Morphological, Histological, and Glyphosate Residue Analysis of Helianthus annuus L. Plants Treated with Glyphosate
Abstract
1. Introduction
1.1. The Impact of Pesticides on the Environment
1.2. The Effect of Glyphosate and Its Metabolites on the Living Environment
2. Materials and Methods
2.1. Materials Used for the Experiment
2.2. Description of the Experiment
2.3. Morphology
2.4. Histology
2.5. Sampling and Sample Preparation Prior to GC-MS Analysis
2.6. Residue Analysis by GC-MS
2.7. Statistical Evaluation
3. Results
3.1. Morphological Evaluation
3.2. Histological Examination
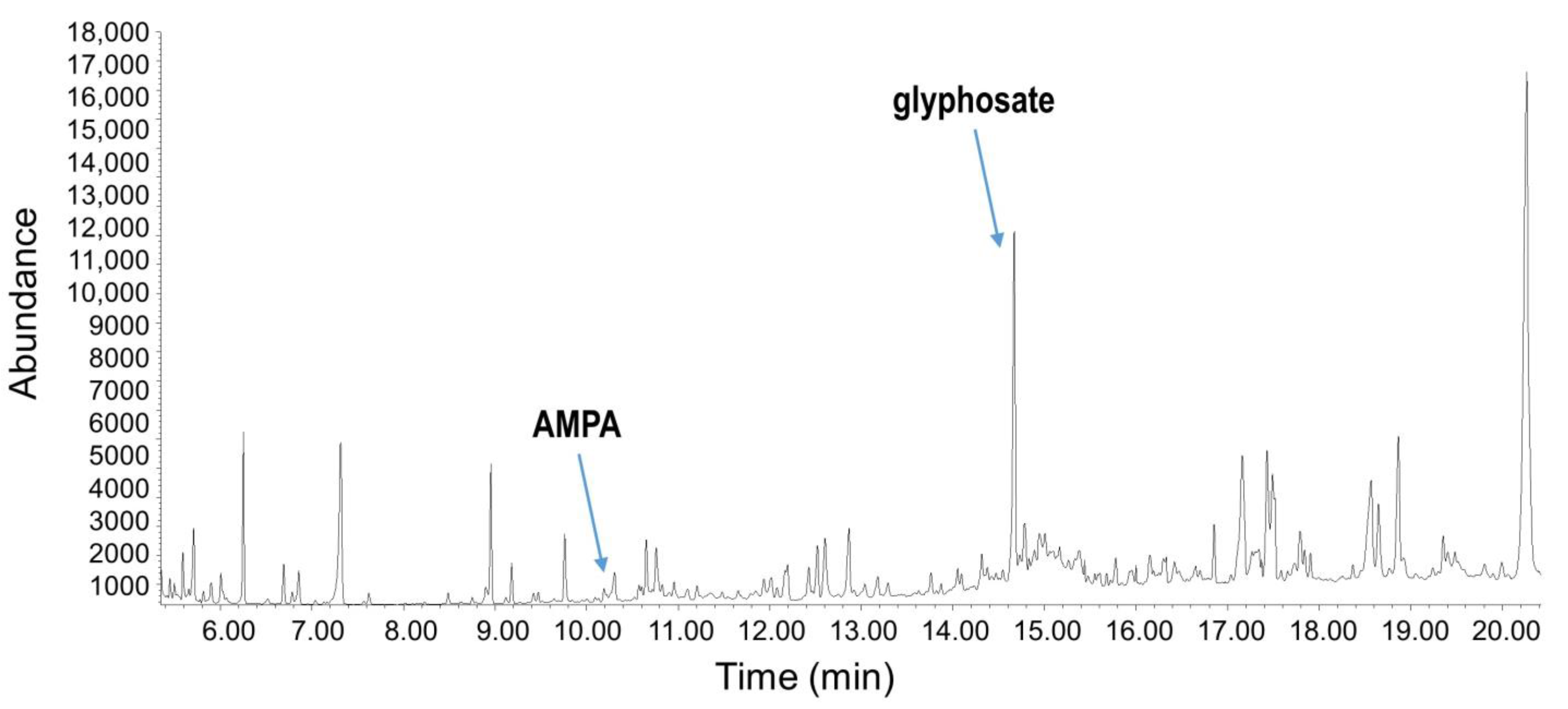
3.3. Residue Assessment
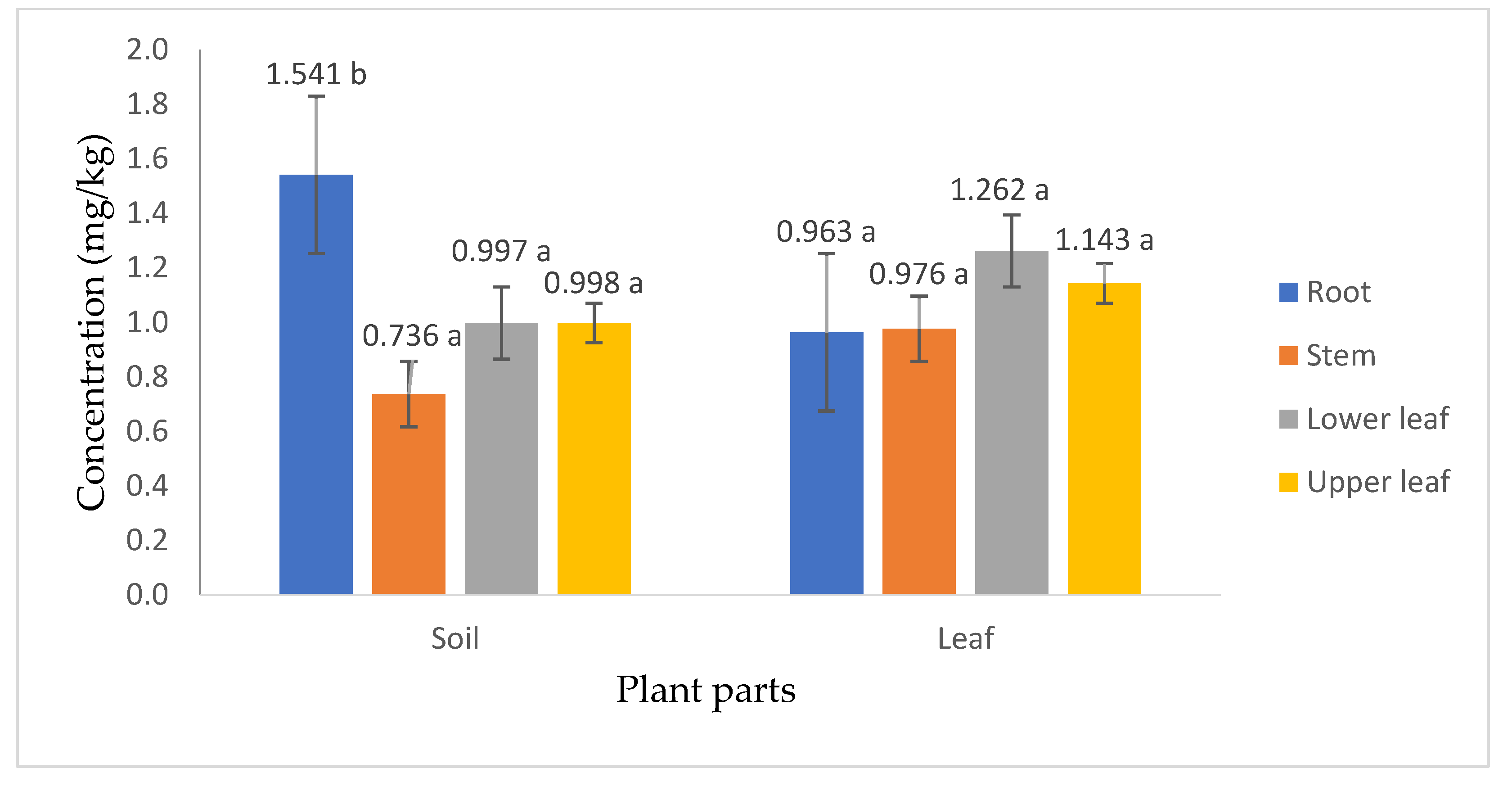
3.4. Glyphosate Content
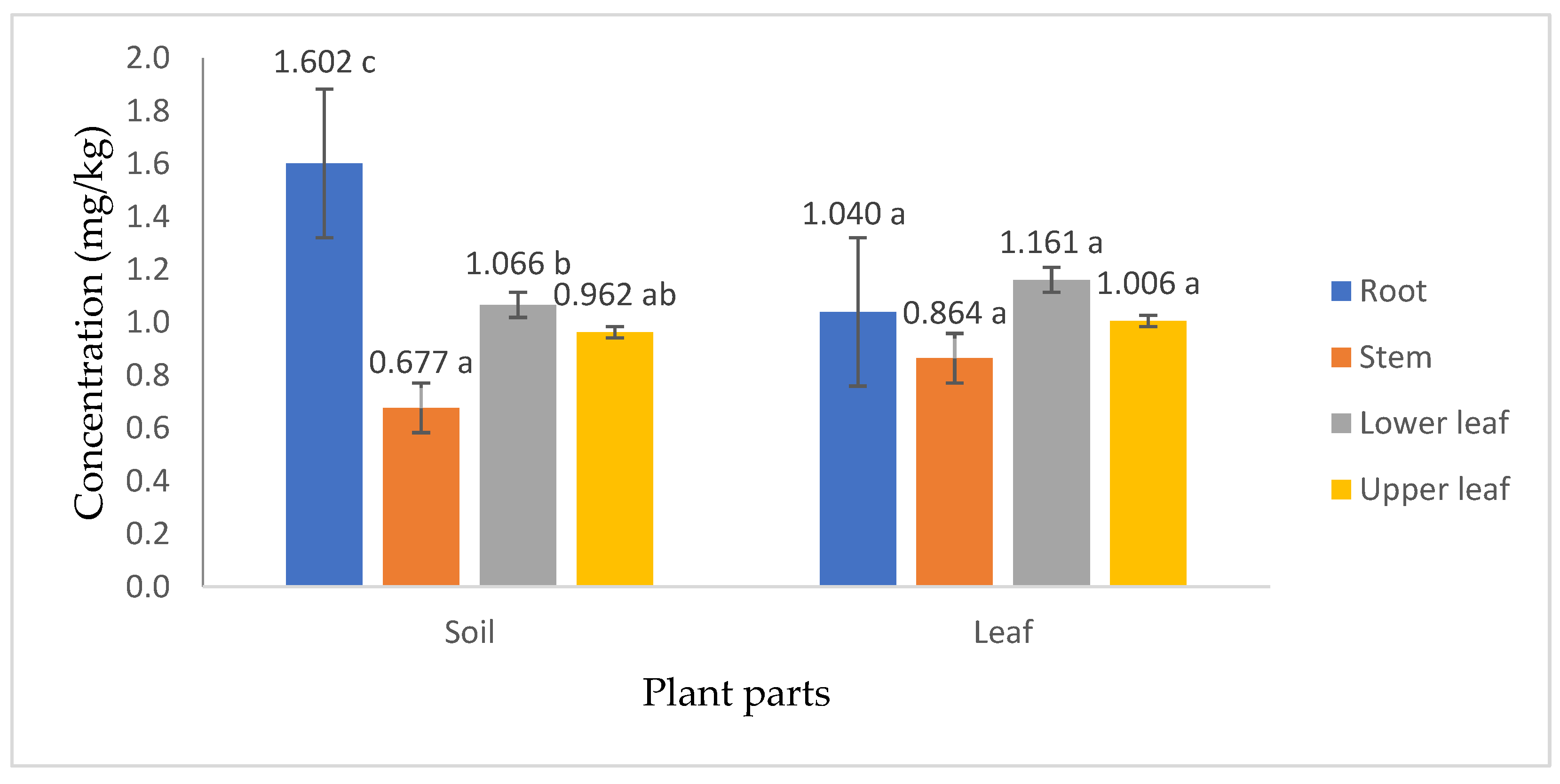
3.5. AMPA Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.Q. Heavy metals and pesticides toxicity in agricultural soil and plants: Ecological risks and human health implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Singh Sidhu, G.P.; Bali, A.S.; Handa, N.; Kapoor, D.; Yadav, P.; Khanna, K.; et al. Photosynthetic response of plants under different abiotic stresses: A review. J. Plant Growth Regul. 2020, 39, 509–531. [Google Scholar] [CrossRef]
- Bhunia, P. Environmental toxicants and hazardous contaminants: Recent advances in technologies for sustainable development. J. Hazard. Toxic Radioact. Waste 2017, 21, 02017001. [Google Scholar] [CrossRef]
- Chin, N.P. Environmental toxins: Physical, social, and emotional. Breastfeed. Med. 2010, 5, 223. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.P.K.; Sethi, N.; Mohan, A. Analysis of the glyphosate herbicide in water, soil and food using derivatising agents. Environ. Chem. Lett. 2017, 15, 85–100. [Google Scholar] [CrossRef]
- Székács, A.; Darvas, B. Forty years with glyphosate. In Herbicides—Properties, Synthesis and Control of Weeds; Hasaneen, M.N.A.E.-G., Ed.; InTech: Rijeka, Croatia, 2012; pp. 247–284. [Google Scholar]
- Székács, A.; Darvas, B. Re-registration challenges of glyphosate in the European Union. Front. Environ. Sci. 2018, 6, 78. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Gill, J.P.K.; Datta, S.; Singh, S.; Dhaka, V.; Kapoor, D.; Wani, A.B.; Dhanjal, D.S.; Kumar, M.; et al. Herbicide glyphosate: Toxicity and microbial degradation. Int. J. Environ. Res. Public Health 2020, 17, 7519. [Google Scholar] [CrossRef]
- Ramula, S.; Kalske, A.; Saikkonen, K.; Helander, M. Glyphosate residues in soil can modify plant resistance to herbivores through changes in leaf quality. Plant Biol. 2022, 24, 979–986. [Google Scholar] [CrossRef]
- Haslam, E. The Shikimate Pathway: Biosynthesis of Natural Products Series, 1st ed.; e-book; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Helander, M.; Pauna, A.; Saikkonen, K.; Saloniemi, I. Glyphosate residues in soil affect crop plant germination and growth. Sci. Rep. 2019, 9, 19653. [Google Scholar] [CrossRef]
- Peillex, C.; Pelletier, M. The impact and toxicity of glyphosate and glyphosate-based herbicides on health and immunity. J. Immun. Toxicol. 2020, 17, 163–174. [Google Scholar] [CrossRef]
- Sviridov, A.V.; Shushkova, T.V.; Ermakova, I.T.; Ivanova, E.V.; Epiktetov, D.O.; Leontievsky, A.A. Microbial degradation of glyphosate herbicides. Appl. Biochem. Microbiol. 2015, 51, 188–195. [Google Scholar] [CrossRef]
- Ledoux, M.L.; Hettiarachchy, N.; Yu, X.; Howard, L.; Lee, S.O. Penetration of glyphosate into the food supply and the incidental impact on the honey supply and bees. Food Control 2020, 109, 106859. [Google Scholar] [CrossRef]
- Fuchs, B.; Saikkonen, K.; Helander, M. Glyphosate-modulated biosynthesis driving plant defense and species interactions. Trends Plant Sci. 2021, 26, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Zulet González, A.; Fernández Escalada, M.; Zabalza Aznárez, A.; Royuela Hernando, M. Enhancement of glyphosate efficacy on Amaranthus palmeri by exogenous quinate application. Pestic. Biochem. Physiol. 2019, 158, 1–11. [Google Scholar] [CrossRef]
- Richmond, M.E. Glyphosate: A review of its global use, environmental impact, and potential health effects on humans and other species. J. Environ. Stud. Sci. 2018, 8, 416–434. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, X.; Luo, J.; Wu, Z.; Wang, L.; Li, B.; Wang, Y.; Sun, G. Degradation dynamics of glyphosate in different types of citrus orchard soils in China. Molecules 2015, 20, 1161–1175. [Google Scholar] [CrossRef]
- Rivas-Garcia, T.; Espinosa-Calderón, A.; Hernández-Vázquez, B.; Schwentesius-Rindermann, R. Overview of Environmental and Health Effects Related to Glyphosate Usage. Sustainability 2022, 14, 6868. [Google Scholar] [CrossRef]
- Strandberg, B.; Sørensen, P.B.; Bruus, M.; Bossi, R.; Dupont, Y.L.; Link, M.; Damgaard, C.F. Effects of glyphosate spray-drift on plant flowering. Environ. Pollut. 2021, 280, 116953. [Google Scholar] [CrossRef]
- De Moraes, C.P.; de Brito, I.P.; Tropaldi, L.; Carbonari, C.A.; Velini, E.D. Hormetic effect of glyphosate on Urochloa decumbens plants. J. Environ. Sci. Health B 2020, 55, 376–381. [Google Scholar] [CrossRef]
- Martins-Gomes, C.; Silva, T.L.; Andreani, T.; Silva, A.M. Glyphosate vs. glyphosate-based herbicides exposure: A review on their toxicity. J. Xenobiot 2022, 12, 3. [Google Scholar] [CrossRef]
- De Troeyer, K.; Casas, L.; Bijnens, E.M.; Bruckers, L.; Covaci, A.; De Henauw, S.; Hond, D.E.; Loots, I.; Nelen, V.; Verheyen, V.J.; et al. Higher proportion of agricultural land use around the residence is associated with higher urinary concentrations of AMPA, a glyphosate metabolite. Int. J. Hyg. Environ. Health 2022, 246, 114039. [Google Scholar] [CrossRef] [PubMed]
- Ojelade, B.S.; Durowoju, O.S.; Adesoye, P.O.; Gibb, S.W.; Ekosse, G.I. Review of Glyphosate-Based Herbicide and Aminomethylphosphonic Acid (AMPA): Environmental and Health Impacts. Appl. Sci. 2022, 12, 8789. [Google Scholar] [CrossRef]
- Sihtmäe, M.; Blinova, I.; Künnis-Beres, K.; Kanarbik, L.; Heinlaan, M.; Kahru, A. Ecotoxicological effects of different glyphosate formulations. Appl. Soil Ecol. 2013, 72, 215–224. [Google Scholar] [CrossRef]
- Qu, M.; Wang, L.; Xu, Q.; An, J.; Mei, Y.; Liu, G. Influence of glyphosate and its metabolite aminomethylphosphonic acid on aquatic plants in different ecological niches. Ecotoxicol. Environ. Saf. 2022, 246, 114155. [Google Scholar] [CrossRef] [PubMed]
- Botten, N.; Wood, L.J.; Werner, J.R. Glyphosate remains in forest plant tissues for a decade or more. For. Ecol. Manag. 2021, 493, 119259. [Google Scholar] [CrossRef]
- Ramula, S.; Mathew, S.A.; Kalske, A.; Nissinen, R.; Saikkonen, K.; Helander, M. Glyphosate residues alter the microbiota of a perennial weed with a minimal indirect impact on plant performance. Plant Soil 2022, 472, 161–174. [Google Scholar] [CrossRef]
- Muola, A.; Fuchs, B.; Laihonen, M.; Rainio, K.; Heikkonen, L.; Ruuskanen, S.; Saikkonen, K.; Helander, M. Risk in the circular food economy: Glyphosate-based herbicide residues in manure fertilizers decrease crop yield. Sci. Total Environ. 2021, 750, 141422. [Google Scholar] [CrossRef]
- Meftaul, I.M.; Venkateswarlu, K.; Annamalai, P.; Parven, A.; Megharaj, M. Glyphosate use in urban landscape soils: Fate, distribution, and potential human and environmental health risks. J. Environ. Manag. 2021, 292, 112786. [Google Scholar] [CrossRef]
- Van Bruggen, A.H.C.; Finckh, M.R.; He, M.; Ritsema, C.J.; Harkes, P.; Knuth, D.; Geissen, V. Indirect effects of the herbicide glyphosate on plant, animal and human health through its effects on microbial communities. Front. Environ. Sci. 2021, 9, 763917. [Google Scholar] [CrossRef]
- Farkas, D.; Horotán, K.; Orlóci, L.; Neményi, A.; Kisvarga, S. New methods for testing/determining the environmental exposure to glyphosate in sunflower (Helianthus annuus L.) plants. Sustainability 2022, 14, 588. [Google Scholar] [CrossRef]
- Fogliatto, S.; Ferrero, A.; Vidotto, F. Current and future scenarios of glyphosate use in Europe: Are there alternatives? Adv. Agron. 2020, 163, 219–278. [Google Scholar]
- Gabonakutató Nonprofit Közhasznú Kft. (Szeged, Hungary). Available online: https://www.gabonakutato.hu/hu/vetomag/napraforgo/olajnapraforgo/gk-milia-cl (accessed on 5 December 2022).
- Clearfield® Herbicide Technology. Available online: https://agriculture.basf.us/crop-protection/products/herbicides/clearfield.html (accessed on 5 December 2022).
- Eker, S.; Ozturk, L.; Yazici, A.; Erenoglu, B.; Romheld, V.; Cakmak, I. Foliar-applied glyphosate substantially reduced uptake and transport of iron and manganese in sunflower (Helianthus annuus L.) plants. J. Agric. Food Chem. 2006, 54, 10019–10025. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Etheredge, L.; McGinty, J.; Morgan, G.; Bagavathiannan, M. First case of glyphosate resistance in weedy sunflower (Helianthus annuus). Pest Manag. Sci. 2020, 76, 3685–3692. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, L.; Yazici, A.; Eker, S.; Gokmen, O.; Römheld, V.; Cakmak, I. Glyphosate inhibition of ferric reductase activity in iron deficient sunflower roots. New Phytol. 2008, 177, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Tesfamariam, T.; Bott, S.; Cakmak, I.; Römheld, V.; Neumann, G. Glyphosate in the rhizosphere—Role of waiting times and different glyphosate binding forms in soils for phytotoxicity to non-target plants. Eur. J. Agron. 2009, 31, 126–132. [Google Scholar] [CrossRef]
- Shaner, D.L. Effects of glyphosate on transpiration. Weed Sci. 1978, 26, 513–516. [Google Scholar] [CrossRef]
- Mesnage, R.; Defarge, N.; De Vendômois, J.S.; Séralini, G.E. Potential toxic effects of glyphosate and its commercial formulations below regulatory limits. Food Chem. Toxicol. 2015, 84, 133–153. [Google Scholar] [CrossRef]
- Gomes, M.P.; Smedbol, E.; Chalifour, A.; Hénault-Ethier, L.; Labrecque, M.; Lepage, L.; Juneau, P. Alteration of plant physiology by glyphosate and its by-product aminomethylphosphonic acid: An overview. J Exp. Bot. 2014, 65, 4691–4703. [Google Scholar] [CrossRef]
- Duke, S.O.; Lydon, J.; Koskinen, W.C.; Moorman, T.B.; Chaney, R.L.; Hammerschmidt, R. Glyphosate effects on plant mineral nutrition, crop rhizosphere microbiota, and plant disease in glyphosate-resistant crops. J. Agric. Food Chem. 2012, 60, 10375–10397. [Google Scholar] [CrossRef]
- D’Agostino, R.B.; Pearson, E.S. Testing for departures from normality. Biometrika 1973, 60, 613–622. [Google Scholar] [CrossRef]
- Junior, W.R.C.; da Costa, Y.K.S.; Carbonari, C.A.; Duke, S.O.; Alves, P.L.D.C.A.; de Carvalho, L.B. Growth, morphological, metabolic and photosynthetic responses of clones of eucalyptus to glyphosate. For. Ecol. Manag. 2020, 470, 118218. [Google Scholar] [CrossRef]
- Smedbol, E.; Lucotte, M.; Maccario, S.; Gomes, M.P.; Paquet, S.; Moingt, M.; Mercier, L.L.C.; Sobarzo, M.R.P.; Blouin, M.A. Glyphosate and Aminomethylphosphonic acid content in glyphosate-resistant soybean leaves, stems, and roots and associated phytotoxicity following a single glyphosate-based herbicide application. J. Agric. Food Chem. 2019, 67, 6133–6142. [Google Scholar] [CrossRef]
- Wood, L.J. The presence of glyphosate in forest plants with different life strategies one year after application. Can. J. For. Res. 2019, 49, 586–594. [Google Scholar] [CrossRef]
- Carrasco Cabrera, L.; Di Piazza, G.; Dujardin, B.; Medina Pastor, P. The 2021 European Union report on pesticide residues in food. EFSA J. 2023, 21, 7939. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA) Review of the existing maximum residue levels for glyphosate according to Article 12 of Regulation (EC) No 396/2005—Revised version to take into account omitted data. EFSA J. 2019, 17, 5862. [CrossRef]
- Benbrook, C.M. How did the US EPA and IARC reach diametrically opposed conclusions on the genotoxicity of glyphosate-based herbicides? Environ. Sci. Eur. 2019, 31, 2. [Google Scholar] [CrossRef]
- Sun, M.; Li, H.; Jaisi, D.P. Degradation of glyphosate and bioavailability of phosphorus derived from glyphosate in a soil-water system. Water Res. 2019, 163, 114840. [Google Scholar] [CrossRef]
- Timms, K.P.; Wood, L.J. Sub-lethal glyphosate disrupts photosynthetic efficiency and leaf morphology in fruit-producing plants, red raspberry (Rubus idaeus) and highbush cranberry (Viburnum edule). GECCO 2020, 24, e01319. [Google Scholar] [CrossRef]
- Dill, G.M.; Sammons, R.D.; Feng, P.C.; Kohn, F.; Kretzmer, K.; Mehrsheikh, A.; Bleeke, M.; Honegger, J.L.; Farmer, D.; Wright, D.; et al. Glyphosate: Discovery, development, applications, and properties. In Glyphosate Resistance in Crops and Weeds; Nandula, V.K., Ed.; Jihn Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; Volume 1, p. 344. [Google Scholar]
- de Freitas-Silva, L.; de Araújo, T.O.; Nunes-Nesi, A.; Ribeiro, C.; Costa, A.C.; da Silva, L.C. Evaluation of morphological and metabolic responses to glyphosate exposure in two neotropical plant species. Ecol. Indicat. 2020, 113, 106246. [Google Scholar] [CrossRef]
- Zhigailov, A.V.; Nizkorodova, A.S.; Sharipov, K.O.; Polimbetova, N.S.; Iskakov, B.K. Glyphosate treatment mediates the accumulation of small discrete 5′-and 3′-terminal fragments of 18S rRNA in plant cells. Vavilov J. Genet. Breed. 2023, 162, 93. [Google Scholar]
- Mahendrakar, K.; Venkategowda, P.M.; Rao, S.M.; Mutkule, D.P. Glyphosate surfactant herbicide poisoning and management. Indian J. Crit. 2014, 18, 328. [Google Scholar]
- Helander, M.; Saloniemi, I.; Saikkonen, K. Glyphosate in northern ecosystems. Trends Plant Sci. 2012, 17, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.B.; Rose, M.T.; Rose, T.J.; Morris, S.G.; Van Zwieten, L. Impact of glyphosate on soil microbial biomass and respiration: A meta-analysis. Soil Biol. Biochem. 2016, 92, 50–57. [Google Scholar] [CrossRef]
- Florencia, F.M.; Carolina, T.; Enzo, B.; Leonardo, G. Effects of the herbicide glyphosate on non-target plant native species from Chaco forest (Argentina). Ecotoxicol. Environ. Safe 2017, 144, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Mamy, L.; Barriuso, E.; Gabrielle, B. Glyphosate fate in soils when arriving in plant residues. Chemosphere 2016, 154, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Rengel, Z.; Chang, H.; Meney, K.; Pantelic, L.; Tomanovic, R. Phytoremediation potential of Juncus subsecundus in soils contaminated with cadmium and polynuclear aromatic hydrocarbons (PAHs). Geoderma 2012, 175, 1–8. [Google Scholar] [CrossRef]
- Haberkon, N.B.R.; Aparicio, V.C.; Mendez, M.J. First evidence of glyphosate and aminomethylphosphonic acid (AMPA) in the respirable dust (PM10) emitted from unpaved rural roads of Argentina. Sci. Total Environ. 2021, 773, 145055. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Control | Soil | Leaf | Unit | |
|---|---|---|---|---|---|
| (a) | Height | 52.508 ± 6.62 (b) | 52.850 ± 3.58 (b) | 47.100 ± 1.71 (a) | cm |
| (b) | Leaves number | 16.83 ± 2.92 (b) | 16.08 ± 2.68 (ab) | 13.83 ± 1.85 (a) | pcs |
| (c) | Fresh shoot and leaf weight | 48.91 ± 5.41 (c) | 36.60 ± 7.58 (b) | 25.02 ± 4.71 (a) | g |
| (d) | Fresh root weight | 11.55 ± 2.62 (b) | 8.48 ± 1.77 (a) | 7.51 ± 1.06 (a) | g |
| (e) | Dried shoot and leaf weight | 10.58 ± 0.98 (b) | 9.07 ± 2.77 (b) | 5.80 ± 0.68 (a) | g |
| (f) | Dried root weight | 1.42 ± 0.42 (b) | 1.23 ± 0.31 (b) | 0.91 ± 0.08 (a) | g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kisvarga, S.; Hamar-Farkas, D.; Horotán, K.; Inotai, K.; Mörtl, M.; Neményi, A.; Székács, A.; Orlóci, L. Morphological, Histological, and Glyphosate Residue Analysis of Helianthus annuus L. Plants Treated with Glyphosate. Agriculture 2023, 13, 1014. https://doi.org/10.3390/agriculture13051014
Kisvarga S, Hamar-Farkas D, Horotán K, Inotai K, Mörtl M, Neményi A, Székács A, Orlóci L. Morphological, Histological, and Glyphosate Residue Analysis of Helianthus annuus L. Plants Treated with Glyphosate. Agriculture. 2023; 13(5):1014. https://doi.org/10.3390/agriculture13051014
Chicago/Turabian StyleKisvarga, Szilvia, Dóra Hamar-Farkas, Katalin Horotán, Katalin Inotai, Mária Mörtl, András Neményi, András Székács, and László Orlóci. 2023. "Morphological, Histological, and Glyphosate Residue Analysis of Helianthus annuus L. Plants Treated with Glyphosate" Agriculture 13, no. 5: 1014. https://doi.org/10.3390/agriculture13051014
APA StyleKisvarga, S., Hamar-Farkas, D., Horotán, K., Inotai, K., Mörtl, M., Neményi, A., Székács, A., & Orlóci, L. (2023). Morphological, Histological, and Glyphosate Residue Analysis of Helianthus annuus L. Plants Treated with Glyphosate. Agriculture, 13(5), 1014. https://doi.org/10.3390/agriculture13051014








