Nematicidal and Toxicity Effects of Eupatorium adenophorum Spreng against the Root-Knot Nematode Meloidogyne incognita in Soil Producing Cucumber
Abstract
1. Introduction
2. Materials and Methods
2.1. Biofumigant Plant
2.2. Identification of Chemical Compounds
2.3. In Vitro Studies
2.4. In Vivo Experiments
2.5. Parameter Measurement
2.6. Data Analysis
3. Results
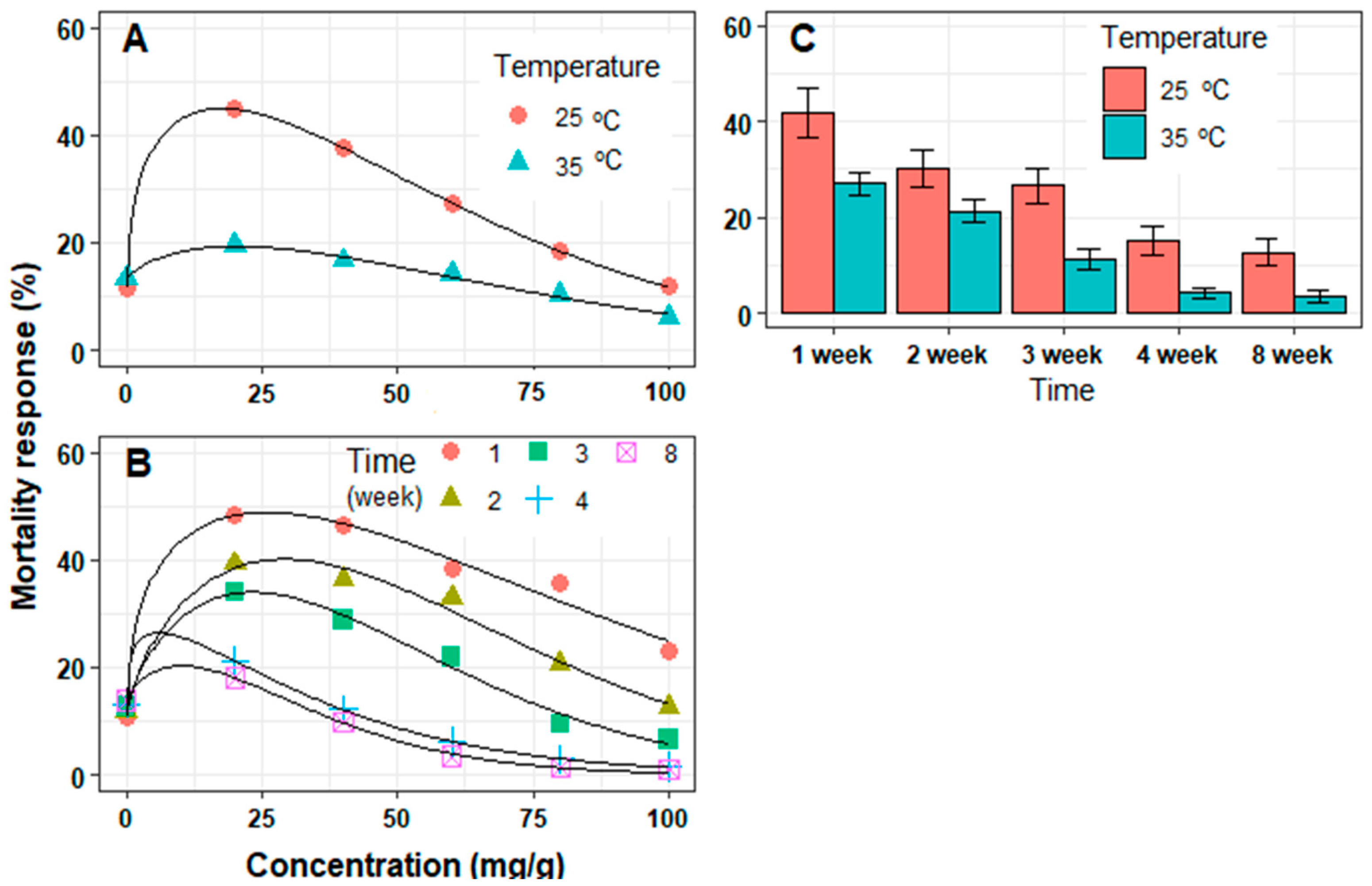
3.1. In Vitro Mortality of J2 Juveniles
3.2. In Vivo Mortality of J2 Juveniles
3.3. Chemical Compounds Extracted
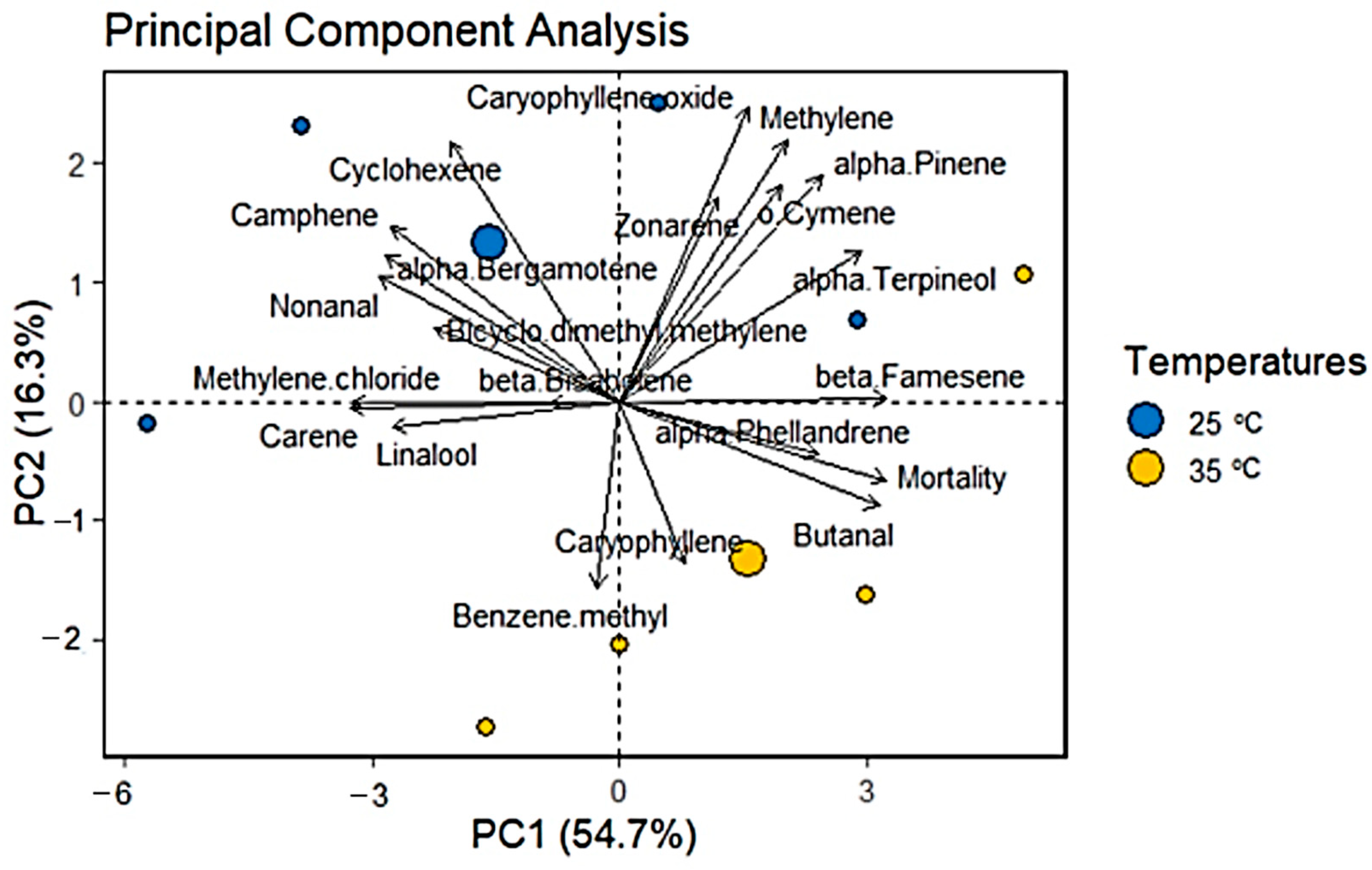
3.4. Principal Component Analysis (PCA)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aydinli, G.; Mennan, S. Identification of root-knot nematodes (Meloidogyne spp.) from greenhouses in the Middle Black Sea Region of Turkey. Turk. J. Zool. 2016, 40, 675–685. [Google Scholar] [CrossRef]
- Jones, T.J.; Haegeman, A.; Danchin, E.G.; Gaur, H.S.; Helder, J.; Jones, M.G.K.; Kikuchi, T.; Manzanilla-Lopez, R.; Palomares-Rius, J.E.; Wesemael, W.M.L.; et al. Top 10 Plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef]
- Ibrahim, I.K.A. Nematode Pests Parasitic on Agricultural Field Crops; Manshaat El-Maaref: Alexandra, Egypt, 2011; p. 250. [Google Scholar]
- Lv, J.; Qi, J.; Shi, Q.; Shen, D.; Zhang, S.; Shao, G.; Li, H.; Sun, Z.; Weng, Y.; Shang, Y.; et al. Genetic diversity and population structure of cucumber (Cucumis sativus L.). PLoS ONE 2012, 7, e46919. [Google Scholar] [CrossRef]
- Pandey, R.; Kalra, A.; Gupta, M.L.; Sharma, P. Phytonematodes: Major pest of MAPs. In Proceedings of the First National Interactive Meet on Medicinal and Aromatic Plants, Lucknow, India, 17–18 May 2002; pp. 188–197. [Google Scholar]
- Escobar, C.; Barcala, M.; Cabrera, J.; Fenoll, C. Chapter one-overview of root-knot nematodes and giant cells. Adv. Bot. Res. 2015, 73, 1–32. [Google Scholar] [CrossRef]
- Schreiner, R.P.; Ivors, K.L.; Pinkerton, J.N. Soil solarization reduces arbuscular mycorrhizal fungi as a consequence of weed suppression. Mycorrhiza 2001, 11, 273–277. [Google Scholar] [CrossRef]
- Cox, C. Fumigant factsheet: Metam sodium. J. Pesti. Ref. 2006, 26, 12–16. [Google Scholar]
- Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef]
- Li, Y.C.; Ji, H.; Li, X.H.; Zhang, H.X.; Li, H.T. Isolation of nematicidal constituents from essential oil of Kaempferia galanga L. rhizome and their activity against Heterodera avenae Wollenweber. Trop. J. Pharm. Res. 2017, 16, 59–65. [Google Scholar] [CrossRef]
- Salem, M.F.; Mahdy, M.E. Suppression of root-knot nematode through innovative mustard bio-fumigation. J. Food. Agric. Soc. 2015, 3, 41–50. [Google Scholar]
- Ntalli, N.G.; Caboni, P. Botanical nematicides: A review. J. Agric. Food Chem. 2012, 60, 9929–9940. [Google Scholar] [CrossRef]
- Carrillo-Hormaza, L.; Mora, C.; Alvarez, R.; Alzate, F.; Osorio, E. Chemical composition and antibacterial activity against Enterobacter cloacae of essential oils from Asteraceae species growing in the Páramos of Colombia. Ind. Crops Prod. 2015, 77, 108–115. [Google Scholar] [CrossRef]
- Katinas, L.; Gutierrez, D.G.; Grossi, M.A.; Crisci, J.V. Panorama de la familia Asteraceae (=Compositae) en la República Argentina. Bol. Soc. Argent. Bot. 2007, 42, 113–129. [Google Scholar]
- King, R.M.; Robinson, H. The Genera of the Eupatorieae (Asteraceae). In Monographs in Systematic Botany; Missouri Botanical Garden 299: St. Louis, MI, USA, 1987; Volume 22, 581p. [Google Scholar]
- Bhattarai, N.; Shrestha, G. Antibacterial and antifungal effect of Eupatorium adenophorum Spreng against bacterial and fungal isolates. Nepal J. Sci. Technol. 2009, 10, 91–95. [Google Scholar] [CrossRef]
- Ito, M.; Watanabe, K.; Kita, Y.; Kawahara, T.; Crawford, D.J.; Yahara, T. Phylogeny and phytogeography of Eupatorium (Eupatorieae, Asteraceae): Insights from sequence data of the nrDNA ITS regions and cpDNA RFLP. J. Plant Res. 2000, 113, 79–89. [Google Scholar] [CrossRef]
- Ouyang, C.; Liu, X.; Yan, D.; Li, Y.; Wang, Q.; Cao, A.; Guo, M. Immunotoxicity assessment of cadinene sesquiterpenes from Eupatorium adenophorum in mice. J. Integr. Agric. 2016, 15, 2319–2325. [Google Scholar] [CrossRef]
- Rondina, R.; Bandoni, A.; Coussio, J. Plantas Silvestres Argentinas Con Reconocidas Propiedades Medicinales o Tóxicas; (CD-ROM); OEA-CYTED: Buenos Aires, Argentina, 2003. [Google Scholar]
- Sasikumar, J.M.; Doss, P.A.; Doss, A. Antibacterial activity of Eupatorium glandulosum leaves. Fitoterapia 2005, 76, 240–243. [Google Scholar] [CrossRef]
- Sanchez, G.E.; Lopez, C.B.R.; Torres, R.d.R.; Pacheco, M.M.M. A revision of Eupatorium (Compositae: Eupatorieae) from Michoacan. FYTON 2011, 80, 139–146. [Google Scholar] [CrossRef]
- Liu, P.Y.; Liu, D.; Li, W.H.; Zhao, T.; Sauriol, F.; Gu, Y.C.; Shi, Q.W.; Zhang, M.L. Chemical constituents of plants from the genus Eupatorium (1904–2014). Chem. Biodivers. 2015, 12, 1481–1515. [Google Scholar] [CrossRef]
- Zhang, M.L.; Wu, M.; Zhang, J.J.; Irwin, D.; Gu, Y.C.; Shi, Q.W. Chemical constituents of plants from the genus Eupatorium. Chem. Biodivers. 2008, 5, 40–55. [Google Scholar] [CrossRef]
- Albuquerque, M.R.J.R.; Silveira, E.R.; Uchôa, D.E.A.; Lemos, T.L.G.; Souza, E.B.; Santiago, G.M.P.; Pessoa, O.D.L. Chemical composition and larvicidal activity of the essential oils from Eupatorium betonicaeforme (DC.) Baker (Asteraceae). J. Agric. Food Chem. 2004, 52, 6708–6711. [Google Scholar] [CrossRef]
- Lang, G.; Passreiter, C.M.; Medinilla, B.E.; Castillo, J.J.; Witte, L. Nontoxic pyrrolizidine alkaloids from Eupatorium semialatum. Biochem. Syst. Ecol. 2001, 29, 143–147. [Google Scholar] [CrossRef]
- Belter, R.K. Petroleum Substitutes from the Essential Oils of Eupatorium Species. U.S. Patent Application 12/287,956, 23 April 2009. [Google Scholar]
- Raj Mohan, D.; Ramaswamy, M. Evaluation of larvicidal activity of the leaf extract of a weed plant, Ageratina adenophora, against two important species of mosquitoes, Aedes aegypti and Culex quinquefasciatus. Afr. J. Biotechnol. 2007, 6, 631–638. [Google Scholar] [CrossRef]
- Kundu, A.; Saha, S.; Walia, S.; Shakil, N.A.; Kumar, J.; Annapurna, K. Cadinene sesquiterpenes from Eupatorium adenophorum and their antifungal activity. J. Environ. Sci. Health. 2013, 48, 516–522. [Google Scholar] [CrossRef]
- Kundu, A.; Saha, S.; Walia, S.; Dutta, T.K. Antinemic potentiality of chemical constituents of Eupatorium adenophorum Spreng leaves against Meloidogyne incognita. Natl. Acad. Sci. Lett. 2016, 39, 145–149. [Google Scholar] [CrossRef]
- Santhosh, J.E.; Beena, B.; Ramana, K.V. Tropical soil microflora of spice-based cropping systems as potential antagonists of root-knot nematodes. J. Invert. Pathol. 2005, 88, 218–225. [Google Scholar]
- Hartman, K.M.; Sasser, J.N. Identification of Meloidogyne species on the basis of differential host test and perineal—Pattern morphology. In An Advanced Treatise on Meloidogyne, Methodology; Barker, K.R., Carter, C.C., Sasser, J.N., Eds.; North Carolina State University Graphics: Raleigh, NC, USA, 1985; Volume 2, pp. 69–77. [Google Scholar]
- Hussey, R.S.; Barker, K. A comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Dis. Rep. 1973, 57, 1025–1028. [Google Scholar]
- Baermann, G. Eine einfache Methode zur Auffindung von Ankylostomum (Nematoden) larven in Erdproben. GTNI 1917, 57, 131–137. [Google Scholar]
- De Ley, I.T.; Karssen, G.; De Ley, P.; Vierstraete, A.; Waeyenberge, L.; Moens, M.; Vanfleteren, J. Phylogenetic analyses of internal transcribed spacer region sequences within Meloidogyne. J. Nematol. 1999, 31, 530–531. [Google Scholar]
- Mao, L.; Yan, D.; Wang, Q.; Li, Y.; Ouyang, C.; Liu, P.; Shen, J.; Guo, M.; Cao, A. Evaluation of the combination of dimethyl disulfide and dazomet as an efficient methyl bromide alternative for cucumber production in China. J. Agric. Food Chem. 2014, 62, 4864–4869. [Google Scholar] [CrossRef]
- Ahmadi Mansourabad, M.; Kargar Bideh, A.; Abdollahi, M. Effects of some micronutrients and macronutrients on the root—Knot nematode, Meloidogyne incognita, in greenhouse cucumber (Cucumis sativus cv. Negin). J. Crop Prot. 2016, 5, 507–517. [Google Scholar] [CrossRef]
- Naserinasab, F.; Sahebani, N.; Etebarian, H.R. Biological control of Meloidogyne javanica by Trichoderma harzianum BI and salicylic acid on tomato. Afr. J. Food Sci. 2011, 5, 276–280. [Google Scholar]
- Parmoon, G.; Moosavi, S.A.; Ataollah Siadat, S. Descriptions of okra seed longevity loss behavior using nonlinear regression models. Adv. Hort. Sci. 2019, 33, 301–312. [Google Scholar] [CrossRef]
- Naserinasab, F.; Heydari, R.; Sanjarian, F.; Rakhshandehroo, F. Investigation of the defense genes expression of phenylalanine ammoniumase and peroxidase in interaction with neem extract of and Meloidogyne javanica nematodes in tomato. Tissue Cell 2018, 9, 360–377. [Google Scholar]
- Gholamnezhad, J. Effect of plant extracts on activity of some defense enzymes of apple fruit in interaction with Botrytis cinerea. J. Integr. Agric. 2019, 18, 115–123. [Google Scholar] [CrossRef]
- Sosa, M.E.; Lancelle, H.G.; Tonn, C.E.; Andres, M.F. Insecticidal and nematicidal essential oils from Argentinean eupatorium and Baccharis spp. Biochem. Syst. Ecol. 2012, 43, 132–138. [Google Scholar] [CrossRef]
- Aminu-Taiwo, B.; Fawole, B.; Claudius-Cole, A.O. Nematicidal potential of extracts from some selected plants against the root-knot nematode, Meloidogyne incognita. IOSR-JAVS 2018, 11, 22–29. [Google Scholar] [CrossRef]
- Bakonyi, G.; Nagy, P.; Kovacs-Lang, E.; Kovacs, E.; Barabas, S.; Répasi, V.; Seres, A. Soil nematode community structure as affected by temperature and moisture in a temperate semiarid shrubland. Appl. Soil Ecol. 2007, 37, 31–40. [Google Scholar] [CrossRef]
- Robinson, C.M.; Hansen, L.D.; Xue, X.; Adams, B.J. Temperature response of metabolic activity of an antarctic nematode. Biology 2023, 12, 109. [Google Scholar] [CrossRef]
- Castorina, G.; Grassi, F.; Consonni, G.; Vitalini, S.; Oberti, R.; Calcante, A.; Ferrari, E.; Bononi, M.; Iriti, M. Characterization of the biogenic volatile organic compounds (BVOCs) and analysis of the PR1 molecular marker in Vitis vinifera L. Inoculated with the nematode Xiphinema index. Int. J. Mol. Sci. 2020, 21, 4485. [Google Scholar] [CrossRef]
- Ardakani, A.S.; Hosseininejad, S.A. Identifcation of chemical components from essential oils and aqueous extracts of some medicinal plants and their nematicidal efects on Meloidogyne incognita. J. Basic Appl. Zool. 2022, 83, 14. [Google Scholar] [CrossRef]
- Li, Y.; Meng, F.; Deng, X.; Wang, X.; Feng, Y.; Zhang, W.; Pan, L.; Zhang, X. Comparative transcriptome analysis of the pinewood nematode Bursaphelenchus xylophilus reveals the molecular mechanism underlying Its defense response to host-derived α-pinene. Int. J. Mol. Sci. 2019, 20, 911. [Google Scholar] [CrossRef]
- Goyal, L.; Kaushal, S.; Dhillon, N.K.; Bajaj, H. Nematicidal potential of Citrus reticulata peel essential oil, isolated major compound and its derivatives against Meloidogyne incognita. Arch. Phytopathol. Plant Prot. 2021, 54, 449–467. [Google Scholar] [CrossRef]
- Buda, V.; Cepulyte-Rakauskiene, R. The effect of linalool on second-stage juveniles of the potato cyst nematodes Globodera rostochiensis and G. pallida. . J. Nematol. 2011, 43, 149–151. [Google Scholar] [CrossRef]
- Groot, P.D. Attraction of Hylastes opacus (Coleoptera: Scolytidae) to nonanal. Can. Entomol. 2003, 135, 309–311. [Google Scholar] [CrossRef]
- Ndomo-Moualeu, A.F.; Ulrichs, C.; Adler, C. Behavioral responses of Callosobruchus maculatus to volatile organic compounds found in the headspace of dried green pea seeds. J. Pest. Sci. 2016, 89, 107–116. [Google Scholar] [CrossRef]


| Source of Variation | DF | Means Square | |
|---|---|---|---|
| Root-Stems | Leaves | ||
| Temperature | 1 | 11,394.75 ** | 6328.48 ** |
| Time | 4 | 1804.91 ** | 4425.35 ** |
| Concentrations | 5 | 27,635.65 ** | 2436.66 ** |
| Temperature × Time | 4 | 931.90 ** | 80.07 ** |
| Temperature × Concentrations | 5 | 620.92 ** | 761.76 ** |
| Time × Concentrations | 20 | 166.30 * | 285.97 ** |
| Temperature × Time × Concentrations | 20 | 48.24 ns | 54.77 ns |
| Error | 120 | 51.55 | 38.48 |
| Coefficient of Variation (%) | - | 9.73 | 32.10 |
| Treatments | Levels | Parameters Model | R2 | RMSE | ||
|---|---|---|---|---|---|---|
| Ymax (%) | Slope | EC50 (mg/g) | ||||
| Temperature (°C) | 25 | 77.4 ± 1.8 | 7.1 ± 1.1 | 11.3 ± 1.5 | 0.989 | 3.54 |
| 35 | 96.8 ± 0.72 | 5.3 ± 0.3 | 10.3 ± 0.5 | 0.999 | 1.43 | |
| Time (week) | 1 | 76.8 ± 1.5 | 5.8 ± 0.7 | 10.3 ± 1.3 | 0.993 | 2.95 |
| 2 | 81.1 ± 0.9 | 5.9 ± 0.4 | 10.4 ± 0.7 | 0.997 | 1.80 | |
| 3 | 87.5 ± 1.9 | 6.0 ± 0.8 | 10.7 ± 1.4 | 0.991 | 3.72 | |
| 4 | 93.2 ± 1.9 | 5.6 ± 0.7 | 10.0 ± 1.4 | 0.992 | 3.76 | |
| 8 | 96.2 ± 0.6 | 4.4 ± 0.3 | 7.8 ± 0.5 | 0.999 | 1.18 | |
| Treatments | Levels | Parameters Model | R2 | RMSE | |||
|---|---|---|---|---|---|---|---|
| Ymax (%) | Slope | c | ECmax (mg/g) | ||||
| Temperature (°C) | 25 | 45.0 ± 0.2 | 57.1 ± 0.3 | 1.3 ± 0.1 | 17.6 ± 0.5 | 0.999 | 0.19 |
| 35 | 19.3 ± 0.8 | 67.2 ± 3.7 | 1.4 ± 0.2 | 20.3 ± 4.8 | 0.986 | 0.88 | |
| Time (week) | 1 | 48.8 ± 2.3 | 82.1 ± 8.3 | 1.3 ± 0.1 | 25.9 ± 7.9 | 0.981 | 3.13 |
| 2 | 40.1 ± 1.8 | 62.8 ± 3.6 | 1.5 ± 0.2 | 29.2 ± 4.4 | 0.984 | 2.46 | |
| 3 | 34.0 ± 1.8 | 51.3 ± 2.9 | 1.5 ± 0.2 | 23.3 ± 4.4 | 0.986 | 2.09 | |
| 4 | 26.4 ± 0.9 | 33.9 ± 0.3 | 1.2 ± 0.1 | 6.1 ± 0.9 | 0.999 | 0.12 | |
| 8 | 20.3 ± 1.9 | 32.0 ± 3.2 | 1.4 ± 0.2 | 10.1 ± 2.9 | 0.997 | 0.57 | |
| Analysis | Source of Variation | DF | Mortality (%) | Plants Height (cm) | Fruit Fresh Weight (kg/h) | |||
|---|---|---|---|---|---|---|---|---|
| Root-Stems | Leaves | Root-Stems | Leaves | Root-Stems | Leaves | |||
| Means square | Concentration | 2 | 3703.9 ** | 242.76 ** | 2084.1 ** | 1276.77 ** | 309,238,018 ** | 108,297,840 ** |
| Error | 6 | 3.23 | 3.57 | 2.44 | 2 | 268,788.2 | 241,007.2 | |
| CV (%) | - | 4.9 | 9.6 | 0.94 | 0.9 | 9.8 | 10.1 | |
| Means | Control | 13.6 ± 1.1 c | 13.9 ± 1.2 c | 136.0 ± 2.1c | 134.7 ± 5.4 c | 39.4 ± 3.3 c | 26.7 ± 1.6 b | |
| 20 mg/g | 46.1 ± 1.6 b | 23.9 ± 0.7 a | 177.3 ± 2.5 b | 174.3 ± 2.3 a | 54.3 ± 1.5 b | 37.9 ± 1. 3 a | ||
| 40 mg/g | 50.9 ± 0.6 a | 21.3 ± 0.7 b | 185.0 ± 3.5 a | 164.3 ± 1.2 b | 58.8 ± 2.6 a | 28.9 ± 0.9 b | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parsiaaref, S.; Cao, A.; Li, Y.; Ebadollahi, A.; Parmoon, G.; Wang, Q.; Yan, D.; Fang, W.; Zhang, M. Nematicidal and Toxicity Effects of Eupatorium adenophorum Spreng against the Root-Knot Nematode Meloidogyne incognita in Soil Producing Cucumber. Agriculture 2023, 13, 1109. https://doi.org/10.3390/agriculture13061109
Parsiaaref S, Cao A, Li Y, Ebadollahi A, Parmoon G, Wang Q, Yan D, Fang W, Zhang M. Nematicidal and Toxicity Effects of Eupatorium adenophorum Spreng against the Root-Knot Nematode Meloidogyne incognita in Soil Producing Cucumber. Agriculture. 2023; 13(6):1109. https://doi.org/10.3390/agriculture13061109
Chicago/Turabian StyleParsiaaref, Shiva, Aocheng Cao, Yuan Li, Asgar Ebadollahi, Ghasem Parmoon, Qiuxia Wang, Dongdong Yan, Wensheng Fang, and Min Zhang. 2023. "Nematicidal and Toxicity Effects of Eupatorium adenophorum Spreng against the Root-Knot Nematode Meloidogyne incognita in Soil Producing Cucumber" Agriculture 13, no. 6: 1109. https://doi.org/10.3390/agriculture13061109
APA StyleParsiaaref, S., Cao, A., Li, Y., Ebadollahi, A., Parmoon, G., Wang, Q., Yan, D., Fang, W., & Zhang, M. (2023). Nematicidal and Toxicity Effects of Eupatorium adenophorum Spreng against the Root-Knot Nematode Meloidogyne incognita in Soil Producing Cucumber. Agriculture, 13(6), 1109. https://doi.org/10.3390/agriculture13061109













