Multi-Year QTL Mapping and RNA-seq Reveal Candidate Genes for Early Floret-Opening Time in Japonica Rice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Statistical Methods for FOT
2.3. QTLseqr Analysis
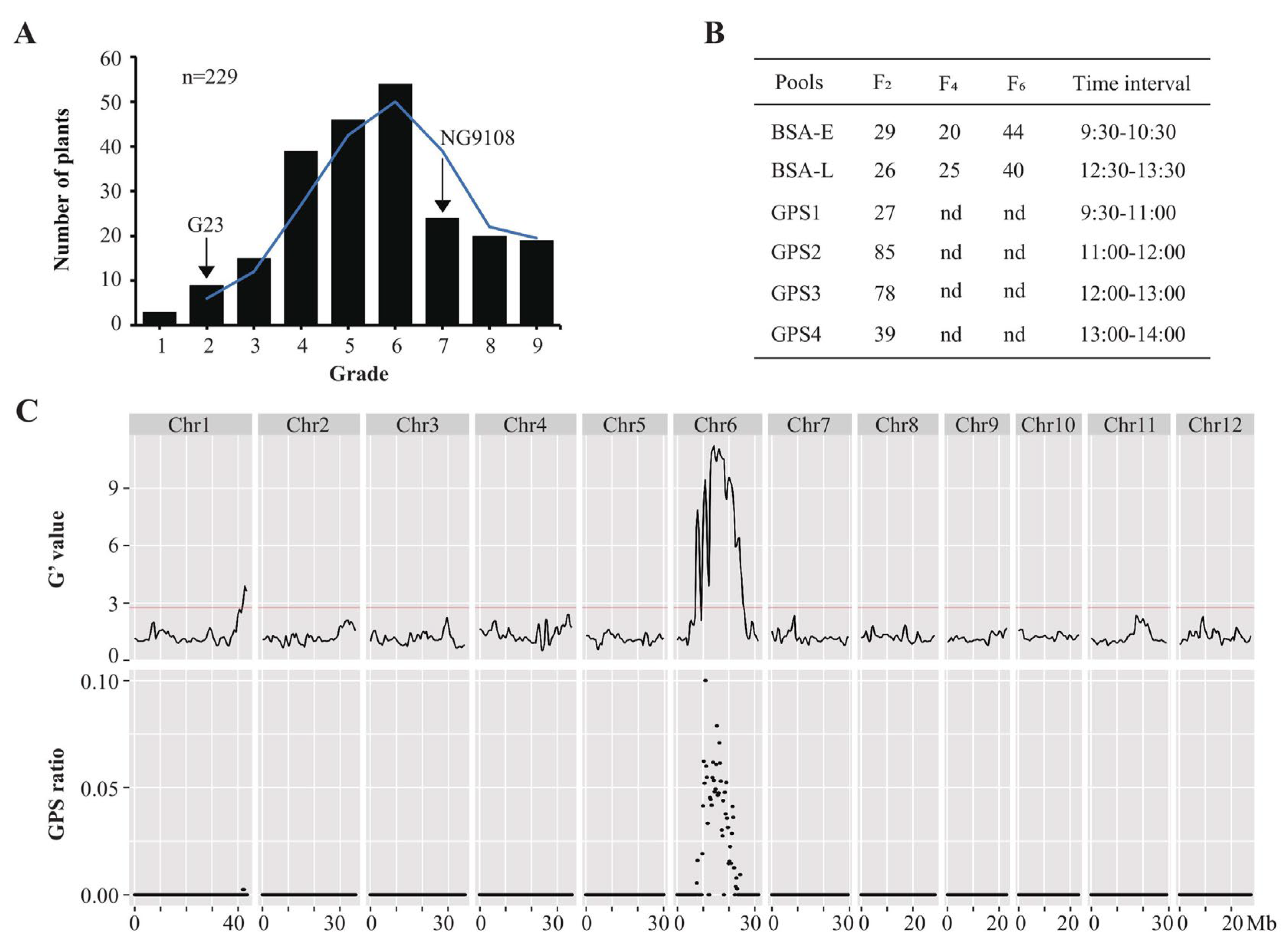
2.4. GradedPool-Seq (GPS) Analysis
2.5. Regional Linkage Mapping Analysis
2.6. RNA-seq Analysis and Candidate Gene Prediction within Target Intervals
3. Results
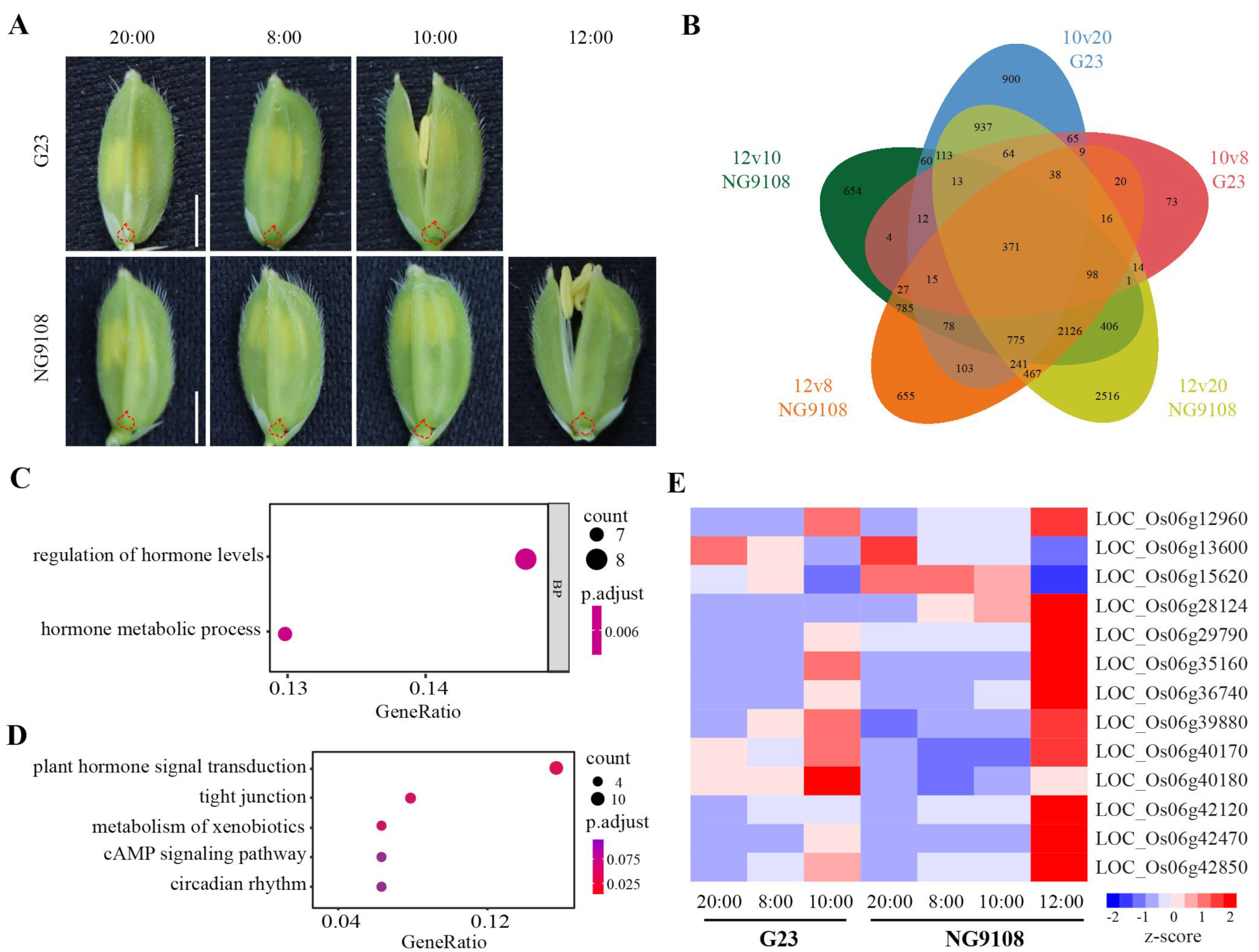
3.1. Floret-Opening Dynamics of G23 and NG9108
3.2. FOT QTL Detection in the F2 Population
3.3. Multiple-Year Verification of qFOT6
3.4. RNA-seq Analysis and Candidate Genes Selection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, G.M.; Tang, J.Y.; Zheng, J.K.; Chu, C.C. Exploration of rice yield potential: Decoding agronomic and physiological traits. Crop. J. 2021, 9, 577–589. [Google Scholar] [CrossRef]
- Zeng, D.L.; Tian, Z.X.; Rao, Y.C.; Dong, G.J.; Yang, Y.L.; Huang, L.C.; Leng, Y.J.; Xu, J.; Sun, C.; Zhang, G.H.; et al. Rational design of high-yield and superior-quality rice. Nat. Plants 2017, 3, 17031. [Google Scholar] [CrossRef] [PubMed]
- Van Doorn, W.G.; Van Meeteren, U. Flower opening and closure: A review. J. Exp. Bot. 2003, 54, 1801–1812. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Nagato, Y. Flower development in rice. J. Exp. Bot. 2011, 62, 4719–4730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baliuag, N.N.A.; Redona, E.D.; Hernandez, J.E.; Cruz, P.C.S.; Ye, C.R. Genetic analysis for heat tolerance and early morning flowering traits at flowering stage in rice (Oryza sativa L.). Philipp. J. Crop. Sci. 2015, 40, 62–72. [Google Scholar] [CrossRef] [Green Version]
- Wan, G.; Feng, Y.; Zhang, F.J.; Xu, Q.; Wang, Y.P.; Yuan, X.P.; Yu, H.Y.; Peng, S.T. Mapping QTLs for flowering time in rice. Acta Agric. Nucleatae Sin. 2013, 27, 562–567. [Google Scholar] [CrossRef]
- Thanh, P.T.; Phan, P.D.T.; Ishikawa, R.; Ishii, T. QTL analysis for flowering time using backcross population between Oryza sativa Nipponbare and O. rufipogon. Genes Genet. Syst. 2010, 85, 273–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Zhang, H.L.; Dai, D.Q.; Li, X.M.; Chen, J.Y.; Bao, J.S.; Ma, L.Y. Identification of QTLs for rice flower opening time in two environments. Euphytica 2017, 213, 181. [Google Scholar] [CrossRef]
- Hirabayashi, H.; Sasaki, K.; Kambe, T.; Gannaban, R.B.; Miras, M.A.; Mendioro, M.S.; Simon, E.V.; Lumanglas, P.D.; Fujita, D.; Takemoto-Kuno, Y.; et al. qEMF3, a novel QTL for the early-morning flowering trait from wild rice, Oryza officinalis, to mitigate heat stress damage at flowering in rice, O. sativa. J. Exp. Bot. 2015, 66, 1227–1236. [Google Scholar] [CrossRef]
- Xu, P.Z.; Wu, T.K.; Ali, A.; Zhang, H.Y.; Liao, Y.X.; Chen, X.Q.; Tian, Y.H.; Wang, W.M.; Fu, X.D.; Li, Y.; et al. EARLY MORNING FLOWERING1 (EMF1) regulates the floret opening time by mediating lodicule cell wall formation in rice. Plant Biotechnol. J. 2022, 20, 1441–1443. [Google Scholar] [CrossRef]
- Wang, M.M.; Zhu, X.P.; Peng, G.Q.; Liu, M.L.; Zhang, S.Q.; Chen, M.H.; Liao, S.T.; Wei, X.Y.; Xu, P.; Tan, X.Y.; et al. Methylesterification of cell-wall pectin controls the diurnal flower-opening times in rice. Mol. Plant 2022, 15, 956–972. [Google Scholar] [CrossRef] [PubMed]
- Bheemanahalli, R.; Sathishraj, R.; Manoharan, M.; Sumanth, H.; Muthurajan, R.; Ishimaru, T.; Krishna, J.S.V. Is early morning flowering an effective trait to minimize heat stress damage during flowering in rice? Field Crop. Res. 2017, 203, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Habib-Ur-Rahman, M.; Ahmad, A.; Raza, A.; Hasnain, M.U.; Alharby, H.F.; Alzahrani, Y.M.; Bamagoos, A.A.; Hakeem, K.R.; Ahmad, S.; Nasim, W.; et al. Impact of climate change on agricultural production; issues, challenges, and opportunities in Asia. Front. Plant Sci. 2022, 13, 925548. [Google Scholar] [CrossRef]
- Ishimaru, T.; Sasaki, K.; Lumanglas, P.D.; Cabral, C.L.U.; Ye, C.; Yoshimoto, M.; Kumar, A.; Henry, A. Effect of drought stress on flowering characteristics in rice (Oryza sativa L.): A study using genotypes contrasting in drought tolerance and flower opening time. Plant Prod. Sci. 2022, 25, 359–370. [Google Scholar] [CrossRef]
- Sun, Q.; Zhao, Y.X.; Zhang, Y.; Chen, S.N.; Ying, Q.; Lv, Z.F.; Che, X.H.; Wang, D.L. Heat stress may cause a significant reduction of rice yield in China under future climate scenarios. Sci. Total. Environ. 2022, 818, 151746. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Wang, L.; Zhou, J.X.; Hu, S.B.; Chen, H.Z.; Xiang, J.; Zhang, Y.K.; Zeng, Y.J.; Shi, Q.H.; Zhu, D.F.; et al. Research progress on heat stress of rice at flowering stage. Rice Sci. 2019, 26, 1–10. [Google Scholar] [CrossRef]
- Song, Y.L.; Wang, C.Y.; Linderholm, H.W.; Fu, Y.; Cai, W.Y.; Xu, J.X.; Zhuang, L.W.; Wu, M.X.; Shi, Y.X.; Wang, G.F.; et al. The negative impact of increasing temperatures on rice yields in southern China. Sci. Total. Environ. 2022, 820, 153262. [Google Scholar] [CrossRef]
- Bheemanahalli, R.; Sathishraj, R.; Tack, J.; Nalley, L.L.; Muthurajan, R.; Jagadish, K.S.V. Temperature thresholds for spikelet sterility and associated warming impacts for subtropical rice. Agric. For. Meteorol. 2016, 221, 122–130. [Google Scholar] [CrossRef]
- Xu, Y.F.; Chu, C.C.; Yao, S.G. The impact of high-temperature stress on rice: Challenges and solutions. Crop. J. 2021, 9, 963–976. [Google Scholar] [CrossRef]
- Jagadish, S.V.K.; Craufurd, P.Q.; Wheeler, T.R. High temperature stress and spikelet fertility in rice (Oryza sativa L.). J. Exp. Bot. 2007, 58, 1627–1635. [Google Scholar] [CrossRef] [Green Version]
- Matsui, T.; Omasa, K.; Horie, T. High temperature at flowering inhibits swelling of pollen grains, a driving force for thecae dehiscence in rice (Oryza sativa L.). Plant Prod. Sci. 2000, 3, 430–434. [Google Scholar] [CrossRef]
- Rang, Z.W.; Jagadish, S.V.K.; Zhou, Q.M.; Craufurd, P.Q.; Heuer, S. Effect of high temperature and water stress on pollen germination and spikelet fertility in rice. Environ. Exp. Bot. 2011, 70, 58–65. [Google Scholar] [CrossRef] [Green Version]
- Ishimaru, T.; Hirabayashi, H.; Kuwagata, T.; Ogawa, T.; Kondo, M. The early-morning flowering trait of rice reduces spikelet sterility under windy and elevated temperature conditions at anthesis. Plant Prod. Sci. 2012, 15, 19–22. [Google Scholar] [CrossRef] [Green Version]
- Jagadish, S.V.K.; Murty, M.V.R.; Quick, W.P. Rice responses to rising temperatures—Challenges, perspectives and future directions. Plant Cell Environ. 2015, 38, 1686–1698. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, T.; Hirabayashi, H.; Sasaki, K.; Ye, C.R.; Kobayashi, A. Breeding efforts to mitigate damage by heat stress to spikelet sterility and grain quality. Plant Prod. Sci. 2016, 19, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Ishimaru, T.; Hlaing, K.T.; Oo, Y.M.; Lwin, T.M.; Sasaki, K.; Lumanglas, P.D.; Simon, E.V.M.; Myint, T.T.; Hairmansis, A.; Susanto, U.; et al. An early-morning flowering trait in rice can enhance grain yield under heat stress field conditions at flowering stage. Field Crop. Res. 2022, 277, 108400. [Google Scholar] [CrossRef]
- Ishimaru, T.; Hirabayashi, H.; Ida, M.; Takai, T.; San-Oh, Y.A.; Yoshinaga, S.; Ando, I.; Ogawa, T.; Kondo, M. A genetic resource for early-morning flowering trait of wild rice Oryza officinalis to mitigate high temperature-induced spikelet sterility at anthesis. Ann. Bot. 2010, 106, 515–520. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Xu, C.X.; He, Y.; Zong, J.; Yang, X.J.; Si, H.M.; Sun, Z.X.; Hu, J.P.; Liang, W.Q.; Zhang, D.B. Mutation in CSA creates a new photoperiod-sensitive genic male sterile line applicable for hybrid rice seed production. Proc. Natl. Acad. Sci. USA 2013, 110, 76–81. [Google Scholar] [CrossRef] [Green Version]
- Lei, Y.Q.; Song, S.F.; Li, X.Q. Development of technologies for heterosis utilization in rice. Hybrid Rice. 2017, 32, 1–4. [Google Scholar] [CrossRef]
- Chu, S.H.; Jiang, W.Z.; Lee, J.; Chin, J.H.; Koh, H.J. QTL analyses of heterosis for grain yield and yield-related traits in indica-japonica crosses of rice (Oryza sativa L.). Genes Genom. 2012, 34, 367–377. [Google Scholar] [CrossRef]
- Hu, N.; Cao, M.H.; Jiang, X.D.; Hu, J.C.; Yao, K.M. Estimating model for rice flowering habit with different rice varieties. Chin. J. Agrometeorol. 2013, 34, 440–446. [Google Scholar] [CrossRef]
- Liu, L.; Zou, Z.S.; Qian, K.; Xia, C.; He, Y.; Zeng, H.L.; Zhou, X.; Riemann, M.; Yin, C.X. Jasmonic acid deficiency leads to scattered floret opening time in cytoplasmic male sterile rice Zhenshan 97A. J. Exp. Bot. 2017, 68, 4613–4625. [Google Scholar] [CrossRef] [Green Version]
- Rani, B.; Sharma, V.K. A modified CTAB method for quick extraction of genomic DNA from rice seed/grain/leaves for PCR analysis. Int. J. Sci. Res. Methodol. 2016, 4, 254–260. [Google Scholar]
- Chen, S.F.; Zhou, Y.Q.; Chen, Y.R.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Heslop-Harrison, Y.H.; Heslop-Harrison, J.S. Lodicule function and filament extension in the grasses: Potassium ion movement and tissue specialization. Ann. Bot. 1996, 77, 573–582. [Google Scholar] [CrossRef]
- Lairson, L.L.; Henrissat, B.; Davies, G.J.; Withers, S.G. Glycosyltransferases: Structures, functions, and mechanisms. Annu. Rev. Biochem. 2008, 77, 521–555. [Google Scholar] [CrossRef] [Green Version]
- Kong, W.L.; Gong, Z.Y.; Zhong, H.; Zhang, Y.; Zhao, G.Q.; Gautam, M.; Deng, X.X.; Liu, C.; Zhang, C.H.; Li, Y.S. Expansion and evolutionary patterns of glycosyltransferase family 8 in gramineae crop genomes and their expression under salt and cold stresses in Oryza sativa ssp. japonica. Biomolecules 2019, 9, 188. [Google Scholar] [CrossRef] [Green Version]
- Hirano, K.; Aya, K.; Morinaka, Y.; Nagamatsu, S.; Sato, Y.; Antonio, B.A.; Namiki, N.; Nagamura, Y.; Matsuoka, M. Survey of genes involved in rice secondary cell wall formation through a co-expression network. Plant Cell Physiol. 2013, 54, 1803–1821. [Google Scholar] [CrossRef] [Green Version]
- Dong, N.Q.; Sun, Y.W.; Guo, T.; Shi, C.L.; Zhang, Y.M.; Kan, Y.; Xiang, Y.H.; Zhang, H.; Yang, Y.B.; Li, Y.C.; et al. UDP-glucosyltransferase regulates grain size and abiotic stress tolerance associated with metabolic flux redirection in rice. Nat. Commun. 2020, 11, 2629. [Google Scholar] [CrossRef]
- Ma, F.F.; Lu, R.; Liu, H.Y.; Shi, B.; Zhang, J.H.; Tan, M.P.; Zhang, A.Y.; Jiang, M.Y. Nitric oxide-activated calcium/calmodulin-dependent protein kinase regulates the abscisic acid-induced antioxidant defence in maize. J. Exp. Bot. 2012, 63, 4835–4847. [Google Scholar] [CrossRef]
- Chen, M.; Ni, L.; Chen, J.; Sun, M.; Qin, C.H.; Zhang, G.; Zhang, A.Y.; Jiang, M.Y. Rice calcium/calmodulin-dependent protein kinase directly phosphorylates a mitogen-activated protein kinase kinase to regulate abscisic acid responses. Plant Cell 2021, 33, 1790–1812. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Fu, X.P.; Zhang, H.; Li, X.; Cai, X.; Zhang, P.P.; Liu, L.; Wang, Q.W.; Sun, M.M.; Wang, Q.W.; et al. Abscisic acid inhibits rice protein phosphatase PP45 via H2O2 and relieves repression of the Ca2+/CaM-dependent protein kinase DMI3. Plant Cell 2019, 31, 128–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]

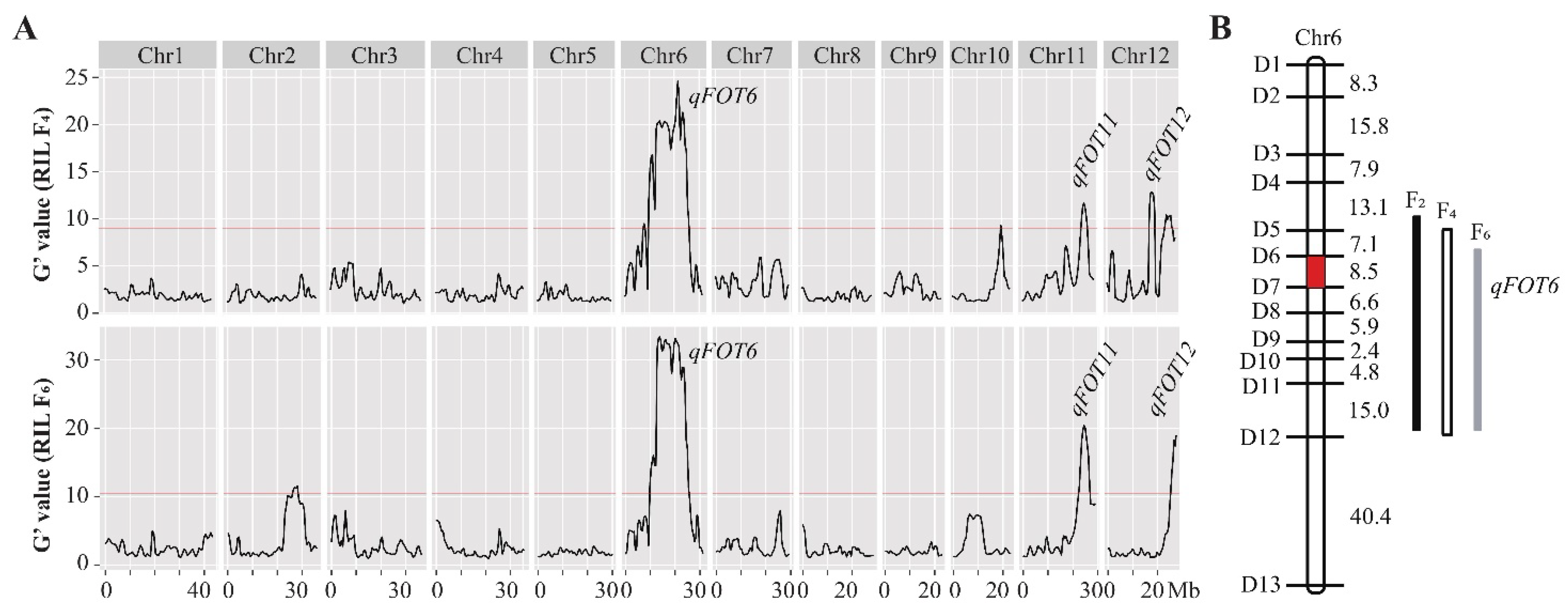
| Chr a | Locus | Left Marker | Right Marker | LOD b Value | PVE (%) c | Add d | Dom e |
|---|---|---|---|---|---|---|---|
| 6 | qFOT6 | D6 | D7 | 23.62 | 37.03 | −0.0329 | −0.0075 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.; Chen, G.; Zhang, R.; Xu, M.; Zhao, L.; Tang, H.; Ni, J.; Zhou, M. Multi-Year QTL Mapping and RNA-seq Reveal Candidate Genes for Early Floret-Opening Time in Japonica Rice. Agriculture 2023, 13, 859. https://doi.org/10.3390/agriculture13040859
Hu X, Chen G, Zhang R, Xu M, Zhao L, Tang H, Ni J, Zhou M. Multi-Year QTL Mapping and RNA-seq Reveal Candidate Genes for Early Floret-Opening Time in Japonica Rice. Agriculture. 2023; 13(4):859. https://doi.org/10.3390/agriculture13040859
Chicago/Turabian StyleHu, Xiaozhou, Guoliang Chen, Rui Zhang, Mengxuan Xu, Ling Zhao, Hailong Tang, Jinlong Ni, and Ming Zhou. 2023. "Multi-Year QTL Mapping and RNA-seq Reveal Candidate Genes for Early Floret-Opening Time in Japonica Rice" Agriculture 13, no. 4: 859. https://doi.org/10.3390/agriculture13040859




