Influence of Plant Growth Retardants and Nitrogen Doses on the Content of Plant Secondary Metabolites in Wheat, the Presence of Pests, and Soil Quality Parameters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Design
2.2. Methods
2.2.1. Secondary Metabolites and Antioxidative Capacity in Plants
Freeze-Drying and the Preparation of Extract from the Above-Ground Part of Wheat
Determination of Total Polyphenolic Compounds
Determination of Antioxidant Capacity Using the FRAP (Ferring Reducing Ability of Plasma) Method
Determination of Chlorogenic Acid
2.2.2. Insect Experiments
2.2.3. Basic Soil Parameters
Soil pH and the Content of Clay, Carbon, and Nitrogen in the Soil
- −
- The clay content using a Mastersizer MS 2000 (Malvern Panalytical, UK) laser particle size analyser;
- −
- The pH value in 1 M KCl—by the potentiometric method [52];
- −
- The content of total organic carbon (TOC) and total nitrogen (TN) expressed in g kg−1 of d.w. of soil was analysed with a Vario Max CN analyser supplied by Elementar (Langenselbold, Germany);
- −
- The content of dissolved organic carbon (DOC) and dissolved nitrogen (DN) were assayed in solutions from an extraction of soil sample using 0.004 mol dm−3 CaCl2, at a soil sample-to-extractant ratio of 1:10 (the extraction took 1 h). The contents of DOC and DTN were assayed using an Analityk Jena Muli N/C 3100 analyser and expressed in mg kg˗1 d.w. of soil sample and as a percentage proportion in the pool of TOC and TN, respectively.
Fractional Composition of Humus and Isolation of Humic Acids
- −
- Decalcification (24 h) with 0.05 M HCl (1:10 w/v), Cd, (Nd)—carbon (nitrogen) in solutions after decalcification;
- −
- Extraction (24 h) of the remaining solid with 0.5 M NaOH (1:10 w/v) with occasional mixing, followed by centrifugation; C(N)HAs + FAs—the sum of the carbon (nitrogen) of humic and fulvic acids.
Extraction and Determination of Phenolic Compounds in Soils
Activity of Enzymes in the Soil
- −
- The catalase (CAT) activity was investigated by the amount of purpurogallin (PPG) formed by the oxidation of pyrogallol in the presence of H2O2. The absorbance of the solution was measured colorimetrically at λ = 460 nm using a spectrophotometer [56].
- −
- The activity of dehydrogenases (DEH) was investigated by the Thalmann method [57] after incubation of the sample with 2,3,5-triphenyltetrazolium chloride and measurement of triphenylformazan (TPF) absorbance at 546 nm and expressed in mg TPF kg−1 24 h−1.
- −
- The activity of peroxidases (PER) was determined according to Barth and Bordeleau [58] by measuring the amount of purpurogallin (PPG) produced by oxidation of pyrogallol in the presence of H2O2.
2.3. Statistical Analyses
3. Results and Discussion
3.1. Secondary Metabolites and Antioxidative Capacity in Plants
3.2. The Density of Insects in Spring Wheat Plants
3.3. Soil Properties
3.3.1. Properties of Soil and Organic Matter
3.3.2. The Content of Phenolic Compounds in Soils
3.3.3. The Activity of Enzymes in the Soil
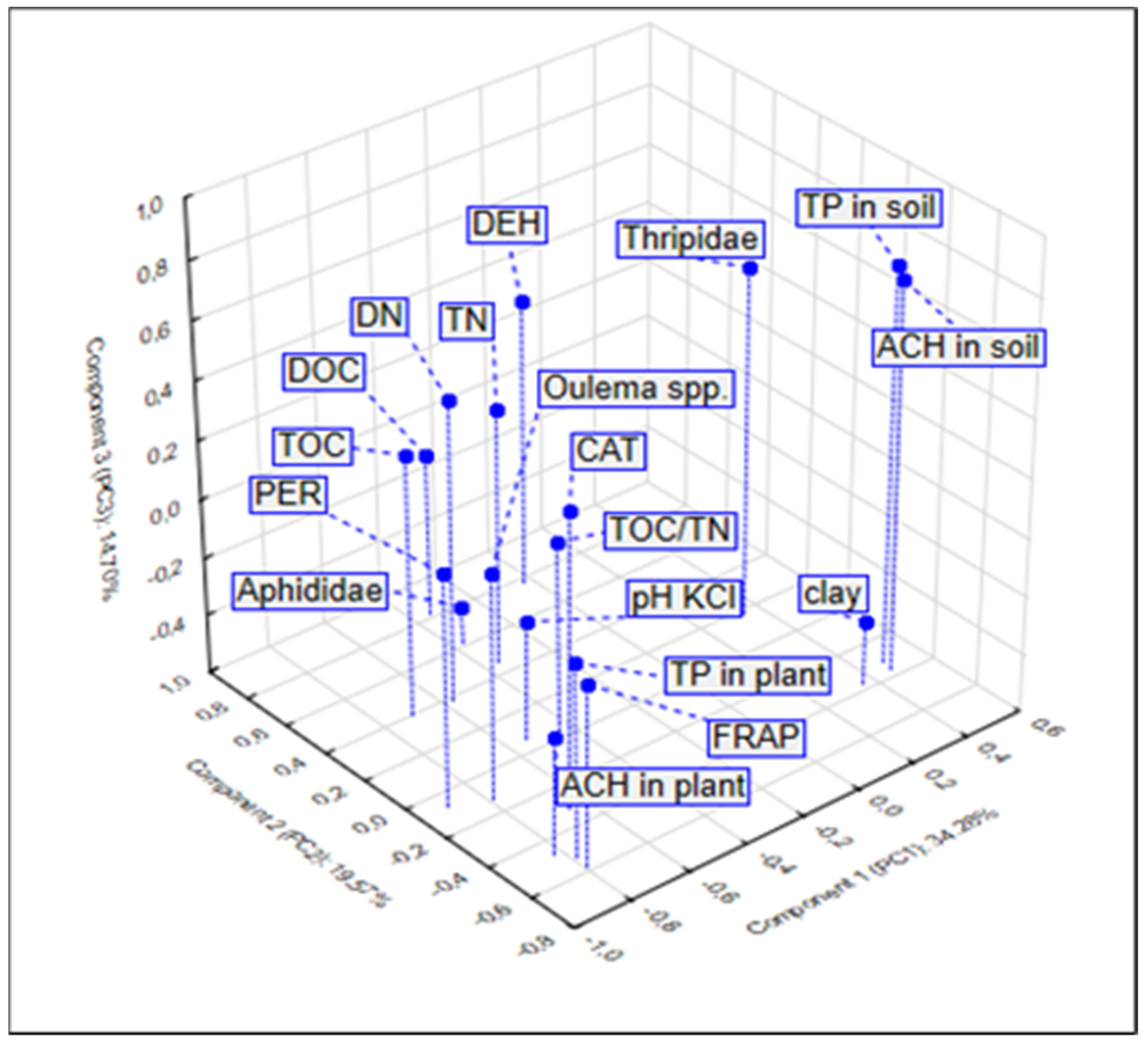
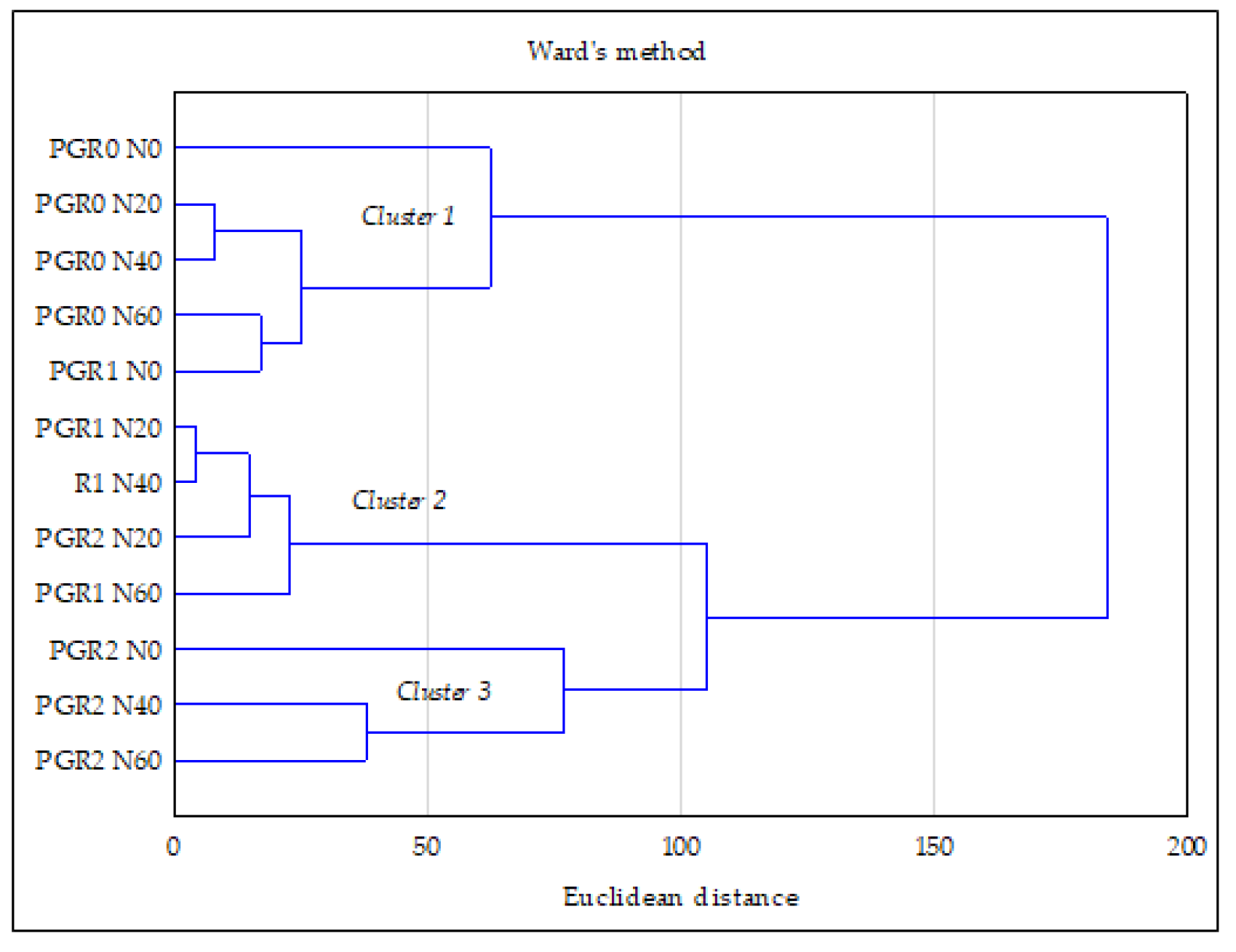
3.4. Relationship between the Studied Properties—PCA and CA Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qin, R.; Noulas, C.; Wysocki, D.; Liang, X.; Wang, G.; Lukas, S. Application of plant growth regulators on soft white winter wheat under different nitrogen fertilizer scenarios in irrigated fields. Agriculture 2020, 10, 305. [Google Scholar] [CrossRef]
- Rademacher, W. Growth retardants: Effects of gibberellin biosynthesis and other metabolic pathways. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 501–531. [Google Scholar] [CrossRef]
- Cycoń, M.; Lewandowska, A.; Piotrowska-Seget, Z. Mineralization dynamics of chlormequat chloride (CCC) in soils of different textures. Pol. J. Environ. Stud. 2012, 21, 595–602. [Google Scholar]
- Karimi, M.; Ahmadi, A.; Hashemi, J.; Abbasi, A.; Tavarini, S.; Pompeiano, A.; Guglielminetti, L.; Angelini, L.G. Plant growth retardants (PGRs) affect growth and secondary metabolite biosynthesis in Stevia rebaudiana Bertoni under drought stress. S. Afr. J. Bot. 2019, 121, 394–401. [Google Scholar] [CrossRef]
- Hancianu, M.; Aprotosoaie, A.C. The effects of pesticides on plant secondary metabolites. In Biotechnological Production of Plant Secondary Metabolites; Bentham Science Publishers: Sharjah, United Arab Emirates, 2012; pp. 176–186. [Google Scholar] [CrossRef]
- Altuntaş, H.; Gwokyalya, R.; Bayram, N. Immunotoxic effects of force-fed ethephon on model organism Galleria mellonella (Lepidoptera: Pyralidae). Drug Chem. Toxicol. 2022, 45, 1761–1768. [Google Scholar] [CrossRef] [PubMed]
- Giron, D.; Frago, E.; Glevarec, G.; Pieterse, C.M.; Dicke, M. Cytokinins as key regulators in plant–microbe–insect interactions: Connecting plant growth and defence. Funct. Ecol. 2013, 27, 599–609. [Google Scholar] [CrossRef]
- Gupta, G.; Bhattacharya, A.K. Assessing toxicity of post-emergence herbicides to the Spilarctia obliqua Walker (Lepidoptera: Arctiidae). J. Pest. Sci. 2008, 81, 9–15. [Google Scholar] [CrossRef]
- Zhao, H.; Cao, H.H.; Pan, M.Z.; Sun, Y.X.; Liu, T.X. The role of plant growth regulators in a plant–aphid–parasitoid tritrophic system. J. Plant Growth Regul. 2017, 36, 868–876. [Google Scholar] [CrossRef]
- Pérez-Ochoa, M.L.; Vera-Guzmán, A.M.; Mondragón-Chaparro, D.M.; Sandoval-Torres, S.; Carrillo-Rodríguez, J.C.; Chávez-Servia, J.L. Effects of growth conditions on phenolic composition and antioxidant activity in the medicinal plant Ageratina petiolaris (Asteraceae). Diversity 2022, 14, 595. [Google Scholar] [CrossRef]
- Sugier, D.; Sugier, P.; Jakubowicz-Gil, J.; Gawlik-Dziki, U.; Zając, A.; Król, B.; Chmiel, S.; Kończak, M.; Pięt, M.; Paduch, R. Nitrogen fertilization and solvents as factors modifying the antioxidant and anticancer potential of Arnica montana L. Flower Head Extracts. Plants 2023, 12, 142. [Google Scholar] [CrossRef]
- Prescott, C.E.; Grayston, S.J.; Helmisaari, H.S.; Kaštovská, E.; Körner, C.; Lambers, H.; Meier, I.C.; Millard, P.; Ostonen, I. Surplus carbon drives allocation and plant–soil interactions. Trends Ecol. Evol. 2020, 35, 1110–1118. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Wu, Z.; Robert, C.A.M.; Ouyang, X.; Züst, T.; Mestrot, A.; Xu, J.; Erb, M. Soil chemistry determines whether defensive plant secondary metabolites promote or suppress herbivore growth. Proc. Natl. Acad. Sci. USA 2021, 118, e2109602118. [Google Scholar] [CrossRef] [PubMed]
- Mithöfer, A.; Boland, W. Plant defense against herbivores: Chemical aspects. Annu. Rev. Plant Biol. 2012, 63, 431–450. [Google Scholar] [CrossRef] [PubMed]
- Mur, L.A.; Simpson, C.; Kumari, A.; Gupta, A.K.; Gupta, K.J. Moving nitrogen to the centre of plant defence against pathogens. Ann. Bot. 2017, 119, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Medina, A.; Van Wees, S.C.; Pieterse, C.M. Airborne signals from Trichoderma fungi stimulate iron uptake responses in roots resulting in priming of jasmonic acid-dependent defences in shoots of Arabidopsis thaliana and Solanum lycopersicum. Plant. Cell Environ. 2017, 40, 2691–2705. [Google Scholar] [CrossRef] [PubMed]
- Kraus, T.E.C.; Dahlgren, R.A.; Zasoski, R.J. Tannins in nutrient dynamics of forest ecosystems—A review. Plant Soil 2003, 256, 41–66. [Google Scholar] [CrossRef]
- Min, K.; Freeman, C.; Kang, H.; Choi, S.U. The regulation by phenolic compounds of soil organic matter dynamics under a changing environment. BioMed Res. Int. 2015, 2015, 825098. [Google Scholar] [CrossRef]
- Ziółkowska, A.; Debska, B.; Banach-Szott, M. Transformations of phenolic compounds in meadow soils. Sci. Rep. 2020, 10, 19330. [Google Scholar] [CrossRef]
- Chen, Y.; Olson, D.M.; Ruberson, J.R. Effects of nitrogen fertilization on tritrophic interactions. Arthropod-Plant Interact. 2010, 4, 81–94. [Google Scholar] [CrossRef]
- Wang, C.; Tian, B.; Yu, Z.; Ding, J. Effect of different combinations of phosphorus and nitrogen fertilization on arbuscular mycorrhizal fungi and aphids in wheat. Insects 2020, 11, 365. [Google Scholar] [CrossRef]
- Kumar, S.; Abedin, M.M.; Singh, A.K.; Das, S. Role of phenolic compounds in plant-defensive mechanisms. Plant Phenolics Sustain. Agric. 2020, 1, 517–532. [Google Scholar] [CrossRef]
- Puri, S.; Singh, S.; Sohal, S.K. Oviposition behaviour and biochemical response of an insect pest, Zeugodacus cucurbitae (Coquillett) (Diptera: Tephritidae) to plant phenolic compound phloroglucinol. Comp. Biochem. Phys. C 2022, 255, 109291. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Beato, M.; Usseglio, V.L.; Camina, J.; Zygadlo, J.A.; Dambolena, J.S.; Zunino, M.P. Phenolic compounds as controllers of Sitophilus zeamais: A look at the structure-activity relationship. J. Stored Prod. Res. 2022, 99, 102038. [Google Scholar] [CrossRef]
- Cipollini, D.; Stevenson, R.; Enright, S.; Eyles, A.; Bonello, P. Phenolic metabolites in leaves of the invasive shrub, Lonicera maackii, and their potential phytotoxic and antiherbivore effects. J. Chem. Ecol. 2008, 34, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Lamparski, R. Entomological and biochemical effects of the application of pro-ecological agrotechnical treatments in spring barley. Wyd. UTP Bydg. 2016, 1–106. [Google Scholar]
- Van Groenigen, J.W.; Van Kessel, C.; Hungate, B.A.; Oenema, O.; Powlson, D.S.; Van Groenigen, K.J. Response to the letter to the editor regarding our viewpoint “sequestering soil organic carbon: A nitrogen dilemma”. Environ. Sci. Technol. 2017, 51, 11503–11504. [Google Scholar] [CrossRef]
- Ouyang, Y.; Norton, J.M. Short-term nitrogen fertilization affects microbial community composition and nitrogen mineralization functions in an agricultural soil. Appl. Environ. Microbiol. 2020, 86, e02278-19. [Google Scholar] [CrossRef]
- Szczepanek, M.; Stypczyńska, Z.; Dziamski, A.; Wichrowska, D. Above- and below-ground part growth in chewings and strong creeping red fescue grown for seed resulting from retardants and N fertilization. Agronomy 2020, 10, 4. [Google Scholar] [CrossRef]
- Zhang, J.; An, T.; Chi, F.; Wei, D.; Zhou, B.; Hao, X.; Jin, L.; Wang, J. Evolution over years of structural characteristics of humic acids in Black Soil as a function of various fertilization treatments. J. Soils Sediments 2019, 19, 1959–1969. [Google Scholar] [CrossRef]
- Ventorino, V.; De Marco, A.; Pepe, O.; De Santo, A.V.; Moschetti, G. Impact of innovative agricultural practices of carbon sequestration on soil microbial community. In Carbon Sequestration in Agricultural Soils; Piccolo, A., Ed.; Springer: Berlin, Germany, 2012; pp. 145–178. [Google Scholar]
- Debska, B.; Kotwica, K.; Banach-Szott, M.; Spychaj-Fabisiak, E.; Tobiašová, E. Soil fertility improvement and carbon sequestration through exogenous organic matter and biostimulant application. Agriculture 2022, 2, 1478. [Google Scholar] [CrossRef]
- Kalbitz, K.; Solinger, S.; Park, J.H.; Michalzik, B.; Matzner, E. Controls on the dynamics of organic matter in soils: A review. Soil Sci. 2000, 165, 277–304. [Google Scholar] [CrossRef]
- Jokubauskaite, I.; Slepetiene, A.; Karcauskiene, D. Influence of different fertilization on the dissolved organic carbon, nitrogen and phosphorus accumulation in acid and limed soils. Eurasian J. Soil Sci. 2015, 4, 137–143. [Google Scholar] [CrossRef]
- Rosa, E.; Debska, B. Seasonal changes in the content of dissolved organic matter in arable soils. J. Soils Sediments 2018, 18, 2703–2714. [Google Scholar] [CrossRef]
- Chantigny, M.H. Dissolved and water-extractable organic matter in soils: A review on the influence of land use and management practice. Geoderma 2003, 113, 357–380. [Google Scholar] [CrossRef]
- Asare, M.O.; Száková, J.; Tlustoš, P. The fate of secondary metabolites in plants growing on Cd-, As-, and Pb-contaminated soils—A comprehensive review. Environ. Sci. Pollut. Res. 2023, 30, 11378–11398. [Google Scholar] [CrossRef] [PubMed]
- Jian, S.; Li, J.; Chen, J.; Wang, G.; Mayes, M.A.; Kudjo, E.M.; Dafeng, H.D.; Luo, Y. Soil extracellular enzyme activities, soil carbon and nitrogen storage under nitrogen fertilization: A meta-analysis. Soil Biol. Biochem. 2016, 101, 32–43. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L. Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol. Biochem. 2010, 42, 391–404. [Google Scholar] [CrossRef]
- Sherene, T. Role of soil enzymes in nutrient transformation: A review. Bio Bull. 2017, 3, 109–131. [Google Scholar]
- Lemanowicz, J.; Bartkowiak, A.; Lamparski, R.; Wojewódzki, P.; Pobereżny, J.; Wszelaczyńska, E.; Szczepanek, M. Physicochemical and enzymatic soil properties influenced by cropping of primary wheat under organic and conventional farming systems. Agronomy 2020, 10, 1652. [Google Scholar] [CrossRef]
- Xiao, Q.; He, B.; Wang, S. Effect of the Different Fertilization Treatments Application on Paddy Soil Enzyme Activities and Bacterial Community Composition. Agronomy 2023, 13, 712. [Google Scholar] [CrossRef]
- USDA. Keys to Soil Taxonomy, 10th ed.; United States Department of Agriculture, Natural Resources Conservation Service: Washington, DC, USA, 2006; pp. 1–332. [Google Scholar]
- Szczepanek, M.; Lemańczyk, G.; Lamparski, R.; Wilczewski, E.; Graczyk, R.; Nowak, R.; Prus, P. Ancient wheat species (Triticum sphaerococcum Perc. and T. persicum Vav.) in organic farming: Influence of sowing density on agronomic traits, pests and diseases occurrence, and weed infestation. Agriculture 2020, 10, 556. [Google Scholar] [CrossRef]
- Keutgen, A.J.; Pawelzik, E. Modifications of strawberry fruit antioxidant pools and fruit quality under NaCl stress. J. Agric. Food Chem. 2007, 55, 4066–4072. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, D.W.; Bain, H.; Dale, M.F.B. Development of rapid colorimetric method for the determination of chlorogenic acid in freeze-dried potato tubers. J. Sci. Food Agric. 1992, 58, 41–48. [Google Scholar] [CrossRef]
- Tratwal, A.; Roik, K.; Horoszkiewicz-Janka, J.; Wielkopolan, B.; Bandyk, A.; Jakubowska, M. Monitorowanie i Prognozowanie Chorób i Szkodników w Uprawie Zbóż i Kukurydzy; Wyd. CDR W Brwinowie: Poznań, Poland, 2015; Volume 63. [Google Scholar]
- Müller, F.P. Mszyce—Szkodniki Roślin. Terenowy Klucz do Oznaczania. Klucze do Oznaczania Bezkręgowców Polski 2; Wyd. PWN: Warszawa, Poland, 1976; pp. 7–79. [Google Scholar]
- Zawirska, I. Wciornastki (Thysanoptera). Diagnostyka Szkodników Roślin i Ich Wrogów Naturalnych; Wyd. SGGW: Warszawa, Poland, 1994; pp. 145–174. [Google Scholar]
- Warchałowski, A. Chrysomelidae. The Leaf Beetles of Europe and the Mediterranean Area; Wyd. Natura Optima Dux Foundation: Warszawa, Poland, 2003; p. 656. [Google Scholar]
- PN-ISO 10390; Chemical and Agricultural Analysis: Determining Soil pH. Polish Standards Committee: Warszawa, Poland, 1997.
- Griffith, S.M.; Schnitzer, M. Analytical characteristics of humic and fulvic acids extracted from tropical volcanic soils. Soil Sci. Soc. Am. Proc. 1975, 39, 861–867. [Google Scholar] [CrossRef]
- Ross, K.A.; Beta, T.; Arntfield, S.D. A comparative study on the phenolic acids identified and quantified in dry beans using HPLC as affected by different extraction and hydrolysis methods. Food Chem. 2009, 113, 336–344. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Wang, Q.; Wang, H.; Duan, B.; Zhang, G. Environmental behaviors of phenolic acids dominated their rhizodeposition in boreal poplar plantation forest soils. J. Soils Sediments 2016, 16, 1858–1870. [Google Scholar] [CrossRef]
- Johnson, J.I.; Temple, K.I. Some variables affecting the measurements of catalase activity in soil. Soil Sci. Soc. Am. 1964, 28, 207–209. [Google Scholar] [CrossRef]
- Thalmann, A. Zur Methodik der Bestimung der Dehydrogenaseaktivität im Boden mittels Triphenyltetrazoliumchlorid (TTC). Landwirtsch. Forsch. 1968, 21, 249–258. [Google Scholar]
- Bartha, R.; Bordeleau, L. Cell-free peroxidases in soil. Soil Biol. Biochem. 1969, 1, 139–143. [Google Scholar] [CrossRef]
- Ward, J.H. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Upreti, K.K.; Sharma, M. Role of plant growth regulators in abiotic stress tolerance. In Abiotic Stress Physiology of Horticultural Crops; Rao, N., Shivashankara, K., Laxman, R., Eds.; Springer: New Delhi, India, 2016. [Google Scholar] [CrossRef]
- Liao, Y.; Zeng, L.; Li, P.; Sun, T.; Wang, C.; Li, F.; Chen, Y.; Du, B.; Yang, Z. Influence of plant growth retardants on quality of codonopsis Radix. Molecules 2017, 22, 1655. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Sun, D.; Li, Y.; Wang, C.; Xie, Y.; Guo, T. effect of nitrogen fertilisation and irrigation on phenolic content, phenolic acid composition, and antioxida;t activity of winter wheat grain. J. Sci. Food Agric. 2015, 95, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Jaenisch, B.; Gui, Y.; Hu, R.; Chen, G.; Lollato, R.P.; Li, Y. Effect of environment and field management strategies on phenolic acid profiles of hard red winter wheat genotypes. J. Sci. Food Agric. 2021, 102, 2424–2431. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, B.; Yan, F.; Honermeier, B. Influence of nitrogen fertilization on yield and phenolic compounds in wheat grains (Triticum aestivum L. ssp. aestivum). J. Plant. Nutr. Soil Sci. 2019, 182, 111–118. [Google Scholar] [CrossRef]
- Tian, W.; Wang, F.; Xu, K.; Zhang, Z.; Yan, J.; Yan, J.; Tian, Y.; Liu, J.; Zhang, Y.; Zhang, Y.; et al. Accumulation of wheat phenolic acids under different nitrogen rates and growing environments. Plants 2022, 11, 2237. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Orozco, R.; Li, L.; Harflett, C.; Shewry, P.R.; Ward, J.L. Effects of environment and genotype on phenolic acids in wheat in the HEALTHGRAIN diversity screen. J. Agric. Food Chem. 2010, 58, 9341–9352. [Google Scholar] [CrossRef]
- Ma, D.; Li, Y.; Zhang, J.; Wang, C.; Qin, H.; Ding, H.; Xie, Y.; Guo, T. accumulation of phenolic compounds and expression profiles of phenolic acid biosynthesis-related genes in developing grains of white, purple, and red wheat. Front. Plant Sci. 2016, 7, 528. [Google Scholar] [CrossRef]
- Engert, N.; John, A.; Henning, W.; Honermeier, B. Effect of sprouting on the concentration of phenolic acids and antioxidative capacity in wheat cultivars (Triticum aestivum ssp. aestivum L.) in dependency of nitrogen fertilization. J. Appl. Bot. Food Qual. 2011, 84, 111–118. [Google Scholar]
- Mikulajová, A.; Takacsova, M.; Alexy, P.; Brindzova, L. Optimization of extraction of phenolic compounds from buckwheat based on an experimental design method. Chemické Listy 2007, 101, 563–568. [Google Scholar]
- Pobereżny, J.; Wszelaczyńska, E.; Lamparski, R.; Lemanowicz, J.; Bartkowiak, A.; Szczepanek, M.; Gościnna, K. The impact of spring wheat species and sowing density on soil biochemical properties, content of secondary plant metabolites and the presence of Oulema ssp. PeerJ 2023, 11, e14916. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xiao, L.; Tong, J.; Liu, F. Foliar application of chlorocholine chloride improves leaf mineral nutrition, antioxidant enzyme activity, and tuber yield of potato (Solanum tuberosum L.). Sci. Hortic. 2010, 125, 521–523. [Google Scholar] [CrossRef]
- Cottrell, T.E.; Wood, B.W.; Ni, X. Application of plant growth regulators mitigates chlorotic foliar injury by the black pecan aphid (Hemiptera: Aphididae). Pest Manag. Sci. 2010, 66, 1236–1242. [Google Scholar] [CrossRef] [PubMed]
- Schutz, K.; Bonkowski, M.; Scheu, S. Effects of Collembola and fertilizers on plant performance (Triticum aestivum) and aphid reproduction (Rhopalosiphum padi). Basic Appl. Ecol. 2008, 9, 182–188. [Google Scholar] [CrossRef]
- Kang, Z.; Liu, F.; Tan, X.; Zhang, Z.; Zhu, J.; Tian, H.; Liu, T. Infection of powdery mildew reduces the fitness of grain aphids (Sitobion avenae) through restricted nutrition and induced defense response in wheat. Front. Plant Sci. 2018, 9, 778. [Google Scholar] [CrossRef]
- Aqueel, M.A.; Leather, S.R. Effect of nitrogen fertilizer on the growth and survival of Rhopalosiphum padi (L.) and Sitobion avenae (F.) (Homoptera: Aphididae) on different wheat cultivars. Crop Prot. 2011, 30, 216–221. [Google Scholar] [CrossRef]
- Long, W.; Xiao-Hui, W.; Tong, H.; Lei, Q.; Li-Kun, L.; Fa-Jun, C. The effect of fertilizer-N on the inter-specific competition among three wheat aphids under elevated CO2. J. Appl. Entomol. 2019, 143, 1032–1042. [Google Scholar] [CrossRef]
- Chantigny, M.H.; Angers, D.A.; Prévost, D.; Simard, R.R.; Chalifour, F.P. Dynamics of soluble organic C and C mineralization in cultivated soils with varying N fertilization. Soil Biol. Biochem. 1999, 31, 543–550. [Google Scholar] [CrossRef]
- Zsolnay, A.; Gorlitz, H. Water extractable organic matter in arable soils effects of drought and long-term fertilization. Soil Biol. Biochem. 1994, 26, 1257–1261. [Google Scholar] [CrossRef]
- Liu, Z.J.; Clay, S.A.; Clay, D.E.; Harper, S.S. Ammonia fertilizer influences atrazine adsorption–desorption characteristics. J. Agric. Food. Chem. 1995, 43, 815–819. [Google Scholar] [CrossRef]
- Homann, P.S.; Grigal, D.F. Molecular weight distribution of soluble organics from laboratory-manipulated soils. Soil Sci. Soc. Am. J. 1992, 56, 1305–1310. [Google Scholar] [CrossRef]
- Embacher, A.; Zsolnay, A.; Gattinger, A.; Munch, J.C. The dynamics of water extractable organic matter (WEOM) in common arable topsoils: II. Influence of mineral and combined mineral and manure fertilization in Haplic Chernozem. Geoderma 2008, 148, 63–69. [Google Scholar] [CrossRef]
- Cao, Z.; Wang, Y.; Li, J.; Zhang, J.; He, N. Soil organic carbon contents, aggregate stability, and humic acid composition in different alpine grasslands in Qinghai-Tibet Plateau. J. Mt. Sci. 2016, 13, 2015–2027. [Google Scholar] [CrossRef]
- Debska, B.; Jaskulska, I.; Jaskulski, D. Method of tillage with the factor determining the quality of organic matter. Agronomy 2020, 10, 1250. [Google Scholar] [CrossRef]
- Pastuszko, A. Soil organic matter. Environ. Prot. Nat. Res. 2007, 30, 83–98. [Google Scholar]
- Holik, L.; Volánek, J.; Vranová, V. Effect of plant growth regulators on protease activity in forest floor of norway spruce stand. Forests 2021, 12, 665. [Google Scholar] [CrossRef]
- Guo, X.; Xu, Y.; Zhang, F.; Yu, S.; Han, L.; Jiang, S. Chlormequat residues and dissipation rates in cotton crops and soil. Ecotoxicol. Environ. Saf. 2010, 73, 642–646. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, M.; Jiang, X.; Guan, D.; Wei, D.; Zhao, B.; Chen, S.; Cao, F.; Li, L.; Yang, X.; et al. Impact of 36 years of nitrogen fertilization on microbial community composition and soil carbon cycling-related enzyme activities in rhizospheres and bulk soils in northeast China. Appl. Soil Ecol. 2019, 136, 148–157. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, C.; Zheng, M.; Jiang, L.; Luo, Y. Patterns and mechanisms of responses by soil microbial communities to nitrogen addition. Soil Biol. Biochem. 2017, 115, 433–441. [Google Scholar] [CrossRef]
- Dong, L.; Berg, B.; Gu, W.; Wang, Z.; Sun, T. Effects of different forms of nitrogen addition on microbial extracellular enzyme activity in temperate grassland soil. Ecol. Processes 2022, 11, 36. [Google Scholar] [CrossRef]
- Sawicka, B.; Krochmal-Marczak, B.; Pszczółkowski, P.; Bielińska, E.J.; Wójcikowska-Kapusta, A.; Barbaś, P.; Skiba, D. Effect of differentiated nitrogen fertilization on the enzymatic activity of the soil for sweet potato (Ipomoea batatas L. [Lam.]) cultivation. Agronomy 2020, 10, 1970. [Google Scholar] [CrossRef]
- Piotrowska, A.; Wilczewski, E. Effects of catch crops cultivated for green manure and mineral nitrogen fertilization on soil enzyme activities and chemical properties. Geoderma 2012, 189–190, 72–80. [Google Scholar] [CrossRef]
- Rutkowski, K.; Łysiak, G.P.; Zydlik, Z. Effect of nitrogen fertilization in the sour cherry orchard on soil enzymatic activities, microbial population, and fruit quality. Agriculture 2022, 12, 2069. [Google Scholar] [CrossRef]
- Liu, C.W.; Lin, K.H.; Kuo, Y.M. Application of factor analysis in the assessment of groundwater quality in a blackfoot disease area in Taiwan. Sci. Total Environ. 2003, 313, 77–89. [Google Scholar] [CrossRef]
- Hoostal, M.J.; Bouzat, J.L. The modulating role of dissolved organic matter on spatial patterns of microbial metabolism in Lake Erie sediments. Microb. Ecol. 2008, 55, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Joanisse, G.D.; Bradley, R.L.; Preston, C.M.; Munson, A.D. Soil enzyme inhibition by condensed litter tannins may drive ecosystem structure and processes: The case of Kalmia angustifolia. New Phytol. 2007, 175, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Shi, W. Soil peroxidase regulates organic matter decomposition through improving the accessibility of reducing sugars and amino acids. Biol. Fertil. Soils 2014, 50, 785–794. [Google Scholar] [CrossRef]
- Zhang, N.; He, X.; Gao, Y.; Li, Y.; Wang, H.; Ma, D.; Zhang, R.; Yang, S. Pedogenic carbonate and soil dehydrogenase activity in response to soil organic matter in artemisia ordosica community. Pedosphere 2010, 20, 229235. [Google Scholar] [CrossRef]
- Bach, C.E.; Warnock, D.D.; Van Horn, D.J.; Weintraub, M.N.; Sinsabaugh, R.L.; Allison, S.D.; German, D.P. Measuring phenol oxidase and peroxidase activities with pyrogallol, l-DOPA, and ABTS: Effect of assay conditions and soil type. Soil Biol. Biochem. 2013, 67, 183–191. [Google Scholar] [CrossRef]
- Błońska, E.; Lasota, J.; Zwydak, M. The relationship between soil properties, enzyme activity and land use. For. Res. Pap. 2017, 78, 39–44. [Google Scholar] [CrossRef]
- Bollag, J.M.; Chen, C.M.; Sarkar, J.M.; Loll, M.J. Extraction and purification of a peroxidase from soil. Soil Biol. Biochem. 1987, 19, 61–67. [Google Scholar] [CrossRef]
- Turner, B.L. Variation in pH optima of hydrolytic enzyme activities in tropical rain forest soils. Appl. Environ. Microbiol. 2010, 76, 6485–6493. [Google Scholar] [CrossRef] [PubMed]

| N Dose ** II Factor | Plant Growth Retardants (PGRs) * I Factor | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PGR0 | PGR1 | PGR2 | Mean | PGR0 | PGR1 | PGR2 | Mean | PGR0 | PGR1 | PGR2 | Mean | |
| TP (µg g−1 DM) | ACH (µg g−1 DM) | FRAP (mM Fe2+ kg−1) | ||||||||||
| N0 | 2.79 b^ ±0.049 | 3.38 a ±0.048 | 3.38 a ±0.023 | 3.182 B ±0.394 | 1241 b ±26.0 | 1284 b ±22.1 | 1340 a ±11.1 | 1288 B ±38.5 | 6.68 b ±0.822 | 7.81 a ±1.056 | 7.82 a ±1.065 | 7.44 B ±0.799 |
| N20 | 3.23 a ±0.435 | 3.48 a ±0.027 | 3.54 a ±0.018 | 3.44 B ±0.342 | 1264 b ±17.4 | 1310 a ±16.4 | 1309 a ±27.0 | 1294 AB ±39.6 | 7.10 b ±0.822 | 7.47 b ±0.600 | 8.19 a ±0.107 | 7.59 B ±0.807 |
| N40 | 3.18 c ±0.097 | 3.52 b ±0.058 | 3.85 a ±0.027 | 3.52 B ±0.128 | 1278 b ±12.5 | 1314 ab ±38.5 | 1348 a ±11.5 | 1313 AB ±11.0 | 7.34 c ±0.355 | 7.78 b ±0.611 | 8.58 a ±0.289 | 7.90 A ±0.940 |
| N60 | 3.24 c ±0.061 | 3.58 b ±0.036 | 3.98 a ±0.005 | 3.60 A ±0.191 | 1284 b ±30.9 | 1318 b ±51.5 | 1380 a ±10.1 | 1330 A ±30.5 | 7.53 b ±0.761 | 7.88 b ±0.412 | 8.82 a ±1.044 | 8.08 A ±1.235 |
| Mean | 3.11 C ±0.280 | 3.49 B ±0.087 | 3.70 A ±0.237 | 3.43 ±0.326 | 1269 B ±34.1 | 1306 A ±51.5 | 1344 C ±23.1 | 1307 ±42.5 | 7.16 c ±1.097 | 7.73 B ±0.961 | 8.35 A ±1.244 | 7.75 ±1.412 |
| N Dose ** II Factor | Plant Growth Retardants (PGRs) * I Factor | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PGR0 | PGR1 | PGR2 | Mean | PGR0 | PGR1 | PGR2 | Mean | PGR0 | PGR1 | PGR2 | Mean | |
| Oulema spp. (ind. Per 22 m2) | Aphididae (ind. Per 22 m2) | Thripidae (ind. Per 22 m2) | ||||||||||
| N0 | 2.25 b^ ±0.500 | 3.50 b ±0.577 | 6.25 a ±0.957 | 4.00 BC ±1.859 | 23.00 c ±0.816 | 25.75 b ±0.957 | 32.75 a ±0.957 | 27.17 A ±4.366 | 7.75 a ±0.500 | 4.50 b ±0.577 | 3.50 c ±0.577 | 5.25 B ±1.960 |
| N20 | 2.50 b ±0.577 | 4.25 a ±1.500 | 5.00 a ±0.816 | 3.92 C ±1.443 | 12.75 c ±0.500 | 20.50 a ±1.000 | 16.75 b ±0.957 | 16.67 C ±3.393 | 5.75 a ±0.816 | 3.75 b ±0.500 | 5.50 a ±0.577 | 5.00 B ±1.044 |
| N40 | 3.25 b ±0.500 | 4.50 b ±1.291 | 7.00 a ±0.816 | 4.92 B ±1.832 | 14.25 b ±0.577 | 21.75 a ±0.577 | 11.50 c ±0.577 | 15.83 C ±4.549 | 4.25 b ±0.500 | 5.75 a ±0.500 | 4.75 b ±0.500 | 4.92 B ±0.793 |
| N60 | 8.25 a ±0.577 | 4.75 c ±0.957 | 6.25 b ±0.957 | 6.42 A ±1.676 | 22.75 a ±0.500 | 22.25 a ±0.957 | 20.50 b ±1.000 | 21.83 B ±1.267 | 8.00 a ±0.816 | 5.75 b ±0.957 | 4.75 c ±0.500 | 6.17 A ±1.530 |
| Mean | 4.06 B ±2.568 | 4.25 B ±1.125 | 6.13 A ±1.088 | 4.81 ±1.942 | 18.19 C ±4.902 | 22.56 A ±2.159 | 20.38 B ±8.123 | 20.38 ±5.786 | 6.44 A ±1.672 | 4.94 B ±0.998 | 4.62 B ±0.885 | 5.33 ±1.449 |
| N Dose ** II Factor | Plant Growth Retardants (PGRs) * I Factor | |||||||
|---|---|---|---|---|---|---|---|---|
| PGR0 | PGR1 | PGR2 | Mean | PGR0 | PGR1 | PGR2 | Mean | |
| pH in KCl | Clay (%) | |||||||
| N0 | 7.24 c^ | 7.30 a | 7.28 b | 7.28 A | 4.56 a | 4.28 b | 4.32 b | 4.38 B |
| ±0.01 | ±0.03 | ±0.01 | ±0.02 | ±0.04 | ±0.02 | ±0.03 | ±0.12 | |
| N20 | 7.14 c | 7.25 a | 7.21 b | 7.20 C | 4.58 ab | 4.49 b | 4.70 a | 4.59 A |
| ±0.01 | ±0.02 | ±0.02 | ±0.05 | ±0.03 | ±0.08 | ±0.06 | ±0.09 | |
| N40 | 7.22 a | 7.22 a | 7.18 b | 7.22 B | 4.30 a | 4.25 a | 4.19 a | 4.25 C |
| ±0.02 | ±0.03 | ±0.02 | ±0.02 | ±0.11 | ±0.05 | ±0.03 | ±0.04 | |
| N60 | 7.25 b | 7.25 b | 7.35 a | 7.29 A | 3.38 b | 4.32 c | 4.55 a | 4.22 C |
| ±0.01 | ±0.01 | ±0.02 | ±0.05 | ±0.04 | ±0.03 | ±0.09 | ±0.51 | |
| Mean | 7.22 B | 7.27 A | 7.26 A | 7.25 | 4.32 B | 4.33 AB | 4.43 A | 4.37 |
| ±0.04 | ±0.04 | ±0.04 | ±0.04 | ±0.49 | ±0.09 | ±0.20 | ±0.14 | |
| N Dose ** II Factor | Plant Growth Retardants (PGRs) * I Factor | |||||||
|---|---|---|---|---|---|---|---|---|
| PGR0 | PGR1 | PGR2 | Mean | PGR0 | PGR1 | PGR2 | Mean | |
| TOC (g g−1) | TN (g g−1) | |||||||
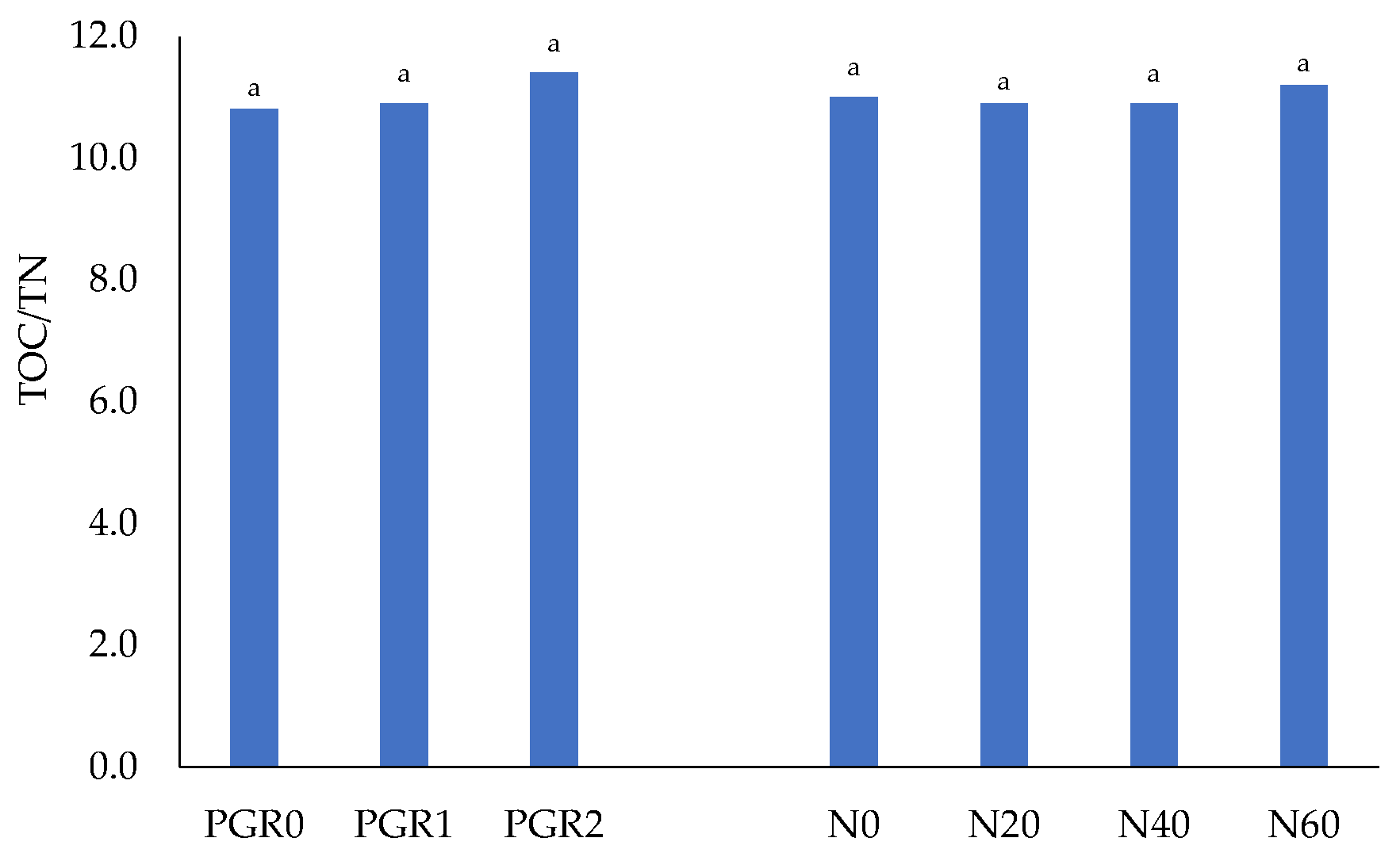
| N0 | 7.46 b^ ±0.10 | 8.50 a ±0.14 | 8.43 a ±0.10 | 8.13 B ±0.12 | 0.70 c ±0.02 | 0.78 b ±0.02 | 0.74 a ±0.02 | 0.74 B ±0.02 |
| N20 | 7.86 b ±0.12 | 8.09 b ±0.21 | 8.67 a ±0.13 | 8.21 B ±0.18 | 0.73 c ±0.02 | 0.81 a ±0.02 | 0.72 b ±0.01 | 0.75 B ±0.01 |
| N40 | 8.16 a ±0.05 | 7.99 a ±0.18 | 8.16 a ±0.17 | 8.10 B ±0.10 | 0.77 a ±0.02 | 0.73 b ±0.02 | 0.73 b ±0.02 | 0.74 B ±0.02 |
| N60 | 8.58 b ±0.15 | 9.12 a ±0.27 | 8.00 c ±0.24 | 8.57 A ±0.20 | 0.78 a ±0.03 | 0.78 a ±0.03 | 0.74 b ±0.01 | 0.77 A ±0.02 |
| Mean | 8.01 B ±0.08 | 8.42 A ±0.25 | 8.31 A ±0.18 | 8.25 ±0.22 | 0.73 B ±0.02 | 0.78 A ±0.02 | 0.73 B ±0.01 | 0.75 ±0.02 |
| DOC (mg g−1) | DN (mg g−1) | |||||||
| N0 | 103.0 b ±5.3 | 109.6 b ±4.7 | 121.1 a ±7.4 | 111.2 AB ±6.1 | 7.40 c ±0.33 | 7.70 b ±0.42 | 8.10 a ±0.28 | 7.70 B ±0.38 |
| N20 | 103.4 b ±5.5 | 117.8 a ±5.7 | 106.6 a ±3.6 | 109.3 AB ±4.8 | 6.60 c ±0.43 | 8.50 a ±0.50 | 7.40 b ±0.65 | 7.50 B ±0.50 |
| N40 | 102.4 b ±5.4 | 115.8 a ±3.9 | 98.2 b ±7.1 | 105.5 B ±5.0 | 6.90 b ±0.28 | 9.10 a ±0.43 | 6.30 b ±0.29 | 7.40 B ±0.33 |
| N60 | 113.1 b ±4.1 | 129.8 a ±6.2 | 91.9 c ±3.5 | 111.6 A ±4.8 | 11.40 a ±0.530 | 10.40 a ±0.29 | 9.80 b ±0.50 | 10.6 A ±0.43 |
| Mean | 105.5 B ±4.9 | 118.2 A ±5.0 | 104.4 B ±5.5 | 109.4 ±4.9 | 8.10 B ±0.39 | 8.90 A ±0.45 | 7.90 B ±0.4 | 8.30 ±0.50 |
| DOC (%) | DN (%) | |||||||
| N0 | 1.38 a ±0.07 | 1.29 b ±0.05 | 1.44 a ±0.08 | 1.37 A ±0.06 | 1.06 a ±0.05 | 0.99 b ±0.05 | 1.09 a ±0.04 | 1.05 B ±0.05 |
| N20 | 1.32 a ±0.07 | 1.46 a ±0.70 | 1.23 b ±0.04 | 1.23 BC ±0.05 | 0.90 c ±0.06 | 1.09 a ±0.06 | 1.03 b ±0.09 | 0.99 C ±0.08 |
| N40 | 1.25 b ±0.07 | 1.45 a ±0.05 | 1.20 b ±0.09 | 1.20 C ±0.06 | 0.90 b ±0.03 | 1.25 a ±0.06 | 0.86 c ±0.09 | 1.00 C ±0.05 |
| N60 | 1.32 ab ±0.06 | 1.42 a ±0.09 | 1.15 b ±0.09 | 1.30 AB ±0.07 | 1.50 a ±0.07 | 1.33 a ±0.04 | 1.32 a ±0.07 | 1.38 A ±0.06 |
| Mean | 1.32 B ±0.07 | 1.40 A ±0.08 | 1.26 B ±0.11 | 1.33 ±0.06 | 1.09 B ±0.06 | 1.16 A ±0.07 | 1.08 B ±0.06 | 1.11 ±0.05 |
| N Dose ** II Factor | Plant Growth Retardants (PGRs) * I Factor | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PGR0 | PGR1 | PGR2 | Mean | PGR0 | PGR1 | PGR2 | Mean | PGR0 | PGR1 | PGR2 | Mean | |
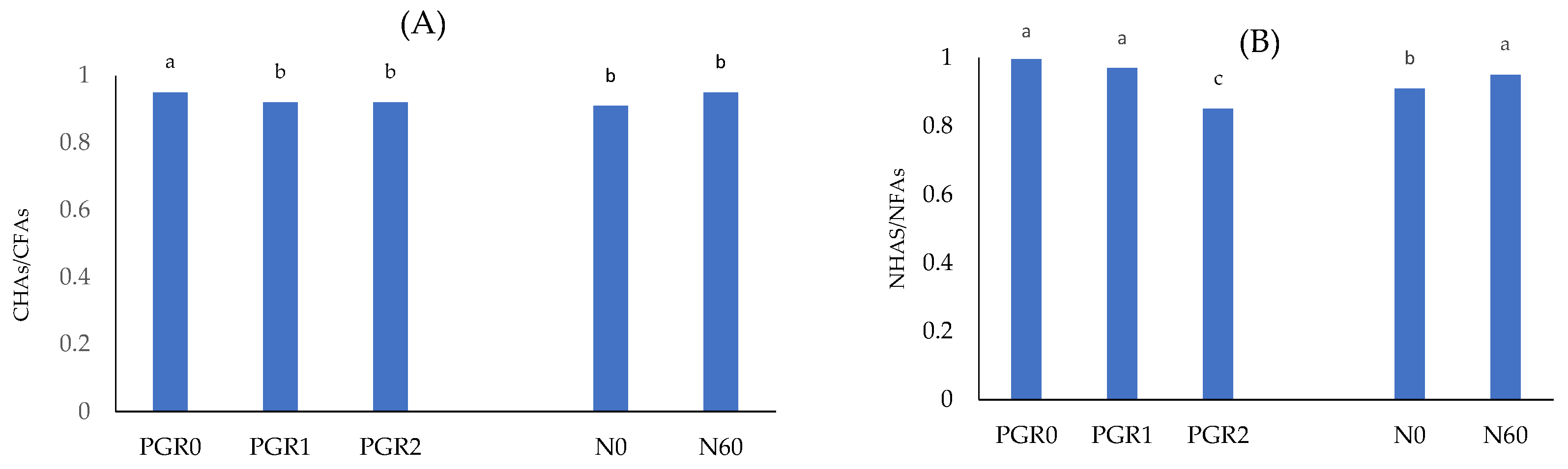
| Cd (mg kg−1) | CHAs (mg kg−1) | CFAs (mg kg−1) | ||||||||||
| N0 | 208 a^ ±2.8 | 177 b ±8.7 | 186 b ±5.0 | 191 B ±8.8 | 1837 a ±50.1 | 1778 b ±37.9 | 1724 b ±39.6 | 1780 B ±40.8 | 1945 a ±18.8 | 1954 a ±23.9 | 1932 b ±23.6 | 1944 B ±20.2 |
| N60 | 203 b ±6.3 | 213 a ±6.0 | 188 c ±6.2 | 201 A ±6.1 | 1844 b ±28.6 | 1919 a ±36.5 | 1842 b ±40.8 | 1969 A ±30.4 | 1926 b ±17.4 | 2052 a ±32.1 | 1948 b ±23.2 | 1975 A ±19.1 |
| Mean | 205 B ±4.8 | 196 A ±7.2 | 187 C ±5.8 | 196 ±6.5 | 1840 A ±35.0 | 1848 A ±36.5 | 1783 B ±40.0 | 1824 ±35.5 | 1935 B ±17.9 | 2003 A ±28.8 | 1940 B ±23.1 | 1960 ±20.1 |
| Nd (mg kg−1) | NHAs (mg kg−1) | NFAs (mg kg−1) | ||||||||||
| N0 | 17.5 a ±1.9 | 12.2 b ±2.2 | 12.7 b ±2.5 | 14.1 B ±2.3 | 135.2 a ±7.2 | 131.8 a ±7.7 | 117.2 b ±5.5 | 128.1 B ±6.6 | 135.8 a ±3.3 | 136.3 a ±4.5 | 137.8 a ±4.3 | 136.6 ±3.9 |
| N60 | 20.1 a ±2.1 | 16.2 b ±2.8 | 17.1 b ±1.8 | 17.8 A ±2.8 | 132.1 a ±6.3 | 134.5 a ±5.4 | 188.8 b ±4.1 | 151.8 A ±5.2 | 133.9 b 3.7 | 140.5 a ±5.7 | 140.2 a ±5.0 | 138.2 ±4.1 |
| Mean | 18.8 A ±2.0 | 14.2 B ±2.6 | 14.9 B ±2.2 | 16.0 ±2.7 | 133.7 B ±6.8 | 133.1 B ±6.5 | 153.0 A ±5.0 | 139.9 ±5.6 | 134.8 B ±3.4 | 138.4 AB ±5.2 | 139.0 A ±4.8 | 137.4 ±4.0 |
| N Dose ** II Factor | Plant Growth Retardants (PGRs) * I Factor | |||||||
|---|---|---|---|---|---|---|---|---|
| PGR0 | PGR1 | PGR2 | Mean | PGR0 | PGR1 | PGR2 | Mean | |
| ACH (mg g−1) | TP (mg g−1) | |||||||
| N0 | 6.62 a^ ±0.38 | 5.60 a ±0.18 | 5.28 b ±0.04 | 5.82 ±0.25 | 75.33 a ±2.72 | 65.73 b ±2.30 | 55.11 c ±2.59 | 65.39 B ±3.40 |
| N20 | 5.92 a ±0.24 | 5.59 b ±0.16 | 5.59 b ±0.22 | 5.70 ±0.20 | 71.76 a ±2.34 | 66.63 b ±2.32 | 68.49 b ±2.95 | 68.96 AB ±3.00 |
| N40 | 5.73 b ±0.11 | 5.55 c ±0.12 | 5.90 a ±0.22 | 5.73 ±0.18 | 72.94 a ±4.00 | 66.14 a ±2.70 | 69.00 a ±2.97 | 69.36 A ±2.87 |
| N60 | 5.65 b ±0.12 | 5.54 c ±0.11 | 5.98 a ±0.26 | 5.75 ±0.20 | 67.72 a ±2.00 | 67.12 a ±1.91 | 67.84 a ±1.82 | 67.56 AB ±1.99 |
| Mean | 5.98 A ±0.23 | 5.54 B ±0.16 | 5.61 B ±0.11 | 5.72 ±0020 | 71.94 A ±2.33 | 66.41 B ±2.55 | 65.11 B ±2.22 | 67.82 ±2.77 |
| N Dose II Factor ** | Plant Growth Retardants (PGRs) * I Factor | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PGR0 | PGR1 | PGR2 | Mean | PGR0 | PGR1 | PGR2 | Mean | PGR0 | PGR1 | PGR2 | Mean | |
| CAT (mg H2O2 kg−1 h−1) | DEH (mg TPF kg−1 24 h−1) | PER (mM PPG kg−1 h−1) | ||||||||||
| N0 | 0.520 b^ ±0.002 | 0.542 b ±0.028 | 0.565 a ±0.014 | 0.542 C ±0.012 | 0.435 c ±0.009 | 0.482 a ±0.006 | 0.458 b ±0.012 | 0.458 B ±0.023 | 1.421 b ±0.002 | 1.599 a ±0.003 | 1.594 a ±0.009 | 1.538 B ±0.089 |
| N20 | 0.536 b ±0.011 | 0.524 c ±0.009 | 0.580 a ±0.012 | 0.546 C ±0.024 | 0.470 b ±0.008 | 0.474 b ±0.008 | 0.485 a ±0.009 | 0.476 A ±0.002 | 1.434 b ±0.003 | 1.575 a ±0.006 | 1.580 a ±0.009 | 1.530 B ±0.070 |
| N40 | 0.606 b ±0.012 | 0.510 c ±0.011 | 0.623 a ±0.009 | 0.580 B ±0.050 | 0.486 a ±0.011 | 0.469 b ±0.012 | 0.420 c ±0.009 | 0.458 B ±0.009 | 1.506 b ±0.002 | 1.570 ab ±0.005 | 1.608 a ±0.012 | 1.561 B ±0.032 |
| N60 | 0.628 b ±0.009 | 0.639 b ±0.012 | 0.657 a ±0.008 | 0.641 A ±0.012 | 0.497 b ±0.013 | 0.509 a ±0.006 | 0.408 c ±0.011 | 0.471 A ±0.006 | 1.590 b ±0.002 | 1.727 a ±0.004 | 1.690 a ±0.003 | 1.669 A ±0.069 |
| Mean | 0.572 B ±0.045 | 0.554 C ±0.050 | 0.606 A ±0.036 | 0.577 ±0.014 | 0.472 B ±0.023 | 0.483 A ±0.015 | 0.443 C ±0.031 | 0.466 ±0.003 | 1.488 B ±0.067 | 1.618 A ±0.064 | 1.618 A ±0.042 | 1.574 ±0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemanowicz, J.; Dębska, B.; Lamparski, R.; Michalska, A.; Pobereżny, J.; Wszelaczyńska, E.; Bartkowiak, A.; Szczepanek, M.; Banach-Szott, M.; Knapowski, T. Influence of Plant Growth Retardants and Nitrogen Doses on the Content of Plant Secondary Metabolites in Wheat, the Presence of Pests, and Soil Quality Parameters. Agriculture 2023, 13, 1121. https://doi.org/10.3390/agriculture13061121
Lemanowicz J, Dębska B, Lamparski R, Michalska A, Pobereżny J, Wszelaczyńska E, Bartkowiak A, Szczepanek M, Banach-Szott M, Knapowski T. Influence of Plant Growth Retardants and Nitrogen Doses on the Content of Plant Secondary Metabolites in Wheat, the Presence of Pests, and Soil Quality Parameters. Agriculture. 2023; 13(6):1121. https://doi.org/10.3390/agriculture13061121
Chicago/Turabian StyleLemanowicz, Joanna, Bożena Dębska, Robert Lamparski, Agata Michalska, Jarosław Pobereżny, Elżbieta Wszelaczyńska, Agata Bartkowiak, Małgorzata Szczepanek, Magdalena Banach-Szott, and Tomasz Knapowski. 2023. "Influence of Plant Growth Retardants and Nitrogen Doses on the Content of Plant Secondary Metabolites in Wheat, the Presence of Pests, and Soil Quality Parameters" Agriculture 13, no. 6: 1121. https://doi.org/10.3390/agriculture13061121
APA StyleLemanowicz, J., Dębska, B., Lamparski, R., Michalska, A., Pobereżny, J., Wszelaczyńska, E., Bartkowiak, A., Szczepanek, M., Banach-Szott, M., & Knapowski, T. (2023). Influence of Plant Growth Retardants and Nitrogen Doses on the Content of Plant Secondary Metabolites in Wheat, the Presence of Pests, and Soil Quality Parameters. Agriculture, 13(6), 1121. https://doi.org/10.3390/agriculture13061121









