Endophytic Effect of the South African Beauveria bassiana Strain PPRI 7598 on the Population Growth and Development of the Russian Wheat Aphid, Diuraphis noxia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Aphid Rearing
2.3. Endophytic Fungal Strain
2.4. Wheat Seed Inoculation and Planting
2.5. In Vivo Bioassays
2.5.1. Experiment 1: Effect of the Endophyte on Aphid Reproduction and Population Growth
2.5.2. Experiment 2: Effect of the Endophyte on Aphid Biomass and T. aestivum Response towards RWA Herbivory
2.5.3. Confirmation of Plant Endophytic Colonisation
2.6. Statistical Analysis
3. Results
3.1. Effect of the Endophyte on RWA Reproduction and Intrinsic Rate of Population Increase
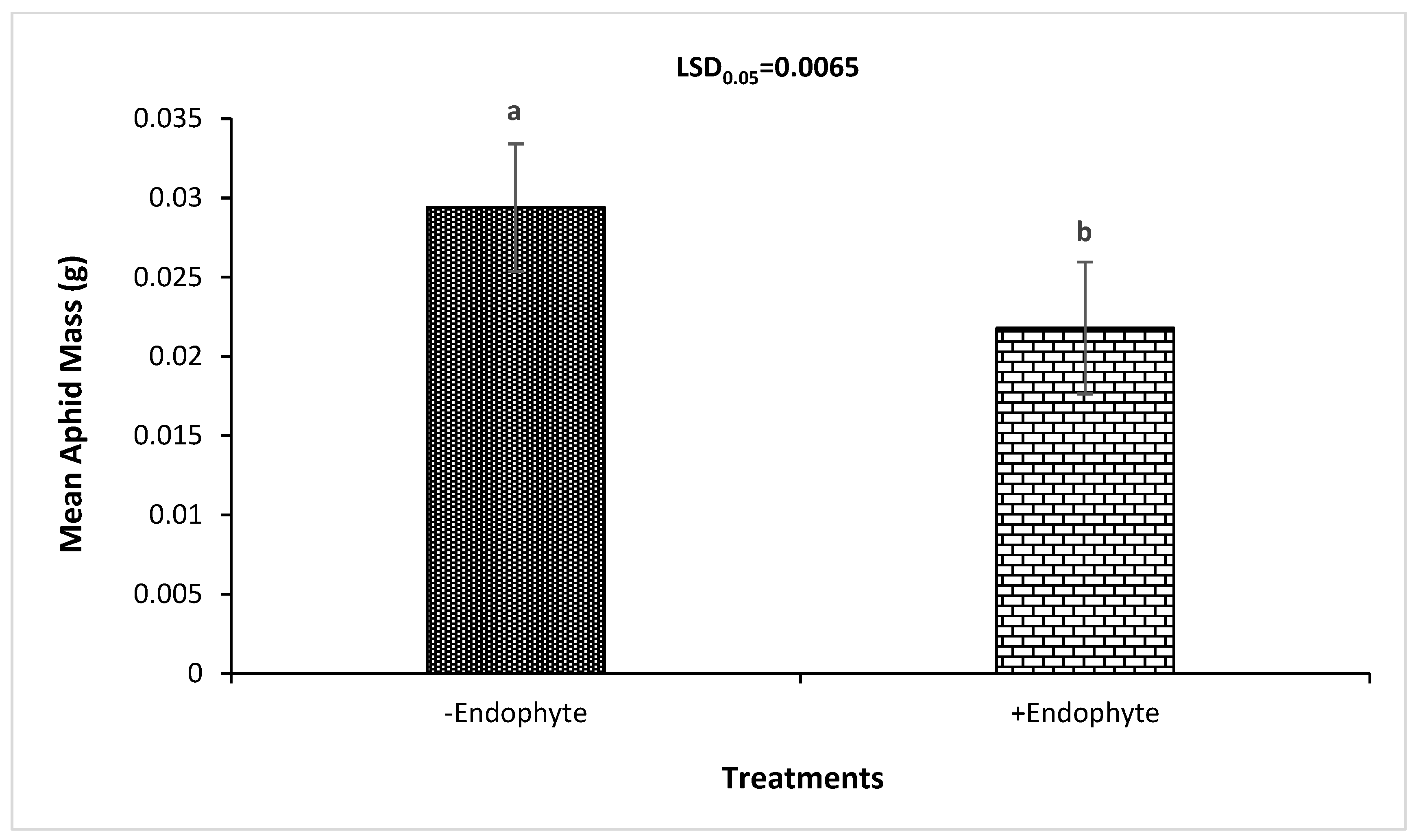
3.2. Effect of the Endophyte on Aphid Mass and T. aestivum Response to RWA Herbivory
3.3. Confirmation of Plant Endophytic Colonisation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bester, M. Dominant Factors Which Influence Wheat Production in South Africa. Doctoral Dissertation, Stellenbosch University, Stellenbosch, South Africa, 2014. [Google Scholar]
- Bhattacharya, S. Brassica-aphid interaction: Challenges and prospects of genetic engineering for integrated aphid management. Physiol. Mol. Plant Pathol. 2019, 108, 101442. [Google Scholar] [CrossRef]
- Hales, D.F.; Tomiuk, J.; Wöhrmann, K.; Sunnucks, P. Evolutionary and genetic aspects of aphid biology: A review. Eur. J. Entomol. 1997, 94, 1–55. [Google Scholar]
- Youssif, M.; Ali, S.; Helaly, W.M. Cereal aphid species (Homoptera: Aphididae) infesting wheat plants and their aphidophagous insects at El-Khattara District, Sharkia Governorate, Egypt. J. Plant Prot. Pathol. 2017, 8, 581–589. [Google Scholar] [CrossRef]
- Marasas, C.; Anandajayasekeram, P.; Tolmay, V.; Martella, D.; Purchase, J.; Prinsloo, G. Socio-Economic Impact of the Russian Wheat Aphid Control Research Program; No. 338; CIMMYT: Stellenbosch, South Africa, 1999. [Google Scholar]
- Kisten, L. Investigation into the Genetic Control of Russian Wheat Aphid Diuraphis noxia Resistance. Doctoral Dissertation, University of Johannesburg, Johannesburg, South Africa, 2022. [Google Scholar]
- Yates, A.D.; Michel, A. Mechanisms of aphid adaptation to host plant resistance. Curr. Opin. Insect Sci. 2018, 26, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Jankielsohn, A. Russian Wheat Aphid Distribution in Wheat Production Areas: Consequences of Management Practices. In Current Trends in Wheat Research; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar]
- Vega, F.E. The use of fungal entomopathogens as endophytes in biological control: A review. Mycologia 2018, 110, 4–30. [Google Scholar] [CrossRef]
- Motholo, L.F.; Booyse, M.; Hatting, J.L.; Tsilo, T.J.; Thekisoe, O.M. Comparison of wheat growth-response to endophytic Beauveria bassiana (Hypocreales: Cordycipitaceae) derived from an insect versus plant host. Aust. J. Crop Sci. 2019, 13, 1793–1802. [Google Scholar] [CrossRef]
- Motholo, L.; Booyse, M.; Tsilo, T.; Hatting, J.; Thekisoe, O. Pathogenicity of Beauveria bassiana (Hypocreales: Cordycipitaceae) against the Russian Wheat Aphid, Diuraphis noxia (Hemiptera: Aphididae). Afr. Entomol. 2020, 28, 455–461. [Google Scholar] [CrossRef]
- Clement, S.; Pike, K.; Kaiser, W.; Wilson, A. Resistance of endophyte-infected plants of tall fescue and perennial ryegrass to the Russian wheat aphid (Homoptera: Aphididae). J. Kans. Entomol. Soc. 1990, 63, 646–648. [Google Scholar]
- Clement, S.; Lester, D.; Wilson, A.; Pike, K. Behavior and performance of Diuraphis noxia (Homoptera: Aphididae) on fungal endophyte-infected and uninfected perennial ryegrass. J. Econ. Entomol. 1992, 85, 583–588. [Google Scholar] [CrossRef]
- Clement, S.; Wilson, A.; Lester, D.; Davitt, C. Fungal endophytes of wild barley and their effects on Diuraphis noxia population development. Entomol. Exp. Appl. 1997, 82, 275–281. [Google Scholar] [CrossRef]
- Gurulingappa, P.; Sword, G.A.; Murdoch, G.; McGee, P.A. Colonization of crop plants by fungal entomopathogens and their effects on two insect pests when in planta. Biol. Control 2010, 55, 34–41. [Google Scholar] [CrossRef]
- Pelizza, S.A.; Mariottini, Y.; Russo, L.M.; Vianna, M.F.; Scorsetti, A.C.; Lange, C.E. Beauveria bassiana (Ascomycota: Hypocreales) introduced as an endophyte in corn plants and its effects on consumption, reproductive capacity, and food preference of Dichroplus maculipennis (Orthoptera: Acrididae: Melanoplinae). J. Insect Sci. 2017, 17, 53. [Google Scholar] [CrossRef]
- Bancole, W.; Laing, M.D.; Yobo, K.S.; Togola, A. Establishment of Beauveria bassiana isolates as endophytes in rice cultivars and their biocontrol efficacy against rice stem borer. Sesamia calamistis. S. Afr. J. Sci. 2020, 116, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rodríguez, A.R.; Raya-Díaz, S.; Zamarreño, Á.M.; García-Mina, J.M.; del Campillo, M.C.; Quesada-Moraga, E. An endophytic Beauveria bassiana strain increases spike production in bread and durum wheat plants and effectively controls cotton leafworm (Spodoptera littoralis) larvae. Biol. Control 2018, 116, 90–102. [Google Scholar] [CrossRef]
- Torkaman, Z.; Talaei-Hassanloui, R.; Khorramnejad, A.; Pashaei, M.R. Effects of endophytism by Beauveria bassiana (Cordycipitaceae) on plant growth, Fusarium (Nectriaceae) disease, and Sunn pest Eurygaster integriceps (Hemiptera: Scutelleridae) in wheat (Poaceae). Can. Entomol. 2023, 155, e12. [Google Scholar] [CrossRef]
- Rohlfs, M.; Churchill, A.C. Fungal secondary metabolites as modulators of interactions with insects and other arthropods. Fungal Genet. Biol. 2011, 48, 23–34. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef]
- Rondot, Y.; Reineke, A. Endophytic Beauveria bassiana in grapevine Vitis vinifera (L.) reduces infestation with piercing-sucking insects. Biol. Control 2018, 116, 82–89. [Google Scholar] [CrossRef]
- Vazquez-de-Aldana, B.R.; García-Ciudad, A.; Garcia-Criado, B.; Vicente-Tavera, S.; Zabalgogeazcoa, I. Fungal endophyte (Epichloë festucae) alters the nutrient content of Festuca rubra regardless of water availability. PLoS ONE 2013, 8, e84539. [Google Scholar] [CrossRef] [PubMed]
- Waqas, M.; Khan, A.L.; Kamran, M.; Hamayun, M.; Kang, S.-M.; Kim, Y.-H.; Lee, I.-J. Endophytic fungi produce gibberellins and indoleacetic acid and promotes host-plant growth during stress. Molecules 2012, 17, 10754–10773. [Google Scholar] [CrossRef]
- González-Mas, N.; Gutiérrez-Sánchez, F.; Sánchez-Ortiz, A.; Grandi, L.; Turlings, T.C.; Muñoz-Redondo, J.M.; Moreno-Rojas, J.M.; Quesada-Moraga, E. Endophytic colonization by the entomopathogenic fungus Beauveria bassiana affects plant volatile emissions in the presence or absence of chewing and sap-sucking insects. Front. Plant Sci. 2021, 12, 660460. [Google Scholar] [CrossRef] [PubMed]
- Hatting, J.L.; Wraight, S.P.; Miller, R.M. Efficacy of Beauveria bassiana (Hyphomycetes) for control of Russian wheat aphid (Homoptera: Aphididae) on resistant wheat under field conditions. Biocontrol Sci. Technol. 2004, 14, 459–473. [Google Scholar] [CrossRef]
- Tolmay, V.; Lindeque, R.; Prinsloo, G. Preliminary evidence of a resistance-breaking biotype of the Russian wheat aphid, Diuraphis noxia (Kurdjumov) (Homoptera: Aphididae), in South Africa. Afr. Entomol. 2007, 15, 228–230. [Google Scholar] [CrossRef]
- Jankielsohn, A. Host associations of Diuraphis noxia (Homoptera: Aphididae) biotypes in South Africa. J. Econ. Entomol. 2013, 106, 2595–2601. [Google Scholar] [CrossRef] [PubMed]
- Chase, A.; Osborne, L.; Ferguson, V. Selective isolation of the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae from an artificial potting medium. Fla. Entomol. 1986, 69, 285–292. [Google Scholar] [CrossRef]
- Akello, J.; Sikora, R. Systemic acropedal influence of endophyte seed treatment on Acyrthosiphon pisum and Aphis fabae offspring development and reproductive fitness. Biol. Control 2012, 61, 215–221. [Google Scholar] [CrossRef]
- Dhingra, O.; Sinclair, J. Basic Plant Pathology Methods, 2nd ed.; Lewis Publishers: Boca Raton, FL, USA, 1995. [Google Scholar]
- Birch, L. The intrinsic rate of natural increase of an insect population. J. Anim. Ecol. 1948, 17, 15–26. [Google Scholar] [CrossRef]
- Tolmay, V.; Van Deventer, C.; Van Der Westhuizen, M. Inheritance of resistance to Russian wheat aphid, Diuraphis noxia (Homoptera: Aphididae) in two wheat lines. S. Afr. J. Plant Soil 1999, 16, 127–130. [Google Scholar] [CrossRef]
- Castillo Lopez, D.; Zhu-Salzman, K.; Ek-Ramos, M.J.; Sword, G.A. The entomopathogenic fungal endophytes Purpureocillium lilacinum (formerly Paecilomyces lilacinus) and Beauveria bassiana negatively affect cotton aphid reproduction under both greenhouse and field conditions. PLoS ONE 2014, 9, e103891. [Google Scholar] [CrossRef]
- Bills, G.F. Isolation and analysis of endophytic fungal communities from woody plants. In Endophytic Fungi in Grasses and Woody Plants: Systematics, Ecology, and Evolution; APS Press: St Paul, MN, USA, 1996; pp. 31–65. [Google Scholar]
- Fisher, P.; Petrini, O. Location of fungal endophytes in tissues of Suaeda fruticosa: A preliminary study. Trans. Br. Mycol. Soc. 1987, 89, 246–249. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Levene, H. Robust tests for equality of variances. In Contributions to Probability and Statistics; Stanford University Press: Stanford, CA, USA, 1960; pp. 278–292. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Vining, G.G.; Kowalski, S.M.; Montgomery, D.C. Response surface designs within a split-plot structure. J. Qual. Technol. 2005, 37, 115–129. [Google Scholar] [CrossRef]
- Ott, L.; Longnecker, M.; Longnecker, M. An Introduction to Statistical Methods and Data Analysis; Cengage Learning: Belmont, CA, USA, 2001. [Google Scholar]
- Martinuz, A.; Schouten, A.; Menjivar, R.; Sikora, R. Effectiveness of systemic resistance toward Aphis gossypii (Hom., Aphididae) as induced by combined applications of the endophytes Fusarium oxysporum Fo162 and Rhizobium etli G12. Biol. Control. 2012, 62, 206–212. [Google Scholar] [CrossRef]
- Bolser, R.C.; Hay, M.E. Are tropical plants better defended? Palatability and defenses of temperate vs. tropical seaweeds. Ecology 1996, 77, 2269–2286. [Google Scholar] [CrossRef]
- Siska, E.L.; Pennings, S.C.; Buck, T.L.; Hanisak, M.D. Latitudinal variation in palatability of salt-marsh plants: Which traits are responsible? Ecology 2002, 83, 3369–3381. [Google Scholar] [CrossRef]
- Pennings, S.C.; Ho, C.-K.; Salgado, C.S.; Więski, K.; Davé, N.; Kunza, A.E.; Wason, E.L. Latitudinal variation in herbivore pressure in Atlantic Coast salt marshes. Ecology 2009, 90, 183–195. [Google Scholar] [CrossRef]
- Ho, C.-K.; Pennings, S.C. Preference and performance in plant–herbivore interactions across latitude—A study in US atlantic salt marshes. PLoS ONE 2013, 8, e59829. [Google Scholar] [CrossRef]
- Argandon̄a, V.H.; Pen̄a, G.F.; Niemeyer, H.M.; Corcuera, L.J. Effect of cysteine on stability and toxicity to aphids of a cyclic hydroxamic acid from Gramineae. Phytochemistry 1982, 21, 1573–1574. [Google Scholar] [CrossRef]
- Leszczynski, B.; Dixon, A.F. Resistance of cereals to aphids: Interaction between hydroxamic acids and the aphid Sitobion avenae (Homoptera: Aphididae). Ann. Appl. Biol. 1990, 117, 21–30. [Google Scholar] [CrossRef]
- Givovich, A.; Niemeyer, H.M. Comparison of the effect of hydroxamic acids from wheat on five species of cereal aphids. Entomol. Exp. Appl. 1995, 74, 115–119. [Google Scholar] [CrossRef]
- Niemeyer, H.M.; Perez, F.J. Potential of Hydroxamic Acids in the Control of Cereal Pests, Diseases, and Weeds; ACS Publications: Washington, DC, USA, 1995. [Google Scholar]
- Dixon, A. Cereal aphids as an applied problem. Agric. Zool. Rev. 1987, 2, 1–57. [Google Scholar]
- Kuldau, G.; Bacon, C. Clavicipitaceous endophytes: Their ability to enhance resistance of grasses to multiple stresses. Biol. Control 2008, 46, 57–71. [Google Scholar] [CrossRef]
- Burd, J.D.; Burton, R.L. Characterization of plant damage caused by Russian wheat aphid (Homoptera: Aphididae). J. Econ. Entomol. 1992, 85, 2017–2022. [Google Scholar] [CrossRef]
- Gutsche, A.R.; Heng-Moss, T.M.; Higley, L.G.; Sarath, G.; Mornhinweg, D.W. Physiological responses of resistant and susceptible barley, Hordeum vulgare to the Russian wheat aphid, Diurpahis noxia (Mordvilko). Arthropod-Plant Interact. 2009, 3, 233–240. [Google Scholar] [CrossRef]
- McGee, P. Reduced growth and deterrence from feeding of the insect pest Helicoverpa armigera associated with fungal endophytes from cotton. Aust. J. Exp. Agric. 2002, 42, 995–999. [Google Scholar] [CrossRef]
- Vega, F.E. Insect pathology and fungal endophytes. J. Invertebr. Pathol. 2008, 98, 277–279. [Google Scholar] [CrossRef]
- Lacey, L.A.; Neven, L.G. The potential of the fungus, Muscodor albus, as a microbial control agent of potato tuber moth (Lepidoptera: Gelechiidae) in stored potatoes. J. Invertebr. Pathol. 2006, 91, 195–198. [Google Scholar] [CrossRef]
- Prado, E.; Tjallingii, W.F. Aphid activities during sieve element punctures. Entomol. Exp. Appl. 1994, 72, 157–165. [Google Scholar] [CrossRef]
- Burd, J.D.; Elliott, N.C. Changes in chlorophyll a fluorescence induction kinetics in cereals infested with Russian wheat aphid (Homopetra: Aphididea). J. Econ. Entomol. 1996, 89, 1332–1337. [Google Scholar] [CrossRef]
- Franzen, L.D.; Gutsche, A.R.; Heng-Moss, T.M.; Higley, L.G.; Sarath, G.; Burd, J.D. Physiological and biochemical responses of resistant and susceptible wheat to injury by Russian wheat aphid. J. Econ. Entomol. 2014, 100, 1692–1703. [Google Scholar] [CrossRef]
- Macedo, T.B.; Peterson, R.K.; Weaver, D.K.; Ni, X. Impact of Diuraphis noxia and Rhopalosiphum padi (Hemiptera: Aphididae) on primary physiology of four near-isogenic wheat lines. J. Econ. Entomol. 2009, 102, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Quisenberry, S.S.; Heng-Moss, T.; Markwell, J.; Higley, L.; Baxendale, F.; Sarath, G.; Klucas, R. Dynamic change in photosynthetic pigments and chlorophyll degradation elicited by cereal aphid feeding. Entomol. Exp. Appl. 2002, 105, 43–53. [Google Scholar] [CrossRef]
- Telang, A.; Sandström, J.; Dyreson, E.; Moran, N.A. Feeding damage by Diuraphis noxia results in a nutritionally enhanced phloem diet. Entomol. Exp. Appl. 1999, 91, 403–412. [Google Scholar] [CrossRef]
- Arnold, A.E. Endophytic fungi: Hidden components of tropical community ecology. In Tropical Forest Community Ecology; Blackwell Scientific, Inc.: Hoboken, NJ, USA, 2008; pp. 178–188. [Google Scholar]
- Gianoli, E.; Niemeyer, H.M. Allocation of herbivory-induced hydroxamic acids in the wild wheat Triticum uniaristatum. Chemoecology 1998, 8, 19–23. [Google Scholar] [CrossRef]
- Ownley, B.H.; Griffin, M.R.; Klingeman, W.E.; Gwinn, K.D.; Moulton, J.K.; Pereira, R.M. Beauveria bassiana: Endophytic colonization and plant disease control. J. Invertebr. Pathol. 2008, 98, 267–270. [Google Scholar] [CrossRef]
- Lohse, R.; Jakobs-Schönwandt, D.; Vidal, S.; Patel, A.V. Evaluation of new fermentation and formulation strategies for a high endophytic establishment of Beauveria bassiana in oilseed rape plants. Biol. Control 2015, 88, 26–36. [Google Scholar] [CrossRef]
- McKinnon, A.; Glare, T.; Ridgway, H.; Holyoake, A. Recovery and detection of an entomopathogenic endophyte: Overcoming the challenges involved. In Proceedings of the 47th Annual Meeting of the Society for Invertebrate Pathology, Mainz, Germany, 3–7 August 2014. [Google Scholar]
- Price, P.W.; Denno, R.F.; Eubanks, M.D.; Finke, D.L.; Kaplan, I. Insect Ecology: Behavior, Populations and Communities; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]


| Trial | Life-Table Parameters | ||
|---|---|---|---|
| Net Reproductive Rate (R0) | Mean Generation Time (T) (Days) | Intrinsic Rate of Increase (rm) | |
| 1 | 29.65 ± 0.32a 1 (1) 2 | 6.0542 ± 0.47b (3) | 0.560 ± 0.13a (1) |
| 2 | 24.52 ± 0.28b (2) | 6.2018 ± 0.59b (2) | 0.504 ± 0.15b (2) |
| 3 | 21.37 ± 0.41b (3) | 7.3061 ± 0.53a (1) | 0.417 ± 0.16c (3) |
| Grand mean | 25.18 ± 2.41 | 6.5207 ± 0.39 | 0.494 ± 0.04 |
| LSD0.05 | 3.592 | 0.5171 | 2.0049 |
| Treatment | R0 | T | rm |
| −Endophyte | 31.39 ± 0.24a (1) | 6.759 ± 0.42a (1) | 0.517 ± 0.12a (1) |
| +Endophyte | 17.21 ± 0.33b (2) | 6.282 ± 0.69b (2) | 0.471 ± 0.20b (2) |
| Grand mean | 24.3 ± 7.09 | 6.521 ± 0.24 | 0.494 ± 0.02 |
| LSD0.05 | 1.8293 | 0.3824 | 0.0282 |
| Trial | Reproduction Rate (R0) | Intrinsic Rate (rm) | ||
|---|---|---|---|---|
| +Endophyte | −Endophyte | +Endophyte | −Endophyte | |
| 1 | 22.33 ± 0.269a 1 (1) 2 | 36.97 ± 0.230a (1) | 0.557 ± 0.023a (1) | 0.563 ± 0.012a (1) |
| 2 | 17.67 ± 0.182b (2) | 31.37 ± 0.240b (2) | 0.483 ± 0.020bc (2) | 0.526 ± 0.018ab (2) |
| 3 | 14.33 ± 0.229b (3) | 28.400 ± 0.201b (3) | 0.372 ± 0.022d (3) | 0.462 ± 0.010c (3) |
| * G. mean | 18.11 ± 2.32 | 32.25 ± 2.51 | 0.471 ± 0.05 | 0.517 ± 0.03 |
| LSD0.05 | 0.6401 | 0.0488 | ||
| Cultivar | Reproduction Rate (R0) | ||
|---|---|---|---|
| +Endophyte | −Endophyte | Average | |
| Kariega | 20.64 ± 0.3a 1 (1) 2 | 34.15 ± 0.268a (1) | 27.39 ± 0.36a (1) |
| Molopo | 16.65 ± 0.234b (2) | 31.11 ± 0.263ab (2) | 23.88 ± 0.37b (2) |
| Gariep | 16.34 ± 0.167b (3) | 28.92 ± 0.191b (3) | 21.63 ± 0.33b (3) |
| * G. mean | 17.88 ± 1.38 | 31.39 ± 1.52 | 24.3 ± 1.68 |
| LSD0.05 | 3.168 | 3.1437 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motholo, L.F.; Booyse, M.; Hatting, J.L.; Tsilo, T.J.; Lekhooa, M.; Thekisoe, O. Endophytic Effect of the South African Beauveria bassiana Strain PPRI 7598 on the Population Growth and Development of the Russian Wheat Aphid, Diuraphis noxia. Agriculture 2023, 13, 1060. https://doi.org/10.3390/agriculture13051060
Motholo LF, Booyse M, Hatting JL, Tsilo TJ, Lekhooa M, Thekisoe O. Endophytic Effect of the South African Beauveria bassiana Strain PPRI 7598 on the Population Growth and Development of the Russian Wheat Aphid, Diuraphis noxia. Agriculture. 2023; 13(5):1060. https://doi.org/10.3390/agriculture13051060
Chicago/Turabian StyleMotholo, Lisemelo Francina, Marde Booyse, Justin Louis Hatting, Toi John Tsilo, Makhotso Lekhooa, and Oriel Thekisoe. 2023. "Endophytic Effect of the South African Beauveria bassiana Strain PPRI 7598 on the Population Growth and Development of the Russian Wheat Aphid, Diuraphis noxia" Agriculture 13, no. 5: 1060. https://doi.org/10.3390/agriculture13051060





