Evaluation of the Differences in the Serum Protein Electrophoretic Pattern in Precolostral Serum of Farm Animal Neonates
Abstract
1. Introduction
2. Material and Methods
2.1. Ethical Declaration
2.2. Animals and Sample Collection
2.3. Laboratory Analyses
2.4. Statistical Analysis
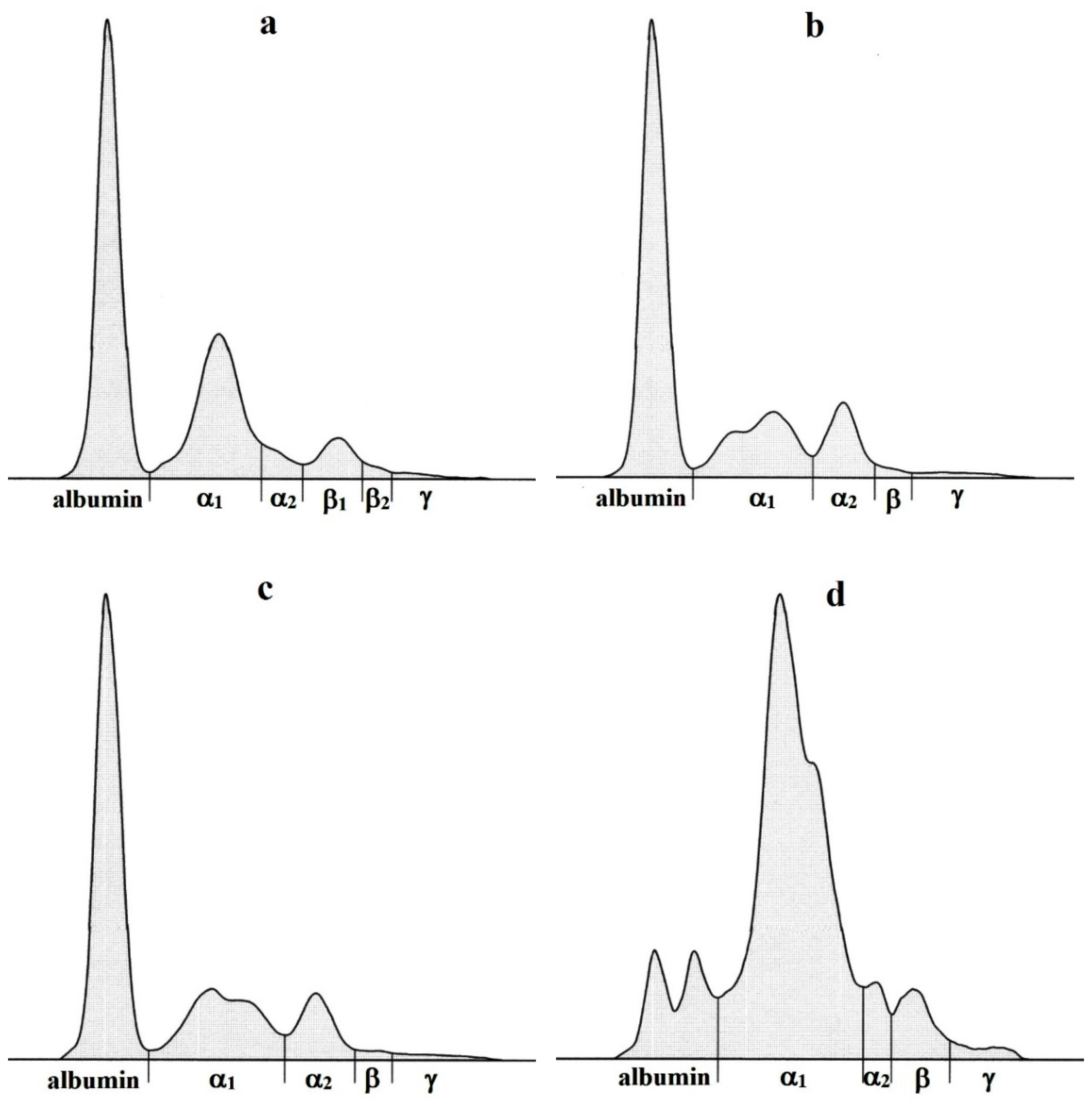
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- King, G.J. Comparative placentation in ungulates. J. Exp. Zool. 1982, 31, 588–602. [Google Scholar] [CrossRef]
- Chucri, T.M.; Monteiro, J.M.; Lima, A.R.; Salvadori, M.L.B.; Kfoury, J.R.; Miglino, M.A. A review of immune transfer by the placenta. J. Reprod. Immunol. 2010, 87, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Leiser, R.; Kaufmann, P. Placental structure: In a comparative aspect. Exp. Clin. Endocrinol. 1994, 102, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Wooding, F.B.P.; Flint, A.P.F.; Heap, R.B.; Morgan, G.; Buttle, H.L.; Young, I.R. Control of binucleate cell immigration in the placenta of ruminants. J. Reprod. Fertil. 1986, 76, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Wooding, F.B.P. Current topic: The synepitheliochorial placenta of ruminants: Binucleate cell fusions and hormone production. Placenta 1992, 13, 101–113. [Google Scholar] [CrossRef]
- Halliday, R.; Williams, M.R. The absorption of immunoglobulin from colostrum by bottle-fed lambs. Ann Rech. Vet. 1979, 10, 549–556. [Google Scholar]
- Constant, S.B.; Leblanc, M.M.; Klaptein, E.F.; Beebe, D.E.; Leneau, H.M.; Nunier, C.J. Serum immunoglobulin G concentration in goat kids fed colostrum or a colostrum substitute. J. Am. Vet. Med. Assoc. 1994, 205, 1759–1762. [Google Scholar]
- Arguello, A.; Castro, N.; Zamorano, M.J.; Castroalonso, A.; Capote, J. Passive transfer of immunity in kid goats fed refrigerated and frozen goat colostrum and commercial sheep colostrum. Small Rum. Res. 2004, 54, 237–241. [Google Scholar] [CrossRef]
- Castro, N.; Capote, J.; Álvarez, S.; Argüello, A. Effects of lyophilized colostrum and different colostrum feeding regimens on passive transfer of immunoglobulin G in Majorera goat kids. J. Dairy Sci. 2005, 88, 3650–3654. [Google Scholar] [CrossRef]
- Moffett, A.; Loke, C. Immunology of placentation in eutherian mammals. Nature 2006, 6, 584–594. [Google Scholar] [CrossRef]
- Wilson, M.E.; Biensen, N.J.; Ford, S.P. Novel insight in to control of litter size in pigs, using placental efficiency as a selection tool. J. Anim. Sci. 1999, 77, 1654–1658. [Google Scholar] [CrossRef] [PubMed]
- Giguère, S.; Polkes, A.C. Immunologic disorders in neonatal foals. Vet. Clin. Equine 2005, 21, 241–272. [Google Scholar] [CrossRef]
- Crisman, M.V.; Scarratt, W.K. Immunodeficiency disorders in horses. Vet. Clin. Equine 2008, 24, 299–310. [Google Scholar] [CrossRef]
- O’Connell, T.X.; Horita, T.J.; Kasravi, B. Understanding and interpreting serum protein electrophoresis. Am. Fam. Physician 2005, 71, 105–112. [Google Scholar] [PubMed]
- Tappin, S.W.; Taylor, S.S.; Tasker, S.; Dodkin, S.J.; Papasouliotis, K.; Murphy, K.F. Serum protein electrophoresis in 147 dogs. Vet. Rec. 2011, 168, 456. [Google Scholar] [CrossRef] [PubMed]
- Piccione, G.; Casella, S.; Giannetto, C.; Vazzana, I.; Niutta, P.P.; Giudice, E. Influence of age on profile of serum proteins in the calf. Acta Vet. 2009, 59, 413–422. [Google Scholar]
- Teixeira, W.T.; Fonteque, G.V.; Ramos, A.F.; da Silva Mariante, A.; do Egito, A.A.; Martins, V.M.V.; Saito, M.E.; Fonteque, J.H. Transfer of passive immunity and serum proteinogram in the first six months of life of Criollo Lageano and Black and White Holstein calves. Pesq. Vet. Bras. 2012, 32, 980–986. [Google Scholar] [CrossRef]
- Piccione, G.; Arfuso, F.; Faggio, C.; Casella, S.; Zumbo, A.; Panzera, M. Serum proteins profile in Comisana lambs during the first month of life. Arch. Tierz. 2013, 56, 742–750. [Google Scholar] [CrossRef]
- Nagy, O.; Tóthová, C.; Nagyová, V.; Kováč, G. Serum protein electrophoretic pattern in goat kids after colostrum intake during the first week of life. J. Vet. Sci. Med. Diagn. 2016, 5, 2. [Google Scholar] [CrossRef]
- Enders, A.C.; Blankenship, T.N. Comparative placental structure. Adv. Drug Deliv. Rev. 1999, 38, 3–15. [Google Scholar] [CrossRef]
- Sato, T.; Oda, K.; Kubo, M. Hematological and biochemical values of thoroughbred foals in the first six months of life. Cornell Vet. 1979, 69, 3–19. [Google Scholar] [PubMed]
- Hammon, H.M.; Schiessler, G.; Nussbaum, A.; Blum, J.W. Feed intake patterns, growth performance and metabolic and endocrine traits in calves fed unlimited amounts of colostrums and milk by automate strating in the neonatal period. J. Dairy Sci. 2002, 85, 3352–3362. [Google Scholar] [CrossRef] [PubMed]
- Franklin, S.T.; Ameral-Philips, D.M.; Jackson, J.A.; Campbell, A.A. Health and performance of Holstein calves that suckled or were hand-fed colostrums and were fed one of three physical forms of starter. J. Dairy Sci. 2003, 86, 2145–2153. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Chang, C.J.; Peh, H.C.; Chen, S.Y. Serum protein levels and neonatal growth rate of Nubian goat kids in Taiwan area. Small Rum. Res. 1999, 32, 153–160. [Google Scholar] [CrossRef]
- Nagy, O.; Tóthová, C.; Nagyová, V.; Kováč, G.; Pošivák, J. Changes in the serum protein electrophoretic pattern in lambs during the first month of life. Acta Vet. Brno 2014, 83, 187–193. [Google Scholar] [CrossRef]
- Ingvarsson, B.I.; Carlsson, R.N.K.; Karlsson, B.W. Synthesis of α-fetoprotein, albumin and total serum protein in neonatal pigs. Biol. Neonate 1978, 34, 259–268. [Google Scholar] [CrossRef]
- Cavanagh, M.E.; Cornelis, M.E.; Dziegielewska, K.M.; Luft, A.J.; Lai, P.C.W.; Lorscheider, F.L.; Saunders, N.R. Proteins in cerebrospinal fluid and plasma of fetal pigs during development. Dev. Neurosci. 1982, 5, 492–502. [Google Scholar] [CrossRef]
- Svendsen, L.S.; Weström, B.R.; Svendsen, J.; Olsson, A.; Karlsson, B.W. Blood serum characteristics of newborn pigs. Comparison of unaffected pigs with pigs belonging to five mortality groups. Acta Vet. Scand. 1991, 32, 287–299. [Google Scholar] [CrossRef]
- Nagyová, V.; Tóthová, C.; Nagy, O. The impact of colostrum intake on the serum protein electrophoretic pattern in newborn ruminants. J. Appl. Anim. Res. 2017, 45, 498–504. [Google Scholar] [CrossRef]
- Souza, D.C.; Silva, D.G.; Rocha, T.G.; Monteiro, B.M.; Pereira, G.T.; Fiori, L.C.; Viana, R.B.; Fagliari, J.J. Serum biochemical profile of neonatal buffalo calves. Arq. Bras. Med. Vet. Zootec. 2019, 71, 187–196. [Google Scholar] [CrossRef]
- Tóthová, C.; Link, R.; Kyzeková, P.; Nagy, O. Serum protein electrophoretic pattern in piglets during the early postnatal period. Sci. Rep. 2021, 11, 17539. [Google Scholar] [CrossRef] [PubMed]
- Bujacz, A.; Talaj, J.A.; Zielinski, K.; Pietrzyk-Brzezinska, A.J.; Neumann, P. Crystal structures of serum albumins from domesticated ruminants and their complexes with 3,5-diiodosalicylic acid. Acta Crystallogr. D Struct. Biol. 2017, 73, 896–909. [Google Scholar] [CrossRef]
- Szymeczko, R.; Kapelaǹski, W.; Piotrowska, A.; Dybala, J.; Boguslawska-Tryk, M.; Burlikowska, K.; Hertig, I.; Sassek, M.; Pruszyǹska-Oszmalek, E.; Maćkowiak, P. Changes in the content of major proteins and selected hormons in the blood serum of piglets during the early postnatal period. Folia Biol. 2009, 57, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Tesouro, M.A.; González-Ramón, N.; Piňeiro, A.; Lampreave, F. Major plasma proteins in pig serum during postnatal development. Reprod. Fertil. Dev. 2005, 17, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Stone, R.T. In vitro liver synthesis and serum levels of alpha-fetoprotein and albumin in the fetal pig. Biol. Reprod. 1981, 20, 573–580. [Google Scholar] [CrossRef]
- Le Dividich, J.; Rooke, J.A.; Herpin, P. Nutritional and immunological importance of colostrum for the new-born pig. J. Agric. Sci. 2005, 143, 469–485. [Google Scholar] [CrossRef]
- Lardinois, R.; Page, L.A. Serum albumin, prealbumin and postalbumin in perinatal pigs. Dev. Biol. 1969, 19, 261–280. [Google Scholar] [CrossRef]
- Lampreave, F.; Piñeiro, A. The major serum protein of fetal and newborn pigs: Biochemical properties and identification as a fetal form of α1-acid glycoprotein. Int. J. Biochem. 1984, 16, 47–53. [Google Scholar] [CrossRef]
- Itoh, H.; Tamura, K.; Izumi, M.; Motoi, Y.; Kidoguchi, K.; Funayama, Y. The influence of age and health status on the serum α1-acid glycoprotein level of conventional and specific pathogen-free pigs. Can. J. Vet. Res. 1993, 57, 74–78. [Google Scholar]
- Heegaard, P.M.H.; Miller, I.; Sorensen, N.S.; Soerensen, K.E.; Skovgaard, K. Pig α1-glycoprotein: Characterization and first description in any species as a negative acute phase protein. PLoS ONE 2013, 8, e68110. [Google Scholar] [CrossRef]
- Lampreave, F.; Piñeiro, A. Concentrations of major plasma proteins in serum and whole-tissue extracts in porcine fetuses during development. J. Reprod. Fertil. 1992, 95, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Weström, B.R.; Karlsson, B.W.; Svendsen, J. Levels of serum protease inhibitors during fetal and postnatal development of the pig. Biol. Neonate 1982, 41, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Gitlin, D.; Boesman, M. Sites of serum α-fetoprotein synthesis in the human and in the rat. J. Clin. Investig. 1967, 46, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- Calvo, M.; Naval, J.; Lampreave, F.; Piñeiro, A. Fatty acids bound to α-fetoprotein and albumin during rat development. Biochim. Biophys. Acta 1988, 959, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, J.J. Serum proteins and the dysproteinemias. In Clinical Biochemistry of Domestic Animals, 5th ed.; Kaneko, J.J., Ed.; Academic Press: London, UK, 1997; pp. 117–138. [Google Scholar]
- Bishop, M.L.; Fody, E.P.; Schoeff, L.E. Clinical Chemistry: Techniques, Principles, Correlations; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2010; pp. 205–245. [Google Scholar]
- Bader, D.; Riskin, A.; Vafsi, O.; Tamir, A.; Peskin, B.; Israel, N.; Merksamer, R.; Dar, H.; David, M. Alpha-fetoprotein in the early neonatal period—A large study and review of the literature. Clin. Chim. Acta 2004, 349, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Bossuyt, X. Advances in serum protein electrophoresis. Adv. Clin. Chem. 2006, 42, 43–80. [Google Scholar]
- Hiss-Pesch, S.; Daniel, F.; Bunkelberg-Denk, S.; Mielenz, M.; Sauerwein, H. Transfer of maternal haptoglobin in suckling piglets. Vet. Immunol. Immunopathol. 2011, 144, 104–110. [Google Scholar] [CrossRef]
- Llamas Moya, S.; Boyle, L.A.; Lynch, P.B.; Arkins, S. Age-related changes in pro-inflammatory cytokines, acute phase proteins and cortisol concentrations in neonatal pigs. Neonatology 2007, 91, 44–48. [Google Scholar] [CrossRef]
- Rocha, T.G.; Silva, F.D.F.; Bortoletto, C.; Silva, D.G.; Buzinaro, M.G.; Zafalon, L.F.; Fagliari, J.J. Serum concentrations of acute phase proteins and immunoglobulins of calves with rotavirus diarrhea. Arq. Bras. Med. Vet. Zootec. 2016, 68, 865–872. [Google Scholar] [CrossRef]
- Erkiliçc, E.E.; Merhan, O.; Kirmizigül, A.H.; Öğün, M.; Akyüz, E.; Çitil, M. Salivary and serum levels of serum amyloid A, haptoglobin, ceruloplasmin and albumin in neonatal calves with diarrhea. Kafkas Univ. Vet. Fak. Derg. 2019, 25, 583–586. [Google Scholar]
- Dinler, C.; Ulutas, B.; Voyvoda, H.; Ulutas, P.A.; Ural, K.; Karagenc, T. Haptoglobin and serum amyloid-A concentrations and their relationship with oocyst count in neonatal lambs experimentally infected with Cryptosporidium parvum. Vet. Parasitol. 2017, 247, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Dinler, C.; Tuna, G.E.; Ay, E.; Ulutas, B.; Voyvoda, H.; Ulutas, P.A. Reference intervals for serum amyloid A, haptoglobin, ceruloplasmin, and fibrinogen in apparently healthy neonatal lambs. Vet. Clin. Pathol. 2020, 49, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Marc, S.; Kirovski, D.; Mircu, C.; Hutu, I.; Otavă, G.; Paul, C.; Boldura, O.M.; Tulcan, C. Serum protein electrophoretic pattern in neonatal calves treated with clinoptilolite. Molecules 2018, 23, 1278. [Google Scholar] [CrossRef] [PubMed]
- Cerón, J.J.; Caldin, M.; Martinez-Subiela, S. Electrophoresis and acute phase protein measurement. In Schalm’s Veterinary Hematology, 6th ed.; Douglas, J., Weiss, K., Wardrop, J., Eds.; Blackwell Publishing Ltd.: Ames, IA, USA, 2010; pp. 1157–1161. [Google Scholar]
- De Jong, G.; van Dijk, J.P.; van Eijk, H.G. The biology of transferrin. Clin. Chim. Acta 1990, 190, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Moser, M.; Pfister, H.; Bruckmaier, R.M.; Rehage, J.; Blum, J.W. Blood serum transferrin concentration in cattle in various physiological states, in veal calves fed different amounts of iron, and in cattle affected by infectious and non-infectious diseases. Zentralbl. Veterinarmed. A 1994, 41, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Thoren-Tholling, K.; Martinsson, K. On the transferrin concentration in serum of sows and growing pigs and in sow colostrum. Acta Vet. Scand. 1974, 15, 120–134. [Google Scholar] [CrossRef]
- Asadi, M.; Toghdory, A.; Hatami, M.; Ghassemi Nejad, J. Milk supplemented with organic iron improves performance, blood hematology, iron metabolism parameters, biochemical and immunological parameters in suckling Dalagh lambs. Animals 2022, 12, 510. [Google Scholar] [CrossRef]
- Atyabi, N.; Gharagozloo, F.; Nassiri, S.M. The necessity of iron supplementation for normal development of commercially reared suckling calves. Comp. Clin. Pathol. 2006, 15, 165–168. [Google Scholar] [CrossRef]
- Li, Y.; Yang, W.; Dong, D.; Jiang, S.; Yang, Z.; Wang, Y. Effect of different sources and levels of iron in the diet of sows on iron status in neonatal pigs. Anim. Nutr. 2018, 4, 197–202. [Google Scholar] [CrossRef]
- Jones, H.N.; Powell, T.L.; Jansson, T. Regulation of placental nutrient transport: A review. Placenta 2007, 28, 763–774. [Google Scholar] [CrossRef]
- Borghesi, J.; Mario, L.C.; Nogueira Rodrigues, M.; Oliveira Favaron, P.; Miglino, M.A. Immunoglobulin transport during gestation in domestic animals and humans—A review. Open J. Anim. Sci. 2014, 4, 323–336. [Google Scholar] [CrossRef]
- Baintner, K. Transmission of antibodies from mother to young: Evolutionary strategies in a proteolytic environment. Vet. Immunol. Immunopathol. 2007, 117, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Poitras, B.J.; Miller, R.B.; Wilkie, B.N.; Bosu, W.T. The maternal to fetal transfer of immunoglobulins associated with placental lesions in sheep. Can. J. Vet. Res. 1986, 50, 68–73. [Google Scholar] [PubMed]
- Gabriel, S.; Geldhof, P.; Phiri, I.K.; Cornillie, P.; Goddeeris, B.M.; Vercruysse, J. Placental transfer of immunoglobulins in cattle infected with Schistosoma mattheei. Vet. Immunol. Immunopathol. 2005, 104, 265–272. [Google Scholar] [CrossRef]


| Parameters | Calves | Lambs | Kids | Piglets | p Value |
|---|---|---|---|---|---|
| Albumin | 58.1 ± 2.8 a | 68.0 ± 1.6 b | 64.4 ± 3.7 c | 16.1 ± 2.9 d | <0.001 |
| α1-globulins | 27.5 ± 1.8 a | 16.6 ± 2.0 b | 21.1 ± 2.9 c | 68.5 ± 3.3 d | <0.001 |
| α2-globulins | 2.93 ± 0.66 a | 11.39 ± 0.92 b | 10.91 ± 1.43 b | 4.69 ± 0.86 c | <0.001 |
| β-globulins | 10.11 ± 1.33 a | 1.93 ± 0.45 b | 1.40 ± 0.31 b | 8.20 ± 1.29 c | <0.001 |
| γ-globulins | 1.40 ± 0.58 a | 2.13 ± 0.43 b | 2.20 ± 1.11 a,b | 2.53 ± 1.11 b | <0.001 |
| A/G | 1.40 ± 0.17 a | 2.13 ± 0.16 b | 1.84 ± 0.30 c | 0.19 ± 0.04 d | <0.001 |
| Parameters | Calves | Lambs | Kids | Piglets | p Value |
|---|---|---|---|---|---|
| TP | 40.0 ± 2.1 a | 41.9 ± 2.7 a | 40.8 ± 4.8 a | 25.9 ± 2.4 b | <0.001 |
| Albumin | 23.2 ± 1.6 a | 28.5 ± 1.8 b | 26.4 ± 3.7 c | 4.2 ± 0.9 d | <0.001 |
| α1-globulins | 11.0 ± 0.9 a | 6.9 ± 1.0 b | 8.5 ± 1.0 c | 17.7 ± 1.8 d | <0.001 |
| α2-globulins | 1.16 ± 0.30 a | 4.79 ± 0.57 b | 4.47 ± 0.89 b | 1.22 ± 0.22 a | <0.001 |
| β-globulins | 4.06 ± 0.62 a | 0.80 ± 0.18 b | 0.57 ± 0.15 b | 2.12 ± 0.37 c | <0.001 |
| γ-globulins | 0.56 ± 0.22 a | 0.89 ± 0.19 b | 0.90 ± 0.53 a,b | 0.65 ± 0.29 a | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tóthová, C.; Link, R.; Glembová, V.; Nagy, O. Evaluation of the Differences in the Serum Protein Electrophoretic Pattern in Precolostral Serum of Farm Animal Neonates. Agriculture 2023, 13, 1035. https://doi.org/10.3390/agriculture13051035
Tóthová C, Link R, Glembová V, Nagy O. Evaluation of the Differences in the Serum Protein Electrophoretic Pattern in Precolostral Serum of Farm Animal Neonates. Agriculture. 2023; 13(5):1035. https://doi.org/10.3390/agriculture13051035
Chicago/Turabian StyleTóthová, Csilla, Róbert Link, Veronika Glembová, and Oskar Nagy. 2023. "Evaluation of the Differences in the Serum Protein Electrophoretic Pattern in Precolostral Serum of Farm Animal Neonates" Agriculture 13, no. 5: 1035. https://doi.org/10.3390/agriculture13051035
APA StyleTóthová, C., Link, R., Glembová, V., & Nagy, O. (2023). Evaluation of the Differences in the Serum Protein Electrophoretic Pattern in Precolostral Serum of Farm Animal Neonates. Agriculture, 13(5), 1035. https://doi.org/10.3390/agriculture13051035





