Use of Bioinoculants Affects Variation in Snap Bean Yield Grown under Deficit Irrigation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Irrigation Regimes
2.3. Microorganisms Treatments
2.4. Morpho-Physiological Parameters
2.5. Experimental Design
2.6. Statistical Analysis
2.7. Climatic and Soil Conditions
3. Results
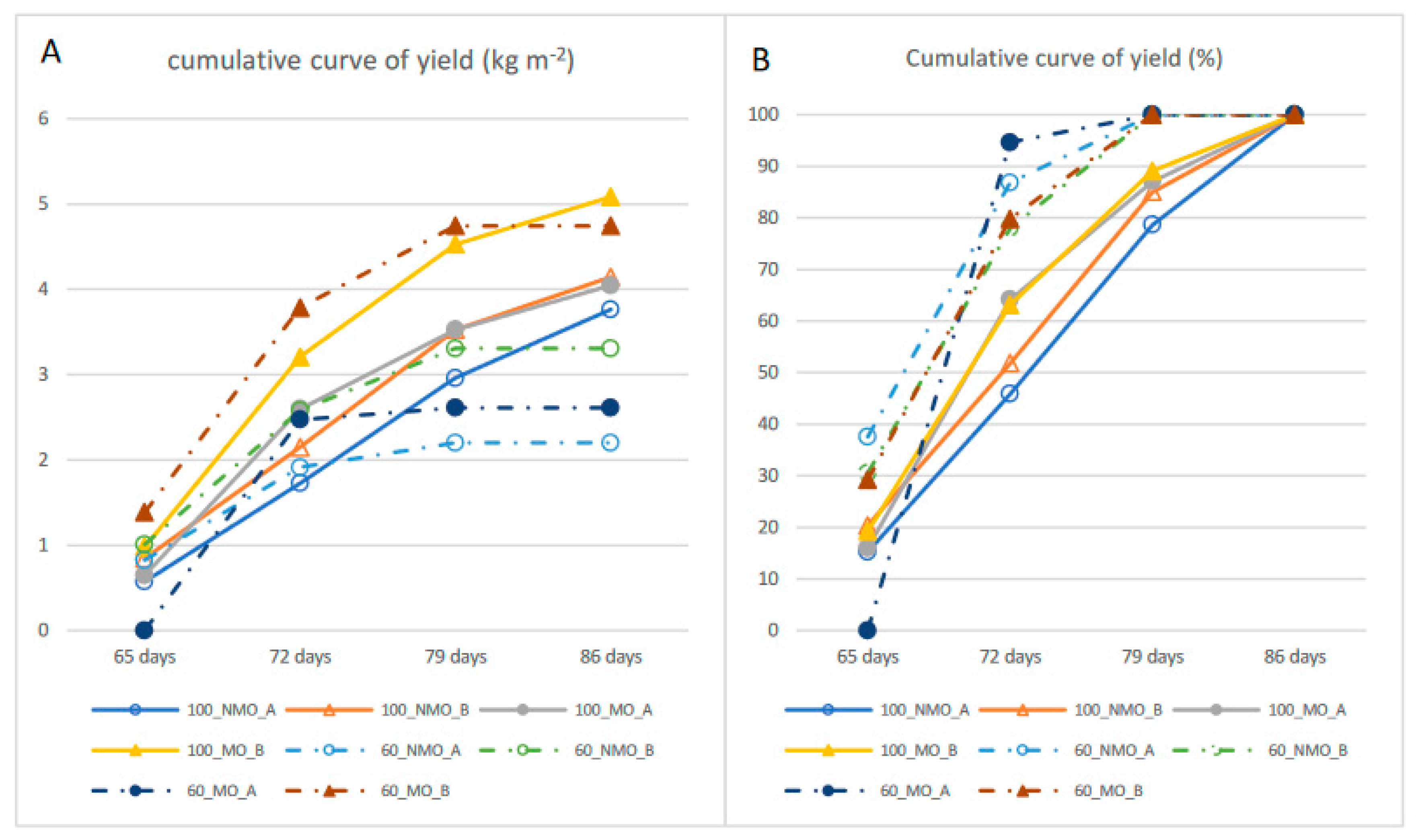
3.1. Production and Plants Characteristics
3.2. Correlations
3.3. Principal Component Analysis (PCA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Giampieri, F.; Quiles, J.L.; Cianciosi, D.; Yuliett Forbes-Hernández, T.; Josè Orantes-Bermejo, F.; Miguel Alvarez-Suarez, J.; Battino, M. Bee Products: An Emblematic Example of Underutilized Sources of Bioactive Compounds. Cite This J. Agric. Food Chem 2022, 2022, 6833–6848. [Google Scholar] [CrossRef]
- Schrama, M.; de Haan, J.J.; Kroonen, M.; Verstegen, H.; van der Putten, W.H. Crop Yield Gap and Stability in Organic and Conventional Farming Systems. Agric. Ecosyst. Env. 2018, 256, 123–130. [Google Scholar] [CrossRef]
- Lal, R. Managing Soils for Resolving the Conflict between Agriculture and Nature: The Hard Talk. Eur. J. Soil. Sci. 2020, 71, 1–9. [Google Scholar] [CrossRef]
- Khangura, R.; Ferris, D.; Wagg, C.; Bowyer, J. Regenerative Agriculture—A Literature Review on the Practices and Mechanisms Used to Improve Soil Health. Sustainability 2023, 15, 2338. [Google Scholar] [CrossRef]
- Di Bella, M.C.; Melilli, M.G.; Treccarichi, S.; Tribulato, A.; Arena, D.; Ruffino, A.; Argento, S.; Branca, F. Influence of irrigation regime on productive and qualitative traits of Kale (Brassica Oleracea Var. Acephala DC) under Organic Farming Dystem. In Proceedings of the Acta Horticulturae; International Society for Horticultural Science: Leuven, Belgium, 2022; Volume 1354, pp. 301–307. [Google Scholar]
- Treccarichi, S.; Infurna, G.M.; Ciulla, A.; Rossitto, A.; Argento, S.; Fallahi, H.R.; Branca, F. Evaluation of innovative growing techniques for organic aaffron production in the Mediterranean countries. In Proceedings of the Acta Horticulturae; International Society for Horticultural Science: Leuven, Belgium, 2022; Volume 1354, pp. 57–62. [Google Scholar]
- Leip, A.; Bodirsky, B.L.; Kugelberg, S. The Role of Nitrogen in Achieving Sustainable Food Systems for Healthy Diets. Glob. Food Sec. 2021, 28, 100408. [Google Scholar] [CrossRef]
- Muller, A.; Ferré, M.; Engel, S.; Gattinger, A.; Holzkämper, A.; Huber, R.; Müller, M.; Six, J. Can Soil-Less Crop Production Be a Sustainable Option for Soil Conservation and Future Agriculture? Land Use Policy 2017, 69, 102–105. [Google Scholar] [CrossRef]
- Massa, D.; Magán, J.J.; Montesano, F.F.; Tzortzakis, N. Minimizing Water and Nutrient Losses from Soilless Cropping in Southern Europe. Agric. Water Manag. 2020, 241, 106395. [Google Scholar] [CrossRef]
- Miedaner, T.; Juroszek, P. Global Warming and Increasing Maize Cultivation Demand Comprehensive Efforts in Disease and Insect Resistance Breeding in North-Western Europe. Plant Pathol. 2021, 70, 1032–1046. [Google Scholar] [CrossRef]
- Wang NG, Y.; Zhang, Y.; Gao, Z.; Yang, W. Breeding for Resistance to Tomato Bacterial Diseases in China: Challenges and Prospects. Hortic. Plant J. 2018, 4, 193–207. [Google Scholar] [CrossRef]
- Argento, S.; Melilli, M.G.; Branca, F. Enhancing Greenhouse Tomato-Crop Productivity by Using Brassica Macrocarpa Guss. Leaves for Controlling Root-Knot Nematodes. Agronomy 2019, 9, 820. [Google Scholar] [CrossRef] [Green Version]
- Prohens, J.T.; Soler, S.; Tripodi, P.; Campanelli, G.; Sestili, S.; Figàs, M.R.; Casanova, C.; Fonseca, R.; Hascöet, E.; Turner, M.; et al. Selection and breeding of tomato for organic conditions. In Proceedings of the Acta Horticulturae; International Society for Horticultural Science: Leuven, Belgium, 2022; Volume 1354, pp. 95–103. [Google Scholar]
- Treccarichi, S.; Ben Ammar, H.; Amari, M.; Cali, R.; Tribulato, A.; Branca, F. Molecular Markers for Detecting Inflorescence Size of Brassica Oleracea L. Crops and B. Oleracea Complex Species (n = 9) Useful for Breeding of Broccoli (B. Oleracea Var. Italica) and Cauliflower (B. Oleracea Var. Botrytis). Plants 2023, 12, 407. [Google Scholar] [CrossRef] [PubMed]
- Asmamaw, D.K.; Janssens, P.; Desse, M.; Tilahun, S.; Adgo, E.; Nyssen, J.; Walraevens, K.; Cornelis, W. Deficit Irrigation as a Sustainable Option for Improving Water Productivity in Sub-Saharan Africa: The Case of Ethiopia a Critical Review. Environ. Res. Commun 2021, 3, 102001. [Google Scholar] [CrossRef]
- Du, T.; Kang, S.; Zhang, J.; Davies, W.J. Deficit Irrigation and Sustainable Water-Resource Strategies in Agriculture for China’s Food Security. J. Exp. Bot. 2015, 66, 2253–2269. [Google Scholar] [CrossRef]
- Castorina, A.; Consoli, S.; Barbagallo, S.; Branca, F.; Farag, A.; Licciardello, F.; Cirelli, G.L. Assessing Environmental Impacts of Constructed Wetland Effluents for Vegetable Crop Irrigation. Int. J. Phytoremediat. 2016, 18, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Ben Ammar, H.; Picchi, V.; Arena, D.; Treccarichi, S.; Bianchi, G.; Lo Scalzo, R.; Marghali, S.; Branca, F. Variation of Bio-Morphometric Traits and Antioxidant Compounds of Brassica Oleracea L. Accessions in Relation to Drought Stress. Agronomy 2022, 12, 2016. [Google Scholar] [CrossRef]
- Branca, F.; Di Bella, M.C.; Arena, D.; Tribulato, A.; Kusznierewicz, B.; Parchem, K.; Bartoszek, A. Chemical characterization of wild populations of brassica oleracea complex Species (N = 9) for the content of their bioactive compounds. In Proceedings of the Acta Horticulturae; International Society for Horticultural Science: Leuven, Belgium, 2022; Volume 1354, pp. 137–143. [Google Scholar]
- Branca, F.; Arena, D.; Argento, S.; Frustaci, F.; Ciccarello, L.; Melilli, M.G. Recovery of healthy compounds in waste bracts of globe artichoke heads. In Proceedings of the Acta Horticulturae; International Society for Horticultural Science: Leuven, Belgium, 2022; Volume 1354, pp. 361–366. [Google Scholar]
- Arena, D.; Treccarichi, S.; Di Bella, M.C.; Achkar, N.; Ben Ammar, H.; Picchi, V.; Lo Scalzo, R.; Amari, M.; Branca, F. Evaluation of brassica Oleracea L. crops and wild relatives for bio-morphometric and biochemical characteristics. In Proceedings of the Acta Horticulturae; International Society for Horticultural Science: Leuven, Belgium, 2022; Volume 1355, pp. 71–79. [Google Scholar]
- Singh, M.; Singh, P.; Singh, S.; Saini, R.K.; Angadi, S.V. A Global Meta-Analysis of Yield and Water Productivity Responses of Vegetables to Deficit Irrigation. Sci. Rep. 2021, 11, 2016. [Google Scholar] [CrossRef]
- Ahmad, H.M.; Fiaz, S.; Hafeez, S.; Zahra, S.; Shah, A.N.; Gul, B.; Aziz, O.; Fakhar, A.; Rafique, M.; Chen, Y.; et al. Plant Growth-Promoting Rhizobacteria Eliminate the Effect of Drought Stress in Plants: A Review. Front. Plant Sci. 2022, 13, 1965. [Google Scholar] [CrossRef]
- De Lima, B.C.; Bonifacio, A.; de Alcantara Neto, F.; Araujo, F.F.; Araujo, A.S.F. Bacillus Subtilis Rhizobacteria Ameliorate Heat Stress in the Common Bean. Rhizosphere 2022, 21, 100472. [Google Scholar] [CrossRef]
- Stopnisek, N.; Shade, A. Persistent Microbiome Members in the Common Bean Rhizosphere: An Integrated Analysis of Space, Time, and Plant Genotype. ISME J. 2021, 15, 2708–2722. [Google Scholar] [CrossRef]
- McNear, D.H. The Rhizosphere-Roots, Soil and Everything In Between Meeting the Global Challenge of Sustainable Food, Fuel and Fiber Production. Nat. Educ. Knowl. 2013, 4, 1–15. [Google Scholar]
- Malgioglio, G.; Rizzo, G.F.; Nigro, S.; Lefebvre Du Prey, V.; Herforth-Rahmé, J.; Catara, V.; Branca, F. Plant-Microbe Interaction in Sustainable Agriculture: The Factors That May Influence the Efficacy of PGPM Application. Sustainability 2022, 14, 2253. [Google Scholar] [CrossRef]
- Chandran, H.; Meena, M.; Swapnil, P. Plant Growth-Promoting Rhizobacteria as a Green Alternative for Sustainable Agriculture. Sustainability 2021, 13, 10986. [Google Scholar] [CrossRef]
- Egamberdieva, D.; De Cal, A.; Pellegrini, M.; Smith, D.L.; Jiao, X.; Takishita, Y.; Zhou, G. Plant Associated Rhizobacteria for Biocontrol and Plant Growth Enhancement. Front. Plant Sci. 2021, 1, 17. [Google Scholar] [CrossRef]
- Martin, R.; Vocciante, M.; Grifoni, M.; Fusini, D.; Petruzzelli, G.; Franchi, E.; SpA, E. The Role of Plant Growth-Promoting Rhizobacteria (PGPR) in Mitigating Plant’s Environmental Stresses. Appl. Sci. 2022, 12, 1231. [Google Scholar] [CrossRef]
- Daniel, A.I.; Fadaka, A.O.; Gokul, A.; Bakare, O.O.; Aina, O.; Fisher, S.; Burt, A.F.; Mavumengwana, V.; Keyster, M.; Klein, A. Microorganisms Review Biofertilizer: The Future of Food Security and Food Safety. Microorganisms 2022, 10, 1220. [Google Scholar] [CrossRef] [PubMed]
- AlAli, H.A.; Khalifa, A.; Al-Malki, M. Plant Growth-Promoting Rhizobacteria from Ocimum Basilicum Improve Growth of Phaseolus Vulgaris and Abelmoschus Esculentus. South Afr. J. Bot. 2021, 139, 200–209. [Google Scholar] [CrossRef]
- Ullah, N.; Ditta, A.; Imtiaz, M.; Li, X.; Jan, A.U.; Mehmood, S.; Rizwan, M.S.; Rizwan, M. Muhammad Appraisal for Organic Amendments and Plant Growth-Promoting Rhizobacteria to Enhance Crop Productivity under Drought Stress: A Review. J. Agro. Crop. Sci. 2021, 207, 783–802. [Google Scholar] [CrossRef]
- Sica, P.; Scariolo, F.; Galvao, A.; Battaggia, D.; Nicoletto, C.; Maucieri, C.; Palumbo, F.; Franklin, D.; Cabrera, M.; Borin, M.; et al. Molecular Hallmarks, Agronomic Performances and Seed Nutraceutical Properties to Exploit Neglected Genetic Resources of Common Beans Grown by Organic Farming in Two Contrasting Environments. Front Plant Sci. 2021, 12, 674985. [Google Scholar] [CrossRef]
- Romanyà, J.; Casals, P. Biological Nitrogen Fixation Response to Soil Fertility Is Species-Dependent in Annual Legumes. J. Soil Sci. Plant Nutr. 2020, 20, 546–556. [Google Scholar] [CrossRef]
- Uebersax, M.A.; Cichy, K.A.; Gomez, F.E.; Porch, T.G.; Heitholt, J.; Osorno, J.M.; Kamfwa, K.; Snapp, S.S.; Bales, S. Dry Beans (Phaseolus vulgaris L.) as a Vital Component of Sustainable Agriculture and Food Security—A Review. Legume Sci. 2022, 5, e155. [Google Scholar] [CrossRef]
- Myers, J.R.; Kmiecik, K. Common bean: Economic importance and relevance to biological science research. In The Common Bean Genome; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- González, A.M.; Yuste-Lisbona, F.J.; Fernández-Lozano, A.; Lozano, R.; Santalla, M. Genetic mapping and QTL analysis in common bean. In The Common Bean Genome; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration-Guidelines for Computing Crop Water Requirements-FAO Irrigation and Drainage Paper 56; Fao: Rome, Italy, 1998; p. D05109. [Google Scholar] [CrossRef]
- Koutsika-Sotiriou, M.; Traka-Mavrona, E. Snap bean. In Vegetables II; Springer: New York, NY, USA, 2008. [Google Scholar]
- Rana, M.K.; Kamboj, N.K. Snap bean. Vegetable Crops Science, CRC Press: Cleveland, OH, USA, 2017; 745-7582017. [Google Scholar]
- Shonnard, G.C.; Gepts, P.; Abate, G.; Resources, N.; Bean, S. Snap Bean Production Introduction Snap Bean. Crop. Sci. 2006, 48824, 1168–1175. [Google Scholar]
- Rivero, R.M.; Suzuki, N.; Sreekumar, P.M.; Ding, N.-Z.; He, M.; He, C.-Q. Abiotic Stresses: General Defenses of Land Plants and Chances for Engineering Multistress Tolerance. Front. Plant Sci. 2018, 9, 1771. [Google Scholar] [CrossRef] [Green Version]
- Myint, S.W.; Aggarwal, R.; Zheng, B.; Wentz, E.A.; Holway, J.; Fan, C.; Selover, N.J.; Wang, C.; Fischer, H.A. Adaptive Crop Management under Climate Uncertainty: Changing the Game for Sustainable Water Use. Atmosphere 2021, 12, 1080. [Google Scholar] [CrossRef]
- Rizzo, G.F.; Ciccarello, L.; Felis, M.D.; Mortada, A.; Cirelli, G.L.; Milani, M.; Branca, F. Agronomic effects of reclaimed water used for tomato (Solanuml L.) and lettuce (Lactuca sativa L.) irrigation. In Proceedings of the Acta Horticulturae; International Society for Horticultural Science: Leuven, Belgium, 2022; Volume 1355, pp. 387–392. [Google Scholar]
- Malgioglio, G.; Infurna, M.G.; Arena, D.; Toscano, S.; Rizzo, G.F.; Nigro, S.; du Prey, V.L.; Branca, F. Effects of microbial consortia and betaines on snapbean grown under water stress conditions. In Proceedings of the Acta Horticulturae; International Society for Horticultural Science: Leuven, Belgium, 2022; Volume 1354, pp. 293–299. [Google Scholar]
- Fadiji, A.E.; Orozco-Mosqueda, M.d.C.; de los Santos-Villalobos, S.; Santoyo, G.; Babalola, O.O. Recent Developments in the Application of Plant Growth-Promoting Drought Adaptive Rhizobacteria for Drought Mitigation. Plants 2022, 11, 3090. [Google Scholar] [CrossRef]
- Christakis, C.A.; Daskalogiannis, G.; Chatzaki, A.; Markakis, E.A.; Mermigka, G.; Sagia, A.; Rizzo, G.F.; Catara, V.; Lagkouvardos, I.; Studholme, D.J.; et al. Endophytic Bacterial Isolates From Halophytes Demonstrate Phytopathogen Biocontrol and Plant Growth Promotion Under High Salinity. Front. Microbiol. 2021, 12, 681567. [Google Scholar] [CrossRef]
- Picchi, V.; Scalzo, R.L.; Tava, A.; Doria, F.; Argento, S.; Toscano, S.; Treccarichi, S.; Branca, F. Phytochemical Characterization and in Vitro Antioxidant Properties of Four Brassica Wild Species from Italy. Molecules 2020, 25, 3495. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.I.; Danish, S.; Sánchez-Moreiras, A.M.; Vicente, Ó.; Jabran, K.; Chaudhry, U.K.; Branca, F.; Reigosa, M.J. Unraveling Sorghum Allelopathy in Agriculture: Concepts and Implications. Plants 2021, 10, 1795. [Google Scholar] [CrossRef]
- Shah, A.; Nazari, M.; Antar, M.; Msimbira, L.A.; Naamala, J.; Lyu, D.; Rabileh, M.; Zajonc, J.; Smith, D.L. PGPR in Agriculture: A Sustainable Approach to Increasing Climate Change Resilience. Front. Sustain. Food Syst. 2021, 5, 667546. [Google Scholar] [CrossRef]
- Scudeletti, D.; Crusciol, C.A.C.; Bossolani, J.W.; Moretti, L.G.; Momesso, L.; Servaz Tubaña, B.; de Castro, S.G.Q.; De Oliveira, E.F.; Hungria, M. Trichoderma Asperellum Inoculation as a Tool for Attenuating Drought Stress in Sugarcane. Front. Plant Sci. 2021, 12, 645542. [Google Scholar] [CrossRef]
- Naamala, J.; Smith, D.L. Relevance of Plant Growth Promoting Microorganisms and Their Derived Compounds, in the Face of Climate Change. Agronomy 2020, 10, 1179. [Google Scholar] [CrossRef]
- Rawal, R.; Scheerens, J.C.; Fenstemaker, S.M.; Francis, D.M.; Miller, S.A.; Benitez, M.S. Novel Trichoderma Isolates Alleviate Water Deficit Stress in Susceptible Tomato Genotypes. Front. Plant Sci. 2022, 13, 869090. [Google Scholar] [CrossRef]
- Kaur, S.; Kumar, P. Ameliorative Effect of Trichoderma, Rhizobium and Mycorrhiza on Internodal Length, Leaf Area and Total Soluble Protein in Mung Bean (Vigna Radiata [L.] R. Wilazek) under Drought Stress. J. Pharm. Phytochem. 2020, 9, 971–977. [Google Scholar]
- Nahrawy, S.E.-; Elbagory, M.; Omara, A.E.-D. Biocompatibility Effect of Bradyrhizobium Japonicum and Trichoderma Strains on Growth, Nodulation and Physiological Traits of Soybean (Glycine max L.) under Water Deficit Conditions. J. Adv. Microbiol. 2020, 20, 52–66. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Fernandes, Â.; Plexida, S.; Chrysargyris, A.; Tzortzakis, N.; Barreira, J.C.M.; Barros, L.; Ferreira, I.C.F.R. Biostimulants Application Alleviates Water Stress Effects on Yield and Chemical Composition of Greenhouse Green Bean (Phaseolus Vulgaris L.). Agronomy 2020, 10, 181. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Luqueño, F.; Dendooven, L.; Munive, A.; Corlay-Chee, L.; Serrano-Covarrubias, L.M.; Espinosa-Victoria, D. Micro-Morphology of Common Bean (Phaseolus Vulgaris L.) Nodules Undergoing Senescence. Acta Physiol. Plant 2008, 30, 545–552. [Google Scholar] [CrossRef]
- Basile, L.A.; Lepek, V.C. Minireview Legume-Rhizobium Dance: An Agricultural Tool That Could Be Improved? Microbial Biotechnology 2021, 14, 1897–1917. [Google Scholar] [CrossRef]

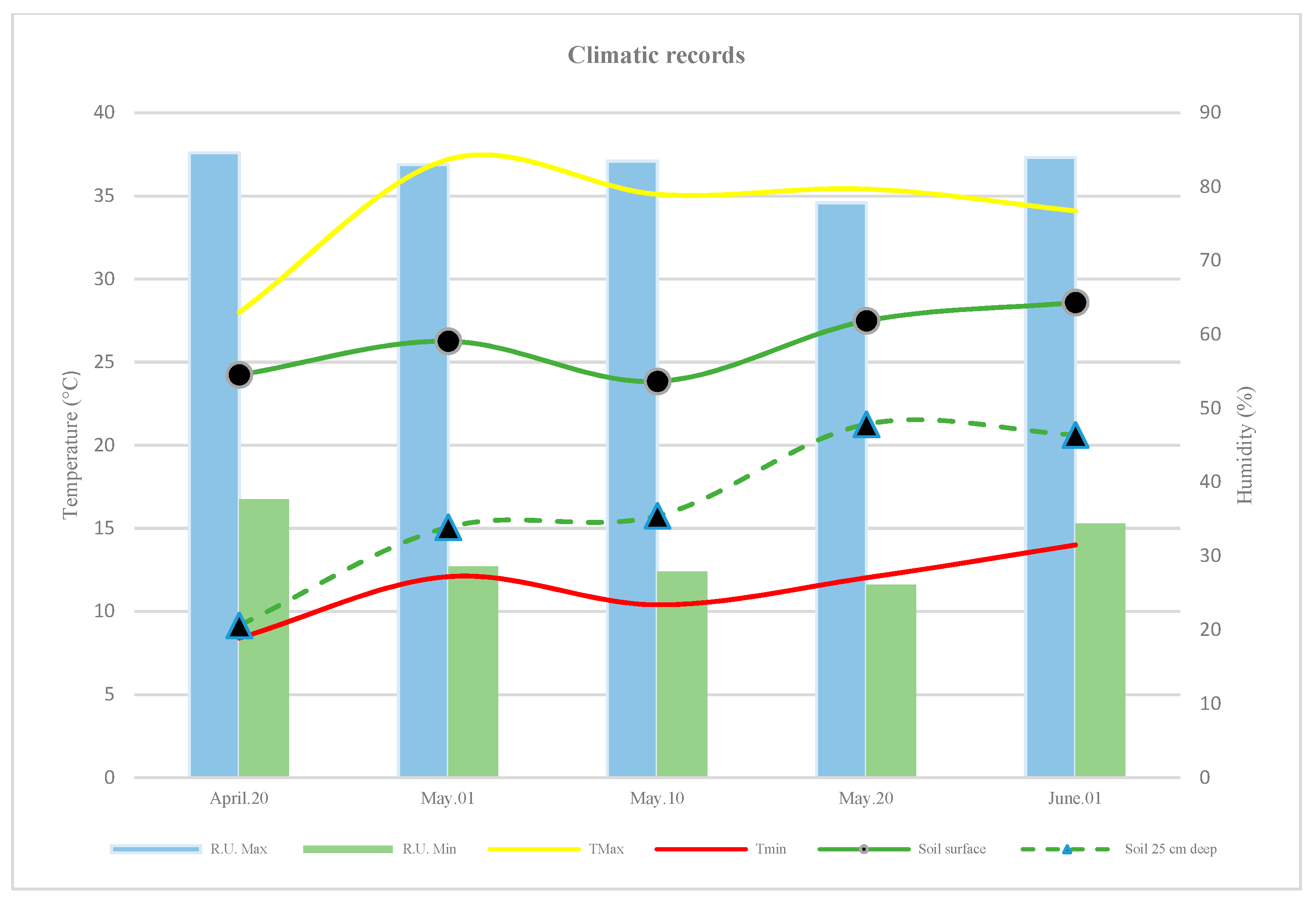


| Soil Analysis | ||
|---|---|---|
| Fine ground (<2 mm) | 989 | g/kg |
| Sand (0.02–2 mm) | 856 | g/kg |
| Silt (0.002–0.02 mm) | 53 | g/kg |
| Clay (<0.002 mm) | 91 | g/kg |
| Total Limestone | 5 | g/kg |
| Total Nitrogen (N) | 1 | g/kg |
| Organic carbon | 6.7 | g/kg |
| C/N Ratio | 6.7 | |
| Assimilable phosphorus (P2O5) | 144 | mg/kg |
| Exchangeable potassium (K2O) | 706 | mg/kg |
| pH | 7.6 | |
| specific conductivity (25 °C) | 3.63 | dS/m |
| Cation exchange capacity (CSC) | 11.5 | meq/100 g |
| Degree of saturation in bases (GDB) | 100 | % |
| Exchangeable Calcium | 7.9 | meq/100 g |
| Exchangeable Magnesium | 1.7 | meq/100 g |
| Exchangeable sodium | 0.4 | meq/100 g |
| Exchangeable potassium (saturated extract) | 1.5 | meq/100 g |
| Calcium | 68.89 | % |
| Magnesium | 14.45 | % |
| Sodium (ESP) | 3.63 | % |
| Potassium | 13.03 | % |
| K/Mg Ratio | 0.9 | |
| Mg/K Ratio | 1.11 | |
| ETc 100 | ETc 60 | MEAN | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NMO | MO | NMO | MO | |||||||||||||||||
| A | B | A | B | A | B | A | B | ETc 100 | ETc 60 | NMO | MO | A | B | TOT | ||||||
| Yield kg m−2 | 3.45 | 3.91 | 3.68 | 3.84 | 5.00 | 4.42 | 2.00 | 2.95 | 2.47 | 2.40 | 4.32 | 3.36 | 4.05 | 2.92 | 3.08 | 3.89 | 2.92 | 4.04 | 3.48 | |
| Pod N° m−2 | 820.0 | 335.9 | 578.0 | 803.6 | 504.6 | 654.1 | 651.2 | 341.4 | 496.3 | 747.1 | 548.9 | 648.0 | 616.0 | 572.1 | 537.1 | 651.0 | 755.5 | 432.7 | 594.1 | |
| Pod Ø (mm) | 5.88 | 6.88 | 6.38 | 6.71 | 9.00 | 7.86 | 6.09 | 5.78 | 5.93 | 6.49 | 8.76 | 7.63 | 7.12 | 6.78 | 6.16 | 7.74 | 6.29 | 7.61 | 6.95 | |
| Pod length (cm) | 11.7 | 11.3 | 11.5 | 12.0 | 14.3 | 13.1 | 10.8 | 9.0 | 9.9 | 12.4 | 13.1 | 12.8 | 12.3 | 11.3 | 10.7 | 12.9 | 11.7 | 11.9 | 11.8 | |
| Pod weight (g) | 4.3 | 12.4 | 8.4 | 5.0 | 10.8 | 7.9 | 3.1 | 7.9 | 5.5 | 3.2 | 7.8 | 5.5 | 8.1 | 5.5 | 6.9 | 6.7 | 3.9 | 9.8 | 6.8 | |
| N° Branch | 4.3 | 4.0 | 4.2 | 5.3 | 4.7 | 5.0 | 6.3 | 5.7 | 6.0 | 6.0 | 5.0 | 5.5 | 4.6 | 5.8 | 5.1 | 5.3 | 5.5 | 4.8 | 5.2 | |
| E.F.W. (g) | 252.0 | 170.7 | 211.3 | 278.7 | 282.0 | 280.3 | 125.0 | 146.7 | 135.8 | 163.3 | 210.0 | 186.7 | 245.8 | 161.3 | 173.6 | 233.5 | 204.8 | 202.3 | 203.5 | |
| I.F.W. (g) | 27.3 | 14.0 | 20.7 | 20.7 | 16.0 | 18.3 | 15.0 | 10.0 | 12.5 | 21.7 | 16.7 | 19.2 | 19.5 | 15.8 | 16.6 | 18.8 | 21.2 | 14.2 | 17.7 | |
| E.D.M. (%) | 16.9 | 17.3 | 17.1 | 16.0 | 15.9 | 16.0 | 31.7 | 40.1 | 35.9 | 34.2 | 33.0 | 33.6 | 16.5 | 34.7 | 26.5 | 24.8 | 24.7 | 26.6 | 25.6 | |
| I.D.M. (%) | 19.6 | 24.1 | 21.8 | 53.3 | 46.8 | 50.0 | 51.4 | 52.3 | 51.9 | 60.3 | 70.5 | 65.4 | 35.9 | 58.6 | 36.9 | 57.7 | 46.2 | 48.4 | 47.3 | |
| N° nodules | 85.0 | 40.7 | 62.8 | 80.0 | 26.3 | 53.2 | 114.7 | 56.0 | 85.3 | 70.3 | 17.7 | 44.0 | 58.0 | 64.7 | 74.1 | 48.6 | 87.5 | 35.2 | 61.3 | |
| SPAD | 43.5 | 44.4 | 44.0 | 45.2 | 45.5 | 45.3 | 43.6 | 45.2 | 44.4 | 46.3 | 48.2 | 47.3 | 44.6 | 45.9 | 44.2 | 46.3 | 44.6 | 45.8 | 45.2 | |
| Analysis of variance—Student-Newman-Keuls | ||||||||||||||||||||
| ETc | MO | GE | ETc × MO | ETc × GE | MO × GE | ETc × MO × GE | ||||||||||||||
| Yield kg m−2 | *** | *** | *** | n.s. | n.s. | n.s. | n.s. | |||||||||||||
| Pod N° m2 | n.s. | * | *** | n.s. | n.s. | n.s. | n.s. | |||||||||||||
| Pod Ø (mm) | n.s. | ** | * | n.s. | n.s. | * | n.s. | |||||||||||||
| Pod length (cm) | n.s. | ** | n.s. | n.s. | n.s. | n.s. | n.s. | |||||||||||||
| Pod weight (g) | *** | n.s. | ** | n.s. | ** | n.s. | n.s. | |||||||||||||
| N° Branch | * | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |||||||||||||
| E.F.W. (g) | ** | * | n.s. | n.s. | n.s. | n.s. | n.s. | |||||||||||||
| I.F.W. (g) | n.s. | n.s. | * | n.s. | n.s. | n.s. | n.s. | |||||||||||||
| E.D.M. (%) | *** | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |||||||||||||
| I.D.M. (%) | *** | *** | n.s. | * | n.s. | n.s. | n.s. | |||||||||||||
| Nod N° | n.s. | ** | *** | n.s. | n.s. | n.s. | n.s. | |||||||||||||
| SPAD | * | *** | * | n.s. | n.s. | n.s. | n.s. | |||||||||||||
| Yield kg m−2 | Pod N° m2 | Pod Ø (mm) | Pod Length (cm) | Pod Weight (g) | N° Branch | E.F.W. (g) | I.F.W. (g) | E.D.M. (%) | I.D.M. (%) | N° Nodules | SPAD | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yield kg m−2 | 1 | |||||||||||
| Pod N° m2 | −0.092 | 1 | ||||||||||
| Pod Ø (mm) | 0.582 ** | −0.177 | 1 | |||||||||
| Pod length (cm) | 0.506 * | 0.257 | 0.730 ** | 1 | ||||||||
| Pod weight (g) | 0.670 ** | −0.745 ** | 0.475 * | 0.169 | 1 | |||||||
| N° Branch | −0.520 ** | 0.229 | −0.163 | −0.150 | −0.523 ** | 1 | ||||||
| E.F.W. (g) | 0.413 * | 0.221 | 0.355 | 0.415 * | 0.102 | −0.035 | 1 | |||||
| I.F.W. (g) | −0.050 | 0.608 ** | −0.137 | 0.317 | −0.391 | 0.182 | 0.502 * | 1 | ||||
| E.D.M. (%) | −0.472 * | −0.189 | −0.142 | −0.260 | −0.269 | 0.423 * | −0.598 ** | −0.353 | 1 | |||
| I.D.M. (%) | −0.050 | 0.076 | 0.359 | 0.168 | −0.233 | 0.432 * | −0.116 | −0.318 | 0.589 ** | 1 | ||
| N° nodules | −0.622 ** | 0.518 ** | −0.604 ** | −0.261 | −0.691 ** | 0.464 * | −0.161 | 0.398 | 0.045 | −0.226 | 1 | |
| SPAD | 0.444 * | −0.068 | 0.432 * | 0.230 | 0.207 | −0.039 | −0.172 | −0.301 | 0.299 | 0.583 ** | −0.446 * | 1 |
| Component Scores | ||
|---|---|---|
| PC1 | PC2 | |
| Yield kg m−2 | 0.976 | 0.068 |
| Pod N° m−2 | −0.299 | 0.722 |
| Pod Ø (mm) | 0.849 | −0.246 |
| Pod length (cm) | 0.705 | 0.229 |
| Pod weight (g) | 0.762 | −0.335 |
| N° Branch | −0.745 | −0.353 |
| E.F.W. (g) | 0.668 | 0.576 |
| I.F.W. (g) | −0.032 | 0.844 |
| E.D.M. (%) | −0.547 | −0.750 |
| I.D.M. (%) | −0.076 | −0.624 |
| N° nodules | −0.850 | 0.458 |
| SPAD | 0.417 | −0.570 |
| % of Variance | 42.00 | 28.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzo, G.F.; Al Achkar, N.; Treccarichi, S.; Malgioglio, G.; Infurna, M.G.; Nigro, S.; Tribulato, A.; Branca, F. Use of Bioinoculants Affects Variation in Snap Bean Yield Grown under Deficit Irrigation. Agriculture 2023, 13, 865. https://doi.org/10.3390/agriculture13040865
Rizzo GF, Al Achkar N, Treccarichi S, Malgioglio G, Infurna MG, Nigro S, Tribulato A, Branca F. Use of Bioinoculants Affects Variation in Snap Bean Yield Grown under Deficit Irrigation. Agriculture. 2023; 13(4):865. https://doi.org/10.3390/agriculture13040865
Chicago/Turabian StyleRizzo, Giulio Flavio, Nicolas Al Achkar, Simone Treccarichi, Giuseppe Malgioglio, Matteo Giuseppe Infurna, Sebastian Nigro, Alessandro Tribulato, and Ferdinando Branca. 2023. "Use of Bioinoculants Affects Variation in Snap Bean Yield Grown under Deficit Irrigation" Agriculture 13, no. 4: 865. https://doi.org/10.3390/agriculture13040865
APA StyleRizzo, G. F., Al Achkar, N., Treccarichi, S., Malgioglio, G., Infurna, M. G., Nigro, S., Tribulato, A., & Branca, F. (2023). Use of Bioinoculants Affects Variation in Snap Bean Yield Grown under Deficit Irrigation. Agriculture, 13(4), 865. https://doi.org/10.3390/agriculture13040865











