Assessing the Role of Crop Rotation in Shaping Foliage Characteristics and Leaf Gas Exchange Parameters for Winter Wheat
Abstract
1. Introduction
2. Materials and Methods
2.1. Site, Soil and Climate
2.2. Experimental Design
- A.
- winter rape + catch crop (blue tansy) − spring barley − field pea − winter wheat (50% cereals including 25% wheat);
- B.
- winter rape − winter wheat + catch crop (blue tansy) − field pea − winter wheat (50% wheat);
- C.
- winter rape + catch crop (blue tansy) − field pea − winter wheat − winter wheat (50% wheat);
- D.
- winter rape − winter wheat + catch crop (blue tansy) − spring barley − winter wheat (75% cereals including 50% wheat).
2.3. Measurements
2.3.1. Foliage Characteristics
2.3.2. Leaf Gas Exchange
2.3.3. Above-Ground Biomass Yield, Crop Growth Rate, and Grain Yield of Wheat
2.4. Statistical Analysis
3. Results and Discussion
3.1. Winter Wheat Foliage Characteristics
3.1.1. Stem Elongation Stage (BBCH 36)
3.1.2. Heading Stage (BBCH 58)
3.2. Leaf Gas Exchange
3.2.1. Stem Elongation Stage (BBCH 36)
3.2.2. Heading Stage (BBCH 58)
3.3. Above-Ground Biomass and Grain Yield
3.3.1. Above-Ground Biomass
3.3.2. Wheat Growth Rate between BBCH 36 and BBCH 58
3.3.3. Grain Yield
3.4. The Relationship between Assimilation and the Foliage Characteristics and Wheat Yield
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. FAO Statistical Yearbook—Word Food and Agriculture; FAO: Rome, Italy, 2022; p. 382. [Google Scholar]
- Bennett, A.J.; Bending, G.D.; Chandler, D.; Hilton, S.; Mills, P. Meeting the demand for crop production: The challenge of yield decline in crops grown in short rotations. Biol. Rev. 2012, 87, 52–71. [Google Scholar] [CrossRef] [PubMed]
- Wanic, M.; Treder, K. Effect of forecrops crops on gas exchange and selected agronomic characteristics of wheat and spelt. J. Elem. 2020, 25, 607–619. [Google Scholar] [CrossRef]
- Pramanick, B.; Kumar, M.; Naik, B.M.; Singh, S.K.; Maitra, S.; Naik, B.; Rajput, V.D.; Minkina, T. Long-Term Conservation Tillage and Precision Nutrient Management in Maize-Wheat Cropping System: Effect on Soil Properties, Crop Production, and Economics. Agronomy 2022, 12, 2766. [Google Scholar] [CrossRef]
- Ball, B.C.; Bingham, I.; Rees, R.M.; Watson, C.A.; Litterick, A. The role of crop rotations in determining soil structure and crop growth conditions. Can. J. Soil Sci. 2005, 85, 557–577. [Google Scholar] [CrossRef]
- Babulicova, M. The influence of fertilization and crop rotation on the winter wheat production. Plant Soil Environ. 2014, 60, 297–302. [Google Scholar] [CrossRef]
- Wozniak, A. Effect of Crop Rotation and Cereal Monoculture on the Yield and Quality of Winter Wheat Grain and on Crop Infestation with Weeds and Soil Properties. Int. J. Plant Prod. 2019, 13, 177–182. [Google Scholar] [CrossRef]
- Gill, K.S. Crop rotations compared with continuous canola and wheat for crop production and fertilizer use over 6 yr. Can. J. Plant Sci. 2018, 98, 1139–1149. [Google Scholar] [CrossRef]
- Lepiarczyk, A.; Kulig, B.; Stepnik, K. The influence of simplified soil cultivation and forecrop on the development LAI of selected cultivars of winter wheat in cereal crop rotation. Fragm. Agron. (Pol.) 2005, 2, 98–105. [Google Scholar]
- Faligowska, A.; Szymanska, G.; Panasiewicz, K.; Szukala, J.; Koziara, W.; Ratajczak, K. The long-term effect of legumes as forecrops on the productivity of rotation (winter rape-winter wheat-winter wheat) with nitrogen fertilization. Plant Soil Environ. 2019, 65, 138–144. [Google Scholar] [CrossRef]
- Sieling, K.; Christen, O. Crop rotation effects on yield of oilseed rape, wheat and barley and residual effects on the subsequent wheat. Arch. Agron. Soil Sci. 2015, 61, 1531–1549. [Google Scholar] [CrossRef]
- Sieling, K.; Stahl, C.; Winkelmann, C.; Christen, O. Growth and yield of winter wheat in the first 3 years of a monoculture under varying N fertilization in NW Germany. Eur. J. Agron. 2005, 22, 71–84. [Google Scholar] [CrossRef]
- Aslam, F.; Khaliq, A.; Matloob, A.; Tanveer, A.; Hussain, S.; Zahir, Z. Allelopathy in agro-ecosystems: A critical review of wheat allelopathy-concepts and implications. Chemoecology 2017, 27, 1–24. [Google Scholar] [CrossRef]
- Kirkegaard, J.A.; Ryan, M.H. Magnitude and mechanisms of persistent crop sequence effects on wheat. Field Crops Res. 2014, 164, 154–165. [Google Scholar] [CrossRef]
- Feng, H.; Abagandura, G.O.; Senturklu, S.; Landblom, D.G.; Lai, L.; Ringwall, K.; Kumar, S. Soil quality indicators as influenced by 5-year diversified and monoculture cropping systems. J. Agric. Sci. 2020, 158, 594–605. [Google Scholar] [CrossRef]
- Wanic, M.; Denert, M.; Treder, K. Effect of forecrops on the yield and quality of common wheat and spelt wheat grain. J. Elem. 2019, 24, 369–383. [Google Scholar] [CrossRef]
- Vitale, L.; Arena, C.; Carillo, P.; Di Tommasi, P.; Mesolella, B.; Nacca, F.; De Santo, A.V.; Fuggi, A.; Magliulo, V. Gas exchange and leaf metabolism of irrigated maize at different growth stages. Plant Biosyst. 2011, 145, 485–494. [Google Scholar] [CrossRef]
- Wang, X.B.; Wang, L.F.; Shangguan, Z. Leaf Gas Exchange and Fluorescence of Two Winter Wheat Varieties in Response to Drought Stress and Nitrogen Supply. PLoS ONE 2016, 11, e0165733. [Google Scholar] [CrossRef]
- Lalarukh, I.; Ashraf, M.A.; Azeem, M.; Hussain, M.; Akbar, M.; Ashraf, M.Y.; Javed, M.T.; Iqbal, N. Growth stage-based response of wheat (Triticum aestivum L.) to kinetin under water-deficit environment: Pigments and gas exchange attributes. Acta Agric. Scan. Sect. B Soil Plant Sci. 2014, 64, 501–510. [Google Scholar] [CrossRef]
- Todorova, D.; Aleksandrov, V.; Anev, S.; Sergiev, I. Photosynthesis Alterations in Wheat Plants Induced by Herbicide, Soil Drought or Flooding. Agronomy, 2022; 12, 390. [Google Scholar] [CrossRef]
- de la Riva, E.G.; Olmo, M.; Poorter, H.; Ubera, J.L.; Villar, R. Leaf Mass per Area (LMA) and Its Relationship with Leaf Structure and Anatomy in 34 Mediterranean Woody Species along a Water Availability Gradient. PLoS ONE 2016, 11, e0148788. [Google Scholar] [CrossRef]
- Ahmad, M.I.; Shah, A.N.; Sun, J.Q.; Song, Y.H. Comparative Study on Leaf Gas Exchange, Growth, Grain Yield, and Water Use Efficiency under Irrigation Regimes for Two Maize Hybrids. Agriculture 2020, 10, 369. [Google Scholar] [CrossRef]
- Perdomo, J.A.; Conesa, M.A.; Medrano, H.; Ribas-Carbo, M.; Galmes, J. Effects of long-term individual and combined water and temperature stress on the growth of rice, wheat and maize: Relationship with morphological and physiological acclimation. Physiol. Plant. 2015, 155, 149–165. [Google Scholar] [CrossRef]
- Bhusal, N.; Lee, M.; Lee, H.; Adhikari, A.; Han, A.R.; Han, A.; Kim, H.S. Evaluation of morphological, physiological, and biochemical traits for assessing drought resistance in eleven tree species. Sci. Total Environ. 2021, 779, 146466. [Google Scholar] [CrossRef] [PubMed]
- Bhusal, N.; Bhusal, S.J.; Yoon, T.M. Comparisons of physiological and anatomical characteristics between two cultivars in bi-leader apple trees (Malus x domestica Borkh.). Sci. Hortic. 2018, 231, 73–81. [Google Scholar] [CrossRef]
- Ren, B.Z.; Liu, W.; Zhang, J.W.; Dong, S.T.; Liu, P.; Zhao, B. Effects of plant density on the photosynthetic and chloroplast characteristics of maize under high-yielding conditions. Sci. Nat. 2017, 104, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Richards, R.A. Selectable traits to increase crop photosynthesis and yield of grain crops. J. Exp. Bot. 2000, 51, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Ewert, F. Modelling plant responses to elevated CO2: How important is leaf area index? Ann. Bot. 2004, 93, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Tshikunde, N.M.; Odindo, A.; Shimelis, H.; Mashilo, J. Leaf gas exchange and water-use efficiency of dry-land wheat genotypes under water stressed and non-stressed conditions. Acta Agric. Scan. Sect. B Soil Plant Sci. 2018, 68, 738–748. [Google Scholar] [CrossRef]
- Asner, G.P.; Martin, R.E.; Tupayachi, R.; Emerson, R.; Martinez, P.; Sinca, F.; Powell, G.V.N.; Wright, S.J.; Lugo, A.E. Taxonomy and remote sensing of leaf mass per area (LMA) in humid tropical forests. Ecol. Appl. 2011, 21, 85–98. [Google Scholar] [CrossRef]
- Orzech, K.; Wanic, M.; Zaluski, D. Gas Exchanges in the Leaves of Silage Maize Depending on the Forecrop and Maize Development Stage. Agronomy 2022, 12, 396. [Google Scholar] [CrossRef]
- Hangs, R.D.; Van Rees, K.C.J.; Schoenau, J.J.; Guo, X. A simple technique for estimating above-ground biomass in short-rotation willow plantations. Biomass Bioenergy 2011, 35, 2156–2162. [Google Scholar] [CrossRef]
- Hikosaka, K. Leaf canopy as a dynamic system: Ecophysiology and optimality in leaf turnover. Ann. Bot. 2005, 95, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, M.X.; Xu, J.S.; Liu, X.Y.; Wang, S.Y.; Shi, L.X. Physiological and metabolomics analyses of young and old leaves from wild and cultivated soybean seedlings under low-nitrogen conditions. BMC Plant Biol. 2019, 19, 1–15. [Google Scholar] [CrossRef]
- Cheng, T.; Rivard, B.; Sanchez-Azofeifa, A.G.; Feret, J.B.; Jacquemoud, S.; Ustin, S.L. Deriving leaf mass per area (LMA) from foliar reflectance across a variety of plant species using continuous wavelet analysis. Isprs J. Photogramm. Remote Sens. 2014, 87, 28–38. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, U.; Poorter, L.; Wright, I.J.; Villar, R. Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis (vol 182, pg 565, 2009). New Phytol. 2009, 183, 1222. [Google Scholar]
- Pan, X.; Lada, R.R.; Caldwell, C.D.; Falk, K.C. Water-stress and N-nutrition effects on photosynthesis and growth of Brassica carinata. Photosynthetica 2011, 49, 309–315. [Google Scholar] [CrossRef]
- Ohsumi, A.; Hamasaki, A.; Nakagawa, H.; Yoshida, H.; Shiraiwa, T.; Horie, T. A model explaining genotypic and ontogenetic variation of leaf photosynthetic rate in rice (Oryza sativa) based on leaf nitrogen content and stomatal conductance. Ann. Bot. 2007, 99, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.Y.; Berninger, F.; Li, C.Y. Photosynthetic responses of Populus przewalski subjected to drought stress. Photosynthetica 2006, 44, 62–68. [Google Scholar] [CrossRef]
- Zhang, S.R.; Dang, Q.L. Effects of carbon dioxide concentration and nutrition on photosynthetic functions of white birch seedlings. Tree Physiol. 2006, 26, 1457–1467. [Google Scholar] [CrossRef]
- Ahmadi, A.; Siosemardeh, A. Investigation on the physiological basis of grain yield and drought resistance in wheat: Leaf photosynthetic rate, stomatal conductance and non-stomatal limitation. Int. J. Agric. Biol. 2005, 7, 807–811. [Google Scholar]
- Radford, P.J. Growth Analysis Formulae—Their Use and Abuse1. Crop Sci. 1967, 7, 171–175. [Google Scholar] [CrossRef]
- Kirkegaard, J.; Christen, O.; Krupinsky, J.; Layzell, D. Break crop benefits in temperate wheat production. Field Crops Res. 2008, 107, 185–195. [Google Scholar] [CrossRef]
- Ma, P.; Lan, Y.; Lyu, T.F.; Zhang, Y.J.; Lin, D.; Li, F.J.; Li, Y.; Yang, Z.Y.; Sun, Y.J.; Ma, J. Improving Rice Yields and Nitrogen Use Efficiency by Optimizing Nitrogen Management and Applications to Rapeseed in Rapeseed-Rice Rotation System. Agronomy 2020, 10, 1060. [Google Scholar] [CrossRef]
- Liu, Z.X.; Gao, F.; Yang, J.Q.; Zhen, X.Y.; Li, Y.; Zhao, J.H.; Li, J.R.; Qian, B.C.; Yang, D.Q.; Li, X.D. Photosynthetic Characteristics and Uptake and Translocation of Nitrogen in Peanut in a Wheat-Peanut Rotation System Under Different Fertilizer Management Regimes. Front. Plant Sci. 2019, 10, 86. [Google Scholar] [CrossRef]
- Reich, P.B.; Walters, M.B.; Ellsworth, D.S. Leaf life-span in relation to leaf, plant, and stand characteristics among diverse ecosystems. Ecol. Monogr. 1992, 62, 365–392. [Google Scholar] [CrossRef]
- Janusauskaite, D.; Feiziene, D.; Feiza, V. Nitrogen-induced variations in leaf gas exchange of spring triticale under field conditions. Acta Physiol. Plant. 2017, 39, 1–12. [Google Scholar] [CrossRef]
- DaMatta, F.M.; Loos, R.A.; Silva, E.A.; Loureiro, M.E.; Ducatti, C. Effects of soil water deficit and nitrogen nutrition on water relations and photosynthesis of pot-grown Coffea canephora Pierre. Trees-Struct. Funct. 2002, 16, 555–558. [Google Scholar] [CrossRef]
- Zhang, X.C.; Yu, X.F.; Ma, Y.F. Effect of nitrogen application and elevated CO2 on photosynthetic gas exchange and electron transport in wheat leaves. Photosynthetica 2013, 51, 593–602. [Google Scholar] [CrossRef]
- Baig, M.J.; Anand, A.; Mandal, P.K.; Bhatt, R.K. Irradiance influences contents of photosynthetic pigments and proteins in tropical grasses and legumes. Photosynthetica 2005, 43, 47–53. [Google Scholar] [CrossRef]
- Sugiharto, B.; Miyata, K.; Nakamoto, H.; Sasakawa, H.; Sugiyama, T. Regulation of expression of carbon-assimilating enzymes by nitrogen in maize leaf. Plant Physiol. 1990, 92, 963–969. [Google Scholar] [CrossRef]
- Hirayama, M.; Wada, Y.; Nemoto, H. Estimation of drought tolerance based on leaf temperature in upland rice breeding. Breed. Sci. 2006, 56, 47–54. [Google Scholar] [CrossRef]
- Lehmann, P.; Or, D. Effects of stomata clustering on leaf gas exchange. New Phytol. 2015, 207, 1015–1025. [Google Scholar] [CrossRef]
- Zhao, W.H.; Liu, L.Z.; Shen, Q.; Yang, J.H.; Han, X.Y.; Tian, F.; Wu, J.N. Effects of Water Stress on Photosynthesis, Yield, and Water Use Efficiency in Winter Wheat. Water 2020, 12, 2127. [Google Scholar] [CrossRef]
- Chapagain, T.; Riseman, A. Nitrogen and carbon transformations, water use efficiency and ecosystem productivity in monocultures and wheat-bean intercropping systems. Nutr. Cycl. Agroecosyst. 2015, 101, 107–121. [Google Scholar] [CrossRef]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef]
- Saeidi, M.; Abdoli, M. Effect of Drought Stress during Grain Filling on Yield and Its Components, Gas Exchange Variables, and Some Physiological Traits of Wheat Cultivars. J. Agr. Sci. Technol. 2015, 17, 885–898. [Google Scholar]
- Setter, T.L.; Flannigan, B.A.; Melkonian, J. Loss of kernel set due to water deficit and shade in maize. Crop Sci. 2001, 41, 1530–1540. [Google Scholar] [CrossRef]
- Agomoh, I.V.; Drury, C.F.; Phillips, L.A.; Reynolds, W.D.; Yang, X.M. Increasing crop diversity in wheat rotations increases yields but decreases soil health. Soil Sci. Soc. Am. J. 2020, 84, 170–181. [Google Scholar] [CrossRef]
- Darguza, M.; Gaile, Z. Yield and quality of winter wheat, depending on crop rotation and soil tillage. Res. Rural Dev. 2019, 2, 29–35. [Google Scholar] [CrossRef]
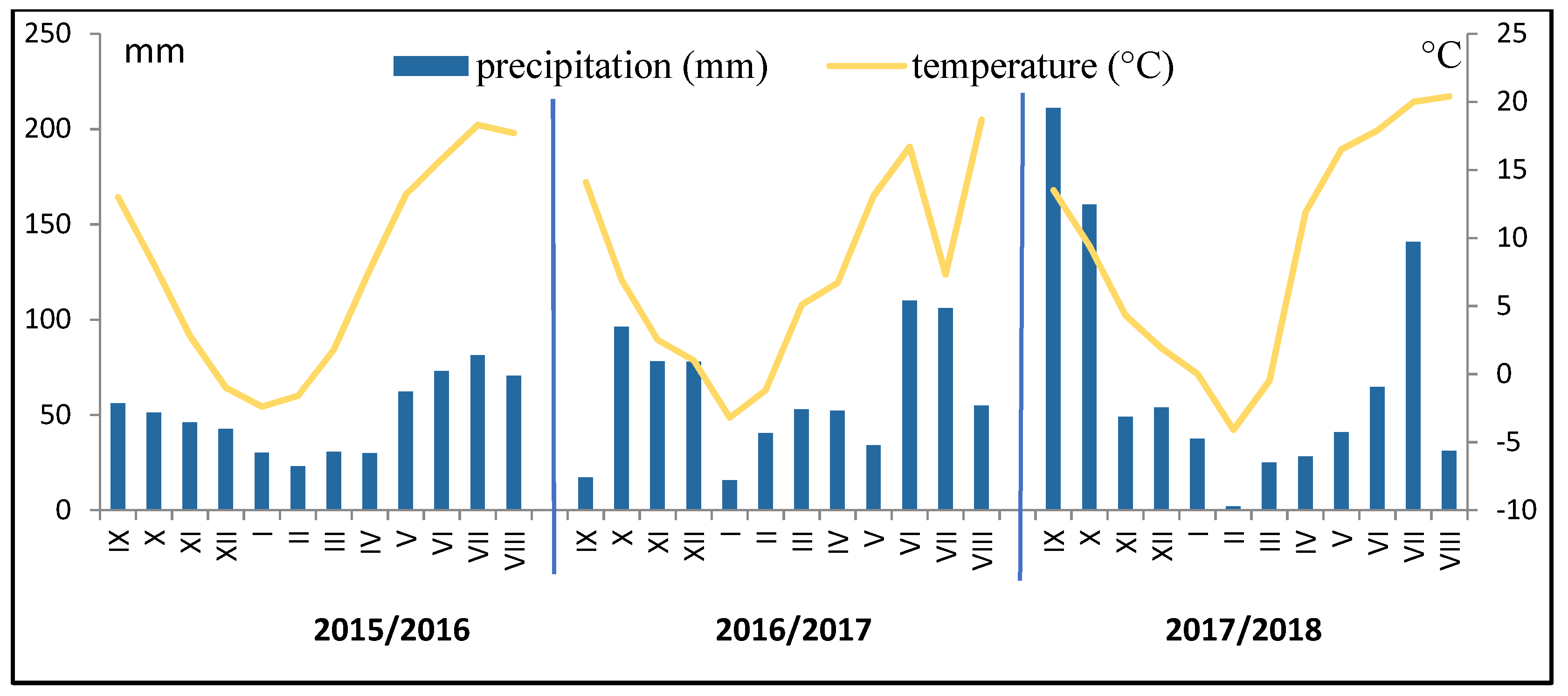
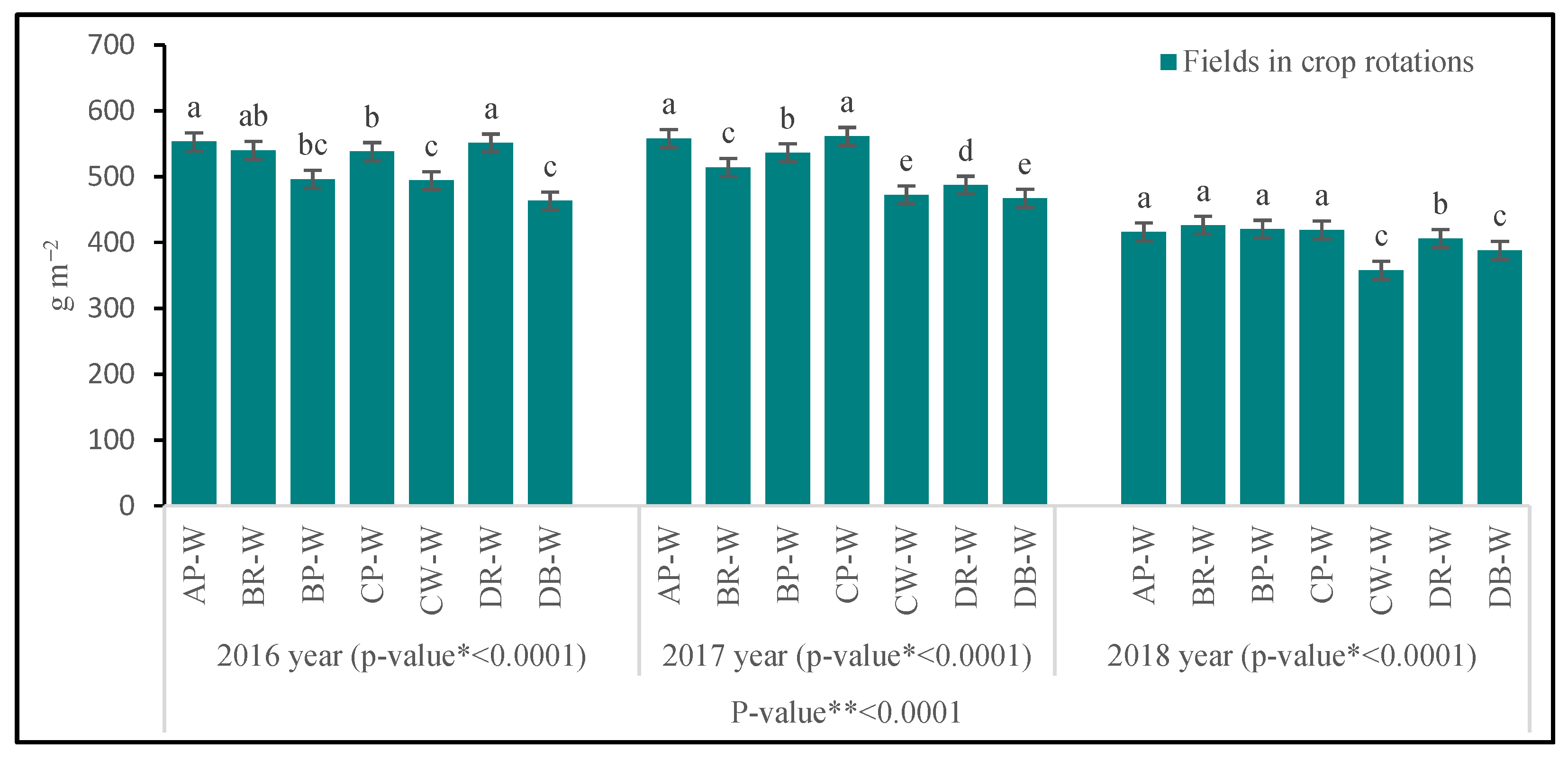
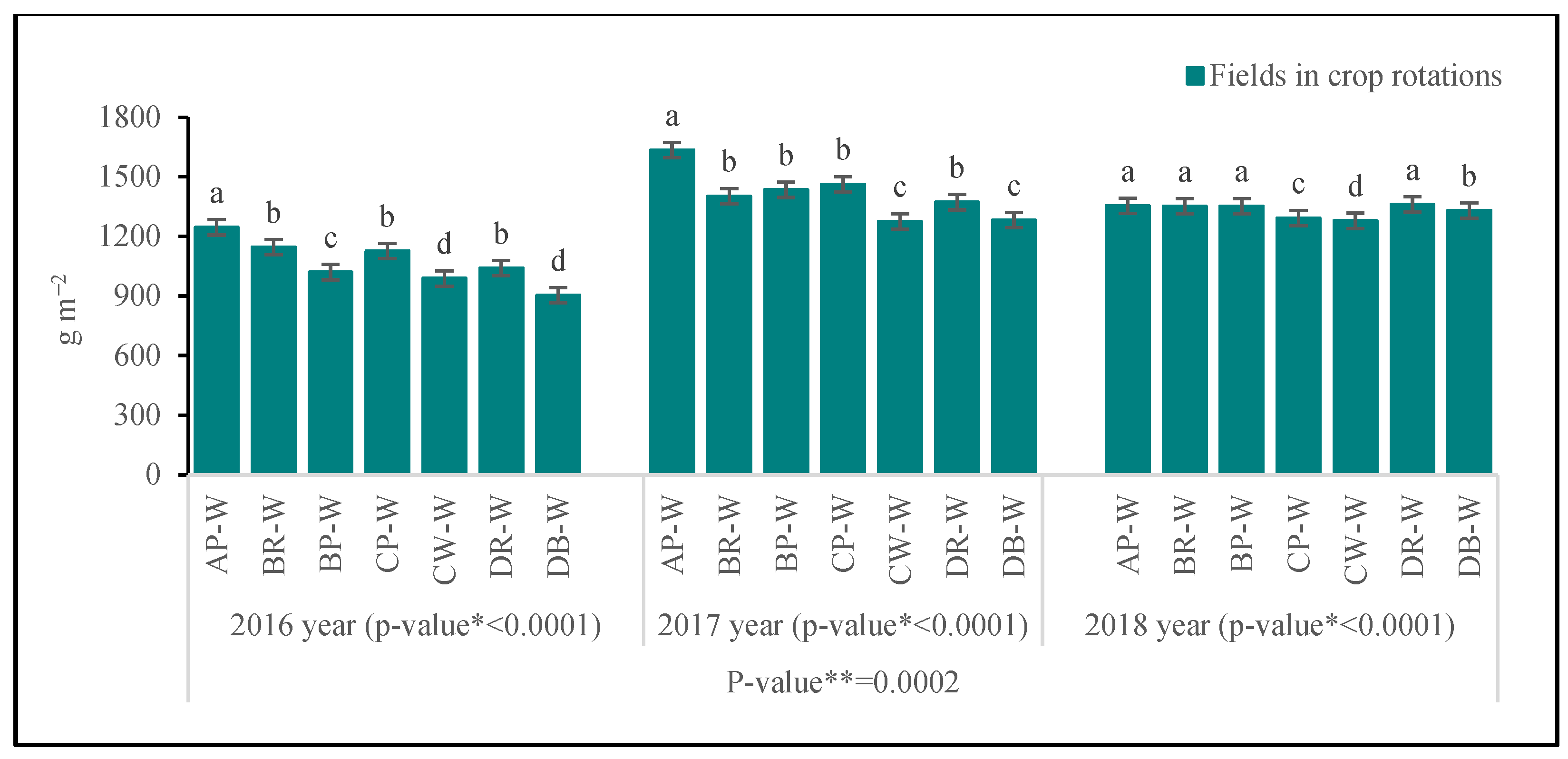

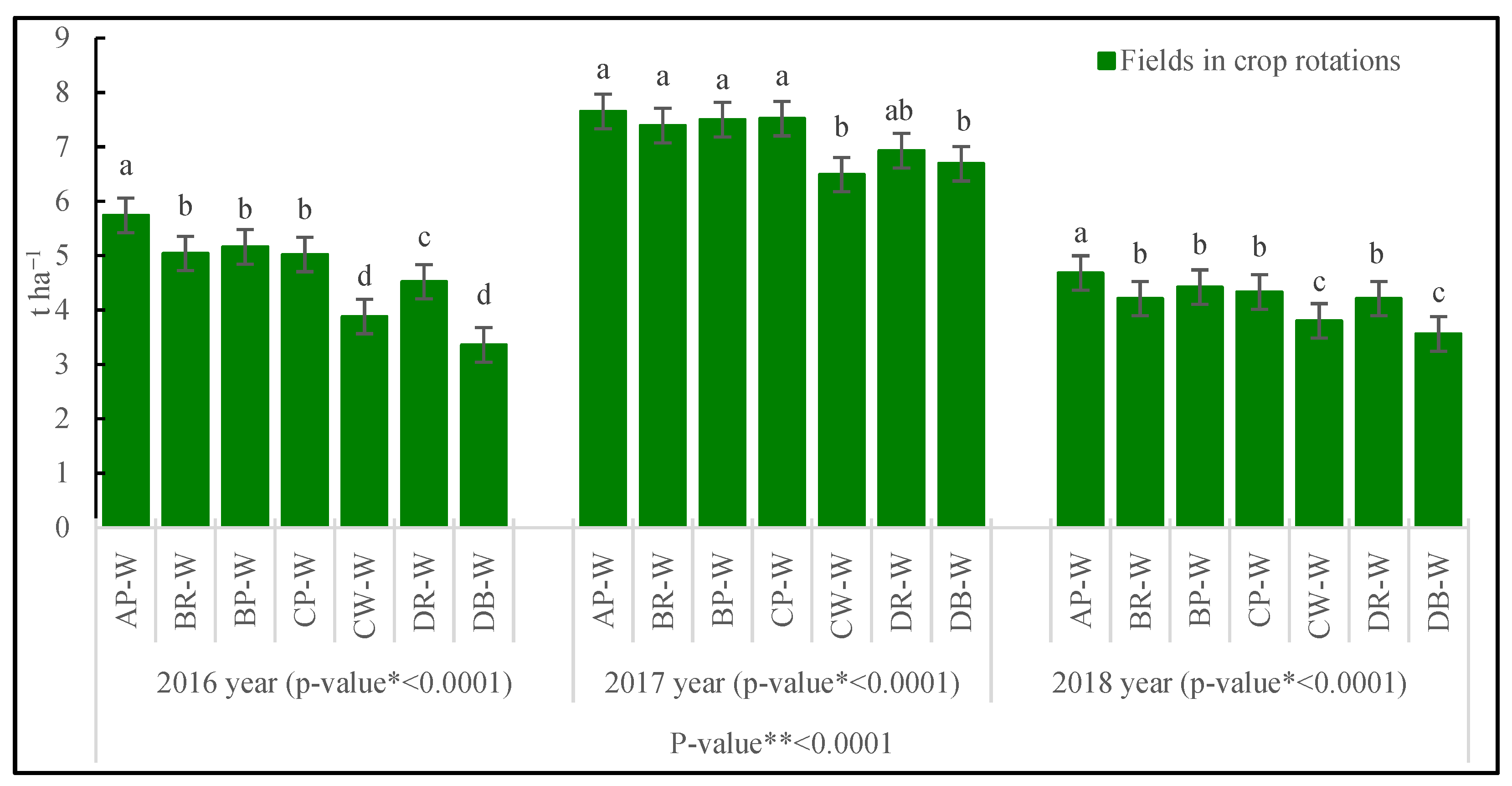
| Fields in Crop Rotations | LA | LAI | LMA | Cl | Narea | T | |
|---|---|---|---|---|---|---|---|
| 2016 year | |||||||
| AP-W | 9.9 a | 3.74 ab | 24.0 bc | 47.4 a | 0.24 a | 15.3 bc | |
| BR-W | 10.0 a | 3.71 ab | 22.4 c | 46.6 b | 0.24 a | 15.2 b | |
| BP-W | 10.4 a | 3.51 ab | 22.8 c | 46.5 b | 0.20 b | 14.7 c | |
| CP-W | 10.4 a | 3.34 b | 23.6 bc | 45.3 b | 0.21 b | 14.5 c | |
| CW-W | 9.5 b | 3.03 c | 27.0 a | 45.2 c | 0.19 c | 16.2 a | |
| DR-W | 10.6 a | 4.09 a | 22.2 c | 46.0 b | 0.22 b | 14.2 c | |
| DB-W | 9.0 b | 2.17 c | 26.3 a | 45.1 c | 0.24 c | 16.5 a | |
| p-value * | 0.005 | 0.0002 | 0.0004 | 0.0136 | 0.0002 | <0.0001 | |
| 2017 year | |||||||
| AP-W | 11.3 a | 3.85 a | 23.1 e | 45.0 a | 0.27 ns | 19.4 b | |
| BR-W | 10.6 b | 3.30 a | 28.8 b | 42.0 b | 0.23 ns | 19.1 b | |
| BP-W | 10.1 b | 3.29 a | 23.0 e | 42.6 b | 0.25 ns | 19.2 b | |
| CP-W | 11.5 a | 3.32 a | 26.8 c | 40.8 b | 0.24 ns | 19.5 b | |
| CW-W | 10.5 b | 2.89 b | 34.4 a | 41.1 b | 0.24 ns | 21.2 a | |
| DR-W | 10.5 b | 3.48 a | 24.7 d | 42.4 b | 0.24 ns | 19.0 c | |
| DB-W | 10.5 b | 2.95 b | 35.1 a | 41.5 b | 0.27 ns | 21.1 a | |
| p-value * | <0.0001 | <0.0001 | <0.0001 | 0.0001 | 0.543 | <0.0001 | |
| 2018 year | |||||||
| AP-W | 12.1 a | 3.16 a | 23.1 c | 54.5 a | 0.41 a | 23.8 b | |
| BR-W | 12.2 a | 2.91 a | 25.1 b | 55.1 ab | 0.42 a | 24.3 b | |
| BP-W | 12.8 a | 3.11 a | 23.0 c | 54.2 ab | 0.37 b | 23.4 b | |
| CP-W | 11.2 b | 3.03 a | 24.7 b | 54.3 ab | 0.31 bc | 23.0 b | |
| CW-W | 11.2 b | 2.36 b | 26.8 a | 53.4 b | 0.27 c | 25.2 a | |
| DR-W | 11.3 b | 2.98 a | 24.7 b | 56.7 a | 0.38 ab | 23.9 b | |
| DB-W | 11.0 b | 2.57 b | 28.8 a | 52.8 c | 0.30 c | 25.6 a | |
| p-value * | 0.0003 | 0.0004 | <0.0001 | 0.0086 | <0.0001 | <0.0001 | |
| p-value ** | <0.0001 | 0.0003 | <0.0001 | 0.0011 | 0.0120 | <0.0001 | |
| Fields in Crop Rotations | LA | LAI | LMA | Cl | Narea | T |
|---|---|---|---|---|---|---|
| 2016 year | ||||||
| AP-W | 13.9 a | 6.29 a | 29.8 e | 42.4 a | 0.14 a | 33.6 c |
| BR-W | 11.8 b | 5.31 b | 40.7 c | 40.3 bc | 0.13 a | 34.4 b |
| BP-W | 11.7 b | 5.40 b | 33.2 d | 41.4 ab | 0.14 a | 34.6 b |
| CP-W | 11.5 b | 5.25 b | 41.6 b | 40.6 bc | 0.14 a | 33.3 c |
| CW-W | 10.2 c | 3.69 c | 43.6 a | 40.3 b | 0.10 b | 35.3 a |
| DR-W | 11.8 b | 5.81 a | 28.3 e | 40.6 c | 0.14 a | 33.7 c |
| DB-W | 10.8 c | 3.58 c | 42.6 a | 39.5 c | 0.10 b | 35.4 a |
| p-value * | <0.0001 | 0.0003 | <0.0001 | 0.0011 | <0.0001 | <0.0001 |
| 2017 year | ||||||
| AP-W | 15.9 b | 6.02 b | 23.9 d | 52.6 a | 0.14 a | 20.7 cd |
| BR-W | 15.3 b | 5.38 b | 26.3 c | 49.4 bc | 1.11 b | 20.9 c |
| BP-W | 17.2 a | 6.45 a | 25.7 c | 49.6 b | 0.11 b | 20.4 d |
| CP-W | 17.3 a | 6.40 a | 27.7 b | 50.3 b | 0.11 b | 21.5 b |
| CW-W | 14.0 c | 4.71 c | 30.0 a | 48.0 d | 0.10 b | 22.3 a |
| DR-W | 16.2 b | 6.30 a | 27.4 b | 48.7 c | 0.11 b | 20.5 d |
| DB-W | 13.7 c | 5.00 c | 28.2 a | 46.8 d | 0.11 b | 22.6 a |
| p-value * | 0.0003 | <0.0001 | 0.0005 | <0.0001 | <0.0001 | <0.0001 |
| 2018 year | ||||||
| AP-W | 12.1 a | 3.53 a | 31.0 b | 54.8 a | 0.20 a | 28.0 ns |
| BR-W | 12.2 a | 3.25 a | 36.1 a | 53.4 b | 0.19 ab | 28.0 ns |
| BP-W | 11.9 b | 3.24 a | 35.6 a | 53.4 b | 0.21 a | 28.0 ns |
| CP-W | 12.2 a | 3.63 a | 35.9 a | 55.3 a | 0.18 b | 27.5 ns |
| CW-W | 11.4 c | 2.64 b | 36.0 a | 51.1 d | 0.16 c | 27.5 ns |
| DR-W | 12.2 a | 3.26 a | 31.9 b | 52.6 c | 0.21 a | 27.8 ns |
| DB-W | 11.3 c | 3.10 ab | 36.1 a | 51.3 d | 0.15 c | 27.4 ns |
| p-value * | <0.0001 | 0.0004 | <0.0001 | 0.0001 | 0.0002 | 0.3612 |
| p-value ** | 0.0018 | <0.0001 | <0.0001 | 0.0550 | 0.0001 | <0.0001 |
| Fields in Crop Rotations | gs | E | ci | An | WUE | WUE1 | ls |
|---|---|---|---|---|---|---|---|
| 2016 year | |||||||
| AP-W | 0.01 ns | 0.07 a | 354.4 a | 16.5 a | 235.7 d | 1650 a | 0.19 e |
| BR-W | 0.01 ns | 0.06 b | 338.9 bc | 15.6 ab | 260.0 c | 1560 ab | 0.31 b |
| BP-W | 0.01 ns | 0.05 c | 342.9 b | 15.8 ab | 316.0 b | 1580 ab | 0.21 d |
| CP-W | 0.01 ns | 0.05 c | 321.3 cd | 15.3 b | 306.0 b | 1530 b | 0.24 cd |
| CW-W | 0.01 ns | 0.04 d | 324.8 bcd | 14.3 c | 357.5 a | 1430 c | 0.27 bc |
| DR-W | 0.01 ns | 0.06 b | 315.4 d | 15.4 b | 256.7 c | 1540 b | 0.72 a |
| DB-W | 0.01 ns | 0.04 d | 301.1 e | 11.5 d | 287.5 bc | 1150 d | 0.20 cd |
| p-value * | - | <0.0001 | 00001 | <0.0001 | 0.0002 | 0.0003 | <0.0001 |
| 2017 year | |||||||
| AP-W | 1.80 a | 8.51 a | 460.3 a | 29.7 a | 3.49 bc | 16.5 bc | 0.10 e |
| BR-W | 1.55 b | 6.16 c | 301.0 cd | 20.6 c | 3.34 bc | 13.3 c | 0.54 ab |
| BP-W | 1.24 d | 5.92 d | 279.0 de | 18.1 d | 3.06 c | 14.6 bc | 0.58 a |
| CP-W | 1.28 c | 7.19 b | 314.8 c | 18.0 d | 2.50 d | 14.1 c | 0.26 d |
| CW-W | 0.84 f | 3.08 f | 320.3 c | 16.8 e | 5.45 a | 20.0 b | 0.41 c |
| DR-W | 1.05 e | 4.79 e | 387.5 b | 21.9 b | 4.57 b | 20.9 b | 0.06 e |
| DB-W | 0.76 g | 4.65 e | 263.0 f | 18.0 d | 3.87 bc | 23.7 a | 0.49 b |
| p-value * | <0.0001 | <0.0001 | <0.0001 | 0.0002 | 0.0005 | 0.0009 | 0.0002 |
| 2018 year | |||||||
| AP-W | 1.83 a | 8.52 a | 180.8 d | 23.8 a | 2.79 c | 13.0 a | 0.50 ns |
| BR-W | 1.51 c | 7.21 b | 180.5 d | 18.1 c | 2.51 c | 12.0 bc | 0.51 ns |
| BP-W | 1.63 b | 6.16 c | 196.5 c | 18.5 c | 3.00 b | 11.3 c | 0.48 ns |
| CP-W | 1.63 b | 6.08 cd | 179.5 d | 20.6 b | 3.39 a | 12.6 b | 0.51 ns |
| CW-W | 1.28 d | 4.78 e | 201.8 a | 17.4 d | 3.64 a | 13.6 a | 0.44 ns |
| DR-W | 1.80 a | 5.91 d | 201.3 b | 21.9 a | 3.71 a | 12.2 b | 0.51 ns |
| DB-W | 1.37 d | 4.74 e | 136.3 e | 15.5 e | 3.33 ab | 11.3 c | 0.51 ns |
| p-value * | 0.0011 | <0.0001 | <0.0001 | 0.0003 | 0.0002 | 0.0073 | 0.5561 |
| p-value ** | <0.0001 | 0.0001 | 0.0001 | <0.0001 | 0.0180 | <0.0001 | <0.0001 |
| Fields in Crop Rotations | gs | E | ci | An | WUE | WUE1 | ls |
|---|---|---|---|---|---|---|---|
| 2016 year | |||||||
| AP-W | 0.93 a | 8.51 a | 336.1 a | 18.5 a | 2.17 b | 19.9 c | 0.06 f |
| BR-W | 0.62 b | 8.19 a | 261.9 c | 17.9 b | 2.19 b | 28.9 b | 0.29 a |
| BP-W | 0.86 a | 8.21 a | 270.1 c | 17.8 b | 2.17 b | 20.7 c | 0.25 b |
| CP-W | 0.72 b | 7.20 c | 325.3 a | 17.7 b | 2.46 b | 24.6 c | 0.10 e |
| CW-W | 0.49 c | 6.20 d | 291.4 b | 16.1 d | 2.60 a | 32.9 b | 0.18 d |
| DR-W | 0.89 a | 6.26 d | 296.8 b | 17.3 bc | 2.76 a | 19.4 c | 0.17 d |
| DB-W | 0.27 d | 5.53 e | 271.0 c | 16.6 d | 3.00 a | 61.5 a | 0.21 c |
| p-value * | 0.0001 | 0.0002 | <0.0001 | <0.0001 | <0.0001 | 0.001 | 0.001 |
| 2017 year | |||||||
| AP-W | 0.85 a | 8.53 a | 445.1 a | 29.1 a | 3.41 c | 34.2 b | 0.06 bc |
| BR-W | 0.56 b | 6.16 c | 301.0 c | 19.2 e | 3.12 c | 34.2 b | 0.06 bc |
| BP-W | 0.53 b | 6.08 c | 390.4 b | 20.6 d | 3.39 d | 38.9 b | 0.05 c |
| CP-W | 0.58 b | 5.91 d | 444.0 a | 23.8 b | 4.03 a | 41.1 a | 0.04 c |
| CW-W | 0.44 c | 4.78 e | 320.1 c | 15.3 g | 3.20 c | 34.8 b | 0.09 a |
| DR-W | 0.80 a | 7.21 b | 387.5 b | 21.9 c | 3.04 c | 27.4 b | 0.07 b |
| DB-W | 0.38 c | 4.65 e | 276.8 d | 17.0 f | 3.66 b | 44.7 a | 0.11 a |
| p-value * | <0.0001 | <0.0001 | <0.0001 | 0.0004 | 0.0086 | 0.0003 | 0.001 |
| 2018 year | |||||||
| AP-W | 0.08 a | 1.42 a | 262.8 a | 13.1 a | 9.23 a | 163.8 b | 0.47 b |
| BR-W | 0.07 b | 1.39 a | 256.3 ab | 12.4 a | 8.92 ab | 177.1 b | 0.45 b |
| BP-W | 0.06 b | 1.08 ab | 250.0 ab | 9.7 c | 8.98 ab | 161.5 b | 0.33 c |
| CP-W | 0.05 b | 1.40 a | 244.6 bc | 9.5 c | 6.79 c | 190.0 a | 0.40 b |
| CW-W | 0.05 b | 1.20 a | 232.7 c | 7.7 d | 6.42 c | 154.0 bc | 0.69 a |
| DR-W | 0.08 a | 1.27 a | 224.5 c | 10.4 b | 8.19 b | 130.0 c | 0.48 b |
| DB-W | 0.05 b | 0.91 b | 217.9 d | 7.4 d | 8.13 b | 148.0 c | 0.63 a |
| p-value * | 0.0004 | 0.0001 | <0.0001 | 0.0002 | 0.0031 | 0.0002 | 0.003 |
| p-value ** | 0.0001 | <0.0001 | <0.0001 | 0.0009 | 0.0003 | 0.0040 | <0.0001 |
| Year | LA | LAI | LMA | Cl | Narea | T | AGB | Yield of Grain |
|---|---|---|---|---|---|---|---|---|
| BBCH 36 | ||||||||
| 2016 | 0.13 | 0.52 * | −0.44 * | 0.22 | 0.55 * | −0.75 * | 0.72 * | × |
| 2017 | 0.29 | 0.01 | −0.38 * | 0.79 * | 0.10 | −0.54 * | 0.55 * | × |
| 2018 | 0.22 | 0.54 * | −0.48 * | 0.45 * | 0.40 * | −0.43 * | 0.31 | × |
| BBCH 58 | ||||||||
| 2016 | 0.76 * | 0.54 * | −0.73 * | 0.58 * | 0.52 * | −0.54 * | 0.78 * | 0.65 * |
| 2017 | 0.31 | 0.71 * | −0.40 * | 0.42 * | 0.45 * | −0.61 * | 0.61 * | 0.62 * |
| 2018 | 0.34 | 0.39 * | −0.42 * | 0.43 * | 0.10 | −0.68 * | 0.43 * | 0.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wanic, M.; Parzonka, M. Assessing the Role of Crop Rotation in Shaping Foliage Characteristics and Leaf Gas Exchange Parameters for Winter Wheat. Agriculture 2023, 13, 958. https://doi.org/10.3390/agriculture13050958
Wanic M, Parzonka M. Assessing the Role of Crop Rotation in Shaping Foliage Characteristics and Leaf Gas Exchange Parameters for Winter Wheat. Agriculture. 2023; 13(5):958. https://doi.org/10.3390/agriculture13050958
Chicago/Turabian StyleWanic, Maria, and Mariola Parzonka. 2023. "Assessing the Role of Crop Rotation in Shaping Foliage Characteristics and Leaf Gas Exchange Parameters for Winter Wheat" Agriculture 13, no. 5: 958. https://doi.org/10.3390/agriculture13050958
APA StyleWanic, M., & Parzonka, M. (2023). Assessing the Role of Crop Rotation in Shaping Foliage Characteristics and Leaf Gas Exchange Parameters for Winter Wheat. Agriculture, 13(5), 958. https://doi.org/10.3390/agriculture13050958






