Identification of Candidate Genes for Drought Resistance during Soybean Seed Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Growth Conditions and Sample Collection
2.2. RNA Isolation and Transcriptome Sequencing
2.3. Identification of Differentially Expressed Genes (DEGs)
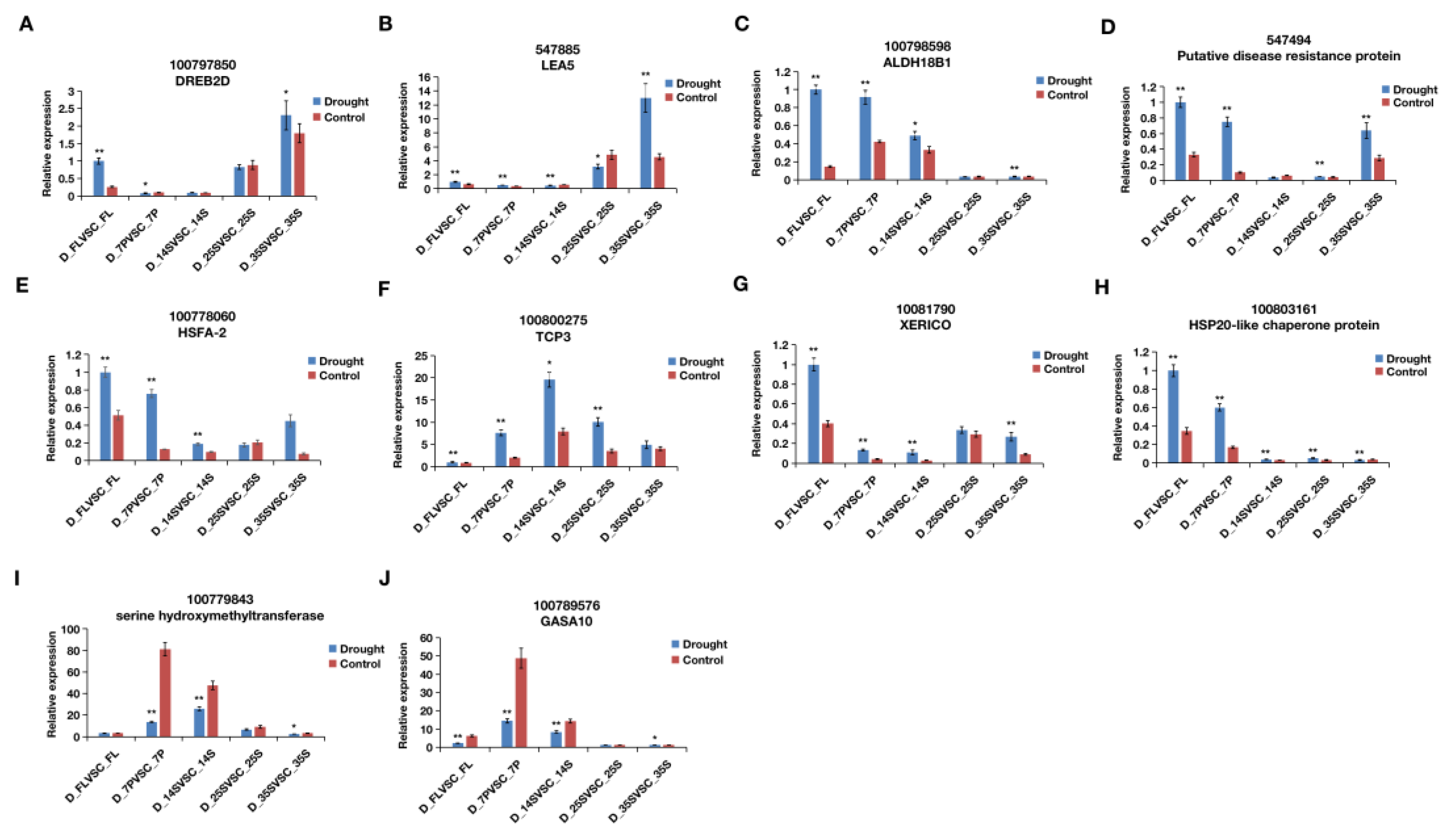
2.4. qRT-PCR Verification
3. Results
3.1. Generation of Transcriptome Data
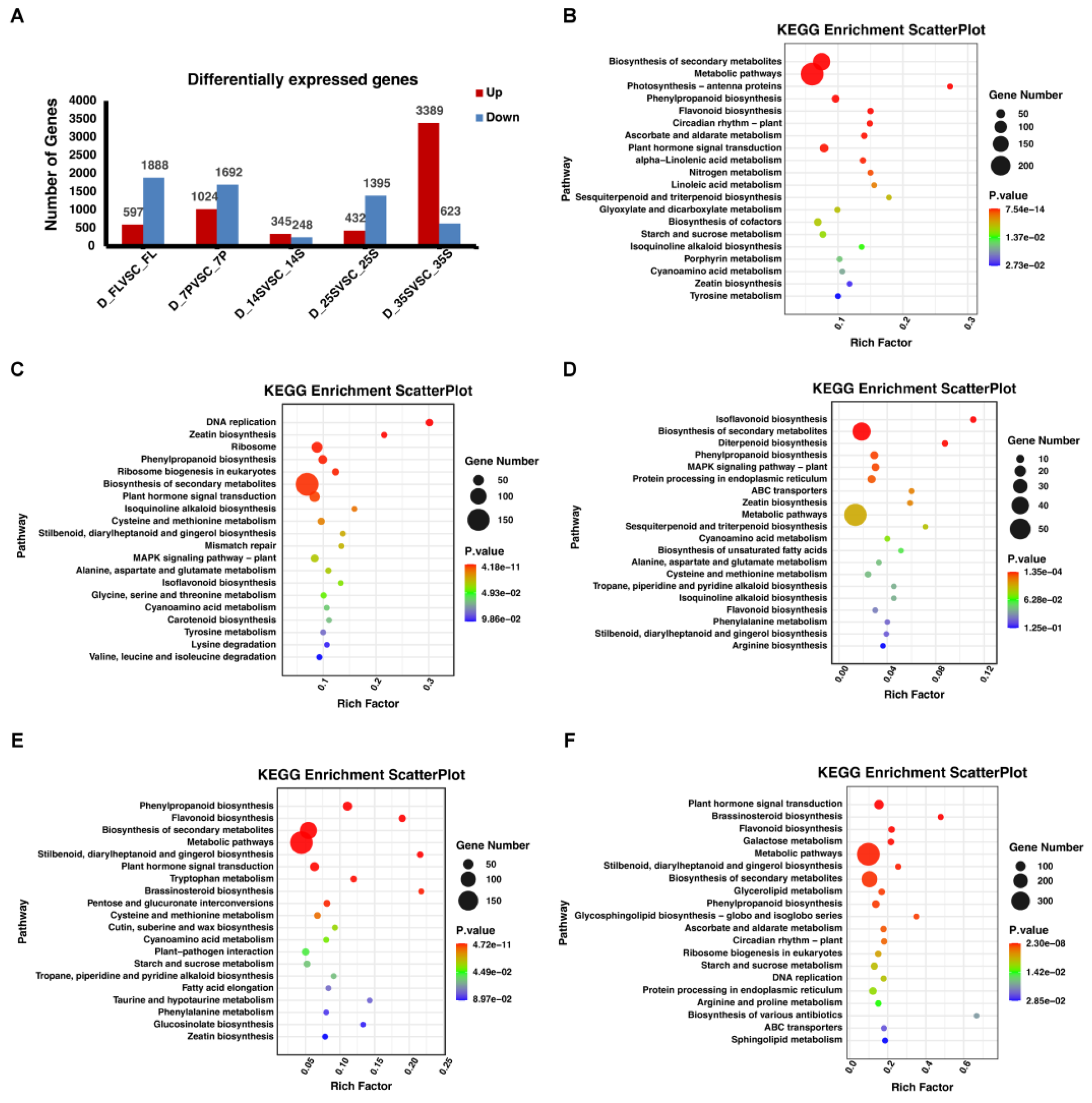
3.2. Differential Expression Genes under Drought Stress
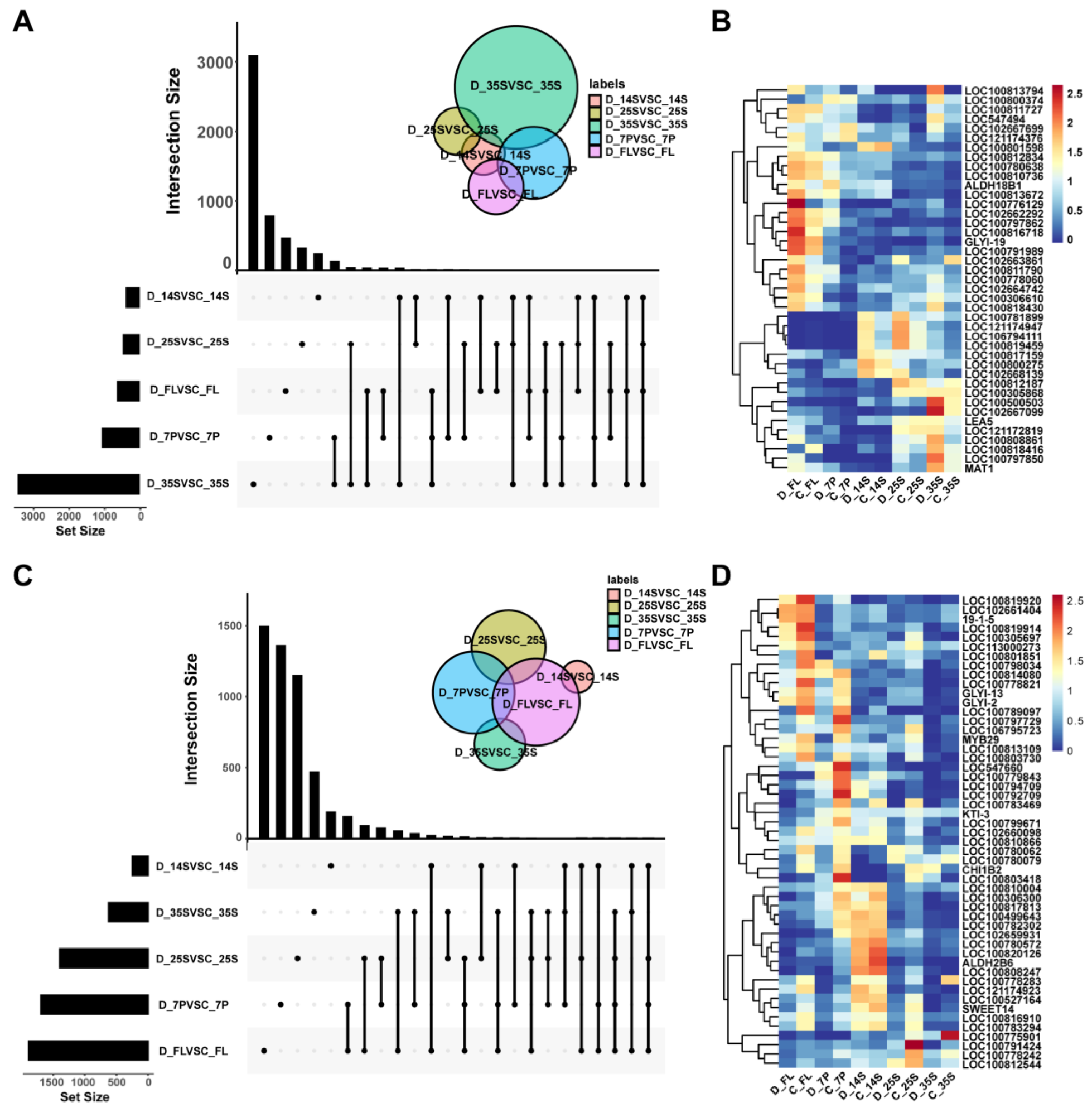
3.3. Identification of Common DEGs in Response to Drought
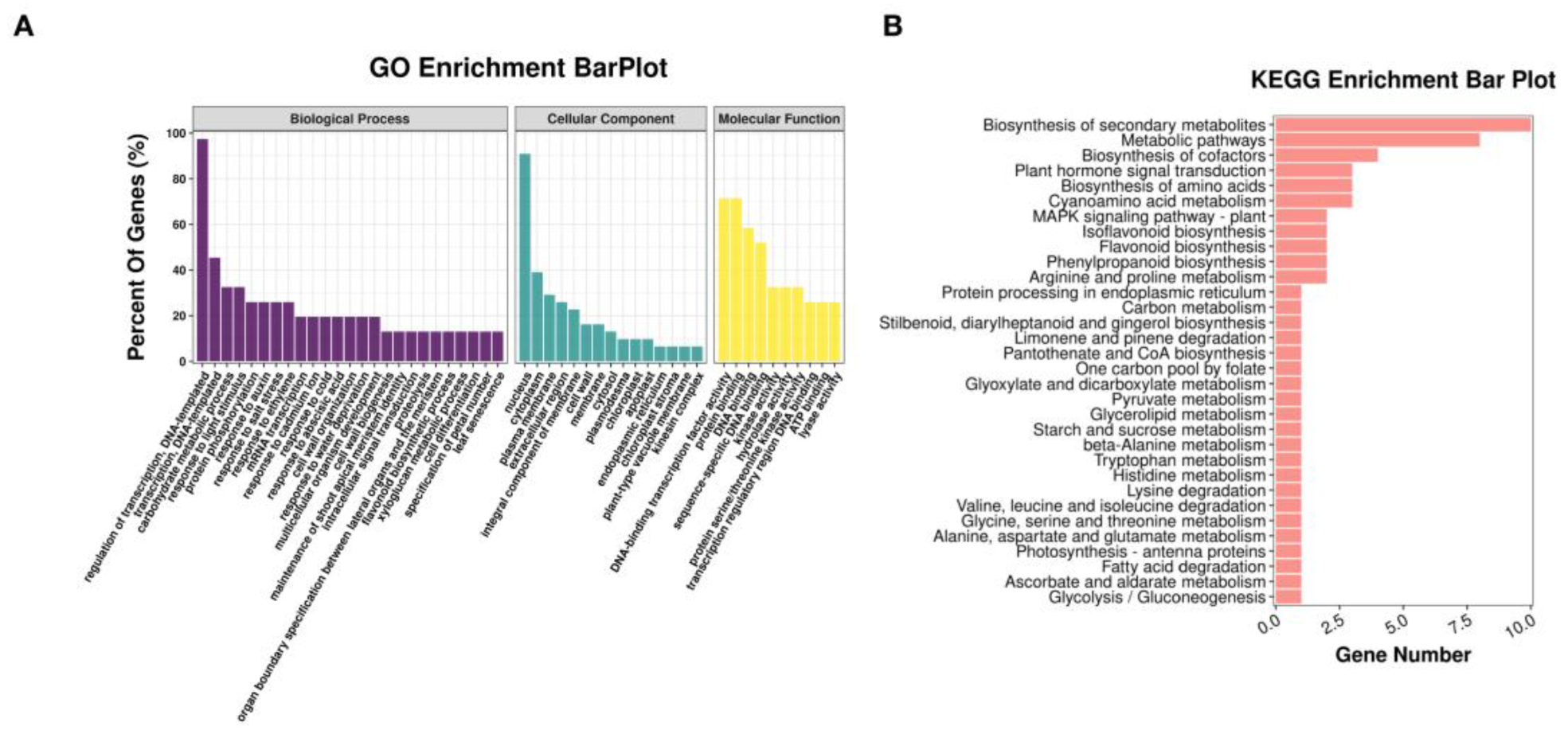
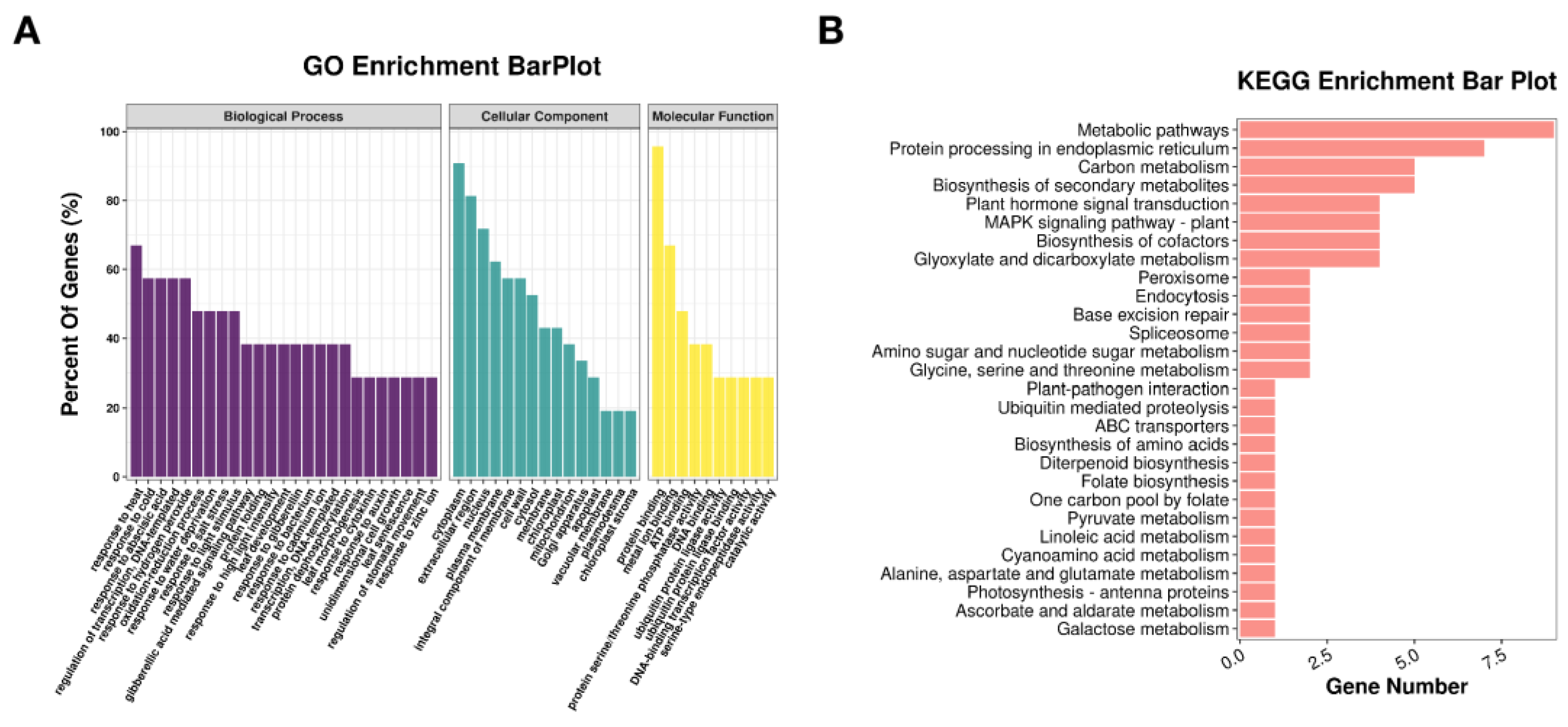
3.4. Identification of Candidate Drought Resistance Genes at Different Stages of Soybean Seed Development
3.5. qRT-PCR
4. Discussion
4.1. Drought-Responsive Genes at Five Stages of Soybean Seed Development
4.2. Identified the Candidate Genes in Response to Drought during Soybean Seed Development
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, J.-K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Wang, T.; Gao, L.; Song, P.; Yang, Y.; Zhi, H.; Li, K. Development of Comprehensive Serological Techniques for Sensitive, Quantitative and Rapid Detection of Soybean Mosaic Virus. Int. J. Mol. Sci. 2022, 23, 9457. [Google Scholar] [CrossRef] [PubMed]
- Kokubun, M.; Shimada, S.; Takahashi, M. Flower Abortion Caused by Preanthesis Water Deficit Is Not Attributed to Impairment of Pollen in Soybean. Crop. Sci. 2001, 41, 1517–1521. [Google Scholar] [CrossRef]
- Ranjan, A.; Jayaraman, D.; Grau, C.; Hill, J.H.; Whitham, S.A.; Ané, J.-M.; Smith, D.L.; Kabbage, M. The Pathogenic Development of Sclerotinia Sclerotiorum in Soybean Requires Specific Host NADPH Oxidases. Mol. Plant. Pathol. 2018, 19, 700–714. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, Z.; Hu, F.; Liu, L.; Huang, X.; Zhao, J.; Wang, H. Aberrant Seed Development in Litchi Chinensis Is Associated with the Impaired Expression of Cell Wall Invertase Genes. Hortic. Res. 2018, 5, 39. [Google Scholar] [CrossRef]
- Xiong, H.; Wang, R.; Jia, X.; Sun, H.; Duan, R. Transcriptomic Analysis of Rapeseed (Brassica napus L.) Seed Development in Xiangride, Qinghai Plateau, Reveals How Its Special Eco-Environment Results in High Yield in High-Altitude Areas. Front. Plant. Sci. 2022, 13, 927418. [Google Scholar] [CrossRef]
- Farooq, M.; Gogoi, N.; Barthakur, S.; Baroowa, B.; Bharadwaj, N.; Alghamdi, S.S.; Siddique, K.H.M. Drought Stress in Grain Legumes during Reproduction and Grain Filling. J. Agro Crop. Sci. 2017, 203, 81–102. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, Q.; Chen, L.; Yao, X.; Zhang, H.; Wu, J.; Xie, F. Effect of Drought Stress during Soybean R2-R6 Growth Stages on Sucrose Metabolism in Leaf and Seed. Int. J. Mol. Sci. 2020, 21, 618. [Google Scholar] [CrossRef]
- Kumari, S.; nee Sabharwal, V.P.; Kushwaha, H.R.; Sopory, S.K.; Singla-Pareek, S.L.; Pareek, A. Transcriptome Map for Seedling Stage Specific Salinity Stress Response Indicates a Specific Set of Genes as Candidate for Saline Tolerance in Oryza sativa L. Funct. Integr. Genomics 2009, 9, 109–123. [Google Scholar] [CrossRef]
- Shan, X.; Li, Y.; Jiang, Y.; Jiang, Z.; Hao, W.; Yuan, Y. Transcriptome Profile Analysis of Maize Seedlings in Response to High-Salinity, Drought and Cold Stresses by Deep Sequencing. Plant. Mol. Biol. Rep. 2013, 31, 1485–1491. [Google Scholar] [CrossRef]
- Waititu, J.K.; Cai, Q.; Sun, Y.; Sun, Y.; Li, C.; Zhang, C.; Liu, J.; Wang, H. Transcriptome Profiling of Maize (Zea mays L.) Leaves Reveals Key Cold-Responsive Genes, Transcription Factors, and Metabolic Pathways Regulating Cold Stress Tolerance at the Seedling Stage. Genes 2021, 12, 1638. [Google Scholar] [CrossRef]
- Conti, V.; Cantini, C.; Romi, M.; Cesare, M.M.; Parrotta, L.; Del Duca, S.; Cai, G. Distinct Tomato Cultivars Are Characterized by a Differential Pattern of Biochemical Responses to Drought Stress. Int. J. Mol. Sci. 2022, 23, 5412. [Google Scholar] [CrossRef]
- Ali, F.; Bano, A.; Fazal, A. Recent Methods of Drought Stress Tolerance in Plants. Plant. Growth Regul. 2017, 82, 363–375. [Google Scholar] [CrossRef]
- Kosová, K.; Vítámvás, P.; Prášil, I.T. Wheat and Barley Dehydrins under Cold, Drought, and Salinity—What Can LEA-II Proteins Tell Us about Plant Stress Response? Front. Plant. Sci. 2014, 5, 343. [Google Scholar] [CrossRef]
- Vihervaara, A.; Duarte, F.M.; Lis, J.T. Molecular Mechanisms Driving Transcriptional Stress Responses. Nat. Rev. Genet. 2018, 19, 385–397. [Google Scholar] [CrossRef]
- Garrigues, J.M.; Tsu, B.V.; Daugherty, M.D.; Pasquinelli, A.E. Diversification of the Caenorhabditis Heat Shock Response by Helitron Transposable Elements. eLife 2019, 8, e51139. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Li, R.; Chang, X.; Yuan, X.; Jing, R. Cloning and Characterization of TaSAP7-A, a Member of the Stress-Associated Protein Family in Common Wheat. Front. Plant. Sci. 2021, 12, 609351. [Google Scholar] [CrossRef]
- Hegde, P.S.; White, I.R.; Debouck, C. Interplay of Transcriptomics and Proteomics. Curr. Opin. Biotechnol. 2003, 14, 647–651. [Google Scholar] [CrossRef]
- Du, J.; Wang, S.; He, C.; Zhou, B.; Ruan, Y.-L.; Shou, H. Identification of Regulatory Networks and Hub Genes Controlling Soybean Seed Set and Size Using RNA Sequencing Analysis. J. Exp. Bot. 2017, 68, 1955–1972. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, C.; Wang, M.; Li, T.; Liu, X.; Jiang, J. Plasma Membrane-Localized SlSWEET7a and SlSWEET14 Regulate Sugar Transport and Storage in Tomato Fruits. Hortic. Res. 2021, 8, 186. [Google Scholar] [CrossRef]
- Xuan, H.; Huang, Y.; Zhou, L.; Deng, S.; Wang, C.; Xu, J.; Wang, H.; Zhao, J.; Guo, N.; Xing, H. Key Soybean Seedlings Drought-Responsive Genes and Pathways Revealed by Comparative Transcriptome Analyses of Two Cultivars. Int. J. Mol. Sci. 2022, 23, 2893. [Google Scholar] [CrossRef]
- Prince, S.J.; Joshi, T.; Mutava, R.N.; Syed, N.; Joao Vitor, M.D.S.; Patil, G.; Song, L.; Wang, J.; Lin, L.; Chen, W.; et al. Comparative Analysis of the Drought-Responsive Transcriptome in Soybean Lines Contrasting for Canopy Wilting. Plant. Sci. 2015, 240, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Shahriari, A.G.; Soltani, Z.; Tahmasebi, A.; Poczai, P. Integrative System Biology Analysis of Transcriptomic Responses to Drought Stress in Soybean (Glycine max L.). Genes 2022, 13, 1732. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, H.; Sun, A.; Wang, L.; Ren, C.; Liu, J.; Gao, X. Transcriptome Analysis Reveals Key Drought-Stress-Responsive Genes in Soybean. Front. Genet. 2022, 13, 1060529. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Vengavasi, K.; Kumar, M.N.; Yadav, S.K.; Pandey, R. Expression of Potential Reference Genes in Response to Macronutrient Stress in Rice and Soybean. Gene 2021, 792, 145742. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Sazegari, S.; Niazi, A.; Ahmadi, F.S. A Study on the Regulatory Network with Promoter Analysis for Arabidopsis DREB-Genes. Bioinformation 2015, 11, 101–106. [Google Scholar] [CrossRef]
- Andrási, N.; Pettkó-Szandtner, A.; Szabados, L. Diversity of Plant Heat Shock Factors: Regulation, Interactions, and Functions. J. Exp. Bot. 2021, 72, 1558–1575. [Google Scholar] [CrossRef]
- Coego, A.; Julian, J.; Lozano-Juste, J.; Pizzio, G.A.; Alrefaei, A.F.; Rodriguez, P.L. Ubiquitylation of ABA Receptors and Protein Phosphatase 2C Coreceptors to Modulate ABA Signaling and Stress Response. Int. J. Mol. Sci. 2021, 22, 7103. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A Multifunctional Amino Acid. Trends Plant. Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Magwanga, R.O.; Lu, P.; Kirungu, J.N.; Lu, H.; Wang, X.; Cai, X.; Zhou, Z.; Zhang, Z.; Salih, H.; Wang, K.; et al. Characterization of the Late Embryogenesis Abundant (LEA) Proteins Family and Their Role in Drought Stress Tolerance in Upland Cotton. BMC Genet. 2018, 19, 6. [Google Scholar] [CrossRef]
- Jung, C.; Nguyen, N.H.; Cheong, J.-J. Transcriptional Regulation of Protein Phosphatase 2C Genes to Modulate Abscisic Acid Signaling. Int. J. Mol. Sci. 2020, 21, 9517. [Google Scholar] [CrossRef]
- Lenk, I.; Fisher, L.H.C.; Vickers, M.; Akinyemi, A.; Didion, T.; Swain, M.; Jensen, C.S.; Mur, L.A.J.; Bosch, M. Transcriptional and Metabolomic Analyses Indicate That Cell Wall Properties Are Associated with Drought Tolerance in Brachypodium Distachyon. Int. J. Mol. Sci. 2019, 20, 1758. [Google Scholar] [CrossRef]
- Jangam, A.P.; Pathak, R.R.; Raghuram, N. Microarray Analysis of Rice D1 (RGA1) Mutant Reveals the Potential Role of G-Protein Alpha Subunit in Regulating Multiple Abiotic Stresses Such as Drought, Salinity, Heat, and Cold. Front. Plant. Sci. 2016, 7, 11. [Google Scholar] [CrossRef]
- Oztur, Z.N.; Talamé, V.; Deyholos, M.; Michalowski, C.B.; Galbraith, D.W.; Gozukirmizi, N.; Tuberosa, R.; Bohnert, H.J. Monitoring Large-Scale Changes in Transcript Abundance in Drought- and Salt-Stressed Barley. Plant. Mol. Biol. 2002, 48, 551–573. [Google Scholar] [CrossRef]
- Hazen, S.P.; Pathan, M.S.; Sanchez, A.; Baxter, I.; Dunn, M.; Estes, B.; Chang, H.-S.; Zhu, T.; Kreps, J.A.; Nguyen, H.T. Expression Profiling of Rice Segregating for Drought Tolerance QTLs Using a Rice Genome Array. Funct. Integr. Genomics 2005, 5, 104–116. [Google Scholar] [CrossRef]
- Guo, P.; Baum, M.; Grando, S.; Ceccarelli, S.; Bai, G.; Li, R.; von Korff, M.; Varshney, R.K.; Graner, A.; Valkoun, J. Differentially Expressed Genes between Drought-Tolerant and Drought-Sensitive Barley Genotypes in Response to Drought Stress during the Reproductive Stage. J. Exp. Bot. 2009, 60, 3531–3544. [Google Scholar] [CrossRef]
- Li, P.; Lin, P.; Zhao, Z.; Li, Z.; Liu, Y.; Huang, C.; Huang, G.; Xu, L.; Deng, Z.; Zhang, Y.; et al. Gene Co-Expression Analysis Reveals Transcriptome Divergence between Wild and Cultivated Sugarcane under Drought Stress. Int. J. Mol. Sci. 2022, 23, 569. [Google Scholar] [CrossRef]
- Opdenakker, K.; Remans, T.; Vangronsveld, J.; Cuypers, A. Mitogen-Activated Protein (MAP) Kinases in Plant Metal Stress: Regulation and Responses in Comparison to Other Biotic and Abiotic Stresses. Int. J. Mol. Sci. 2012, 13, 7828–7853. [Google Scholar] [CrossRef]
- Mathesius, U. Flavonoids Induced in Cells Undergoing Nodule Organogenesis in White Clover Are Regulators of Auxin Breakdown by Peroxidase. J. Exp. Bot. 2001, 52, 419–426. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed]
- Cavaiuolo, M.; Cocetta, G.; Ferrante, A. The Antioxidants Changes in Ornamental Flowers during Development and Senescence. Antioxidants 2013, 2, 132–155. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Feng, Y.; Yu, S.; Fan, Z.; Li, X.; Li, J.; Yin, H. The Flavonoid Biosynthesis Network in Plants. Int. J. Mol. Sci. 2021, 22, 12824. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Peng, C.; Li, T.; Huang, J.; Song, H.; Zhu, Q.; Wang, M. The Effects of Drought and Re-Watering on Non-Structural Carbohydrates of Pinus Tabulaeformis Seedlings. Biology 2021, 10, 281. [Google Scholar] [CrossRef]
- Kannenberg, S.A.; Phillips, R.P. Non-Structural Carbohydrate Pools Not Linked to Hydraulic Strategies or Carbon Supply in Tree Saplings during Severe Drought and Subsequent Recovery. Tree Physiol. 2020, 40, 259–271. [Google Scholar] [CrossRef]
- Ruan, Y.-L.; Jin, Y.; Yang, Y.-J.; Li, G.-J.; Boyer, J.S. Sugar Input, Metabolism, and Signaling Mediated by Invertase: Roles in Development, Yield Potential, and Response to Drought and Heat. Mol. Plant. 2010, 3, 942–955. [Google Scholar] [CrossRef]
- Li, S.; Zachgo, S. TCP3 Interacts with R2R3-MYB Proteins, Promotes Flavonoid Biosynthesis and Negatively Regulates the Auxin Response in Arabidopsis Thaliana. Plant. J. 2013, 76, 901–913. [Google Scholar] [CrossRef]
- Feng, K.; Hou, X.-L.; Xing, G.-M.; Liu, J.-X.; Duan, A.-Q.; Xu, Z.-S.; Li, M.-Y.; Zhuang, J.; Xiong, A.-S. Advances in AP2/ERF Super-Family Transcription Factors in Plant. Crit. Rev. Biotechnol. 2020, 40, 750–776. [Google Scholar] [CrossRef]
- He, X.; Wang, T.; Zhu, W.; Wang, Y.; Zhu, L. GhHB12, a HD-ZIP I Transcription Factor, Negatively Regulates the Cotton Resistance to Verticillium Dahliae. Int. J. Mol. Sci. 2018, 19, 3997. [Google Scholar] [CrossRef]
- Wang, W.; Vinocur, B.; Altman, A. Plant Responses to Drought, Salinity and Extreme Temperatures: Towards Genetic Engineering for Stress Tolerance. Planta 2003, 218, 1–14. [Google Scholar] [CrossRef]
- Gong, Z.; Yang, S. Drought Meets SWEET. Nat. Plants 2022, 8, 25–26. [Google Scholar] [CrossRef]
- Bauwe, H.; Kolukisaoglu, U. Genetic Manipulation of Glycine Decarboxylation. J. Exp. Bot. 2003, 54, 1523–1535. [Google Scholar] [CrossRef]
- Diniz, A.L.; da Silva, D.I.R.; Lembke, C.G.; Costa, M.D.-B.L.; Ten-Caten, F.; Li, F.; Vilela, R.D.; Menossi, M.; Ware, D.; Endres, L.; et al. Amino Acid and Carbohydrate Metabolism Are Coordinated to Maintain Energetic Balance during Drought in Sugarcane. Int. J. Mol. Sci. 2020, 21, 9124. [Google Scholar] [CrossRef]
- Ye, Y.; Ding, Y.; Jiang, Q.; Wang, F.; Sun, J.; Zhu, C. The Role of Receptor-like Protein Kinases (RLKs) in Abiotic Stress Response in Plants. Plant. Cell. Rep. 2017, 36, 235–242. [Google Scholar] [CrossRef]
- Chen, F.; Fang, P.; Peng, Y.; Zeng, W.; Zhao, X.; Ding, Y.; Zhuang, Z.; Gao, Q.; Ren, B. Comparative Proteomics of Salt-Tolerant and Salt-Sensitive Maize Inbred Lines to Reveal the Molecular Mechanism of Salt Tolerance. Int. J. Mol. Sci. 2019, 20, 4725. [Google Scholar] [CrossRef]
- Zhang, Y.-X.; Xu, H.-H.; Liu, S.-J.; Li, N.; Wang, W.-Q.; Møller, I.M.; Song, S.-Q. Proteomic Analysis Reveals Different Involvement of Embryo and Endosperm Proteins during Aging of Yliangyou 2 Hybrid Rice Seeds. Front. Plant. Sci. 2016, 7, 1394. [Google Scholar] [CrossRef]
- Nahirñak, V.; Almasia, N.I.; Hopp, H.E.; Vazquez-Rovere, C. Snakin/GASA Proteins: Involvement in Hormone Crosstalk and Redox Homeostasis. Plant. Signal. Behav. 2012, 7, 1004–1008. [Google Scholar] [CrossRef]
- Kadam, N.N.; Tamilselvan, A.; Lawas, L.M.F.; Quinones, C.; Bahuguna, R.N.; Thomson, M.J.; Dingkuhn, M.; Muthurajan, R.; Struik, P.C.; Yin, X.; et al. Genetic Control of Plasticity in Root Morphology and Anatomy of Rice in Response to Water Deficit. Plant. Physiol. 2017, 174, 2302–2315. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, X.; Xue, Y.; Cao, D.; Luan, X.; Zhao, K.; Liu, Q.; Ren, Y.; Zhu, Z.; Li, Y.; Liu, X. Identification of Candidate Genes for Drought Resistance during Soybean Seed Development. Agriculture 2023, 13, 949. https://doi.org/10.3390/agriculture13050949
Tang X, Xue Y, Cao D, Luan X, Zhao K, Liu Q, Ren Y, Zhu Z, Li Y, Liu X. Identification of Candidate Genes for Drought Resistance during Soybean Seed Development. Agriculture. 2023; 13(5):949. https://doi.org/10.3390/agriculture13050949
Chicago/Turabian StyleTang, Xiaofei, Yongguo Xue, Dan Cao, Xiaoyan Luan, Kezhen Zhao, Qi Liu, Yang Ren, Zifei Zhu, Yong Li, and Xinlei Liu. 2023. "Identification of Candidate Genes for Drought Resistance during Soybean Seed Development" Agriculture 13, no. 5: 949. https://doi.org/10.3390/agriculture13050949
APA StyleTang, X., Xue, Y., Cao, D., Luan, X., Zhao, K., Liu, Q., Ren, Y., Zhu, Z., Li, Y., & Liu, X. (2023). Identification of Candidate Genes for Drought Resistance during Soybean Seed Development. Agriculture, 13(5), 949. https://doi.org/10.3390/agriculture13050949





