Effect of Microbial Preparation and Biomass Incorporation on Soil Biological and Chemical Properties
Abstract
:1. Introduction
| Treatments Applied | Experimental Variants | Effects of the Treatment on Soil Properties | References |
|---|---|---|---|
| 5 straw utilization and fertilization modes | SM—straw mulching; SC—straw crushing; CM—cattle manure; NSR—control with no straw return; CK—control without fertilizers | Cattle manure—the most beneficial effects on soil fertility and bacterial diversity. | [36] |
| 3 fertilization treatments | CK—no-fertilizer; NPK—nitrogen; phosphorus, and potassium fertilizers; NPKS—NPK plus straw | Straw addition—bacterial abundance unchanged, Actinomycetes abundance decreased, fungal abundance significantly increased. | [37] |
| 2 planting patterns, 6 nitrogen fertilizer doses | SR—straw returning; TP—traditional planting (straw removed from the field after harvested) N fertilizer doses: 0, 100, 150, 200, 250, and 300 kg N ha−1 | SR—significantly increased soil fertility, enzymatic activities, community diversity, and composition of bacterial and fungal communities compared to TP; TN, SOC—closely correlated with bacterial community composition. | [38] |
| 3 mulching treatments | CK—no mulching; SM—straw mulching; PM—plastic film mulching | SM—no effect on bacterial diversity and richness; enhanced fungal diversity and richness compared to CK in subsoil layers; enhanced soil C and N fractions compared to PM and CK. Bacterial diversity correlated with soil C and N fractions. | [39] |
| 4 fertilization treatments | CK—only chemical NPK fertilizers; the N substituted 20% by organic manure OM, straw SW, organic manure and straw (1:1) OMSW | Organic manure and straw application (especially in the OMSW) can increase soil N contents, but reduce N loss. | [40] |
| 6 straw utilization and nitrogen fertilization modes | straw return rates (50%, 100%); N fertilizer doses (270, 360, 450, 540 kg N ha−1 yr−1) | 50% straw return combined with 450 or 540 kg N ha−1 yr−1—increased soil nitrogen, available potassium, and available phosphorus contents. The long-term combined application of 100% straw returning and higher N fertilizer (>450 kg ha−1 yr−1)—not appropriate for soil health; the risk of disease and pollution in soil. | [41] |
| 6 fertilization treatments | CK—unfertilized control; N—nitrogen fertilizer; NP—nitrogen and phosphorus (P) fertilizers; NP + S—straw plus N and P fertilizers; FYM—farmyard manure; NP + FYM—farmyard manure plus N and P fertilizers | Organic manure with inorganic fertilizers—increased SOC and TN contents; without straw or manure—soil available K content declined. | [42] |
| 3 EM and fertilization treatments | EM compost treatment; traditional compost treatment; unfertilized control. | Soil organic matter, total N, available P, available K content—higher in the EM compost plot than in the traditional compost plot. | [43] |
| 2 EM treatments | soil fertilization with EM; unfertilized control. | EM application—reduction in the content of organic carbon (TOC) | [29] |
| 4 EM and fertilization treatments | T1 control—mineral fertilizers; T2—a diluted solution of EM sprayed on the soil surface; T3—green manure added as soil mulch above the ground; T4— mixture of EM1 and green manure. | T2 significantly increased the availability of phosphorous, potassium, and total nitrogen compared to T1. EM1 and green manure improved forage yield and soil properties. | [44] |
| 7 EM treatments | (i) the spraying agent EMA; (ii) EMA with the EM enriched organic substrate Bokashi; (iii) EMA with Bokashi and farmyard manure; controls to (i)–(iii)—the same treatments with sterilized EM preparations; control without EM application. | (i)—no significant differences to the untreated control (treatment without EM application) for the investigated soil parameters. ((ii) and (iii)—significant differences to the untreated control for soil microbial parameters (not consistent throughout the parameters and sampling times). Treatments with living EM compared with its sterilised control treatments—no differences on any of the parameters. EMA did not improve soil quality. | [45] |
2. Materials and Methods
2.1. Experiment Location and Layout
- A1—
- No manure introduction and no straw incorporation;
- A2—
- No manure introduction and straw incorporation;
- A3—
- Manure introduction and no straw incorporation;
- A4—
- Manure introduction and straw incorporation.
- B1—
- Without biopreparation EM application (EM × 0);
- B2—
- Biopreparation EM single application, added to the soil during post-harvest treatment in October; dose: 40 dm3·ha−1 (EM × 1);
- B3—
- Biopreparation EM dual application: added to the soil the soil during post-harvest cultivation in autumn at a dose of 20 dm3·ha−1 and EM sprayed on leaves; dose: 20 dm3·ha−1 at BBCH 21–23 (EM × 2).
2.2. Soil Samples
2.3. Microbial Analyses
2.4. Soil Chemical Analyses
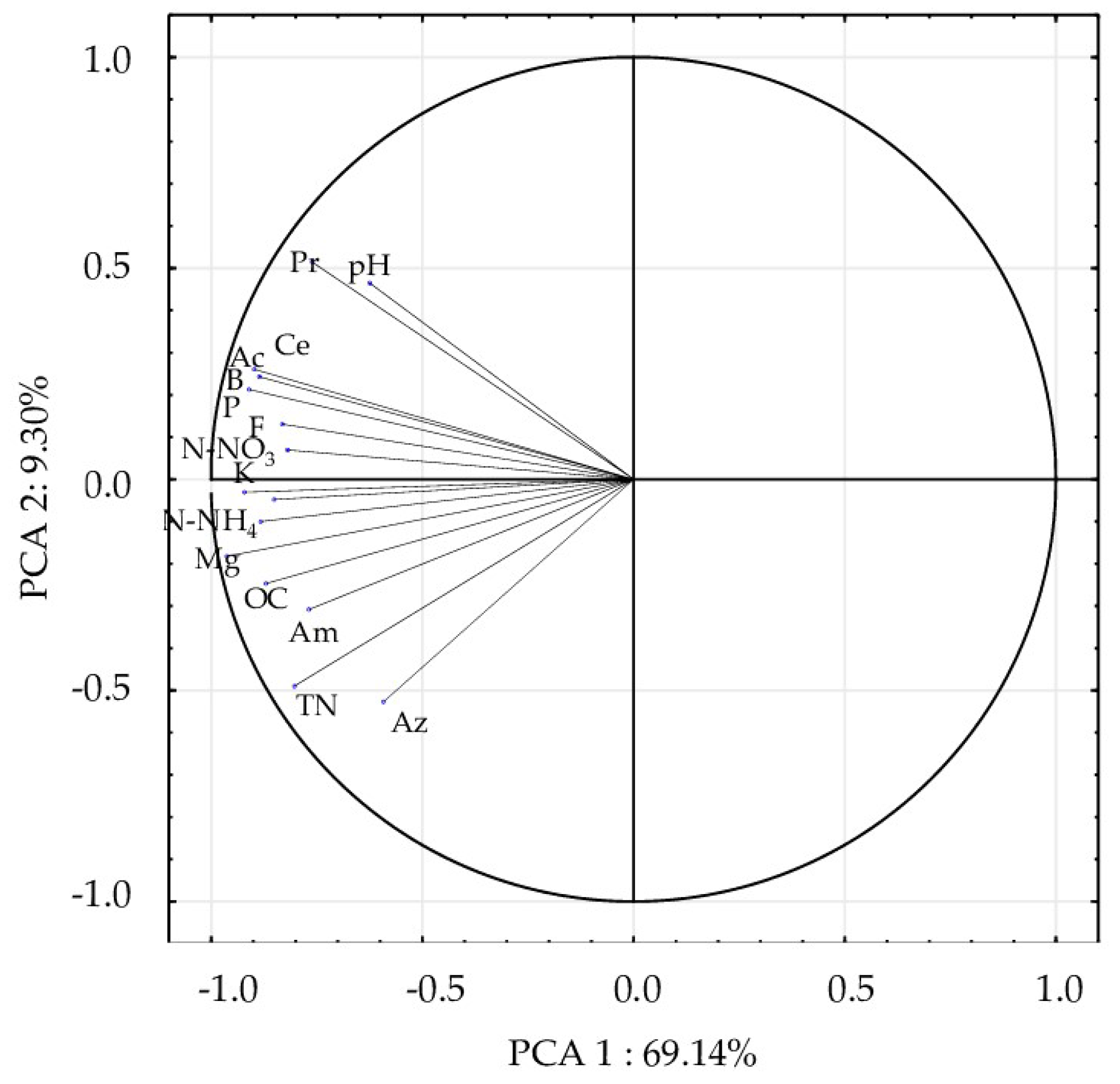
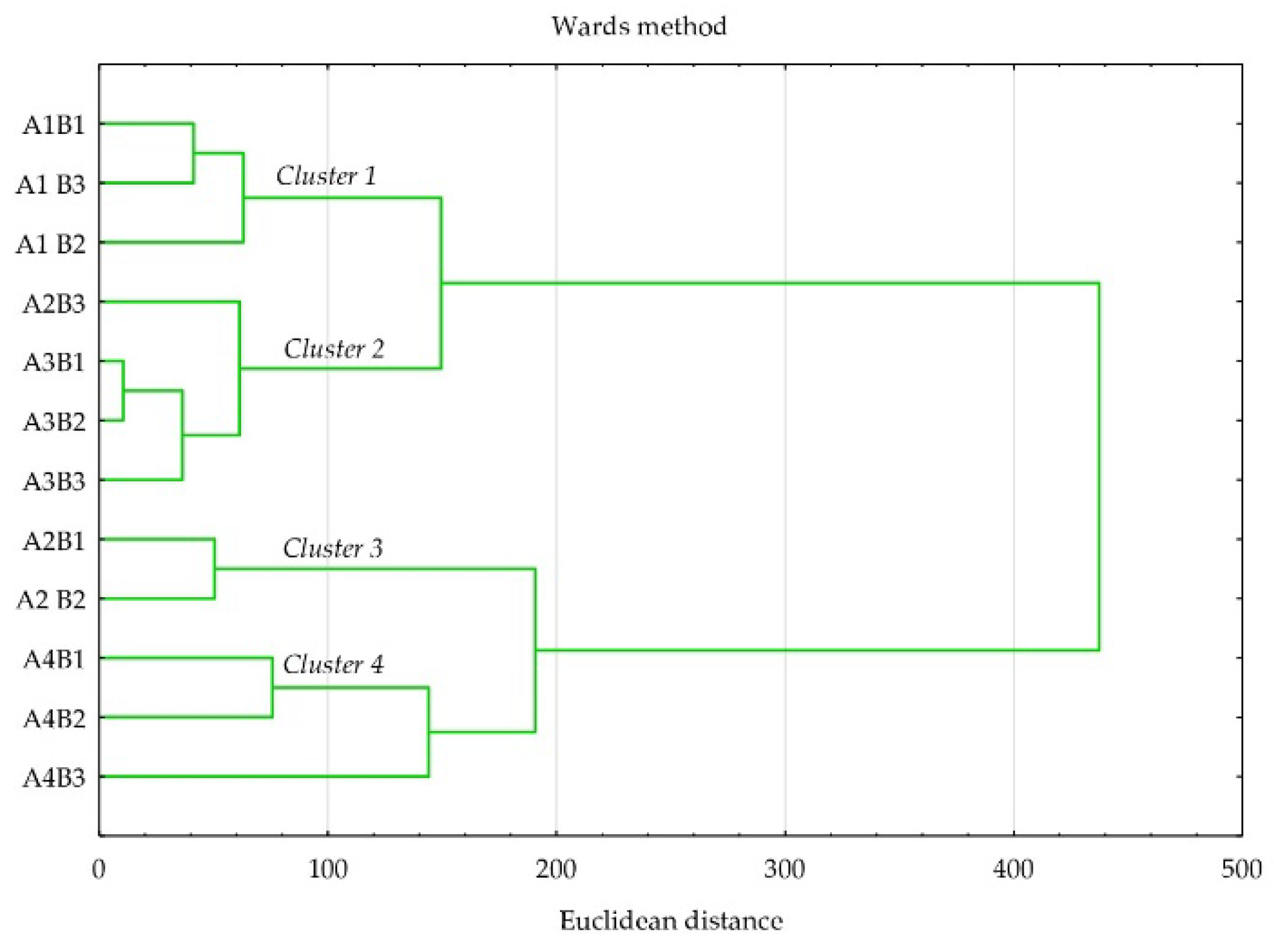
2.5. Data Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cui, Z.; Zhang, H.; Chen, X.; Zhang, C.; Ma, W.; Huang, C.; Zhang, W.; Mi, G.; Miao, Y.; Li, X.; et al. Pursuing sustainable productivity with millions of smallholder farmers. Nature 2018, 555, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D. Benefits of intensive agricultural intercropping. Nat. Plants 2020, 6, 604–605. [Google Scholar] [CrossRef]
- Baaken, M.C. Sustainability of agricultural practices in Germany: A literature review along multiple environmental domains. Reg. Environ. Chang. 2022, 22, 39. [Google Scholar] [CrossRef]
- Hou, D.; Bolan, N.S.; Tsang, D.C.W.; Kirkham, M.B.; O’Connor, D. Sustainable soil use and management: An interdisciplinary and systematic approach. Sci. Total Environ. 2020, 729, 138961. [Google Scholar] [CrossRef] [PubMed]
- Rojas, R.V.; Achouri, M.; Maroulis, J.; Caon, L. Healthy soils: A prerequisite for sustainable food security. Environ. Earth Sci. 2016, 75, 180. [Google Scholar] [CrossRef]
- Montanarella, L.; Panagos, P. The relevance of sustainable soil management within the European Green Deal. Land Use Policy 2021, 100, 104950. [Google Scholar] [CrossRef]
- Björnsson, L.; Prade, T. Sustainable Cereal Straw Management: Use as Feedstock for Emerging Biobased Industries or Cropland Soil Incorporation? Waste Biomass Valoriz. 2021, 12, 5649–5663. [Google Scholar] [CrossRef]
- Iriti, M.; Scarafoni, A.; Pierce, S.; Castorina, G.; Vitalini, S. Soil Application of Effective Microorganisms (EM) Maintains Leaf Photosynthetic Efficiency, Increases Seed Yield and Quality Traits of Bean (Phaseolus vulgaris L.) Plants Grown on Different Substrates. Int. J. Mol. Sci. 2019, 20, 2327. [Google Scholar] [CrossRef]
- Ogura, T.; Date, Y.; Masukujane, M.; Coetzee, T.; Akashi, K.; Kikuchi, J. Improvement of physical, chemical and biological properties of aridisol from Botswana by the incorporation of torrefied biomass. Sci. Rep. 2016, 6, 28011. [Google Scholar] [CrossRef]
- Sanchez, F.G.; Carter, E.A.; Klepac, J.F. Enhancing the soil organic matter pool through biomass incorporation. Biomass Bioenergy 2003, 24, 337–349. [Google Scholar] [CrossRef]
- Soto Boni, T.; Pujol Pereira, E.I.; Avelino Santos, A.; Rodrigues Cassiolato, A.M.; Maltoni, K.L. Biomass residues improve soil chemical and biological properties reestablishing native species in an exposed subsoil in Brazilian Cerrado. PLoS ONE 2022, 17, e0270215. [Google Scholar] [CrossRef] [PubMed]
- Coonan, E.C.; Richardson, A.E.; Kirkby, C.A.; Kirkegaard, J.A.; Amidy, M.R.; Strong, C.L. Soil fertility and nutrients mediate soil carbon dynamics following residue incorporation. Nutr. Cycl. Agroecosyst. 2020, 116, 205–221. [Google Scholar] [CrossRef]
- Voltr, V.; Menšík, L.; Hlisnikovský, L.; Hruška, M.; Pokorný, E.; Pospíšilová, L. The Soil Organic Matter in Connection with Soil Properties and Soil Inputs. Agronomy 2021, 11, 779. [Google Scholar] [CrossRef]
- Jensen, J.L.; Thomsen, I.K.; Eriksen, J.; Christensen, B.T. Spring Barley Grown for Decades with Straw Incorporation and Cover Crops: Effects on Crop Yields and N Uptake. Field Crop. Res. 2021, 270, 108228. [Google Scholar] [CrossRef]
- Ray, R.L.; Griffin, R.W.; Fares, A.; Elhassan, A.; Awal, R.; Woldesenbet, S.; Risch, E. Soil CO2 Emission in Response to Organic Amendments, Temperature, and Rainfall. Sci. Rep. 2020, 10, 5849. [Google Scholar] [CrossRef] [PubMed]
- Paustian, K.; Larson, E.; Kent, J.; Marx, E.; Swan, A. Soil C sequestration as a biological negative emissions strategy. Front. Clim. 2019, 1, 8. [Google Scholar] [CrossRef]
- Monforti, F.; Lugato, E.; Motola, V.; Bódis, K.; Scarlat, N.; Dallemand, J. Optimal Energy Use of Agricultural Crop Residues Preserving Soil Organic Carbon Stocks in Europe. Renew. Sustain. Energy Rev. 2015, 44, 519–529. [Google Scholar] [CrossRef]
- Cuong, T.T.; Le, H.A.; Khai, N.M.; Hung, P.A.; Linh, L.T.; Thanh, N.V.; Tri, N.D.; Huan, N.X. Renewable energy from biomass surplus resource: Potential of power generation from rice straw in Vietnam. Sci. Rep. 2021, 11, 792. [Google Scholar] [CrossRef]
- Krause, H.M.; Stehle, B.; Mayer, J.; Mayer, M.; Steffens, M.; Mäder, P.; Fliessbach, A. Biological soil quality and soil organic carbon change in biodynamic, organic, and conventional farming systems after 42 years. Agron. Sustain. Dev. 2022, 42, 117. [Google Scholar] [CrossRef]
- Bickel, S.; Or, D. Soil bacterial diversity mediated by microscale aqueous-phase processes across biomes. Nat. Commun. 2020, 11, 116. [Google Scholar] [CrossRef]
- Ray, P.; Lakshmanan, V.; Labbé, J.L.; Craven, K.D. Microbe to Microbiome: A Paradigm Shift in the Application of Microorganisms for Sustainable Agriculture. Front. Microbiol. 2020, 11, 622926. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.M.; Khan, I.M.; Shah, T.I.; Bangroo, S.A.; Kirmani, N.A.; Nazir, S.; Malik, A.R.; Aezum, A.M.; Mir, Y.H.; Hilal, A.; et al. Soil Microbiome: A Treasure Trove for Soil Health Sustainability under Changing Climate. Land 2022, 11, 1887. [Google Scholar] [CrossRef]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The Role of Soil Microorganisms in Plant Mineral Nutrition-Current Knowledge and Future Directions. Front. Plant Sci. 2017, 8, 1617. [Google Scholar] [CrossRef] [PubMed]
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.E.; De Deyn, G.; de Goede, R.; Fleskens, L.; Geissen, V.; Kuyper, T.W.; Mäder, P.; et al. Soil quality—A critical review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Suman, J.; Rakshit, A.; Ogireddy, S.D.; Singh, S.; Gupta, C.; Chandrakala, J. Microbiome as a Key Player in Sustainable Agriculture and Human Health. Front. Soil Sci. 2022, 2, 821589. [Google Scholar] [CrossRef]
- Golec, A.F.C.; Pérez, P.G.; Lokare, C. Effective microorganisms: Myth or reality? Rev. Peru. Biol. 2007, 14, 315–319. [Google Scholar]
- Sawicka, B.; Pszczółkowski, P.; Kiełtyka-Dadasiewicz, A.; Barbaś, P.; Ćwintal, M.; Krochmal-Marczak, B. The Effect of Effective Microorganisms on the Quality of Potato Chips and French Fries. Appl. Sci. 2021, 11, 1415. [Google Scholar] [CrossRef]
- Pierce, S.; Quaglino, F.; Montagna, M.; Spada, A.; Casati, P.; Iriti, M. Evaluation of effective microorganisms® efficacy on ‘Candidatus Phytoplasma solani’-infected and healthy periwinkle plants. Mitt. Klosterneubg. Rebe Wein Obstbau Früchteverwert. 2016, 66, 89–92. [Google Scholar]
- Hu, C.; Qi, Y. Long-term effective microorganisms application promote growth and increase yields and nutrition of wheat in China. Eur. J. Agron. 2013, 46, 63–67. [Google Scholar] [CrossRef]
- Allahverdiev, S.R.; Minkova, N.O.; Yarigin, D.V.; Gündüz, G. The Silent Heroes: Effective microorganisms. Orman. Derg. 2015, 10, 24–28. [Google Scholar]
- Yang, X.; Lai, J.L.; Zhang, Y.; Luo, X.G. Reshaping the microenvironment and bacterial community of TNT- and RDX-contaminated soil by combined remediation with vetiver grass (Vetiveria ziznioides) and effective microorganism (EM) flora. Sci. Total Environ. 2022, 815, 152856. [Google Scholar] [CrossRef] [PubMed]
- Tsui, L.; Paul, A.; Chen, Y.-T.; Tz-Chi, E. Potential mechanisms contributing to the high cadmium removal efficiency from contaminated soil by using effective microorganisms as novel electrolyte in electrokinetic remediation applications. Environ. Res. 2022, 215, 114239. [Google Scholar] [CrossRef] [PubMed]
- Talaat, N.B. Effective microorganisms: An innovative tool for inducing common bean (Phaseolus vulgaris L.) salt-tolerance by regulating photosynthetic rate and endogenous phytohormones production. Sci. Hortic. 2019, 250, 254–265. [Google Scholar] [CrossRef]
- Abd El-Mageed, T.A.; Rady, M.M.; Taha, R.S.; Abd El Azeam, S.; Simpson, C.R.; Semida, W.M. Effects of integrated use of residual sulfur-enhanced biochar with effective microorganisms on soil properties, plant growth and short-term productivity of Capsicum annuum under salt stress. Sci. Hortic. 2020, 261, 108930. [Google Scholar] [CrossRef]
- Cui, Q.; Xia, J.B.; Yang, H.J.; Liu, J.T.; Shao, P.S. Biochar and effective microorganisms promote Sesbania cannabina growth and soil quality in the coastal saline-alkali soil of the Yellow River Delta, China. Sci. Total Environ. 2021, 756, 143801. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, H.; Xie, W.; Yang, Z.; Lv, Q. Long-term effects of maize straw return and manure on the microbial community in cinnamon soil in Northern China using 16S rRNA sequencing. PLoS ONE 2021, 16, e0249884. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, S. Linkages between straw decomposition rate and the change in microbial fractions and extracellular enzyme activities in soils under different long-term fertilization treatments. PLoS ONE 2018, 13, e0202660. [Google Scholar] [CrossRef]
- Yang, L.; Muhammad, I.; Chi, Y.X.; Wang, D.; Zhou, X.B. Straw Return and Nitrogen Fertilization to Maize Regulate Soil Properties, Microbial Community, and Enzyme Activities Under a Dual Cropping System. Front. Microbiol. 2022, 13, 823963. [Google Scholar] [CrossRef]
- Fu, X.; Wang, J.; Sainju, U.M.; Zhao, F.; Liu, W. Soil microbial community and carbon and nitrogen fractions responses to mulching under winter wheat. Appl. Soil Ecol. 2019, 139, 64068. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, Q.; Liu, X.; Jing, X.; Shi, C.; Zheng, L. Organic manure input and straw cover improved the community structure of nitrogen cycle function microorganism driven by water erosion. Int. Soil Water Conserv. Res. 2022, 10, 129–142. [Google Scholar] [CrossRef]
- Yu, M.; Wang, Q.; Su, Y.; Xi, H.; Qiao, Y.; Guo, Z.; Wang, Y.; Shen, A. Response of Soil Environment and Microbial Community Structure to Different Ratios of Long-Term Straw Return and Nitrogen Fertilizer in Wheat–Maize System. Sustainability 2023, 15, 1986. [Google Scholar] [CrossRef]
- Liu, E.; Yan, C.; Mei, X.; He, W.; Bing, S.H.; Ding, L.; Liu, Q.; Liu, S.; Fan, T. Longterm effect of chemical fertilizer, straw, and manure on soil chemical and biological properties in northwest China. Geoderma 2010, 158, 173–180. [Google Scholar] [CrossRef]
- Tołoczko, W.; Trawczynska, A.; Niewiadomski, A. Content of organic compounds in soils fertilized with EM preparation. Soil Sci. Ann. 2009, 60, 97–101. [Google Scholar]
- Ismail, S.M. Influence of effective microorganisms and green manure on soil properties and productivity of pearl millet alfalfa grown on sandy loam in Saudi Arabia. Afr. J. Microb. Res. 2013, 7, 375–382. [Google Scholar]
- Mayer, J.; Scheid, S.; Widmer, F.; Fließach, A.; Oberholzer, H.-R. How effective are Effective microorganisms (EM)? Results from a field study in temperate climate. Appl. Soil Ecol. 2010, 46, 230–239. [Google Scholar] [CrossRef]
- FAO IUSSWorking Group WRB. World References Base for Soil Resources; FAO: Rome, Italy, 2015; p. 132. [Google Scholar]
- United States Department of Agriculture. Soil Mechanics Level I Module 3 USDA Soil Textural Classification Study Guide; United States Department of Agriculture: Washington, DC, USA, 1987.
- Lamparski, R.; Kotwica, K. Effect of the use of pro-ecological treatments and previous crop straw on the weed infestation of winter wheat and spring barley cultivated as short-term monoculture. Acta Sci. Pol. Agric. 2020, 19, 201–212. [Google Scholar]
- Kotwica, K.; Jaskulska, I.; Gałęzewski, L.; Jaskulski, D.; Lamparski, R. Spring wheat yield in short-term monoculture depending on the tillage method, use of organic matter and a biostimulant. Acta Sci. Pol. Agric. 2014, 13, 19–28. [Google Scholar]
- Atlas, R.M. Handbook of Microbiological Media; CRC Press: Boca Raton, FL, USA, 2010; ISBN 978-0-429-13049-6. [Google Scholar]
- Crawford, D.L.; Lynch, J.M.; Whipps, J.M.; Ousley, M.A. Isolation and Characterization of Actinomycete Antagonists of a Fungal Root Pathogen. Appl. Environ. Microbiol. 1993, 59, 3899–3905. [Google Scholar] [CrossRef]
- Gupta, P.; Samant, K.; Sahu, A. Isolation of Cellulose-Degrading Bacteria and Determination of Their Cellulolytic Potential. Int. J. Microbiol. 2012, 2012, 578925. [Google Scholar] [CrossRef]
- Alef, K.; Nannipieri, P. Methods in Applied Soil Microbiology and Biochemistry; Academic Press: London, UK, 1995; ISBN 9780125138406. [Google Scholar]
- Aquilanti, L.; Favilli, F.; Clemeti, F. Comparison of different strategies for isolation and preliminary identification of Azotobacter from soil samples. Soil. Biol. Biochem. 2004, 36, 1475–1483. [Google Scholar] [CrossRef]
- PN-ISO 10390. Chemical and Agricultural Analysis—Determining Soil pH; Polish Standards Committee: Warszawa, Poland, 1997. [Google Scholar]
- PN-R-04023. Agricultural Analysis—Determination of the Content of Available Phosphorus in Mineral Soils; Polish Standards Committee: Warszawa, Poland, 1996. [Google Scholar]
- PN-R-04022. Agricultural Analysis—Determination of the Content Available Potassium in Mineral Soils; Polish Standards Committee: Warszawa, Poland, 1996. [Google Scholar]
- PN-R-04020. Agricultural Analysis. Determination of the Content Available Magnesium; Polish Standards Committee: Warszawa, Poland, 1994. [Google Scholar]
- Kotwica, K.; Breza-Boruta, B.; Bauza-Kaszewska, J.; Kanarek, P.; Jaskulska, I.; Jaskulski, D. The Cumulative Effect of Various Tillage Systems and Stubble Management on the Biological and Chemical Properties of Soil in Winter Wheat Monoculture. Agronomy 2021, 11, 1726. [Google Scholar] [CrossRef]
- Statistica, Data Analysis Software System; Version 12; TIBCO Software Inc.: Palo Alto, CA, USA, 2019. Available online: https://www.tibco.com/products/data-science (accessed on 6 March 2023).
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Gebska, M.; Grontkowska, A.; Swiderek, W.; Golebiewska, B. Farmer Awareness and Implementation of Sustainable Agriculture Practices in Different Types of Farms in Poland. Sustainability 2020, 12, 8022. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M. Simplifying the complexity of the soil microbiome to guide the development of next-generation SynComs. J. Sustain. Agric. Environ. 2022, 1, 9–15. [Google Scholar] [CrossRef]
- Zhen, Z.; Liu, H.; Wang, N.; Guo, L.; Meng, J.; Ding, N.; Wu, G.; Jiang, G. Effects of manure compost application on soil microbial community diversity and soil microenvironments in a temperate cropland in China. PLoS ONE 2014, 9, e108555. [Google Scholar] [CrossRef]
- Watts, D.B.; Torbert, H.A.; Feng, Y.; Prior, S.A. Soil Microbial Community Dynamics as Influenced by Composted Dairy Manure, Soil Properties, and Landscape Position. Soil Sci. 2010, 175, 474–486. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, X.-X.; Guo, X.; Wang, D.; Chu, H. Bacterial diversity in soils subjected to long-term chemical fertilization can be more stably maintained with the addition of livestock manure than wheat straw. Soil Biol. Biochem. 2015, 88, 9–18. [Google Scholar] [CrossRef]
- Suyal, D.C.; Soni, R.; Singh, D.K.; Goel, R. Microbiome change of agricultural soil under organic farming practices. Biologia 2021, 76, 1315–1325. [Google Scholar] [CrossRef]
- Guan, X.; Wei, L.; Turner, N.C.; Ma, S.; Yang, M.; Wang, T. Improved straw management practices promote in situ straw decomposition and nutrient release, and increase crop production. J. Clean. Prod. 2020, 250, 119514. [Google Scholar] [CrossRef]
- Higa, T.; Parr, J.F. Beneficial and Effective Microorganisms for a Sustainable Agriculture and Environment. 1994. Available online: http://www.em-la.com/archivos-de-usuario/base_datos/ (accessed on 10 March 2023).
- Abd El-Mageed, T.A.; Gyushi, M.A.H.; Hemida, K.A.; El-Saadony, M.T.; Abd El-Mageed, S.A.; Abdalla, H.; AbuQamar, S.F.; El-Tarabily, K.A.; Abdelkhalik, A. Coapplication of Effective Microorganisms and Nanomagnesium Boosts the Agronomic, Physio-Biochemical, Osmolytes, and Antioxidants Defenses Against Salt Stress in Ipomoea batatas. Front. Plant Sci. 2022, 13, 883274. [Google Scholar] [CrossRef]
- Pranagal, J.; Ligęza, S.; Smal, H. Impact of Effective Microorganisms (EM) Application on the Physical Condition of Haplic Luvisol. Agronomy 2020, 10, 1049. [Google Scholar] [CrossRef]
- Gross, A.; Glaser, B. Meta-analysis on how manure application changes soil organic carbon storage. Sci. Rep. 2021, 11, 5516. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Kapoor, K.K.; Gupta, A.P. Impact of organic manures with and without mineral fertilizers on soil chemical and biological properties under tropical conditions. J. Plant. Nutr. Soil Sci. 2005, 168, 117–122. [Google Scholar] [CrossRef]
- Han, S.H.; An, J.Y.; Hwang, J.; Bin Kim, S.; Park, B.B. The effects of organic manure and chemical fertilizer on the growth and nutrient concentrations of yellow poplar (Liriodendron tulipifera Lin.) in a nursery system. For. Sci. Technol. 2016, 12, 137–143. [Google Scholar]
- Powlson, D.; Bhogal, A.; Chambers, B.; Coleman, K.; Macdonald, A.; Goulding, K.; Whitmore, A. The potential to increase soil carbon stocks through reduced tillage or organic material additions in England and Wales: A case study. Agric. Ecosyst. Environ. 2012, 146, 23–33. [Google Scholar] [CrossRef]
- Xia, L.; Lam, S.K.; Wolf, B.; Kiese, R.; Chen, D.; Butterbach-Bahl, K. Trade-offs between soil carbon sequestration and reactive nitrogen losses under straw return in global agroecosystems. Glob. Chang. Biol. 2018, 24, 5919–5932. [Google Scholar] [CrossRef]
- Akhtar, K.; Wang, W.; Ren, G.; Khan, A.; Feng, Y.; Yang, G. Changes in Soil Enzymes, Soil Properties, and Maize Crop Productivity under Wheat Straw Mulching in Guanzhong, China. Soil Tillage Res. 2018, 182, 94–102. [Google Scholar] [CrossRef]
- Wei, T.; Zhang, P.; Wang, K.; Ding, R.; Yang, B.; Nie, J.; Jia, Z.; Han, Q. Effects of wheat straw incorporation on the availability of soil nutrients and enzyme activities in semiarid areas. PLoS ONE 2015, 10, e012099. [Google Scholar]
- Wang, X.; Jia, Z.; Liang, L.; Zhao, Y.; Yang, B.; Ding, R.; Wang, J.; Nie, J. Changes in Soil Characteristics and Maize Yield under Straw Returning System in Dryland Farming. Field Crops Res. 2018, 218, 11–17. [Google Scholar] [CrossRef]
- Li, J.; Li, H.; Zhang, Q.; Shao, H.; Gao, C.; Zhang, X. Effects of Fertilization and Straw Return Methods on the Soil Carbon Pool and CO2 Emission in a Reclaimed Mine Spoil in Shanxi Province, China. Soil Tillage Res. 2019, 195, 104361. [Google Scholar] [CrossRef]
- Han, P.; Zhang, W.; Wang, G.; Sun, W.; Huang, Y. Changes in soil organic carbon in croplands subjected to fertilizer management: A global meta-analysis. Sci. Rep. 2016, 6, 27199. [Google Scholar] [CrossRef] [PubMed]
- Szymanek, M.; Dziwulska-Hunek, A.; Zarajczyk, J.; Michałek, S.; Tanaś, W. The Influence of Red Light (RL) and Effective Microorganism (EM) Application on Soil Properties, Yield, and Quality in Wheat Cultivation. Agronomy 2020, 10, 1201. [Google Scholar] [CrossRef]

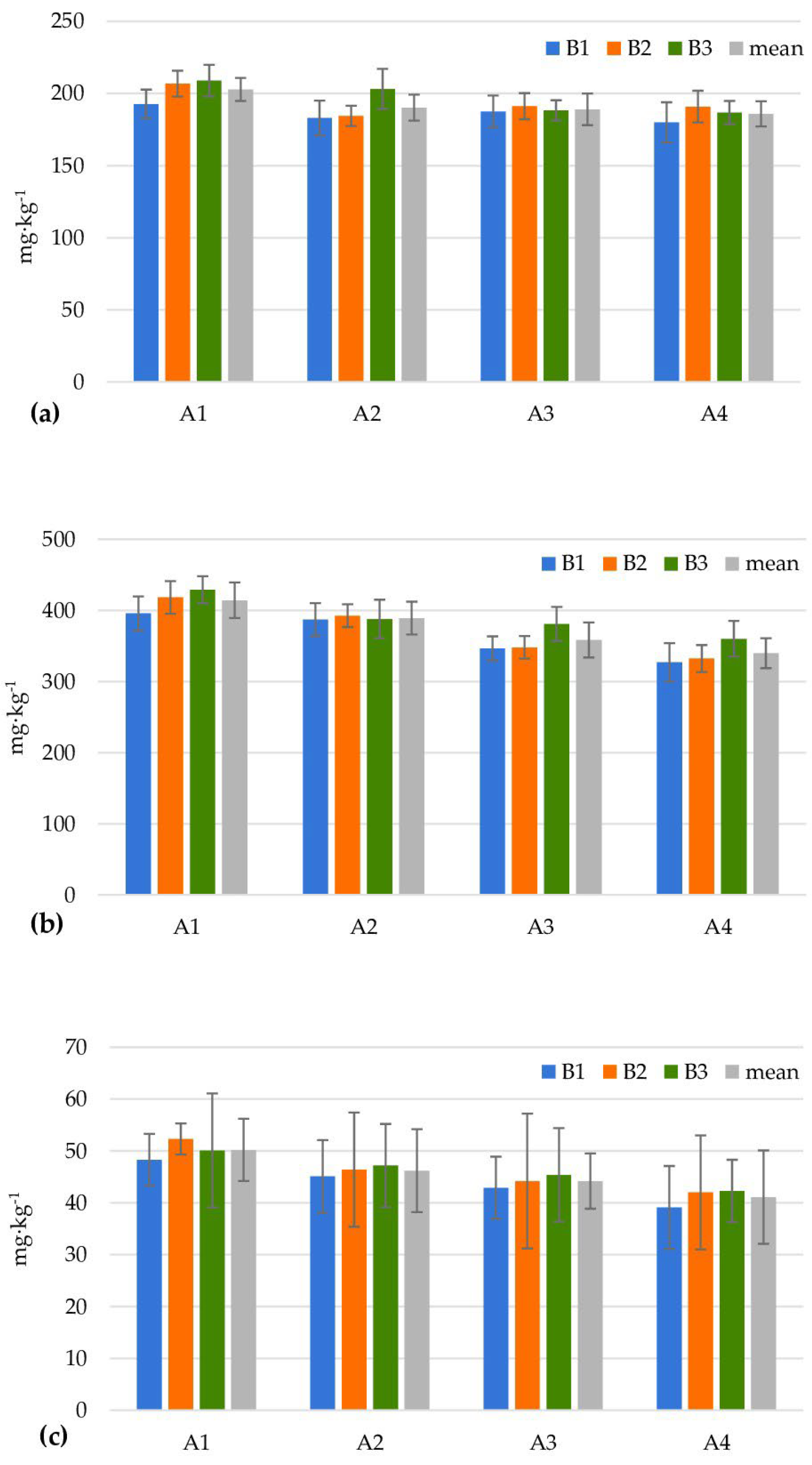
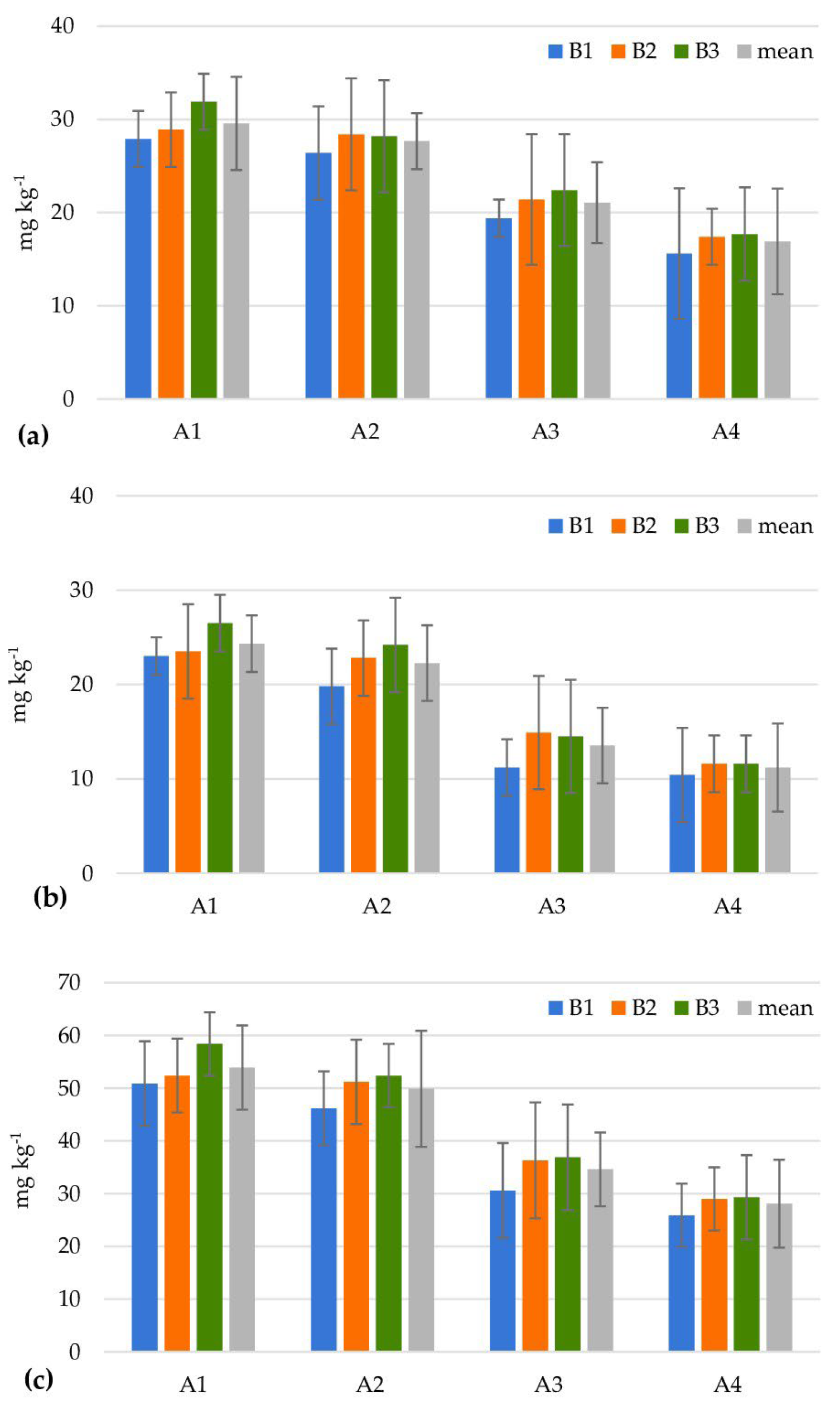
| Levels of Factor A | Levels of Factor B | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B1 (EM × 0) | B2 (EM × 1) | B3 (EM × 2) | ||||||||||
| Date of Analysis | ||||||||||||
| I | II | III | Mean | I | II | III | Mean | I | II | III | Mean | |
| Heterotrophic bacteria (106 cfu g−1) | ||||||||||||
| A1 | 25.0 ± 4.1 | 41.7 ± 2.4 | 16.7 ± 1.2 | 27.8 | 22.3 ± 0.9 | 40.3 ± 2.4 | 22.3 ± 1.7 | 28.3 | 16.3 ± 3.3 | 44.0 ± 6.2 | 27.7 ± 2.6 | 29.3 |
| A2 | 23.3 ± 6.8 | 39.7 ± 2.6 | 31.0 ± 1.4 | 31.3 | 20.0 ± 0.1 | 33.7 ± 1.9 | 48.0 ± 4.3 | 33.9 | 52.3 ± 2.6 | 71.0 ± 0.8 | 47.3 ± 3.1 | 56.9 |
| A3 | 60.0 ± 4.2 | 26.0 ± 0.8 | 26.7 ± 0.9 | 37.6 | 22.7 ± 1.2 | 56.0 ± 3.3 | 36.0 ± 2.9 | 38.2 | 21.0 ± 2.8 | 113.0 ± 6.7 | 31.3 ± 1.9 | 55.1 |
| A4 | 18.3 ± 1.2 | 42.0 ± 2.2 | 134.0 ± 9.4 | 64.8 | 20.7 ± 0.5 | 283.3 ± 7.0 | 74.0 ± 8.5 | 126.0 | 42.3 ± 1.7 | 390.7 ± 19.3 | 62.3 ± 4.0 | 165.1 |
| Mean | 31.7 | 37.4 | 52.1 | 21.4 | 103.3 | 45.1 | 33.0 | 154.7 | 42.2 | |||
| LSD0.05 for Factor A = 13.44; Factor B = 3.37; Interaction A/B = 23.09; B/A = 15.73 | ||||||||||||
| Filamentous fungi (104 cfu g−1) | ||||||||||||
| A1 | 22.3 ± 0.9 | 18.3 ± 1.6 | 10.0 ± 0.5 | 16.9 | 20.7 ± 1.9 | 18.0 ± 1.2 | 15.7 ± 0.9 | 18.1 | 28.7 ± 4.0 | 22.7 ± 4.1 | 12.7 ± 1.4 | 21.3 |
| A2 | 16.7 ± 0.5 | 14.3 ± 0.5 | 15.0 ± 0.9 | 15.3 | 34.3 ± 2.6 | 15.0 ± 0.5 | 15.0 ± 0.5 | 21.4 | 67.3 ± 5.7 | 13.3 ± 0.5 | 8.7 ± 0.6 | 29.8 |
| A3 | 36.0 ± 2.9 | 6.7 ± 1.1 | 14.3 ± 1.4 | 19.0 | 28.7 ± 2.3 | 20.3 ± 0.9 | 15.3 ± 0.9 | 21.4 | 32.0 ± 2.8 | 20.3 ± 0.9 | 13.0 ± 0.9 | 21.8 |
| A4 | 33.3 ± 0.9 | 22.7 ± 1.7 | 17.7 ± 1.8 | 24.6 | 20.3 ± 2.1 | 57.0 ± 0.8 | 13.7 ± 1.1 | 30.3 | 31.7 ± 2.5 | 24.3 ± 0.5 | 28.3 ± 2.4 | 28.1 |
| Mean | 27.1 | 15.5 | 14.3 | 26.0 | 27.6 | 14.9 | 39.9 | 20.2 | 15.7 | |||
| LSD0.05 for Factor A = 5.26; Factor B = 1.71; Interaction A/B = 8.88; B/A = 6.12 | ||||||||||||
| Actinobacteria (105 cfu g−1) | ||||||||||||
| A1 | 24.7 ± 3.9 | 24.3 ± 3.3 | 14.3 ± 1.2 | 21.1 | 22.7 ± 2.7 | 24.3 ± 2.0 | 29.3 ± 2.9 | 25.4 | 27.7 ± 2.1 | 27.7 ± 2.5 | 19.3 ± 1.9 | 24.9 |
| A2 | 21.7 ± 0.5 | 37.3 ± 5.2 | 23.0 ± 2.6 | 27.3 | 36.7 ± 2.1 | 29.5 ± 1.6 | 26.0 ± 3.6 | 30.7 | 28.3 ± 3.4 | 29.0 ± 2.2 | 27.0 ± 3.2 | 28.1 |
| A3 | 23.3 ± 3.3 | 36.7 ± 2.4 | 14.0 ± 0.8 | 24.7 | 17.3 ± 0.5 | 34.0 ± 5.2 | 31.7 ± 3.6 | 27.7 | 56.0 ± 9.4 | 21.7 ± 1.2 | 23.0 ± 0.1 | 33.6 |
| A4 | 39.3 ± 7.4 | 25.3 ± 3.1 | 32.3 ± 3.2 | 32.3 | 26.3 ± 3.5 | 34.3 ± 3.7 | 37.7 ± 4.5 | 32.8 | 46.0 ± 8.5 | 57.3 ± 4.2 | 26.3 ± 2.1 | 43.2 |
| Mean | 27.3 | 30.9 | 20.9 | 25.8 | 30.5 | 31.2 | 39.5 | 33.9 | 23.9 | |||
| LSD0.05 for Factor A = 9.55; Factor B = 2.62; Interaction A/B = 14.38; B/A = 9.21 | ||||||||||||
| Levels of Factor A * | Levels of Factor B | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B1 (EMx0) | B2 (EMx1) | B3 (EMx2) | ||||||||||
| Date of Analysis | ||||||||||||
| I | II | III | Mean | I | II | III | Mean | I | II | III | Mean | |
| Cellulolytic microorganisms (106 cfu g−1) | ||||||||||||
| A1 | 1.7 ± 0.5 | 13.7 ± 2.4 | 6.0 ± 0.6 | 7.1 | 4.7 ± 0.0 | 13.3 ± 1.0 | 8.0 ± 0.5 | 8.7 | 13.5 ± 1.3 | 7.7 ± 0.9 | 7.0 ± 0.9 | 9.4 |
| A2 | 5.7 ± 0.0 | 11.7 ± 1.1 | 11.3 ± 0.9 | 9.6 | 9.0 ± 0.9 | 13.3 ± 1.3 | 9.3 ± 0.9 | 10.6 | 6.0 ± 0.0 | 24.7 ± 1.8 | 10.3 ± 1.2 | 13.7 |
| A3 | 5.3 ± 0.7 | 13.3 ± 0.9 | 9.0 ± 0.8 | 9.2 | 9.0 ± 1.1 | 11.7 ± 1.2 | 10.7 ± 0.9 | 10.4 | 12.0 ± 1.4 | 9.0 ± 1.0 | 8.7 ± 0.9 | 9.9 |
| A4 | 7.7 ± 0.7 | 9.0 ± 0.9 | 28.0 ± 1.9 | 14.9 | 8.3 ± 0.6 | 20.0 ± 1.2 | 9.3 ± 0.9 | 12.6 | 13.7 ± 1.3 | 26.3 ± 2.3 | 11.7 ± 0.9 | 17.2 |
| Mean | 5.1 | 11.9 | 13.6 | 7.8 | 14.6 | 9.3 | 11.3 | 16.9 | 9.4 | |||
| LSD0.05 for Factor A = 5.71; Factor B = 4.70; Interaction A/B = 9.93; B/A = 8.08 | ||||||||||||
| Amylolytic microorganisms (106 cfu g−1) | ||||||||||||
| A1 | 12.0 ± 0.8 | 8.3 ± 0.7 | 10.0 ± 1.2 | 10.1 | 5.3 ± 0.2 | 19.0 ± 1.4 | 10.0 ± 0.8 | 11.4 | 11.5 ± 1.2 | 16.3 ± 1.2 | 7.7 ± 0.7 | 11.8 |
| A2 | 8.0 ± 0.0 | 15.0 ± 1.0 | 14.0 ± 0.9 | 12.3 | 15.3 ± 1.6 | 13.0 ± 0.9 | 13.7 ± 1.2 | 14.0 | 22.7 ± 1.2 | 11.7 ± 0.9 | 7.7 ± 0.8 | 14.0 |
| A3 | 8.0 ± 0.4 | 21.7 ± 2.4 | 15.0 ± 1.2 | 14.9 | 8.7 ± 0.7 | 23.7 ± 1.9 | 11.7 ± 1.1 | 14.7 | 14.7 ± 1.2 | 22.3 ± 1.9 | 14.3 ± 0.9 | 17.1 |
| A4 | 7.7 ± 0.5 | 19.7 ± 2.1 | 21.3 ± 1.9 | 16.2 | 14.3 ± 1.1 | 95.3 ± 7.5 | 20.3 ± 1.2 | 43.3 | 13.3 ± 0.5 | 47.0 ± 4.2 | 12.0 ± 1.4 | 24.1 |
| Mean | 8.9 | 16.2 | 15.1 | 10.9 | 37.8 | 13.9 | 15.6 | 24.3 | 10.4 | |||
| LSD0.05 for Factor A = 6.03; Factor B = 3.67; Interaction A/B = 8.47; B/A = 6.05 | ||||||||||||
| Proteolytic microorganisms (106 cfu g−1) | ||||||||||||
| A1 | 7.3 ± 0.7 | 13.3 ± 1.1 | 11.3 ± 0.9 | 10.7 | 19.7 ± 0.9 | 21.7 ± 1.2 | 12.3 ± 0.8 | 17.9 | 10.7 ± 0.5 | 18.0 ± 2.2 | 13.7 ± 0.5 | 14.1 |
| A2 | 9.3 ± 0.9 | 27.0 ± 1.7 | 12.0 ± 1.1 | 16.1 | 17.0 ± 0.8 | 17.3 ± 0.9 | 26.0 ± 2.2 | 20.1 | 31.0 ± 0.8 | 21.0 ± 0.8 | 17.3 ± 0.5 | 23.1 |
| A3 | 18.0 ± 1.2 | 29.3 ± 3.3 | 13.7 ± 0.9 | 20.3 | 13.7 ± 1.1 | 25.0 ± 2.4 | 13.0 ± 1.2 | 17.2 | 26.3 ± 2.0 | 14.0 ± 0.8 | 15.7 ± 1.2 | 18.7 |
| A4 | 9.0 ± 0.0 | 16.3 ± 1.2 | 42.0 ± 2.2 | 22.4 | 16.7 ± 1.2 | 41.3 ± 6.4 | 31.7 ± 2.8 | 29.9 | 21.0 ± 0.2 | 206.7 ± 9.4 | 18.3 ± 0.5 | 82.0 |
| Mean | 10.9 | 21.5 | 19.8 | 16.8 | 26.3 | 20.8 | 22.3 | 64.9 | 16.3 | |||
| LSD0.05 for Factor A = 8.01; Factor B = 2.28; Interaction A/B = 14.49; B/A = 10.19 | ||||||||||||
| Azotobacter spp. (101 cfu g−1) | ||||||||||||
| A1 | 6.7 ± 0.4 | 35.7 ± 1.3 | 5.3 ± 0.2 | 15.9 | 6.6 ± 0.0 | 13.7 ± 0.9 | 8.7 ± 0.7 | 9.6 | 16.7 ± 1.2 | 15.0 ± 1.1 | 9.3 ± 0.5 | 13.7 |
| A2 | 5.0 ± 0.0 | 34.0 ± 1.5 | 45.7 ± 1.7 | 28.2 | 6.7 ± 0.4 | 28.7 ± 1.7 | 34.7 ± 2.3 | 23.3 | 26.7 ± 2.7 | 26.0 ± 2.5 | 7.7 ± 0.7 | 20.1 |
| A3 | 10.0 ± 0.0 | 22.7 ± 1.4 | 15.0 ± 0.9 | 15.9 | 6.6 ± 0.0 | 30.5 ± 1.8 | 8.7 ± 0.9 | 15.3 | 8.3 ± 0.4 | 35.0 ± 1.8 | 6.7 ± 0.2 | 16.7 |
| A4 | 23.3 ± 1.4 | 44.7 ± 4.7 | 18.0 ± 1.1 | 28.7 | 5.0 ± 0.0 | 46.0 ± 4.9 | 41.3 ± 3.9 | 30.8 | 25.0 ± 0.5 | 23.0 ± 0.9 | 17.7 ± 0.9 | 21.9 |
| Mean | 11.3 | 34.3 | 21.0 | 6.2 | 29.7 | 23.4 | 19.2 | 24.8 | 10.4 | |||
| LSD0.05 for Factor A = 6.91; Factor B = 3.72; Interaction A/B = 12.92; B/A = 9.68 | ||||||||||||
| Levels of Factor A | Index Irc | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| pH | OC | TN | |||||||
| B1 | B2 | B3 | B1 | B2 | B3 | B1 | B2 | B3 | |
| A1 | 0.99 | 0.99 | 0.99 | 1.01 | 1.03 | 1.02 | 0.96 | 1.00 | 0.99 |
| A2 | 1.00 | 1.03 | 1.03 | 1.04 | 1.09 | 1.12 | 1.04 | 1.06 | 1.09 |
| A3 | 0.96 | 0.99 | 0.98 | 0.91 | 1.00 | 1.00 | 0.89 | 0.96 | 0.99 |
| A4 | 0.99 | 1.00 | 1.00 | 1.01 | 1.04 | 1.06 | 1.01 | 1.03 | 1.03 |
| Levels of Factor A | Index Irc | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| P | K | Mg | |||||||
| B1 | B2 | B3 | B1 | B2 | B3 | B1 | B2 | B3 | |
| A1 | 0.99 | 1.02 | 1.01 | 1.01 | 1.01 | 1.04 | 0.99 | 1.00 | 1.03 |
| A2 | 1.06 | 1.11 | 1.11 | 1.10 | 1.20 | 1.21 | 1.03 | 1.06 | 1.03 |
| A3 | 0.97 | 0.99 | 0.99 | 0.99 | 1.03 | 1.03 | 0.98 | 0.99 | 0.99 |
| A4 | 1.00 | 1.00 | 1.03 | 1.05 | 1.12 | 1.05 | 1.00 | 1.01 | 1.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breza-Boruta, B.; Bauza-Kaszewska, J. Effect of Microbial Preparation and Biomass Incorporation on Soil Biological and Chemical Properties. Agriculture 2023, 13, 969. https://doi.org/10.3390/agriculture13050969
Breza-Boruta B, Bauza-Kaszewska J. Effect of Microbial Preparation and Biomass Incorporation on Soil Biological and Chemical Properties. Agriculture. 2023; 13(5):969. https://doi.org/10.3390/agriculture13050969
Chicago/Turabian StyleBreza-Boruta, Barbara, and Justyna Bauza-Kaszewska. 2023. "Effect of Microbial Preparation and Biomass Incorporation on Soil Biological and Chemical Properties" Agriculture 13, no. 5: 969. https://doi.org/10.3390/agriculture13050969





