.D.; Collinge, D.B.; Nielsen, L.R. Fungal Communities Associated with Species of Fraxinus Tolerant to Ash Dieback, and Their Potential for Biological Control. Fungal Biol. 2018, 122, 110–120. [Google Scholar] [CrossRef]
- Royse, D.J. The Influence of Fungi Isolated from Peach Twigs on the Pathogenicity of Cytospora Cincta. Phytopathology 1978, 68, 603. [Google Scholar] [CrossRef]
- Larena, I.; Torres, R.; De Cal, A.; Liñán, M.; Melgarejo, P.; Domenichini, P.; Bellini, A.; Mandrin, J.F.; Lichou, J.; De Eribe, X.O.; et al. Biological Control of Postharvest Brown Rot (Monilinia Spp.) of Peaches by Field Applications of Epicoccum Nigrum. Biol. Control 2005, 32, 305–310. [Google Scholar] [CrossRef]
- Latz, M.A.C.; Jensen, B.; Collinge, D.B.; Jørgensen, H.J.L. Endophytic Fungi as Biocontrol Agents: Elucidating Mechanisms in Disease Suppression. Plant Ecol. Divers. 2018, 11, 555–567. [Google Scholar] [CrossRef]
- Swinburne, T.R.; Barr, J.G.; Brown, A.E. Production of Antibiotics by Bacillus Subtilis and Their Effect on Fungal Colonists of Apple Leaf Scars. Trans. Br. Mycol. Soc. 1975, 65, 211–217. [Google Scholar] [CrossRef]
- Walter, M.; Campbell, R.E.; Amponsah, N.T.; Scheper, R.W.A.; Butler, R.C. Evaluation of Biological and Agrichemical Products for Control of Neonectria Ditissima Conidia Production. N. Z. Plant Prot. 2017, 70, 87–96. [Google Scholar] [CrossRef]
- Papp-Rupar, M.; Karlstrom, A.; Passey, T.; Deakin, G.; Xu, X. The Influence of Host Genotypes on the Endophytes in the Leaf Scar Tissues of Apple Trees and Correlation of the Endophytes with Apple Canker (Neonectria ditissima) Development. Phytobiomes J. 2022, 6, 127–138. [Google Scholar] [CrossRef]
- Fávaro, L.C.D.L.; de Melo, F.L.; Aguilar-Vildoso, C.I.; Araújo, W.L. Polyphasic Analysis of Intraspecific Diversity in Epicoccum Nigrum Warrants Reclassification into Separate Species. PLoS ONE 2011, 6, e14828. [Google Scholar] [CrossRef] [PubMed]
- Bruton, B.D. Postharvest Decay of Cantaloupe Caused by Epicoccum Nigrum. Plant Dis. 1993, 77, 1060. [Google Scholar] [CrossRef]
- Busby, P.E.; Peay, K.G.; Newcombe, G. Common Foliar Fungi of Populus Trichocarpa Modify Melampsora Rust Disease Severity. New Phytol. 2016, 209, 1681–1692. [Google Scholar] [CrossRef]
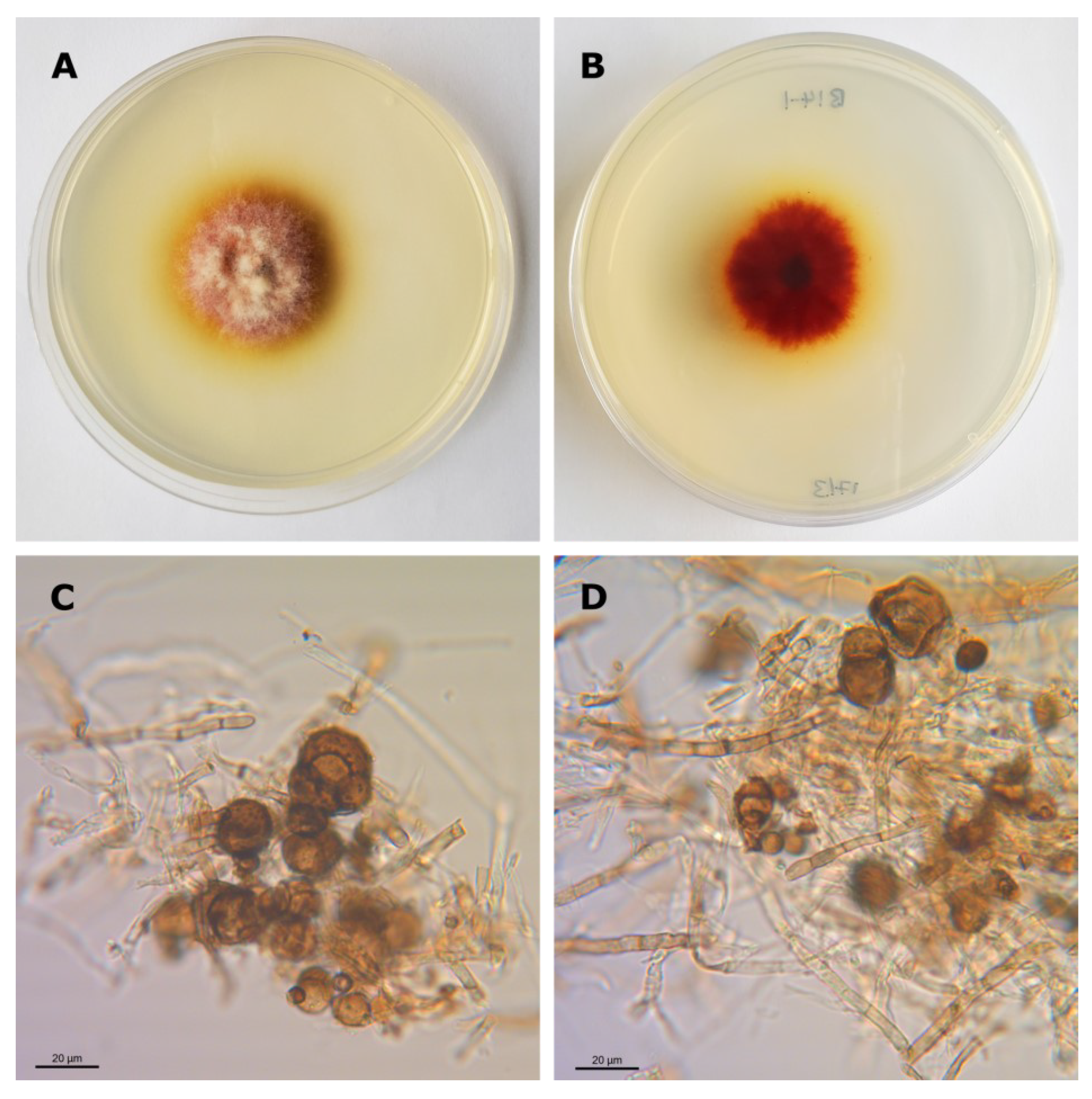
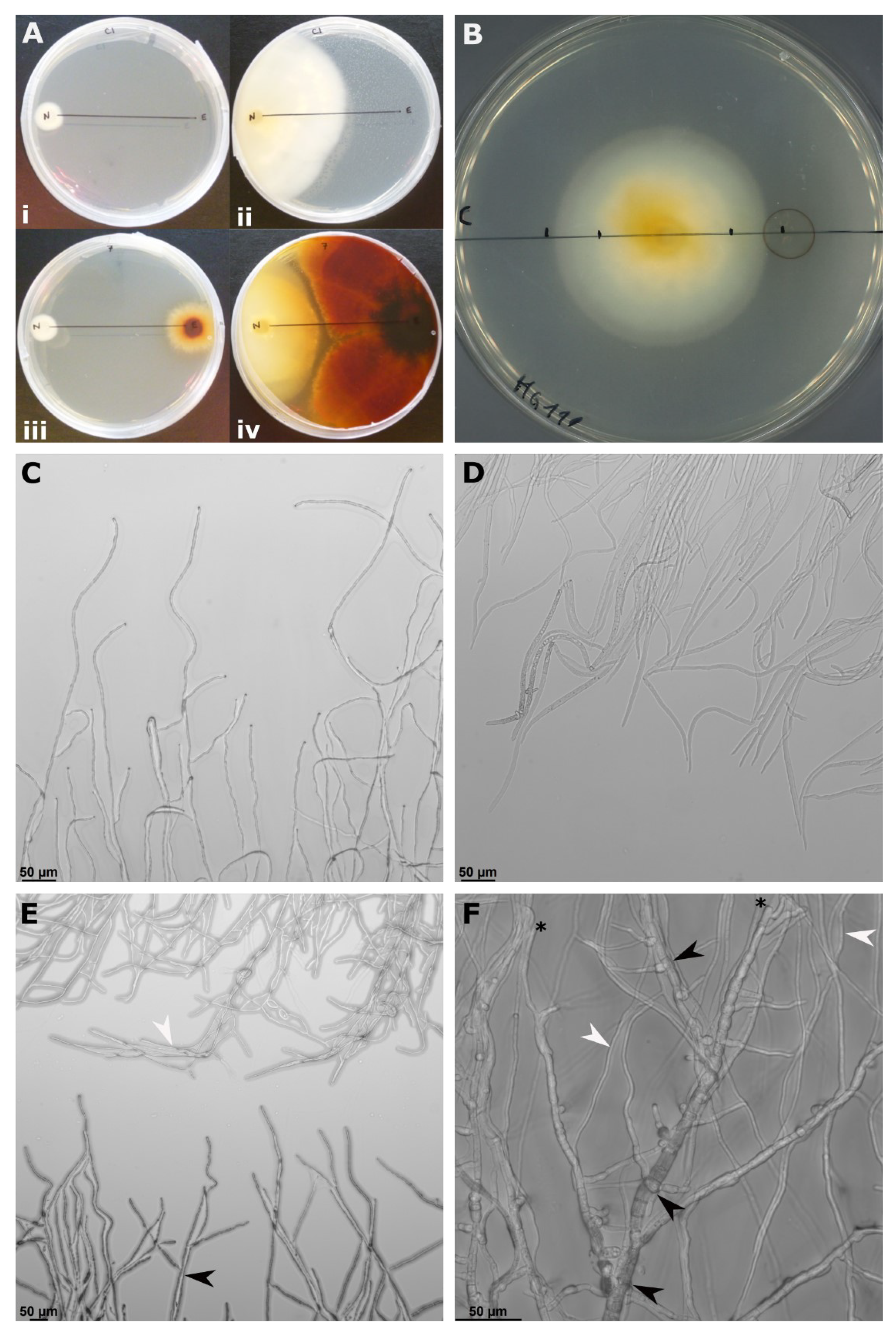


| Epicoccum spp. Isolate | B14-1 | C15 | C29 | |
|---|---|---|---|---|
| Isolated from Apple cv.: | Royal Gala | Queen Cox | Royal Gala | |
| NCBI accession: | ITS | OM650677 | OM650678 | OM650679 |
| TEF | OP753363 | n.t. | n.t. | |
| ACT | OP753362 | n.t. | n.t. | |
| Taxonomic classification | Epicoccum nigrum | Epicoccum spp. | Epicoccum spp. | |
| Reduction of N. ditissima growth (%) in vitro: | Co-culture assay | 52.0 ± 1.4 * | 45.7 ± 1.5 * | 55.8 ± 2.6 * |
| Soluble metabolite assay | 20.0 ± 2.2 * | n.t. | n.t. | |
| Volatile metabolite assay | 3.1 ± 1.4 | n.t. | n.t. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papp-Rupar, M.; Olivieri, L.; Saville, R.; Passey, T.; Kingsnorth, J.; Fagg, G.; McLean, H.; Xu, X. From Endophyte Community Analysis to Field Application: Control of Apple Canker (Neonectria ditissima) with Epicoccum nigrum B14-1. Agriculture 2023, 13, 809. https://doi.org/10.3390/agriculture13040809
Papp-Rupar M, Olivieri L, Saville R, Passey T, Kingsnorth J, Fagg G, McLean H, Xu X. From Endophyte Community Analysis to Field Application: Control of Apple Canker (Neonectria ditissima) with Epicoccum nigrum B14-1. Agriculture. 2023; 13(4):809. https://doi.org/10.3390/agriculture13040809
Chicago/Turabian StylePapp-Rupar, Matevz, Leone Olivieri, Robert Saville, Thomas Passey, Jennifer Kingsnorth, Georgina Fagg, Hamish McLean, and Xiangming Xu. 2023. "From Endophyte Community Analysis to Field Application: Control of Apple Canker (Neonectria ditissima) with Epicoccum nigrum B14-1" Agriculture 13, no. 4: 809. https://doi.org/10.3390/agriculture13040809
APA StylePapp-Rupar, M., Olivieri, L., Saville, R., Passey, T., Kingsnorth, J., Fagg, G., McLean, H., & Xu, X. (2023). From Endophyte Community Analysis to Field Application: Control of Apple Canker (Neonectria ditissima) with Epicoccum nigrum B14-1. Agriculture, 13(4), 809. https://doi.org/10.3390/agriculture13040809









