Selection of Faba Bean (Vicia faba L.) Genotypes for High Yield, Essential Amino Acids and Low Anti-Nutritional Factors
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Experiments
2.3. Agronomical Managements
2.4. Agro-Morphological and Phenological Traits
2.5. Protein and Amino Acid Analyses
2.6. Soil Properties
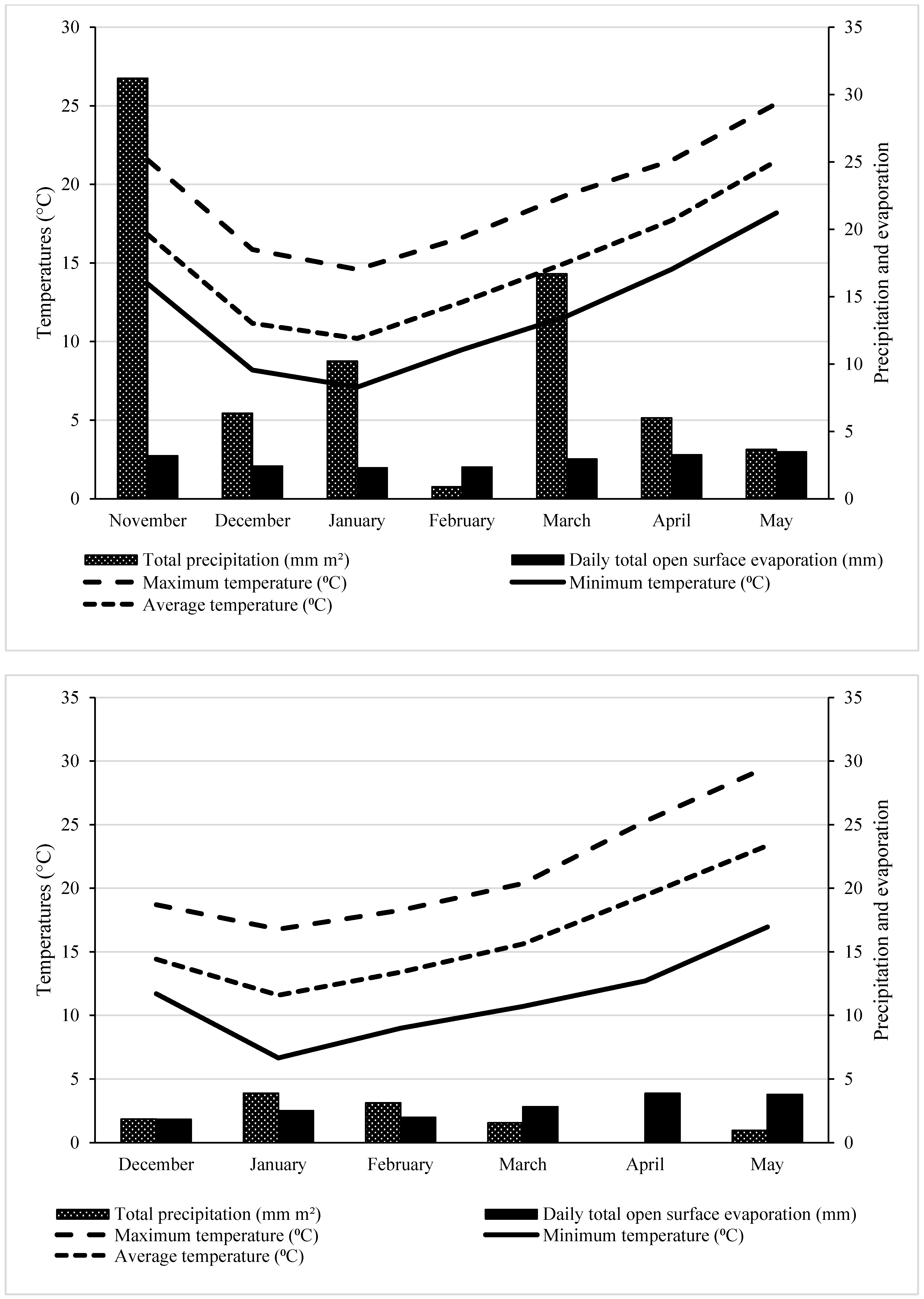
2.7. Climatic Conditions
2.8. Statistical Analyses
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Cillis, F.; Leoni, B.; Massaro, M.; Renna, M.; Santamaria, P. Yield and quality of faba bean (Vicia faba L. var. major) genotypes as a vegetable for fresh consumption: A comparison between Italian landraces and commercial varieties. Agriculture 2019, 9, 253. [Google Scholar] [CrossRef]
- Gardiner, E.E.; Marquardt, R.R.; Kemp, G. Variation in vicine and convicine concentration of faba bean genotypes. Can. J. Plant Sci. 1982, 62, 589–592. [Google Scholar] [CrossRef]
- Gnanasambandam, A.; Paull, J.; Torres, A.; Kaur, S.; Leonforte, T.; Li, H.; Zong, X.; Yang, T.; Materne, M. Impact of molecular technologies on faba bean (Vicia faba L.) breeding strategies. Agronomy 2012, 2, 132–166. [Google Scholar] [CrossRef]
- Inci, N.; Toker, C. Screening and selection of faba beans (Vicia faba L.) for cold tolerance and comparison to wild relatives. Genet. Resour. Crop Evol. 2011, 58, 1169–1175. [Google Scholar] [CrossRef]
- Khalil, A.H.; Mansour, E.H. The effect of cooking, autoclaving and germination on the nutritional quality of faba beans. Food Chem. 1995, 54, 177–182. [Google Scholar] [CrossRef]
- Khan, M.A.; Ammar, M.H.; Migdadi, H.M.; El-Harty, E.H.; Osman, M.A.; Farooq, M.; Alghamdi, S.S. Comparative nutritional profiles of various faba bean and chickpea genotypes. Int. J. Agric. Biol. 2015, 17, 449–457. [Google Scholar] [CrossRef]
- Khazaei, H.; Vandenberg, A. Seed mineral composition and protein content of faba beans (Vicia faba L.) with contrasting tannin contents. Agronomy 2020, 10, 511. [Google Scholar] [CrossRef]
- Pulkkinen, M.; Gautam, M.; Lampi, A.M.; Ollilainen, V.; Stoddard, F.; Sontag-Strohm, T.; Salovaara, H.; Piironen, V. Determination of vicine and convicine from faba bean with an optimized high- performance liquid chromatographic method. Food Res. Int. 2015, 76, 168–177. [Google Scholar] [CrossRef]
- Serafin-Andrzejewska, M.; Jama-Rodzeńska, A.; Helios, W.; Kotecki, A.; Kozak, M.; Białkowska, M.; Bártá, J.; Bártová, V. Accumulation of Minerals in Faba Bean Seeds and Straw in Relation to Sowing Density. Agriculture 2023, 13, 147. [Google Scholar] [CrossRef]
- van der Maesen, L.J.G.; Somaatmadyja, S. Plant Resources of South-East Asia; Prosea Foundation, Bogor, and Pudoc-DLO: Wageningen, The Netherlands, 1992; Volume 1, pp. 15–32. [Google Scholar]
- Vilarino, M.; Métayer, J.P.; Crépon, K.; Duc, G. Effects of varying vicine, convicine and tannin contents of faba bean seeds (Vicia faba L.) on nutritional values for broiler chicken. Anim. Feed. Sci. Technol. 2009, 150, 114–121. [Google Scholar] [CrossRef]
- Yahia, Y.; Elfalleh, W.; Tlili, N.; Hannachi, H.; Loumerem, M.; Ferchichi, A. Phytochemical contents and antioxidant activities of some Tunisian faba bean populations. Rom. Agric. Res. 2013, 30, 65–74. [Google Scholar]
- Lombardo, S.; Pandino, G.; Pesce, G.R.; Anastasi, U.; Tuttobene, R.; Mauromicale, G. Variation in seed mineral elements profile and yield in field bean (Vicia faba L. var. minor) genotypes. Ital. J. Agron. 2016, 11, 261–267. [Google Scholar] [CrossRef]
- Duc, G. Faba bean (Vicia faba L.). Field Crops Res. 1997, 53, 99–109. [Google Scholar] [CrossRef]
- FAOSTAT. 2020. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 6 October 2021).
- TUIK. Turkish Statistical Institute. 2020. Available online: https://biruni.tuik.gov.tr/medas/?kn=92&locale=tr (accessed on 6 October 2021).
- Sellami, M.H.; Lavini, A.; Calandrelli, D.; De Mastro, G.; Pulvento, C. Evaluation of Genotype, Environment, and Management Interactions on Fava Beans under Mediterranean Field Conditions. Agronomy 2021, 11, 1088. [Google Scholar] [CrossRef]
- Gotor, A.A.; Marraccini, E. Innovative pulses for Western European temperate regions: A review. Agronomy 2022, 12, 170. [Google Scholar] [CrossRef]
- Kosinska, A.; Karamec, M.; Penkacik, K.; Urbalewicz, A.; Amarowicz, R. Interactions between tannins and proteins isolated from broad bean seeds (Vicia faba Major) yield soluble and non-soluble complexes. Eur. Food Res. Technol. 2011, 233, 213–222. [Google Scholar] [CrossRef]
- Martín, A.; Cabrera, A.; Medina, J.L. Antinutritional factors in faba bean. Tannin content in Vicia faba: Possibilities for plant breeding. In Present Status and Future Prospects of Faba Bean Production and Improvement in the Mediterranean Countries; Cubero, J.I., Saxena, M.C., Eds.; CIHEAM: Zaragoza, Spain, 1991; pp. 105–110. [Google Scholar]
- Crepon, K.; Marget, P.; Peyronnet, C.; Carrouee, B.; Arese, P.; Duc, G. Nutritional value of faba bean (Vicia faba L.) seeds for feed and food. Field Crops Res. 2010, 115, 329–339. [Google Scholar] [CrossRef]
- Oomah, B.D.; Luc, G.; Leprelle, C.; Drover, J.C.G.; Harrison, J.E.; Olson, M. Phenolics, Phytic Acid, and Phytase in Canadian-Grown Low-Tannin Faba Bean (Vicia faba L.) Genotypes. J. Agric. Food Chem. 2011, 59, 3763–3771. [Google Scholar] [CrossRef]
- Arese, P.; Bosia, A.; Naitana, A.; Gaetani, S.; D’Aquino, M.; Gaetani, G.F. Effect of divicine and isouramil on red cell metabolism in normal and G6PD-deficient (Mediterranean variant) subjects. Possible role in the genesis of favism. Prog. Clin. Biol. Res. 1981, 55, 725–746. [Google Scholar]
- Gutierrez, N.; Avila, C.M.; Duc, G.; Marget, P.; Suso, M.J.; Moreno, M.T.; Torres, A.M. CAPs markers to assist selection for low vicine and convicine contents in faba bean (Vicia faba L.). Theor. Appl. Genet. 2006, 114, 59–66. [Google Scholar] [CrossRef]
- Khamassi, K.; Jeddi, F.B.; Hobbs, D.; Irigoyen, J.; Stoddard, F.; O’Sullivan, D.M.; Jones, H. A baseline study of vicine–convicine levels in faba bean (Vicia faba L.) germplasm. Plant Genet. Resour. 2013, 11, 250–257. [Google Scholar] [CrossRef]
- Canci, H.; Toker, C. Selection for resistance to ascochyta blight (Ascochyta fabae Speg) and assessment of yield and yield criteria in faba bean (Vicia faba L.) Populations. In Proceedings of the Grain Legumes and the Environment: How to Assess Benefits and Impacts? Zurich, Switzerland, 18–19 November 2004; pp. 213–215. [Google Scholar]
- Prandi, B.; Faccini, A.; Lambertini, F.; Bencivenni, M.; Jorba, M.; van Droogenbroek, B.; Bruggeman, G.; Schöber, J.; Petrusan, J.; Elst, K.; et al. Food wastes from agrifood industry as possible sources of proteins: A detailed molecular view on the composition of the nitrogen fraction, amino acid profile and racemisation degree of 39 food waste streams. Food Chem. 2019, 286, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Kivrak, I.; Kivrak, S.; Harmandar, M. Free amino acid profiling in the giant puffball mushroom (Calvatia gigantea) using UPLC-MS/MS. Food Chem. 2014, 158, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Tene, T.M.; Sari, H.; Canci, H.; Maaruf, A.; Eker, T.; Toker, C. Traits Related to Heat Stress in Phaseolus Species. Agriculture, 2023; 13, accepted. [Google Scholar]
- Eyibilir, I.; Canci, H. Evaluation of some faba bean (Vicia faba L.) genotypes with low tannin content for agronomical characteristics. EC Agric. 2019, 5, 296–303. [Google Scholar]
- Toker, C. Estimates of broad-sense heritability for seed yield and yield criteria in faba bean (Vicia faba L.). Hereditas 2004, 140, 222–225. [Google Scholar] [CrossRef]
- Toker, C.; Canci, H.; Cagirgan, M.I. Selection for resistance to Ascochyta Blight (Ascochyta fabae Speg.f.sp. fabae Gossen et al.) in faba bean (Vicia faba L.) populations and assessment of cold tolerance and yield criteria. Turk. J. Field Crops 2004, 9, 78–86. [Google Scholar]
- Malek, N.; Aci, M.M.; Khamassi, K.; Lupini, A.; Rouissi, M.; Hanifi-Mekliche, L. Agro-Morphological and Molecular Variability among Algerian Faba Bean (Vicia faba L.) Accessions. Agronomy 2021, 11, 1456. [Google Scholar] [CrossRef]
- Shabbir, A.; Widderick, M.; Walsh, M.J. An evaluation of growth characteristics of Faba bean cultivars. Agronomy 2021, 11, 1166. [Google Scholar] [CrossRef]
- Olle, M.; Williams, I.H.; Rosa, E. Selecting appropriate faba bean var. minor varieties for production under Northern European environmental conditions. Soil Plant Sci. 2019, 69, 432–438. [Google Scholar] [CrossRef]
- Dewangan, N.K.; Dahiya, G.S.; Janghel, D.K.; Dohare, S. Diversity analysis for seed yield and its component traits among faba bean (Vicia faba L.) germplasm lines. Legume Res.-Int. J. 2022, 45, 689–694. [Google Scholar] [CrossRef]
- Singh, A.; Bhakta, N. Diversity Analysis of Faba Bean (Vicia faba L.) Germplasm of Bihar Using Agro-Morphological Characteristics. Bangladesh J. Bot. 2017, 46, 1249–1257. [Google Scholar] [CrossRef]
- Celmeli, T.; Sari, H.; Canci, H.; Sari, D.; Adak, A.; Eker, T.; Toker, C. The nutritional content of common bean (Phaseolus vulgaris L.) landraces in comparison to modern varieties. Agronomy 2018, 8, 166. [Google Scholar] [CrossRef]
- Karakoy, T.; Baloch, F.S.; Toklu, F.; Ozkan, H. Variation for selected morphological and quality-related traits among 178 faba bean landraces collected from Turkey. Plant Genet. Resour. 2014, 12, 5–13. [Google Scholar] [CrossRef]
- Kokten, K.; Kocak, A.; Bagci, E.; Akcura, M.; Celik, S. Tannin, protein contents and fatty acid compositions of the seeds of several Vicia L. species from Turkey. Grasas y Aceites 2010, 61, 404–408. [Google Scholar] [CrossRef]
- Kumari, P.V.; Sangeetha, N. Nutritional significance of cereals and legumes based food mix-A review. Int. J. Agric. Life Sci.-IJALS 2017, 3, 115–122. [Google Scholar]
| Name | Pedigree | Origin |
|---|---|---|
| FLIP03-005FB | HBP/SOF/03Fam.91/WH | ICARDA |
| FLIP08-027FB | HBP/SOF/2003, Fam.4/WH | ICARDA |
| FLIP08-030FB | HBP/SOF/2003, Fam.7/BH | ICARDA |
| WBR1-3 | WhiteflowerXILB1270-BC/WH | ICARDA |
| FLIP12-20FB | HBP/SOF/2003, Fam.54WH | ICARDA |
| FLIP08-015FB | HBP/SOF/2003, Fam.64/WH | ICARDA |
| FLIP08-016FB | HBP/SOF/2003, Fam.65/BH | ICARDA |
| FLIP08-019FB | HBP/SOF/2003, Fam.74/WH | ICARDA |
| Antalya local | Local Check | Turkiye |
| Atlidere local | Local Check | Turkiye |
| Elisar | FLIP85-98 FB | Lebanon |
| Ica white | HBP/S0C/2003-Fan54B | ICARDA |
| Soil Parameters | Results | Interpretation |
|---|---|---|
| pH | 7.90 | Moderately alkaline |
| EC (%) | 0.01 | No salinity effects |
| CaCO3 (%) | 42.30 | High calcareous |
| Texture | Clay loam | |
| Organic matter (%) | 1.10 | Low |
| Total N (%) | 0.10 | Medium |
| Available P (kg da−1) | 3.80 | Low |
| Exchangeable K (kg da−1) | 70.30 | Optimum |
| Sources of Variation | df | DF-First | DF-50% | MAT | PH | FPH | BN | PN | BY | SY | 100-SW | PL | PW | S/P | HI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Years (Y) | 1 | 0.24 | 0.24 | 2.10 | 24.62 ** | 61.40 ** | 10.75 * | 19.03 ** | 16.33 ** | 27.16 ** | 186.63 ** | 38.45 ** | 4.18 | 2.03 | 52.70 ** |
| Blocks | 2 | 0.53 | 16.45 ** | 2.09 | 4.70 * | 2.90 | 1.27 | 0.14 | 1.28 | 1.08 | 0.68 | 0.42 | 3.68 | 0.38 | 1.63 |
| Genotypes (G) | 11 | 12.44 ** | 1.36 | 1.10 | 1.97 | 3.90 ** | 3.57 ** | 1.14 | 0.70 | 0.80 | 10.34 ** | 8.11 ** | 2.16 | 2.13 | 1.35 |
| Y × G | 11 | 0.60 | 0.02 | 0.06 | 0.36 | 0.89 | 1.10 | 0.47 | 0.69 | 0.74 | 3.14 * | 2.48 * | 1.54 | 0.45 | 0.24 |
| Error | 44 |
| Genotypes | 2016–2017 | 2017–2018 | ||
|---|---|---|---|---|
| Pod Length (mm) | 100-Seed Weight (g) | Pod Length (mm) | 100-Seed Weight (g) | |
| FLIP08-027FB | 61.83 c | 98.69 abc | 92.50 a | 78.63 abcd |
| FLIP08-015FB | 69.04 bc | 75.66 c | 97.08 a | 70.17 cd |
| FLIP08-016FB | 71.21 bc | 84.78 bc | 87.92 a | 65.53 d |
| FLIP03-005FB | 74.97 bc | 96.79 abc | 86.67 a | 67.42 cd |
| FLIP08-019FB | 78.27 bc | 96.40 abc | 93.33 a | 73.92 cd |
| FLIP08-030FB | 86.02 bc | 107.03 ab | 101.08 a | 71.53 cd |
| FLIP12-20FB | 86.08 bc | 97.64 abc | 105.00 a | 73.33 cd |
| Antalya local | 89.61 b | 117.74 a | 102.92 a | 91.25 a |
| Atlidere local | 91.11 b | 118.80 a | 94.58 a | 75.58 bcd |
| WBR1-3 | 120.58 a | 101.36 ab | 110.83 a | 80.25 abc |
| Ica white | * | * | 99.58 a | 77.18 bcd |
| Elisar | * | * | 102.50 a | 88.68 ab |
| Genotypes | Days to First Flowering | Days to 50% Flowering | Days to Maturity | Plant Height (cm) | First Pod Height (cm) | Branches per Plant |
|---|---|---|---|---|---|---|
| FLIP03-005FB | 73.80 ± 10.60 | 86.50 ± 12.43 | 146.33 ± 1.75 | 57.87 ± 15.13 | 13.37 ± 4.34 | 2 ± 0.60 |
| FLIP08-027FB | 74.75 ± 11.50 | 86.50 ± 14.65 | 144.00 ± 1.26 | 53.70 ± 6.98 | 14.79 ± 5.42 | 2 ± 0.60 |
| FLIP08-030FB | 68.05 ± 10.13 | 86.17 ± 12.62 | 143.83 ± 2.31 | 55.16 ± 11.94 | 13.54 ± 4.04 | 2 ± 1.07 |
| WBR1-3 | 81.00 ± 1.75 | 84.50 ± 2.25 | 148.83 ± 2.85 | 62.75 ± 16.41 | 16.54 ± 6.83 | 3 ± 1.12 |
| FLIP12-20FB | 73.58 ± 1.37 | 77.00 ± 2.09 | 145.17 ± 4.62 | 54.45 ± 7.06 | 12.70 ± 5.46 | 2 ± 0.34 |
| FLIP08-015FB | 70.03 ± 10.67 | 87.00 ± 10.58 | 146.50 ± 1.76 | 59.95 ± 11.45 | 14.62 ± 4.85 | 3 ± 0.92 |
| FLIP08-016FB | 73.06 ± 9.35 | 86.00 ± 13.98 | 144.67 ± 4.08 | 58.33 ± 13.19 | 15.08 ± 5.94 | 2 ± 1.20 |
| FLIP08-019FB | 71.87 ± 1.28 | 75.17 ± 2.31 | 143.67 ± 3.20 | 48.91 ± 6.49 | 12.29 ± 3.31 | 2 ± 0.73 |
| Antalya local | 78.75 ± 1.89 | 85.25 ± 3.20 | 147.83 ± 4.75 | 72.83 ± 19.84 | 25.00 ± 13.60 | 4 ± 1.06 |
| Atlidere local | 80.65 ± 0.73 | 83.83 ± 1.47 | 144.83 ± 6.17 | 57.45 ± 15.94 | 15.33 ± 6.25 | 4 ± 1.50 |
| Elisar | 77.40 ± 10.08 | 89.33 ± 15.94 | 147.33 ± 3.21 | 31.00 ± 34.87 | 9.75 ± 10.69 | 1 ± 1.37 |
| Ica white | 68.47 ± 11.03 | 84.06 ± 9.95 | 146.67 ± 2.51 | 29.25 ± 32.21 | 7.37 ± 8.19 | 1 ± 0.95 |
| Genotypes | Pods per Plant | Pod Width (mm) | Seeds per Pod | Biological Yield (kg per ha) | Seed Yield (kg per ha) | Harvest Index (%) |
|---|---|---|---|---|---|---|
| FLIP03-005FB | 10 ± 4.27 | 14.46 ± 2.39 | 3 ± 0.46 | 1838.5 c | 1048.1 cd | 42.44 ± 10.16 |
| FLIP08-027FB | 8 ± 4.18 | 13.83 ± 2.94 | 3 ± 0.40 | 1833.3 c | 794.1 ef | 41.13 ± 22.61 |
| FLIP08-030FB | 10 ± 4.02 | 15.32 ± 2.52 | 3 ± 1.44 | 1585.4 bbc | 834.8 cde | 50.99 ± 9.91 |
| WBR1-3 | 8 ± 3.38 | 17.36 ± 1.00 | 4 ± 0.34 | 2101.0 bc | 993.3 def | 42.44 ± 10.16 |
| FLIP12-20FB | 8 ± 2.86 | 15.82 ± 1.11 | 3 ± 0.25 | 1752.1 c | 878.7 f | 45.79 ± 9.57 |
| FLIP08-015FB | 12 ± 4.47 | 14.25 ± 1.80 | 3 ± 0.44 | 2152.1 bc | 1093.3 c | 48.85 ± 10.04 |
| FLIP08-016FB | 9 ± 5.94 | 14.28 ± 1.08 | 4 ± 0.40 | 2219.8 b | 1221.0 b | 50.36 ± 10.40 |
| FLIP08-019FB | 8 ± 4.50 | 16.06 ± 1.80 | 3 ± 0.25 | 1713.5 c | 1016.6 cd | 61.94 ± 0.48 |
| Antalya local | 6 ± 2.06 | 15.28 ± 2.76 | 4 ± 0.92 | 1758.3 c | 898.1 ef | 40.66 ± 17.58 |
| Atlidere local | 11 ± 5.14 | 14.65 ± 2.03 | 3 ± 0.60 | 2830.2 a | 1498.3 a | 46.11 ± 9.68 |
| Elisar | 3 ± 2.90 | 8.91 ± 9.77 | 2 ± 1.97 | 535.4 d | 481.8 ddef | 19.93 ± 21.95 |
| Ica white | 3 ± 3.16 | 15.83 ± 1.01 | 2 ± 1.82 | 613.5 d | 326.0 g | 23.43 ± 27.03 |
| Genotypes | Protein Content | Arginine | Aspartic Acid | Glutamic Acid | Phenylalanine |
|---|---|---|---|---|---|
| FLIP03-005FB | 22.58 ± 1.05 | 70.65 ± 8.61 | 18.51 ± 12.38 | 22.07 ± 14.02 | 18.46 ± 9.60 |
| FLIP08-027FB | 26.51 ± 9.36 | 159.62 ± 10.00 | 33.19 ± 6.58 | 24.86 ± 16.31 | 14.50 ± 11.46 |
| FLIP08-030FB | 25.46 ± 8.20 | 157.59 ± 0.65 | 18.76 ± 10.05 | 21.23 ± 9.56 | 13.36 ± 9.71 |
| WBR1-3 | 24.85 ± 0.95 | 147.70 ± 23.05 | 18.54 ± 12.20 | 23.52 ± 8.91 | 12.88 ± 8.32 |
| FLIP12-20FB | 25.03 ± 4.65 | 114.93 ± 11.87 | 18.78 ± 11.36 | 20.54 ± 15.42 | 13.11 ± 6.91 |
| FLIP08-015FB | 25.29 ± 3.79 | 102.49 ± 9.46 | 22.14 ± 0.69 | 18.50 ± 3.94 | 11.65 ± 10.03 |
| FLIP08-016FB | 28.00 ± 17.02 | 106.93 ± 0.45 | 19.41 ± 13.00 | 19.23 ± 11.23 | 11.29 ± 5.13 |
| FLIP08-019FB | 29.75 ± 9.58 | 91.36 ± 8.39 | 17.00 ± 9.47 | 22.50 ± 5.82 | 11.45 ± 8.25 |
| Antalya local | 27.13 ± 0.14 | 125.67 ± 17.02 | 20.38 ± 13.11 | 18.70 ± 9.31 | 11.20 ± 5.16 |
| Atlidere local | 28.26 ± 8.37 | 152.71 ± 24.31 | 22.85 ± 20.74 | 25.22 ± 17.46 | 14.42 ± 10.37 |
| Elisar | 31.50 ± 6.47 | 112.66 ± 30.59 | 19.05 ± 5.67 | 18.16 ± 11.20 | 10.44 ± 9.68 |
| Ica white | 28.26 ± 2.61 | 92.00 ± 14.67 | 16.79 ± 8.39 | 19.92 ± 6.71 | 11.10 ± 4.72 |
| Genotypes | Arg | Asp | Cys | Glu | His | Ile + Leu | Lys | Met | Phe | Pro | Ser | Thr | Tyr | Val |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FLIP03-005FB | 621 ± 9.25 | 970 ± 20.09 | 105 ± 6.49 | 993 ± 20.33 | 283 ± 13.47 | 694 ± 10.11 | 541 ± 21.48 | 11 ± 5.20 | 604 ± 27.39 | 451 ± 23.00 | 797 ± 14.02 | 797 ± 28.03 | 258 ± 16.36 | 389 ± 16.79 |
| FLIP08-027FB | 661 ± 5.99 | 1101 ± 25.91 | 118 ± 12.05 | 1137 ± 11.57 | 327 ± 22.69 | 775 ± 9.73 | 598 ± 15.61 | 13 ± 0.86 | 648 ± 13.46 | 453 ± 18.35 | 1038 ± 25.61 | 640 ± 15.27 | 209 ± 13.45 | 453 ± 24.30 |
| FLIP08-030FB | 1019 ± 7.41 | 1317 ± 18.57 | 132 ± 9.47 | 2051 ± 21.74 | 442 ± 15.82 | 927 ± 8.62 | 716 ± 10.23 | 18 ± 1.22 | 771 ± 16.82 | 704 ± 10.92 | 1058 ± 18.70 | 598 ± 16.54 | 297 ± 12.64 | 587 ± 15.83 |
| WBR1-3 | 634 ± 24.80 | 1335 ± 17.68 | 113 ± 6.57 | 1156 ± 15.67 | 299 ± 10.29 | 884 ± 7.15 | 603 ± 9.57 | 12 ± 8.05 | 750 ± 11.20 | 407 ± 16.45 | 1090 ± 16.13 | 612 ± 20.01 | 206 ± 17.58 | 471 ± 16.07 |
| FLIP12-20FB | 635 ± 18.02 | 924 ± 15.92 | 71 ± 8.61 | 1032 ± 14.85 | 310 ± 18.47 | 581 ± 10.00 | 539 ± 16.30 | 10 ± 2.36 | 531 ± 17.64 | 453 ± 12.38 | 573 ± 18.19 | 542 ± 11.30 | 211 ± 19.02 | 331 ± 8.25 |
| FLIP08-015FB | 705 ± 25.00 | 1396 ± 26.30 | 121 ± 10.58 | 1248 ± 23.50 | 360 ± 23.16 | 938 ± 12.06 | 714 ± 18.25 | 12 ± 5.18 | 789 ± 29.37 | 455 ± 17.79 | 989 ± 10.08 | 589 ± 17.85 | 198 ± 10.34 | 496 ± 13.17 |
| FLIP08-016FB | 744 ± 11.36 | 1506 ± 19.28 | 164 ± 9.26 | 1199 ± 19.27 | 341 ± 17.02 | 1099 ± 6.71 | 721 ± 11.24 | 14 ± 6.47 | 934 ± 15.55 | 416 ± 24.68 | 1189 ± 17.89 | 614 ± 16.99 | 236 ± 11.05 | 705 ± 16.24 |
| FLIP08-019FB | 693 ± 17.82 | 1085 ± 30.51 | 93 ± 11.48 | 1114 ± 16.57 | 342 ± 9.37 | 788 ± 13.65 | 628 ± 19.67 | 10 ± 5.61 | 670 ± 20.04 | 431 ± 11.14 | 846 ± 14.00 | 595 ± 10.20 | 209 ± 16.42 | 469 ± 18.05 |
| Antalya local | 867 ± 29.73 | 1382 ± 14.36 | 62 ± 5.97 | 1781 ± 25.48 | 393 ± 11.20 | 691 ± 9.30 | 623 ± 23.54 | 12 ± 3.80 | 568 ± 15.82 | 614 ± 20.01 | 978 ± 15.86 | 498 ± 13.67 | 228 ± 17.59 | 399 ± 17.29 |
| Atlidere local | 692 ± 32.18 | 1148 ± 9.07 | 110 ± 8.09 | 1040 ± 21.69 | 314 ± 14.01 | 656 ± 18.45 | 578 ± 21.00 | 11 ± 5.11 | 507 ± 16.49 | 388 ± 17.80 | 782 ± 11.20 | 587 ± 10.59 | 185 ± 18.36 | 415 ± 21.03 |
| Elisar | 700 ± 57.03 | 1130 ± 21.37 | 102 ± 14.57 | 1014 ± 9.72 | 308 ± 10.30 | 830 ± 23.79 | 559 ± 17.83 | 11 ± 2.52 | 687 ± 23.72 | 388 ± 15.07 | 698 ± 9.67 | 581 ± 19.25 | 203 ± 13.04 | 486 ± 19.62 |
| Ica white | 609 ± 19.68 | 861 ± 19.45 | 60 ± 19.35 | 1028 ± 10.46 | 330 ± 8.11 | 567 ± 15.08 | 525 ± 14.68 | 10 ± 3.01 | 517 ± 20.08 | 455 ± 19.07 | 775 ± 12.53 | 594 ± 12.46 | 197 ± 17.75 | 324 ± 9.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akgun, D.; Canci, H. Selection of Faba Bean (Vicia faba L.) Genotypes for High Yield, Essential Amino Acids and Low Anti-Nutritional Factors. Agriculture 2023, 13, 932. https://doi.org/10.3390/agriculture13050932
Akgun D, Canci H. Selection of Faba Bean (Vicia faba L.) Genotypes for High Yield, Essential Amino Acids and Low Anti-Nutritional Factors. Agriculture. 2023; 13(5):932. https://doi.org/10.3390/agriculture13050932
Chicago/Turabian StyleAkgun, Didem, and Huseyin Canci. 2023. "Selection of Faba Bean (Vicia faba L.) Genotypes for High Yield, Essential Amino Acids and Low Anti-Nutritional Factors" Agriculture 13, no. 5: 932. https://doi.org/10.3390/agriculture13050932
APA StyleAkgun, D., & Canci, H. (2023). Selection of Faba Bean (Vicia faba L.) Genotypes for High Yield, Essential Amino Acids and Low Anti-Nutritional Factors. Agriculture, 13(5), 932. https://doi.org/10.3390/agriculture13050932






