Discontinuous Hydration Cycles with Elicitors Improve Germination, Growth, Osmoprotectant, and Salt Stress Tolerance in Zea mays L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Location and Acquisition of Seeds
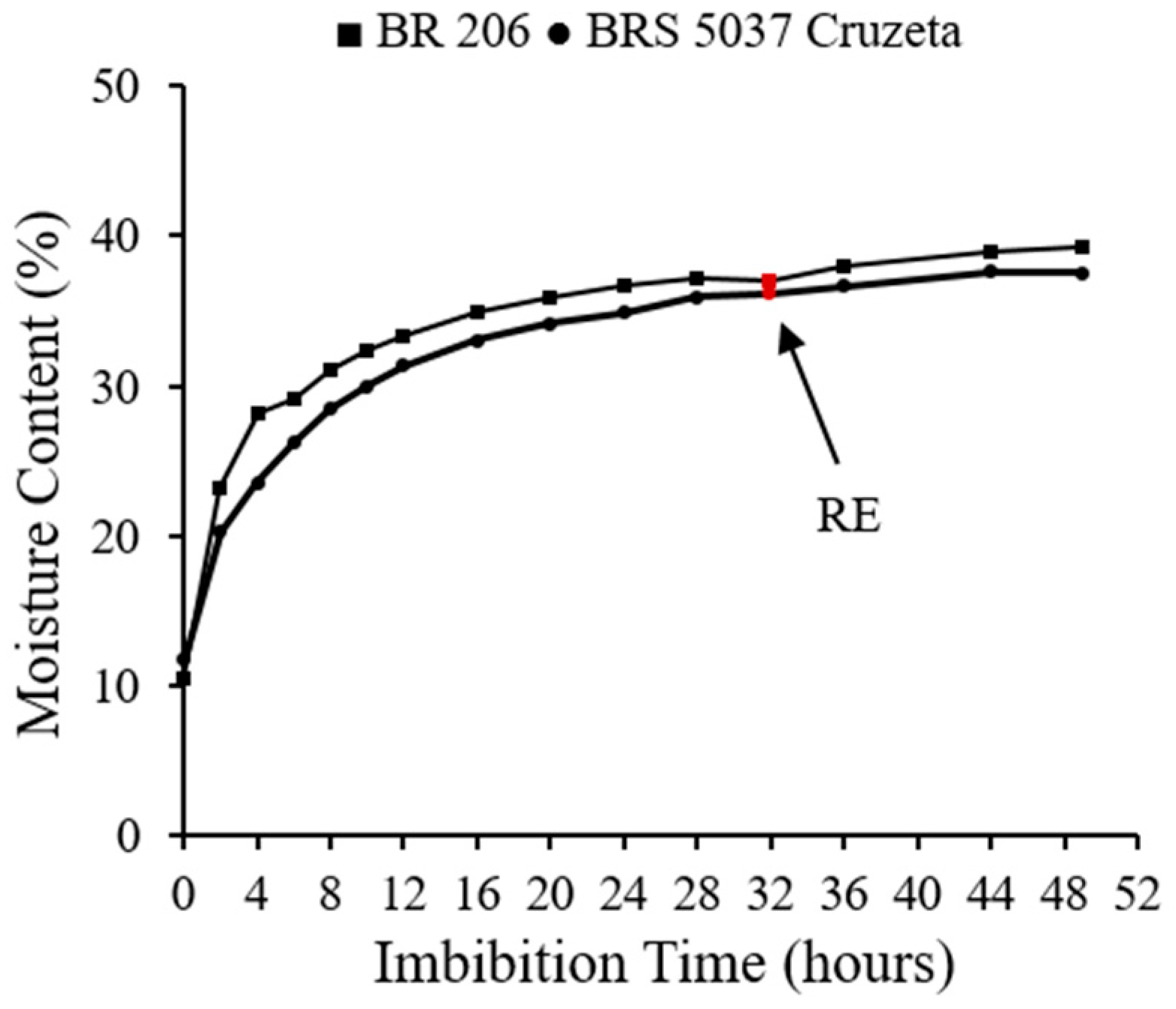
2.2. Imbibition Curve and Experimental Design
2.3. Germination and Seedling Length
2.4. Dry Mass of Seedlings and Salinity Tolerance index
2.5. Osmotic Homeostasis
2.6. Statistical Analysis
3. Results
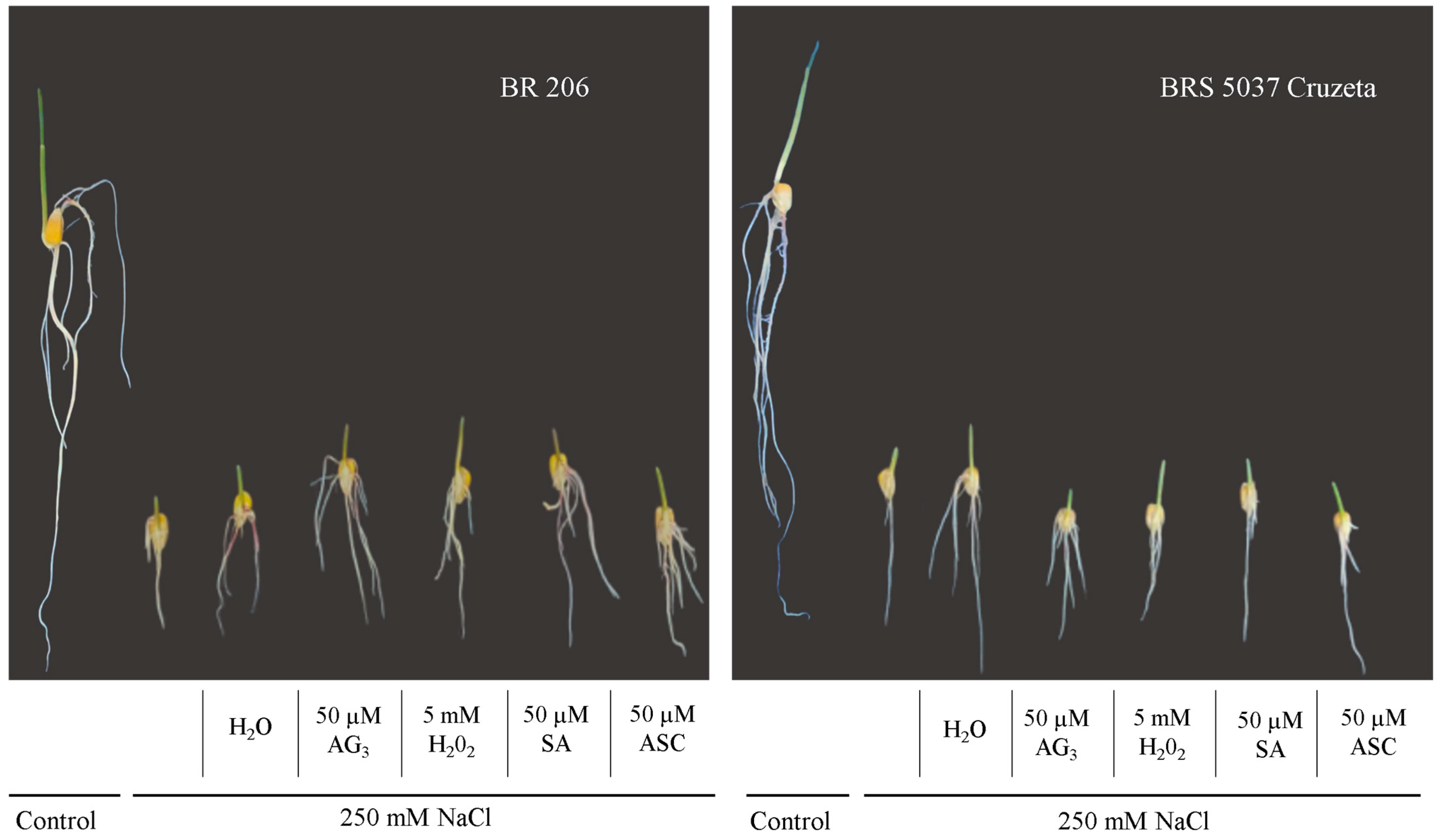
3.1. Germination and Seedling Length
3.2. Dry Mass of Seedlings and Salinity Tolerance Index
3.3. Osmotic Homeostasis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations Statistics (FAOSTAT). Estimative. 2020. Available online: http://www.fao.org/faostat/en/#data (accessed on 10 March 2022).
- Food and Agriculture Organization of the United Nations (FAO). Global Information and Early Warmning System 2021 (GIEWS). Country Briefs. Available online: https://www.fao.org/giews/countrybrief/country.jsp?code=BRA (accessed on 10 March 2022).
- Companhia Nacional de Abastecimento (CONAB). Acompanhamento da Safra 2021/22, Brasileira de Grãos. Brasília, DF, Sexto Levantamento. Available online: https://www.conab.gov.br/info-agro/safras/graos/boletim-da-safra-de-graos (accessed on 15 March 2022).
- Saxena, R.; Kumar, M.; Tomar, R.S. Plant responses and resilience towards drought and salinity stress. Plant Arch. 2019, 19, 50–58. Available online: http://www.plantarchives.org/SPL%20ISSUE%20SUPP%202,2019/50-58.pdf (accessed on 25 May 2022).
- Ferreira, P.A.; Silva, J.B.L.; Ruiz, H.A. Aspectos físicos e químicos de solos em regiões áridas e semiáridas. In Manejo da Salinidade na Agricultura: Estudo Básico e Aplicado, 2nd ed.; Gheyi, H.R., Dias, N.S., Lacerda, C.F., Gomes Filho, E., Eds.; INCTSal: Fortaleza, Brasil, 2016; Volume 1, pp. 17–34. [Google Scholar]
- Liang, W.; Ma, X.; Wan, P.; Liu, L. Plant salt-tolerance mechanism: A review. Biochem. Biophys. Res. Commun. 2018, 495, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Ludwiczak, A.; Osiak, M.; Cárdenas-Pérez, S.; Lubínska-Mielínska, S.; Piernik, A. Osmotic stress or ionic composition: Which affects the early growth of crop species more? Agronomy 2021, 11, 435. [Google Scholar] [CrossRef]
- Silva, H.A.; Oliveira, D.F.A.; Avelino, A.P.; Macêdo, C.E.C.; Barros-Galvão, T.; Voigt, E.L. Salt stress differentially regulates mobilisation of carbon and nitrogen reserves during seedling establishment of Pityrocarpa moniliformis. Plant Biol. 2019, 21, 1110–1118. [Google Scholar] [CrossRef] [PubMed]
- Andrade, W.L.; Melo, A.S.; Melo, Y.L.; Sá, F.V.S.; Rocha, M.M.; Oliveira, A.P.S.; Fernandes Júnior, P.I. Bradyrhizobium inoculation plus foliar application of salicylic acid mitigates water deficit effects on cowpea. J. Plant Growth Regul. 2021, 40, 656–667. [Google Scholar] [CrossRef]
- Sidhu, G.P.S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Tolerance and hyperaccumulation of cadmium by a wild, unpalatable herb Coronopus didymus (L.) Sm. (Brassicaceae). Ecotoxicol. Environ. Saf. 2017, 135, 209–215. [Google Scholar] [CrossRef]
- Ghaffari, H.; Tadayon, M.R.; Nadeem, M.; Cheema, M.; Razmjoo, J. Proline-mediated changes in antioxidant enzymatic activities and the physiology of sugar beet under drought stress. Acta Physiol. Plant. 2019, 41, 23. [Google Scholar] [CrossRef]
- Meena, M.; Divyanshu, K.; Kumar, S.; Swapnil, P.; Zehra, A.; Shukla, V.; Yadav, M.; Upadhyay, R.S. Regulation of L-proline biosynthesis, signal transduction, transport, accumulation and its vital role in plants during variable environmental conditions. Heliyon 2019, 5, e02952. [Google Scholar] [CrossRef]
- Akter, L.; Fakir, O.A.; Alam, M.K.; Islam, M.U.; Chakraborti, P.; Alam, M.J.; Rashid, M.H.; Begum, M.; Kader, M.A. Amelioration of salinity stress in maize seed germination and seedling growth attributes through seed priming. Open J. Soil Sci. 2018, 8, 137–146. [Google Scholar] [CrossRef]
- Costa, A.A.; Paiva, E.P.; Torres, S.B.; Souza Neta, M.L.; Pereira, K.T.O.; Leite, M.S.; Sá, F.V.S. Seed priming improves Salvia hispanica L. seed performance under salt stress. Acta Sci. Agron. 2021, 43, e52006. [Google Scholar] [CrossRef]
- Singh, P.K.; Shahi, S.K.; Singh, A.P. Effects of salt stress on physico-chemical changes in maize (Zea mays L.) plants in response to salicylic acid. Indian J. Plant Sci. 2015, 4, 69–77. Available online: https://www.cibtech.org/J-Plant-Sciences/PUBLICATIONS/2015/VOL-4-NO-1/13-JPS-DEC-016-2014-PRAMOD-EFFECTS-SALICYLIC.pdf (accessed on 15 March 2022).
- Lima, A.; Meiado, M. Discontinuous hydration alters seed germination under stress of two populations of cactus that occur in different ecosystems in Northeast Brazil. Seed Sci. Res. 2017, 27, 292–302. [Google Scholar] [CrossRef]
- Lima, A.T.; Cunha, P.H.J.; Dantas, B.F.; Meiado, M.V. Does discontinuous hydration of Senna spectabilis (DC.) H.S. Irwin & Barneby var. excelsa (Schrad.) H.S. Irwin & Barneby (Fabaceae) seeds confer tolerance to water stress during seed germination? J. Seed Sci. 2018, 40, 36–43. [Google Scholar] [CrossRef]
- Nicolau, P.B.; Silva, F.E.; Felix, F.C.; Torres, S.B.; Pacheco, M.V.; Pereira, M.D. Discontinuous hydration on the germination of Mimosa caesalpiniifolia and Pityrocarpa moniliformis seeds under water stress. Rev. Caatinga 2020, 33, 555–561. [Google Scholar] [CrossRef]
- Sarmento, E.C.S.; Oliveira, F.S.; Cabral, F.A.S.; Oliveira, D.F.; Dutra, A.D. Physiological potential of sorghum seeds under discontinuous hydration and water deficiency conditions. Rev. Ciência Agronômica 2020, 51, e20207200. [Google Scholar] [CrossRef]
- Santos Júnior, J.L.; Freitas, R.S.; Silva, E.C. Discontinuous hydration improves germination and drought tolerance in Annona squamosa seedlings. Res. Soc. Dev. 2021, 10, e56710313706. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, M.; Wakeel, A.; Siddique, K.H.M. Salt stress in maize: Effects, resistance mechanisms, and management. A review. Agron. Sustain. Dev. 2015, 35, 461–481. [Google Scholar] [CrossRef]
- Shi, R.; Tong, L.; Du, T.; Shukla, M.K. Response and modeling of hybrid maize seed vigor to water deficit at different growth stages. Water 2020, 12, 3289. [Google Scholar] [CrossRef]
- Ministério da Agricultura, Pecuária e Abastecimento (MAPA). Regras Para Análise de Sementes; MAPA/SDA: Brasília, Brazil, 2009. [Google Scholar]
- Pereira, K.T.O.; Sá, F.V.S.; Torres, S.B.; Paiva, E.P.; Alves, T.R.C.; Oliveira, R.R. Exogenous application of organic acids in maize seedlings under salt stress. Braz. J. Biol. 2021, 84, e250727. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef]
- Yemm, E.W.; Armar, E.C.; Ricketts, R.E. The determination of amino acid with ninhydrin. Analyst 1955, 80, 209–213. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Ferreira, D.F. Sisvar: A computer analysis system to fixed effects split plot type designs. Rev. Bras. Biom. 2019, 37, 529–535. [Google Scholar] [CrossRef]
- Asad, F.; Dilawar, N.; Rahman, N.; Wisal; Shakeel, M.; Ghazal, M. Effect of indole-3- acetic acid and gibberellic acid (GA3) on seeds germination, growth performance and biochemical constituents of Zea mays L. growing under the salt stress. Pure Appl. Biol. 2022, 11, 639–650. [Google Scholar] [CrossRef]
- Khan, A.; Bilal, S.; Khan, A.L.; Imran, M.; Shahzad, R.; Harrasi, A.A.; Rawahi, A.; Al-Azhri, M.; Mohanta, T.K.; Lee, I.-J. Silicon and gibberellins: Synergistic function in harnessing ABA signaling and heat stress tolerance in date palm (Phoenix dactylifera L.). Plants 2020, 9, 620. [Google Scholar] [CrossRef]
- Sá, F.V.S.; Brito, M.E.B.; Silva, L.A.; Moreira, R.C.L.; Paiva, E.P.; Souto, L.S. Exogenous application of phytohormones mitigates the effect of salt stress on Carica papaya plants. Rev. Bras. Eng. Agríc. Ambient. 2020, 24, 170–175. [Google Scholar] [CrossRef]
- Rodrigues, M.H.B.S.; Silva, J.N.; Alves, E.U.; Bruno, R.L.A. Hydrogen peroxide as a mitigation of salt stress on the germination of Myracroduon urundeuva (Allemão) Engl. Seeds. Sci. For. 2021, 49, e3557. [Google Scholar] [CrossRef]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Braz, R.S.; Lacerda, C.F.; Assis Júnior, R.N.; Ferreira, J.F.S.; Oliveira, A.C.; Ribeiro, A.A. Growth and physiology of maize under water salinity and nitrogen fertilization in two soils. Rev. Bras. Eng. Agríc. Ambient. 2019, 23, 907–913. [Google Scholar] [CrossRef]
- Catão, H.C.R.M.; Caixeta, F.; Lopes, A.M.; Nery-Silva, F.A.; Sá-Júnior, A. Antioxidant activity and physiological performance of popcorn seed after saline stress and analysis of seedling images. Ciência Agrotecnol. 2020, 44, e005020. [Google Scholar] [CrossRef]
- Soothar, M.K.; Hamani, A.K.M.; Sootaha, R.M.K.; Sun, J.; Yang, G.; Bhatti, S.M.; Traore, A. Assessment of acidic biochar on the growth, physiology and nutrients uptake of maize (Zea Mays L.) seedlings under salinity stress. Sustainability 2021, 13, 3150. [Google Scholar] [CrossRef]
- Roy, S.J.; Negrão, S.; Tester, M. Plantas de cultivo resistentes ao sal. Curr. Opin. Biotechnol. 2014, 26, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; Sikron, N.; Gendler, T.; Kazachkova, Y.; Barak, S.; Grafi, G.; Khozin-Goldberg, I.; Fait, A. Ecotypic variability in the metabolic response of seeds to diurnal hydration-dehydration cycles and its relationship to seed vigor. Plant Cell Physiol. 2012, 53, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, B.G.; Ribeiro, L.M.; Dias, D.S.; Mazzottini-dos-Santos, H.C.; Martins, C.P.S.; Lopes, P.S.N.; Mercadante-Simões, M.O. Embryo responses to extreme water events provide insights into the behavior of Butia capitata (Arecaceae) seed banks during hydration cycles. Environ. Exp. Bot. 2020, 169, 103904. [Google Scholar] [CrossRef]
- Hu, X.-L.; Yu, X.-M.; Chen, H.-Y.; Li, W.-Q. Turnover of glycerolipid metabolite pool and seed viability. Int. J. Mol. Sci. 2018, 19, 1417. [Google Scholar] [CrossRef]
- Chen, K.; Arora, R. Priming memory invokes seed stress-tolerance. Environ. Exp. Bot. 2013, 94, 33–45. [Google Scholar] [CrossRef]
- Bowne, J.; Bacic, A.; Tester, M.; Roessner, U. Abiotic stress and metabolomics. Annu. Plant Rev. Biol. Plant Metab. 2011, 43, 61–85. [Google Scholar] [CrossRef]
- Wan, Q.; Hongbo, S.; Zhaolong, X.; Jia, L.; Dayong, Z.; Yihong, H. Salinity tolerance mechanism of osmotin and osmotin-like proteins: A promising candidate for enhancing plant salt tolerance. Curr. Genom. 2017, 18, 553–556. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef]
- Sharma, L.; Priya, M.; Kaushal, N.; Bhandhari, K.; Chaudhary, S.; Dhankher, O.P.; Prasad, P.V.; Siddique, K.H.; Nayyar, H. Plant growth-regulating molecules as thermoprotectants: Functional relevance and prospects for improving heat tolerance in food crop. J. Exp. Bot. 2020, 71, 569–594. [Google Scholar] [CrossRef]

| F-Test (p-Value) | |||||
| Variation Sources | FGC | G | SL | RL | |
| DHCs | 0.0000 | 0.0000 | 0.0000 | 0.0000 | |
| Cultivars (C) | 0.0000 | 0.0000 | 0.0145 | 0.0000 | |
| DHCs × C | 0.000 | 0.0021 | 0.0215 | 0.0007 | |
| Means-test | |||||
| Cultivars | DHCs | FGC (%) | G (%) | SL(cm) | RL (cm) |
| BR 206 | 1 (control) | 100 ± 0.0 aA | 100 ± 0.0 aA | 7.8 ± 0.38 aA | 16.5 ± 0.12aA |
| 2 | 47 ± 4.2 dA | 95 ± 1.0 aA | 1.8 ± 0.08 bA | 3.8 ± 0.09 cA | |
| 3 | 77 ± 4.5 cA | 97 ± 1.3 aA | 2.1 ± 0.15 bA | 4.7 ± 0.31 bA | |
| 4 | 85 ± 3.3 bA | 97 ± 1.0 aA | 1.9 ± 0.10 bA | 5.2 ± 0.33 bA | |
| 5 | 81 ± 1.9 bA | 96 ± 0.8 aA | 2.1 ± 0.08 bA | 5.0 ± 0.11 bA | |
| 6 | 76 ± 1.5 cA | 98 ± 0.0 aA | 2.2 ± 0.01 bA | 4.9 ± 0.33 bA | |
| 7 | 70 ± 5.5 cA | 98 ± 0.8 aA | 1.9 ± 0.13 bA | 5.0 ± 0.14 bA | |
| BRS 5037 Cruzeta | 1 (control) | 98 ± 1.3 aA | 99 ± 0.5 aA | 7.9 ± 0.13aA | 14.0 ± 0.51 aB |
| 2 | 21 ± 3.1 dB | 85 ± 1.7 cB | 1.1 ± 0.13 cB | 3.5 ± 0.30 cA | |
| 3 | 45 ± 1.3 bB | 92 ± 1.0 bB | 1.7 ± 0.15 bA | 4.5 ± 0.33 bA | |
| 4 | 45 ± 3.3 bB | 92 ± 1.7 bB | 1.3 ± 0.08 cB | 4.9 ± 0.24 bA | |
| 5 | 45 ± 2.9 bB | 92 ± 1.7 bB | 1.8 ± 0.07 bA | 3.7 ± 0.47 cB | |
| 6 | 32 ± 1.4 cB | 86 ± 2.2 cB | 2.2 ± 0.10 bA | 4.8 ± 0.22 bA | |
| 7 | 38 ± 3.8 bB | 92 ± 1.0 bB | 2.1 ± 0.14 bA | 3.8 ± 0.19 cB | |
| F-Test (p-Value) | |||||
| Variation Sources | SDM | RDM | TDM | STI | |
| DHCs | 0.0000 | 0.0000 | 0.0000 | 0.0000 | |
| Cultivars (C) | 0.0000 | 0.0000 | 0.0000 | 0.0000 | |
| DHCs × C | 0.0000 | 0.0000 | 0.0000 | 0.0000 | |
| Means-test | |||||
| Cultivars | DHCs | SDM | RDM | TDM | STI |
| mg plant−1 | mg plant−1 | mg plant−1 | % | ||
| BR 206 | 1 (control) | 35.8 ± 0.5 aA | 50.6 ± 1.9 aA | 86.5 ± 1.8 aA | 100.0 ± 0.0 aA |
| 2 | 12.6 ± 1.0 dA | 15.9 ± 0.5 dA | 28.5 ± 1.3 dA | 32.9 ± 1.5 dA | |
| 3 | 21.5 ± 0.5 bA | 21.5 ± 1.1 cA | 43.1 ± 0.7 cA | 49.8 ± 0.8 cA | |
| 4 | 13.6 ± 0.4 dA | 34.6 ± 1.6 bA | 48.2 ± 1.8 bA | 55.8 ± 2.1 bA | |
| 5 | 15.8 ± 0.8 cA | 32.5 ± 0.8 bA | 48.3 ± 1.3 bA | 55.9 ± 1.5 bA | |
| 6 | 15.0 ± 1.2 cA | 31.7 ± 2.0 bA | 46.7 ± 1.3 bA | 54.0 ± 1.5 bA | |
| 7 | 13.9 ± 1.1 dA | 29.6 ± 1.2 bA | 43.5 ± 1.9 cA | 50.3 ± 2.2 cA | |
| BRS 5037 Cruzeta | 1 (control) | 25.8 ± 0.6 aB | 25.9 ± 0.7 aB | 51.7 ± 1.1 aB | 100.0 ± 0.0 aA |
| 2 | 5.5 ± 0.6 cB | 10.2 ± 0.4 bB | 15.8 ± 0.9 cB | 30.5 ± 1.7 eA | |
| 3 | 9.7 ± 0.4 bB | 13.3 ± 0.8 bB | 23.0 ± 1.0 bB | 44.5 ± 1.8 cB | |
| 4 | 6.5 ± 0.2 cB | 12.5 ± 0.5 bB | 19.1 ± 0.7 cB | 36.8 ± 1.4 dB | |
| 5 | 10.1 ± 0.4 bB | 12.0 ± 0.9 bB | 22.1 ± 1.2 bB | 42.7 ± 2.3 cB | |
| 6 | 11.6 ± 0.3 bB | 13.8 ± 0.7 bB | 25.4 ± 0.8 bB | 49.0 ± 1.6 bB | |
| 7 | 10.3 ± 0.1 bB | 11.6 ± 1.1 bB | 21.9 ± 1.1 bB | 42.4 ± 2.1 cB | |
| F-Test (p-Value) | ||||
| Variation Sources | TSS | AA | PRO | |
| DHCs | 0.0000 | 0.0000 | 0.0000 | |
| Cultivars (C) | 0.0000 | 0.0000 | 0.0000 | |
| DHCs × C | 0.0000 | 0.0001 | 0.0000 | |
| Means-test | ||||
| Cultivars | DHCs | TSS mg GLU g−1 FM | AA µmol GLY g−1 FM | PRO µmol PRO g−1 FM |
| BR 206 | 1 (control) | 50. 1 ± 2.0 aA | 47.5 ± 2.3 dA | 0.7 ± 0.2 eA |
| 2 | 51.2 ± 0.5 aA | 68.9 ± 4.4 bA | 59.7 ± 0.8 aA | |
| 3 | 47.1 ± 0.4 bA | 65.1 ± 5.1 cA | 48.5 ± 1.7 cA | |
| 4 | 45.1 ± 1.5 bA | 79.9 ± 1.5 aA | 48.3 ± 1.9 cA | |
| 5 | 44.2 ± 2.1 bA | 57.7 ± 1.1 cA | 41.7 ± 2.4 dA | |
| 6 | 49.5 ± 1.4 aA | 78.3 ± 1.9 aA | 58.2 ± 1.5 aA | |
| 7 | 47.5 ± 3.0 bA | 61.3 ± 2.9 cA | 53.2 ± 2.7 bA | |
| BRS 5037 Cruzeta | 1 (control) | 25.8 ± 0.9 cB | 22.6 ± 1.1 dB | 0.3 ± 0.01 cA |
| 2 | 39.9 ± 0.6 bB | 46.9 ± 1.8 cB | 34.8 ± 4.0 bB | |
| 3 | 35.7 ± 1.2 bB | 55.7 ± 1.5 bB | 30.5 ± 0.4 bB | |
| 4 | 39.0 ± 0.7 bB | 68.8 ± 1.5 aB | 45.4 ± 0.9 aA | |
| 5 | 39.7 ± 1.1 bB | 56.5 ± 5.0 bB | 29.8 ± 1.8 bB | |
| 6 | 49.6 ± 1.1 aA | 62.5 ± 5.1 aB | 33.6 ± 0.5 bB | |
| 7 | 37.0 ± 1.0 bB | 67.8 ± 2.5 aA | 32.5 ± 0.7 bB | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, K.T.O.; Torres, S.B.; de Paiva, E.P.; Alves, T.R.C.; de Souza Neta, M.L.; Venâncio, J.B.; Souto, L.S.; Benedito, C.P.; Peixoto, T.D.C.; Ferreira Neto, M.; et al. Discontinuous Hydration Cycles with Elicitors Improve Germination, Growth, Osmoprotectant, and Salt Stress Tolerance in Zea mays L. Agriculture 2023, 13, 964. https://doi.org/10.3390/agriculture13050964
Pereira KTO, Torres SB, de Paiva EP, Alves TRC, de Souza Neta ML, Venâncio JB, Souto LS, Benedito CP, Peixoto TDC, Ferreira Neto M, et al. Discontinuous Hydration Cycles with Elicitors Improve Germination, Growth, Osmoprotectant, and Salt Stress Tolerance in Zea mays L. Agriculture. 2023; 13(5):964. https://doi.org/10.3390/agriculture13050964
Chicago/Turabian StylePereira, Kleane Targino Oliveira, Salvador Barros Torres, Emanoela Pereira de Paiva, Tatianne Raianne Costa Alves, Maria Lilia de Souza Neta, Jefferson Bittencourt Venâncio, Lauter Silva Souto, Clarisse Pereira Benedito, Tayd Dayvison Custódio Peixoto, Miguel Ferreira Neto, and et al. 2023. "Discontinuous Hydration Cycles with Elicitors Improve Germination, Growth, Osmoprotectant, and Salt Stress Tolerance in Zea mays L." Agriculture 13, no. 5: 964. https://doi.org/10.3390/agriculture13050964





