Development of a Low-Power Automatic Monitoring System for Spodoptera frugiperda (J. E. Smith)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Traditional Monitoring Methods for S. frugiperda
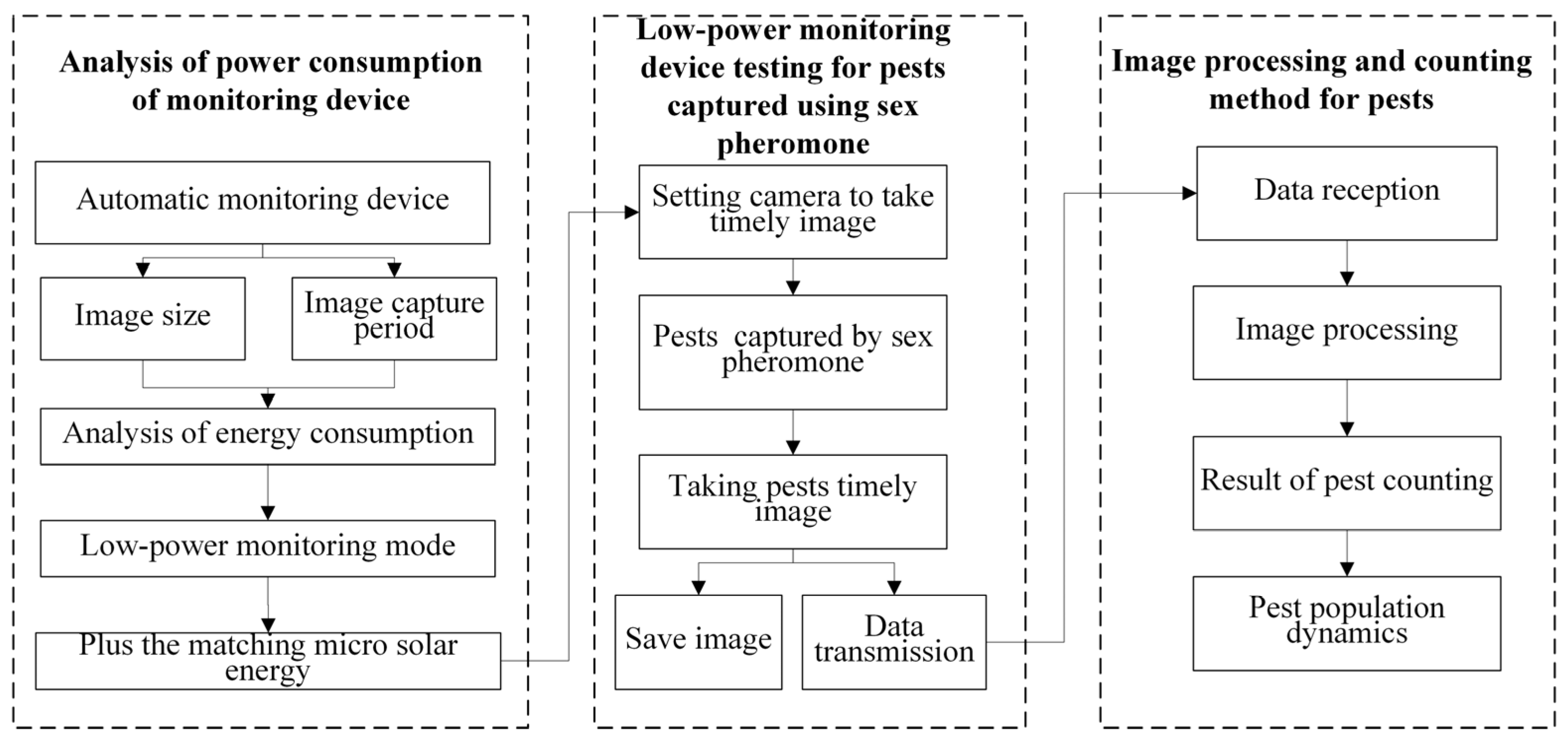
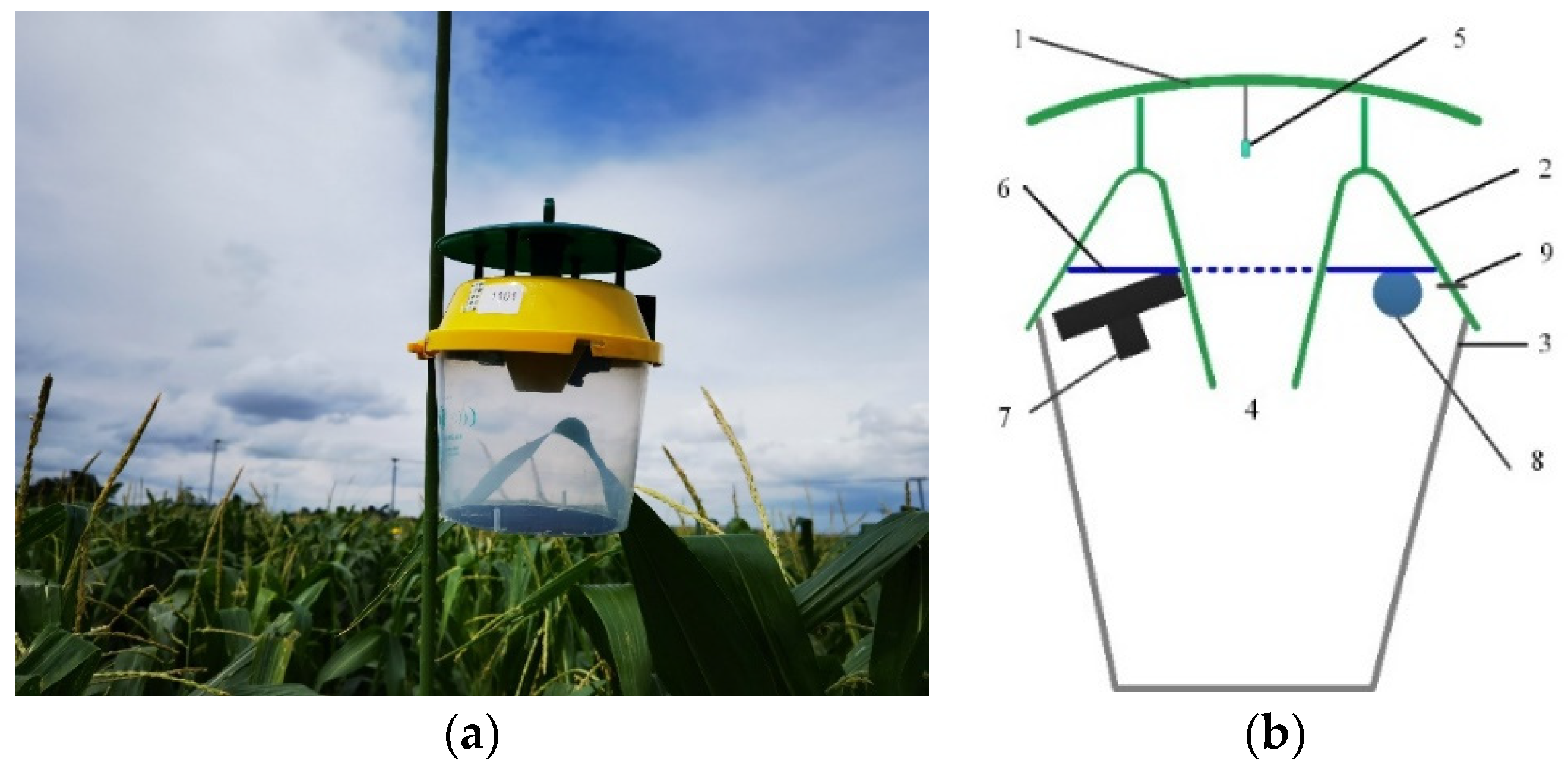
2.2. Architecture of Low-Power Monitoring and Counting System for Pests Captured Using a Sex Pheromone Lure
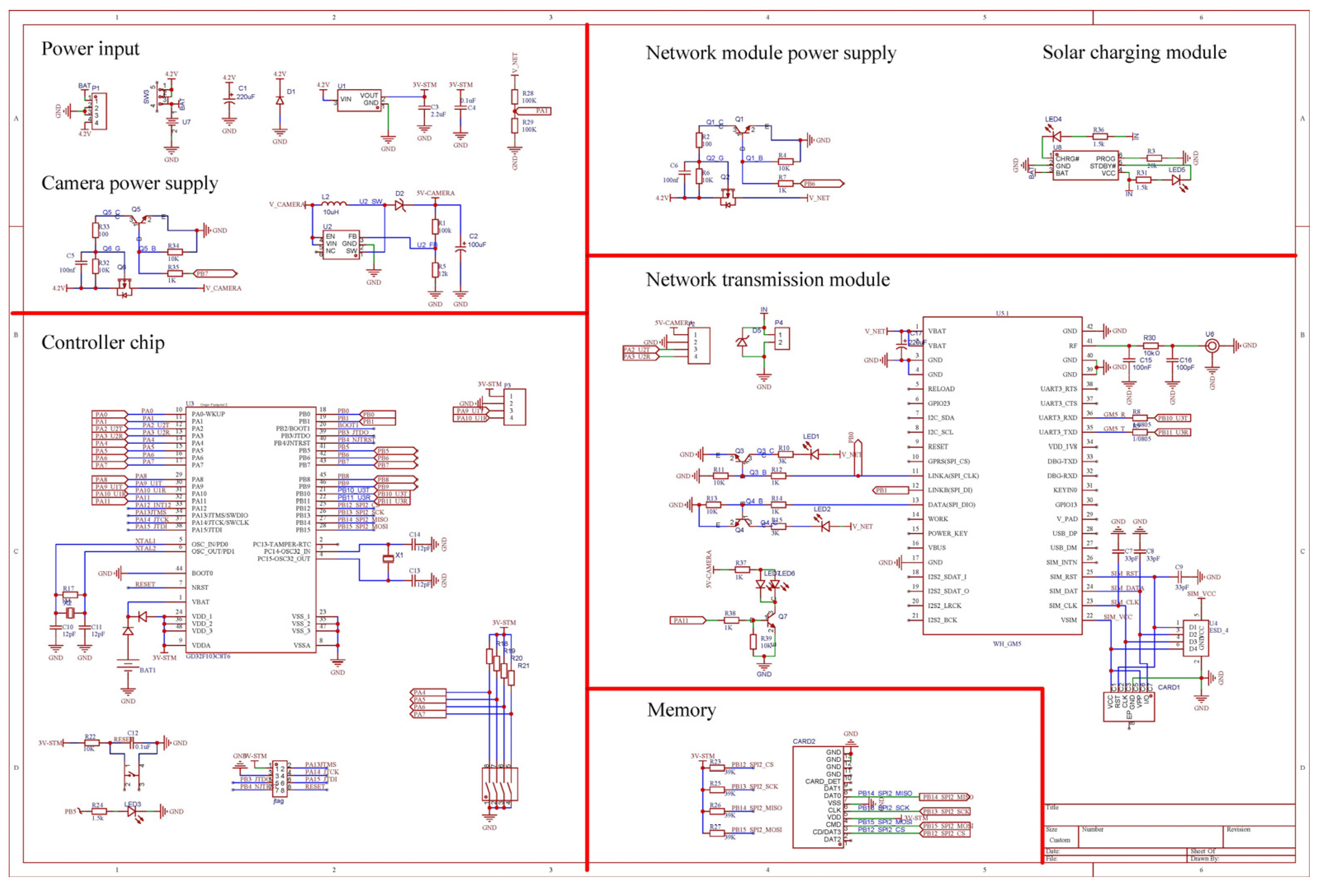
2.3. Software and Hardware Design of Low-Power Automatic Monitoring System for Pests Captured by a Sex Pheromone Lure
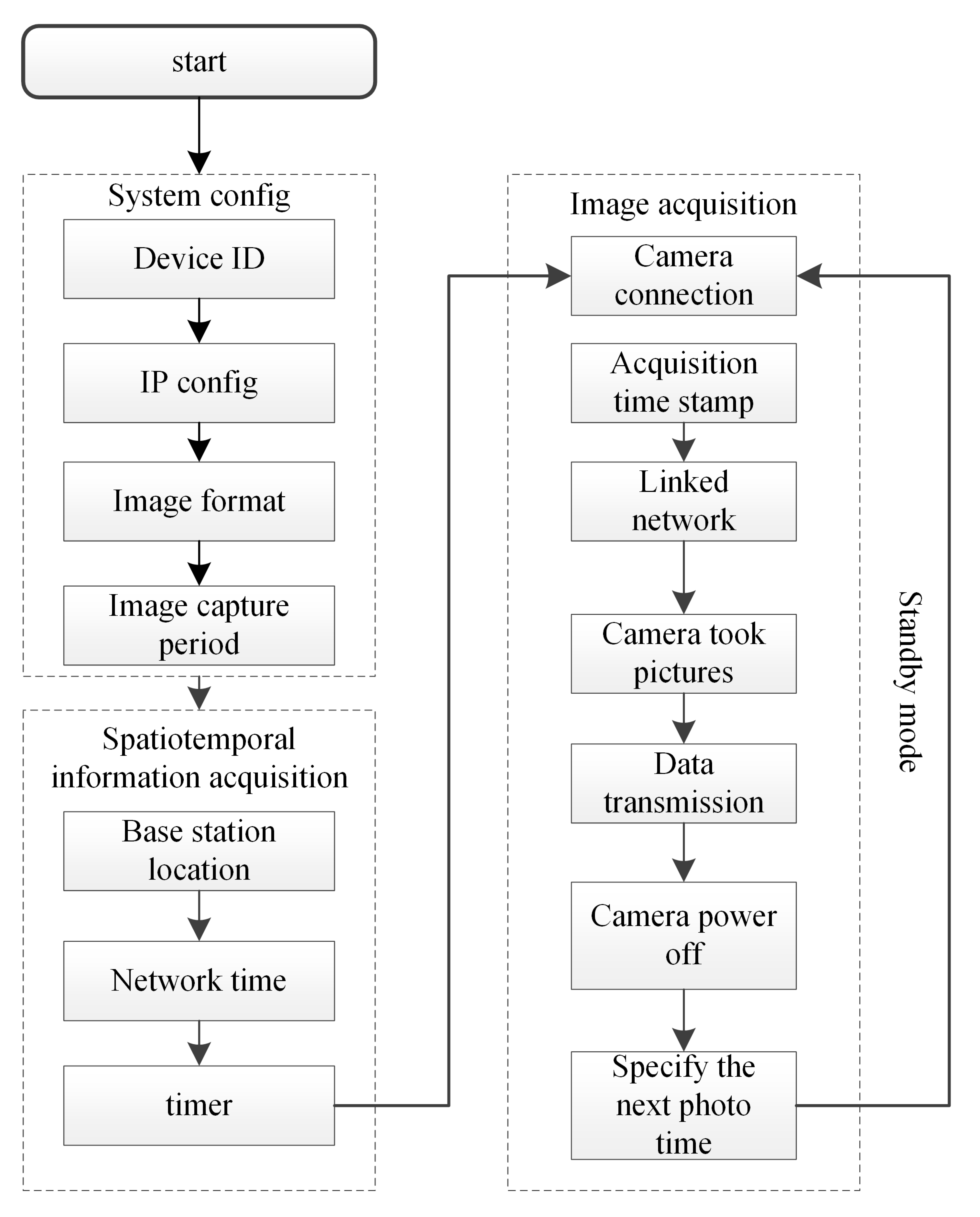
2.4. Working Principle
- (1)
- Device initialization: The device entered working mode from standby mode and checked the time and external ports.
- (2)
- Waiting to connect the network: WH-GM5 started to connect the network and monitor the network connection port.
- (3)
- Image acquisition: When the network connection was successful, the camera began to take photos and read the image size.
- (4)
- Image transmission: The image was sliced by 0.5 KB, and the image data were read and transmitted to the network.
- (5)
- After the image was read, the device waited for the data buffer to empty and closed the network.
- (6)
- Specifying the next specific shooting time.
- (7)
- The device entered standby mode.
2.5. Power Consumption Analysis of Monitoring Device
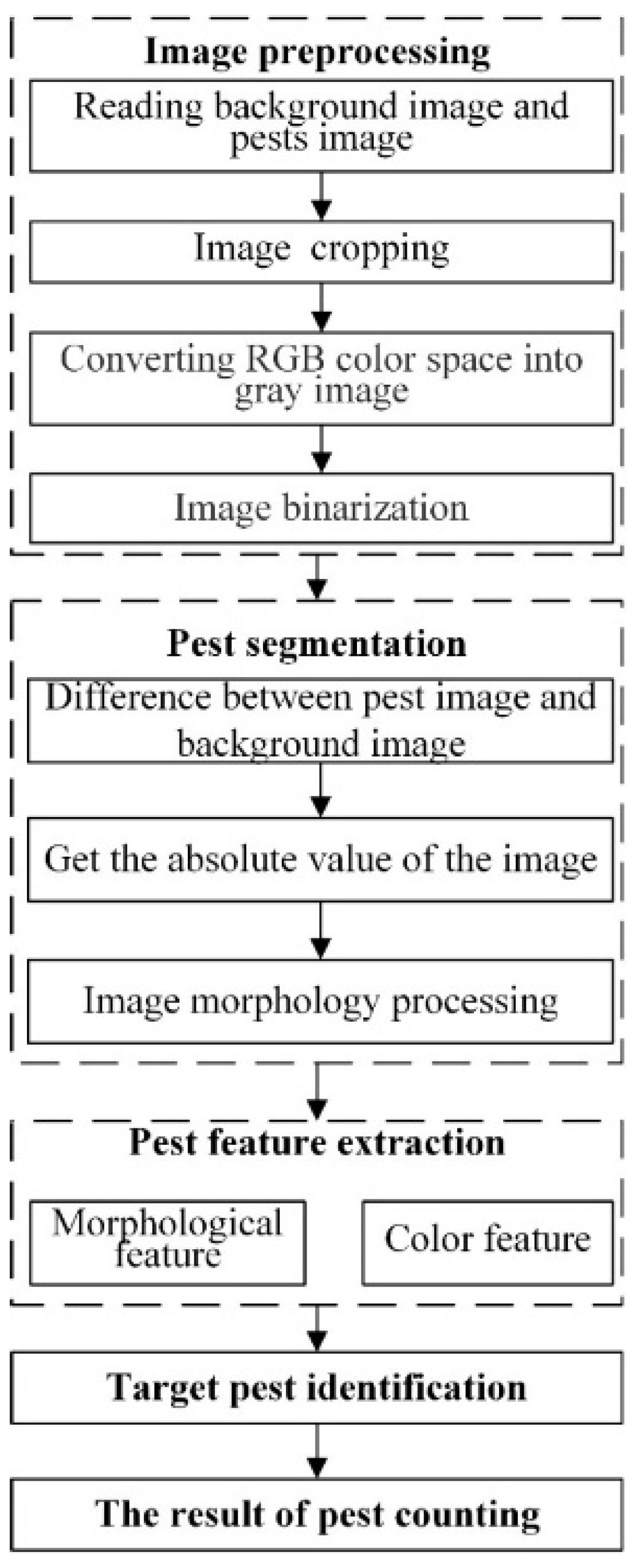
2.6. Image Preprocessing
2.6.1. Image Preprocessing
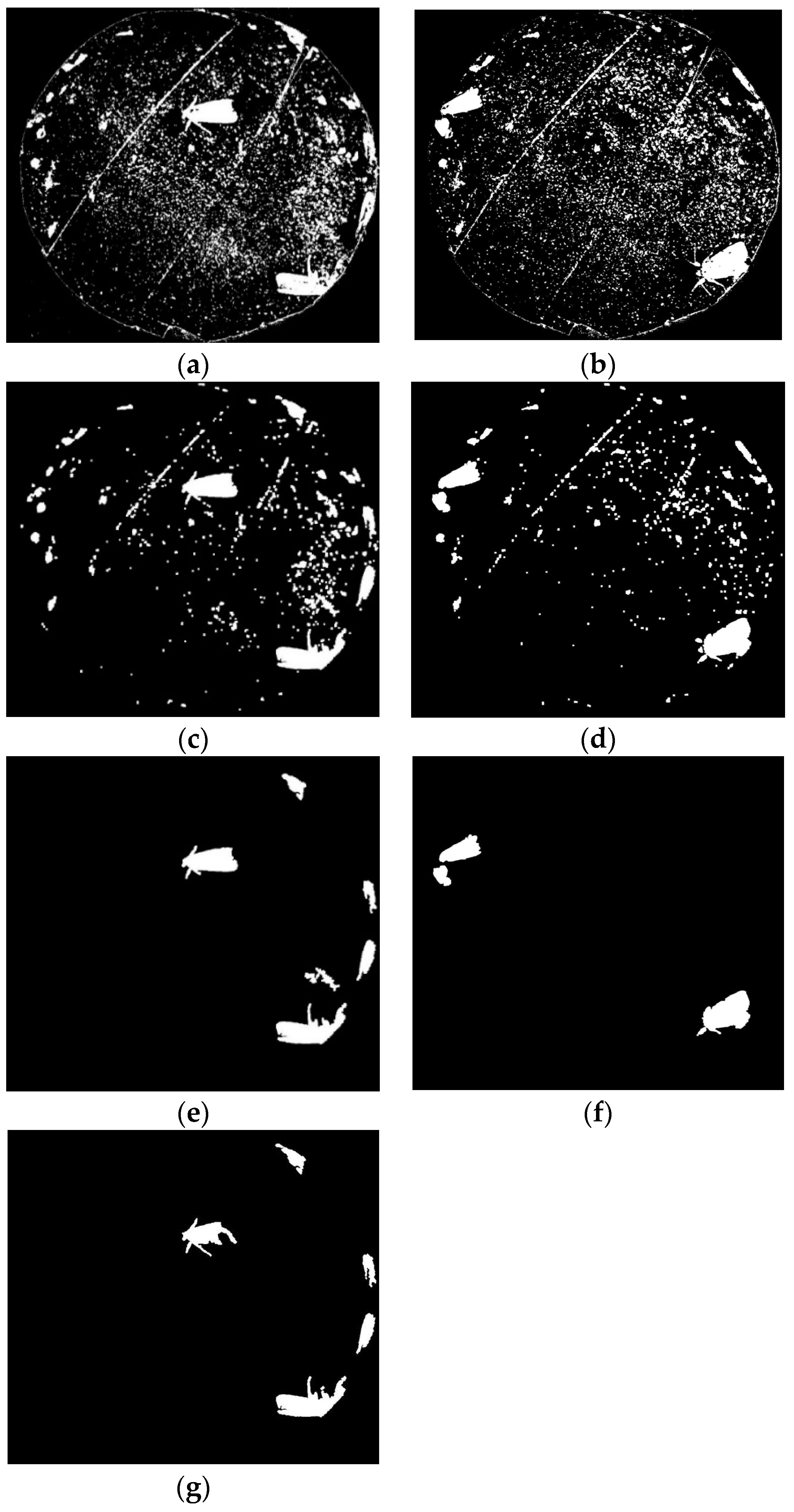
2.6.2. Pest Image Segmentation
2.6.3. Feature Extraction
2.6.4. Target Pest Identification
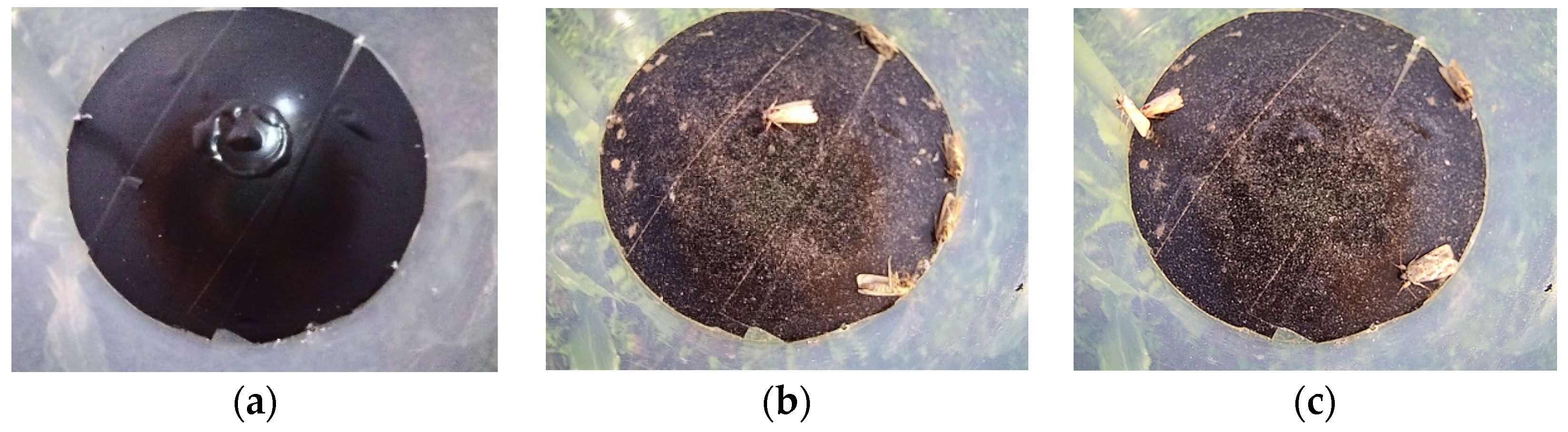
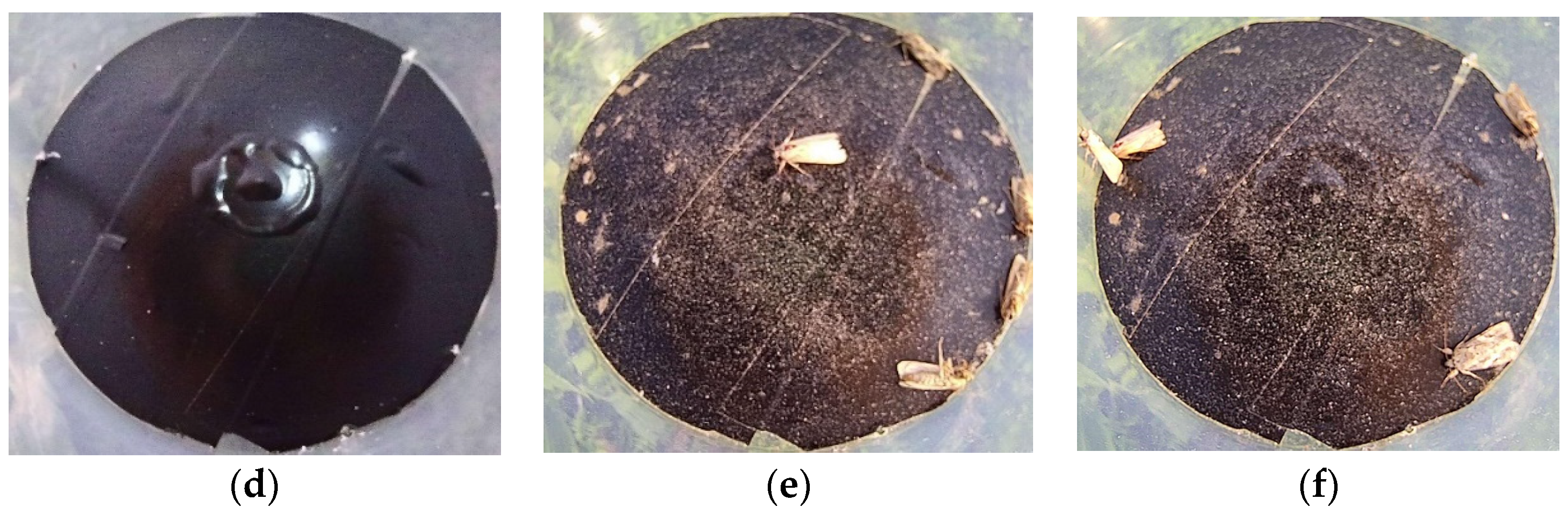
2.7. Field Test and Evaluation Method
3. Results
3.1. Power Consumption Analysis
3.1.1. Analysis of Acquisition and Transmission Time of Images with Different Resolutions
3.1.2. Image Acquisition and Transmission Power Consumption Analysis with Different Resolutions and Capture Periods
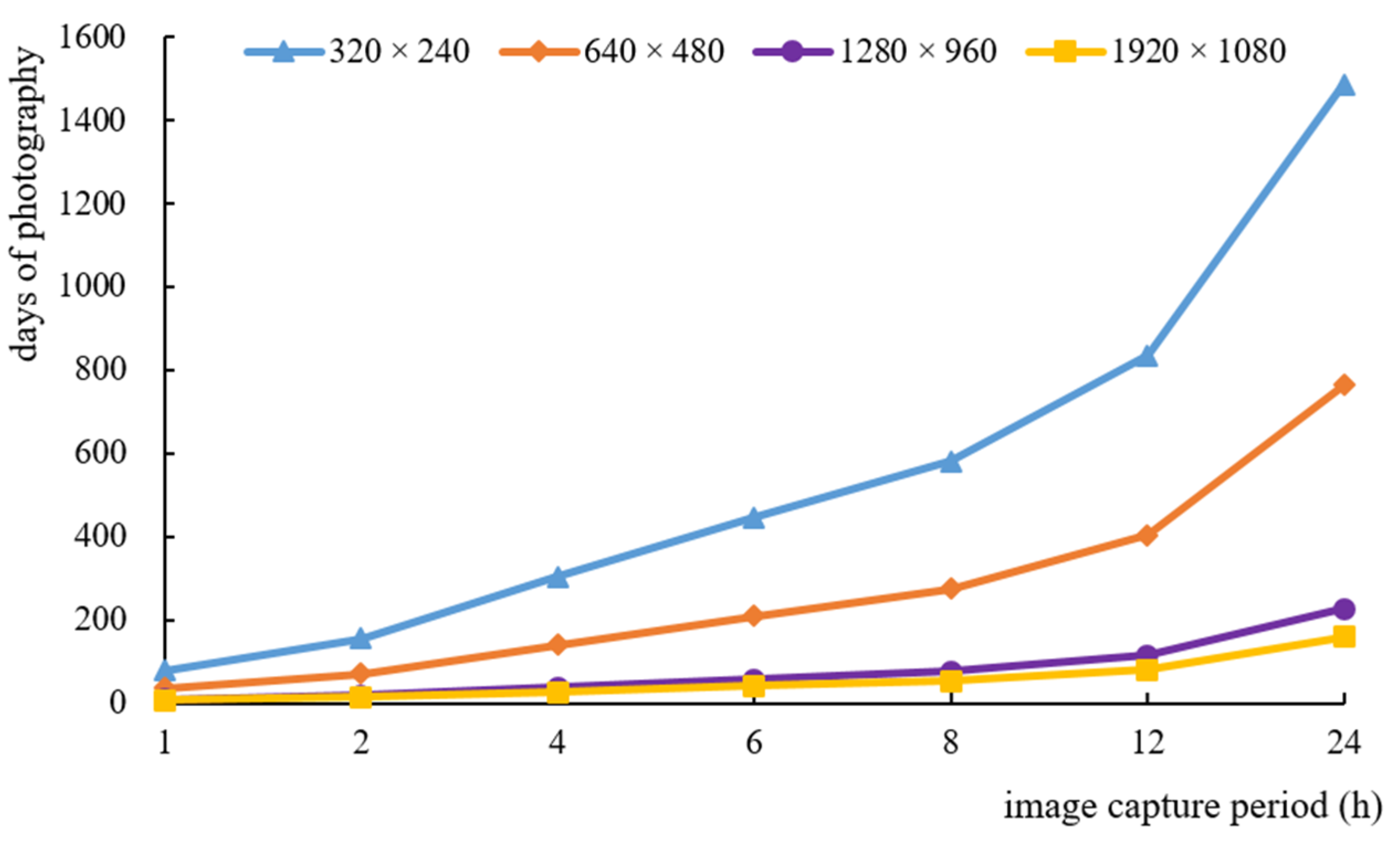
3.1.3. Analysis of Monitoring Duration with Different Image Resolutions
3.2. Image Processing
3.2.1. Image Preprocessing
3.2.2. Image Segmentation
3.2.3. Feature Extraction and Target Pest Identification
4. Discussion
5. Conclusions
- (1)
- The time taken by the monitoring device to capture and transmit images was directly proportional to the image size. The average power consumption of 320 × 240, 640 × 480, 1280 × 960, and 1920 × 1080-pixel images with seven capture periods was 7.24 mWh, 15.27 mWh, 54.50 mWh, and 77.69 mWh, respectively. The higher the image resolution, the higher the power consumption of image acquisition and transmission; the longer the interval between photos, the higher the power consumption of a single image.
- (2)
- The image numbers with four resolutions and seven capture periods were significantly different; the lower the image resolution, the more images were obtained, and the shorter the photo interval, the more images could be taken. The monitoring device could capture 1280 × 960 and 1920 × 1080-pixel images for a minimum of 10 and 7 days, respectively, with a 1 h capture period, and it could capture 1280 × 960 and 1920 × 1080-pixel images for a maximum of 226 and 160 days, respectively, with a 24 h capture period. The number of image capture days was inversely proportional to the image resolution and capture frequency. Long-term acquisition of high-resolution images can be achieved by installing micro-solar panels in the field.
- (3)
- The images of S. frugiperda were processed using image preprocessing, segmentation, feature extraction, and target pest identification. The feature parameters of complexity and H/V were used to identify S. frugiperda; the accuracy of automatic counting of S. frugiperda was 94.10%, and the coefficient of determination R2 was 0.9799.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, Y.; Wu, Q.L.; Zhang, H.W.; Wu, K.M. Spread of invasive migratory pest Spodoptera frugiperda and management practices throughout China. J. Integr. Agric. 2021, 20, 637–645. [Google Scholar] [CrossRef]
- Matova, P.M.; Kamutando, C.N.; Magorokosho, C.; Kutywayo, D.; Gutsa, F.; Labuschagne, M. Fall-armyworm invasion, control practices and resistance breeding in Sub-Saharan Africa. Crop. Sci. 2020, 60, 2951–2970. [Google Scholar] [CrossRef] [PubMed]
- Murúa, G.; Virla, E. Population parameters of Spodoptera frugiperda (Smith) (Lep.: Noctuidae) fed on corn and two predominant grasess in Tucuman (Argentina). Acta Zool. Mex. 2004, 20, 199–210. [Google Scholar] [CrossRef]
- Goergen, G.; Kumar, P.L.; Sankung, S.B.; Togola, A.; Tamo, M. First Report of Outbreaks of the Fall Armyworm Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae), a New Alien Invasive Pest in West and Central Africa. PLoS ONE 2016, 11, e0165632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stokstad, E. FOOD SECURITY New crop pest takes Africa at lightning speed. Science 2017, 356, 473–474. [Google Scholar] [CrossRef]
- Prasanna, B.M.; Huesing, J.E.; Eddy, R.; Peschke, V.M. Fall Armyworm in Africa: A Guide for Integrated Pest Management; Cimmyt: CDMX, Mexico, 2018; pp. 1–28. [Google Scholar]
- Sharanabasappa; Kalleshwaraswamy, C.M.; Asokan, R.; Swamy, H.M.; Maruthi, M.S.; Pavithra, H.B.; Hegde, K.; Navi, S.; Prabhu, S.T.; Goergen, G. First report of the Fall armyworm, Spodoptera frugiperda (J E Smith) (Lepidoptera: Noctuidae), an alien invasive pest on maize in India. Pest Manag. Hortic. Ecosyst. 2018, 24, 23–29. [Google Scholar]
- Sun, X.X.; Hu, C.X.; Jia, H.R.; Wu, Q.L.; Shen, X.J.; Zhao, S.Y.; Jiang, Y.Y.; Wu, K.M. Case study on the first immigration of fall armyworm, Spodoptera frugiperda invading into China. J. Integr. Agric. 2021, 20, 664–672. [Google Scholar] [CrossRef]
- Yang, X.M.; Song, Y.F.; Sun, X.X.; Shen, X.J.; Wu, Q.L.; Zhang, H.W.; Zhang, D.D.; Zhao, S.Y.; Liang, G.M.; Wu, K.M. Population occurrence of the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae), in the winter season of China. J. Integr. Agric. 2021, 20, 772–782. [Google Scholar] [CrossRef]
- Montezano, D.G.; Specht, A.; Sosa-Gómez, D.R.; Roque-Specht, V.F.; Sousa-Silva, J.C.; Paula-Moraes, S.V.; Peterson, J.A.; Hunt, T.E. Host Plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef] [Green Version]
- Overton, K.; Maino, J.L.; Day, R.; Umina, P.A.; Bett, B.; Carnovale, D.; Ekesi, S.; Meagher, R.; Reynolds, O.L. Global crop impacts, yield losses and action thresholds for fall armyworm (Spodoptera frugiperda): A review. Crop Prot. 2021, 145, 105641. [Google Scholar] [CrossRef]
- Tepa-Yotto, G.T.; Meagher, R.L.; Winsou, J.K.; Dahoueto, B.T.A.; Tamò, M.; Sæthre, M.-G.; Nagoshi, R.N. Monitoring Spodoptera frugiperda in Benin: Assessing the influence of trap type, pheromone blends, and habitat on pheromone trapping. Fla. Entomol. 2022, 105, 71–78. [Google Scholar] [CrossRef]
- Chen, M.X.; Yang, X.T.; Shi, B.C.; Li, W.Y.; Du, X.W.; Li, M.; Sun, C.H. Research progress and prospect of technologies for automatic identifying and counting of pests. J. Environ. Entomol. 2015, 37, 176–183. [Google Scholar]
- Meagher, R.L.; Agboka, K.; Tounou, A.K.; Koffi, D.; Agbevohia, K.A.; Amouze, T.R.; Adjevi, K.M.; Nagoshi, R.N. Comparison of pheromone trap design and lures for Spodoptera frugiperda in Togo and genetic characterization of moths caught. Entomol. Exp. Appl. 2019, 167, 507–516. [Google Scholar] [CrossRef]
- Zeng, J.; Du, Y.J.; Jiang, Y.Y.; Liu, W.C. Development and application of sex pheromone in population monitoring of crop insect pests in China. Plant Prot. 2015, 41, 9–15. [Google Scholar]
- Rizvi, S.A.H.; George, J.; Reddy, G.V.P.; Zeng, X.; Guerrero, A. Latest developments in insect sex pheromone research and its application in agricultural pest management. Insects 2021, 12, 484. [Google Scholar] [CrossRef]
- Muthukumar, M.; Kennedy, J.S. A review of the scientific literature on the use of reproductive pheromones in the management of Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Entomol. Sci. 2021, 56, 475–486. [Google Scholar] [CrossRef]
- Eliopoulos, P.A.; Potamitis, I.; Kontodimas, D.C. Estimation of population density of stored grain pests via bioacoustic detection. Crop Prot. 2016, 85, 71–78. [Google Scholar] [CrossRef]
- Jiang, J.A.; Lin, T.S.; Yang, E.C.; Tseng, C.L.; Chen, C.P.; Yen, C.W.; Zheng, X.Y.; Liu, C.Y.; Liu, R.H.; Chen, Y.F.; et al. Application of a web-based remote agro-ecological monitoring system for observing spatial distribution and dynamics of Bactrocera dorsalis in fruit orchards. Precis Agric. 2013, 14, 323–342. [Google Scholar] [CrossRef]
- Han, R.Z.; He, Y. Remote automatic identification system of field pests based on computer vision. Trans. Chin. Soc. Agric. Eng. 2013, 29, 156–162. [Google Scholar]
- Ebrahimi, M.A.; Khoshtaghaz, M.H.; Minaei, S.; Jamshidi, B. Vision-based pest detection based on SVM classification method. Comput. Electron. Agric. 2017, 137, 52–58. [Google Scholar] [CrossRef]
- Suto, J. Codling moth monitoring with camera-equipped automated traps: A review. Agriculture 2022, 12, 1721. [Google Scholar] [CrossRef]
- Lima, M.C.F.; Leandro, M.E.D.D.; Valero, C.; Coronel, L.C.P.; Bazzo, C.O.G. Automatic detection and monitoring of insect pests—A review. Agriculture 2020, 10, 161. [Google Scholar] [CrossRef]
- Xue, D.X.; Zhang, R.R.; Chen, L.P.; Chen, L.P.; Chen, M.X.; Xu, G. Faster R-CNN based image recognition research of Hyphantria cunea. J. Environ. Entomol. 2020, 42, 1502–1509. [Google Scholar]
- Yao, Q.; Xian, D.; Liu, Q.; Yang, B.; Diao, G. Automated counting of rice planthoppers in paddy fields based on image processing. J. Integr. Agric. 2014, 13, 1736–1745. [Google Scholar] [CrossRef]
- Wen, C.; Guyer, D. Image-based orchard insect automated identification and classification method. Comput. Electron. Agric. 2012, 89, 110–115. [Google Scholar] [CrossRef]
- Li, W.Y.; Li, M.; Chen, M.X.; Qian, J.P.; Sun, C.H.; Du, S.F. Feature extraction and classification method of multi-pose pests using machine vision. Trans. Chin. Soc. Agric. Eng. 2014, 30, 154–162. [Google Scholar]
- Chen, J.; Fan, Y.Y.; Wang, T.; Zhang, C.; Qiu, Z.J.; He, Y. Automatic segmentation and counting of aphid nymphs on leaves using convolutional neural networks. Agronomy 2018, 8, 129. [Google Scholar] [CrossRef] [Green Version]
- Boniecki, P.; Koszela, K.; Piekarska-Boniecka, H.; Weres, J.; Zaborowicz, M.; Kujawa, S.; Majewski, A.; Raba, B. Neural identification of selected apple pests. Comput. Electron. Agric. 2015, 110, 9–16. [Google Scholar] [CrossRef]
- Wang, F.Y.; Wang, R.J.; Xie, C.J.; Yang, P.; Liu, L. Fusing multi-scale context-aware information representation for automatic in-field pest detection and recognition. Comput. Electron. Agric. 2020, 169, 10552. [Google Scholar] [CrossRef]
- Guarnieri, A.; Maini, S.; Molari, G.; Rondelli, V. Automatic trap for moth detection in integrated pest management. Bull. Insectology 2011, 64, 247–251. [Google Scholar]
- Fukatsu, T.; Watanabe, T.; Hu, H.; Yoichi, H.; Hirafuji, M. Field monitoring support system for the occurrence of Leptocorisa chinensis Dallas (Hemiptera: Alydidae) using synthetic attractants, Field Servers, and image analysis. Comput. Electron. Agric. 2012, 80, 8–16. [Google Scholar] [CrossRef]
- Ding, W.; Taylor, G. Automatic moth detection from trap images for pest management. Comput. Electron. Agric. 2016, 123, 17–28. [Google Scholar] [CrossRef] [Green Version]
- López, O.; Rach, M.M.; Migallon, H.; Malumbres, M.P.; Bonastre, A.; Serrano, J.J. Monitoring pest insect traps by means of low-power image sensor technologies. Sensors 2012, 12, 15801–15819. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Rong, E.; Li, S.; Zhang, L.; Zhang, Z.; Guo, Y.; Ma, R. Potential application of digital image-processing method and fitted logistic model to the control of oriental fruit moths (Grapholita molesta Busck). Bull. Entomol. Res. 2016, 106, 457–463. [Google Scholar] [CrossRef]
- Doitsidis, L.; Fouskitakis, G.N.; Varikou, K.N.; Rigakis, I.I.; Chatzichristofis, S.A.; Papafilippaki, A.K.; Birouraki, A.E. Remote monitoring of the Bactrocera oleae (Gmelin) (Diptera: Tephritidae) population using an automated McPhail trap. Comput. Electron. Agric. 2017, 137, 69–78. [Google Scholar] [CrossRef]
- Chen, M.X.; Guo, J.Y.; Xu, J.P.; Zhao, L.; Li, W.Y.; Zhang, R.K.; Bi, L.L.; Wen, D.M.; Li, M. Research of automatic monitoring device and counting system for Grapholita molesta (Busck). J. Environ. Entomol. 2018, 40, 1164–1174. [Google Scholar]
- Ünlü, L.; Akdemir, B.; Ögür, E.; Şahin, İ. Remote monitoring of european grapevine moth, Lobesia botrana (Lepidoptera: Tortricidae) population using camera-based pheromone traps in vineyards. Turk. J. Agric.-Food Sci. Technol. 2019, 7, 652–657. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.J.; Nam, I.; Kim, S.Y.; Kim, E.; Lee, C.H.; Ahn, S.; Park, I.K.; Kim, G. Automatic pest counting from pheromone trap images using deep learning object detectors for Matsucoccus thunbergianae Monitoring. Insects 2021, 12, 342. [Google Scholar] [CrossRef]
- Preti, M.; Favaro, R.; Knight, A.L.; Angeli, S. Remote monitoring of Cydia pomonella adults among an assemblage of nontargets in sex pheromone-kairomone-baited smart traps. Pest Manag. Sci. 2021, 77, 4084–4090. [Google Scholar] [CrossRef]
- Schrader, M.J.; Smytheman, P.; Beers, E.H.; Khot, L.R. An open-source low-cost imaging system plug-in for pheromone traps aiding remote insect pest population monitoring in fruit crops. Machines 2022, 10, 52. [Google Scholar] [CrossRef]
- ZL 201921202577.5; Qiu, R.Z.; Zhao, J.; Chi, M.X.; Weng, Q.Y.; Chen, S.X. An Automatic Pest Counting System Based on Image Recognition. Institute of Plant Protection, Fujian Academy of Agricultural Sciences: Fuzhou, China, 2020.
- Qiu, R.Z.; Zhao, J.; He, Y.X.; Chen, S.P.; Huang, M.L.; Chi, M.X.; Liang, Y.; Weng, Q.Y. An automatic identification and counting method of Spodoptera frugiperda (Lepidoptera:Noctuidae) adults based on sex pheromone trapping and deep learning. Acta Entomol. Sin. 2021, 64, 1444–1454. [Google Scholar]
- Koffi, D.; Agboka, K.; Adjevi, A.K.M.; Assogba, K.; Fening, K.O.; Osae, M.; Aboagye, E.; Meagher, R.L.; Nagoshi, R.N. Trapping Spodoptera frugiperda (Lepidoptera: Noctuidae) moths in different crop habitats in Togo and Ghana. J. Econ. Entomol. 2021, 114, 1138–1144. [Google Scholar] [CrossRef]
- Qiu, G.Y. Electrical Circuits, 5th ed.; Higher Education Press: Beijing, China, 2006. [Google Scholar]
- Gonzalez, R.; Woods, R.; Eddins, S. Digital Image Processing Using MATLAB; Publishing House of Electronics Industry: Beijing, China, 2013; pp. 515–519. [Google Scholar]
- Li, W.Y.; Li, M.; Qian, J.P.; Sun, C.H.; Du, D.S.; Chen, M.X. Segmentation method for touching pest images based on shape factor and separation points location. Trans. Chin. Soc. Agric. Eng. 2015, 31, 175–180. [Google Scholar]
- Wang, Z.B.; Wang, K.Y.; Zhang, S.F.; Liu, Z.Q.; Mu, C.X. Whiteflies counting with K-means clustering and ellipse fitting. Trans. Chin. Soc. Agric. Eng. 2014, 30, 105–112. [Google Scholar]
- Preti, M.; Verheggen, F.; Angeli, S. Insect pest monitoring with camera-equipped traps: Strengths and limitations. J. Pest Sci. 2020, 94, 203–217. [Google Scholar] [CrossRef]



| Image Resolution (Pixel) | Number of Images | Image Capture Period (h) | Average Image Sizes (KB) | Time of Photo Transmission (s) |
|---|---|---|---|---|
| 1920 × 1080 | 336 | 2 | 248 | 121.58 |
| 1280 × 960 | 336 | 2 | 170 | 83.13 |
| 640 × 480 | 336 | 2 | 38 | 18.48 |
| 320 × 240 | 336 | 2 | 11 | 5.46 |
| Image Capture Period (h) | Image Resolution (Pixel) | |||
|---|---|---|---|---|
| 320 × 240 | 640 × 480 | 1280 × 960 | 1920 × 1080 | |
| 1 | 6.68 | 14.71 | 53.95 | 77.13 |
| 2 | 6.76 | 14.79 | 54.03 | 77.21 |
| 4 | 6.92 | 14.94 | 54.18 | 77.37 |
| 6 | 7.07 | 15.10 | 54.34 | 77.52 |
| 8 | 7.23 | 15.26 | 54.49 | 77.68 |
| 12 | 7.54 | 15.57 | 54.81 | 77.99 |
| 24 | 8.48 | 16.50 | 55.74 | 78.93 |
| Image Capture Period (h) | Resolution (Pixel) | |||
|---|---|---|---|---|
| 320 × 240 | 640 × 480 | 1280 × 960 | 1920 × 1080 | |
| 1 | 1885 | 857 | 234 | 163 |
| 2 | 1863 | 852 | 233 | 163 |
| 4 | 1821 | 843 | 233 | 163 |
| 6 | 1781 | 834 | 232 | 163 |
| 8 | 1743 | 826 | 231 | 162 |
| 12 | 1671 | 809 | 230 | 162 |
| 24 | 1486 | 763 | 226 | 160 |
| Feature Parameters | Threshold Range | |
|---|---|---|
| Lower Limit | Upper Limit | |
| H/V | 0.06 | 0.22 |
| Complexity | 1.35 | 8.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Chen, L.; Yi, T.; Zhang, R.; Xia, L.; Qu, C.; Xu, G.; Wang, W.; Ding, C.; Tang, Q.; et al. Development of a Low-Power Automatic Monitoring System for Spodoptera frugiperda (J. E. Smith). Agriculture 2023, 13, 843. https://doi.org/10.3390/agriculture13040843
Chen M, Chen L, Yi T, Zhang R, Xia L, Qu C, Xu G, Wang W, Ding C, Tang Q, et al. Development of a Low-Power Automatic Monitoring System for Spodoptera frugiperda (J. E. Smith). Agriculture. 2023; 13(4):843. https://doi.org/10.3390/agriculture13040843
Chicago/Turabian StyleChen, Meixiang, Liping Chen, Tongchuan Yi, Ruirui Zhang, Lang Xia, Cheng Qu, Gang Xu, Weijia Wang, Chenchen Ding, Qing Tang, and et al. 2023. "Development of a Low-Power Automatic Monitoring System for Spodoptera frugiperda (J. E. Smith)" Agriculture 13, no. 4: 843. https://doi.org/10.3390/agriculture13040843





