Ionic Response and Sorghum Production under Water and Saline Stress in a Semi-Arid Environment
Abstract
1. Introduction
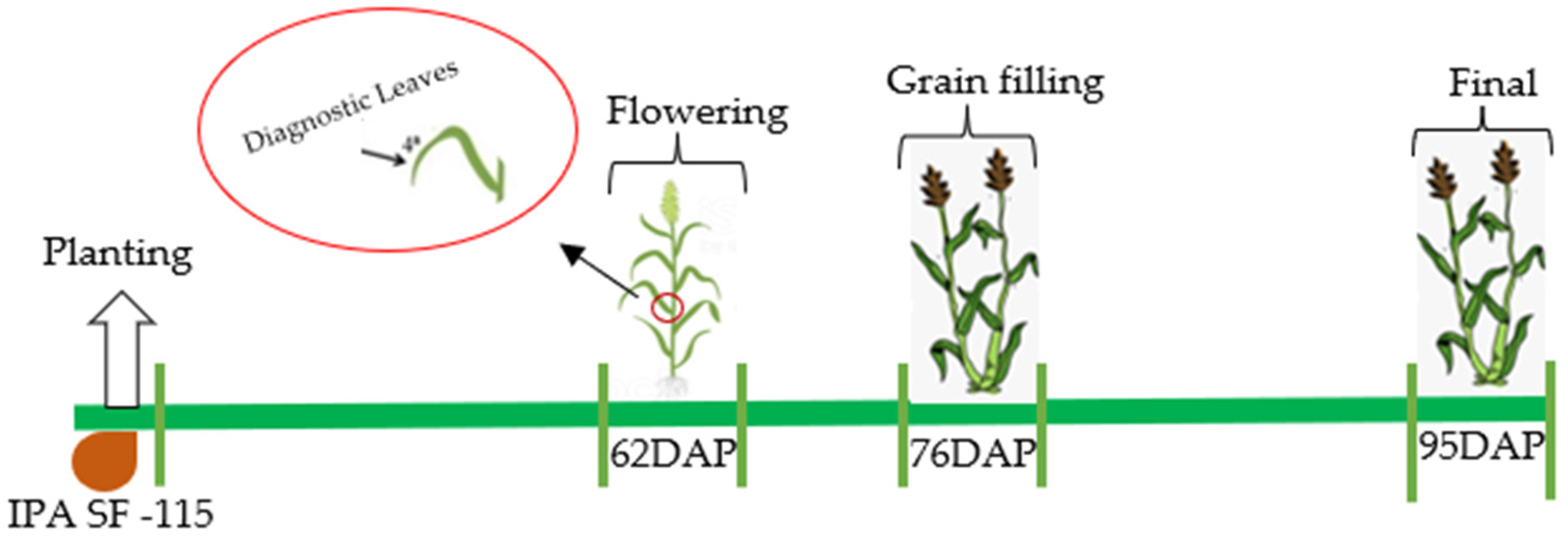
2. Materials and Methods
3. Results
3.1. Content of Mineral Elements in Sorghum Diagnosis Leaf
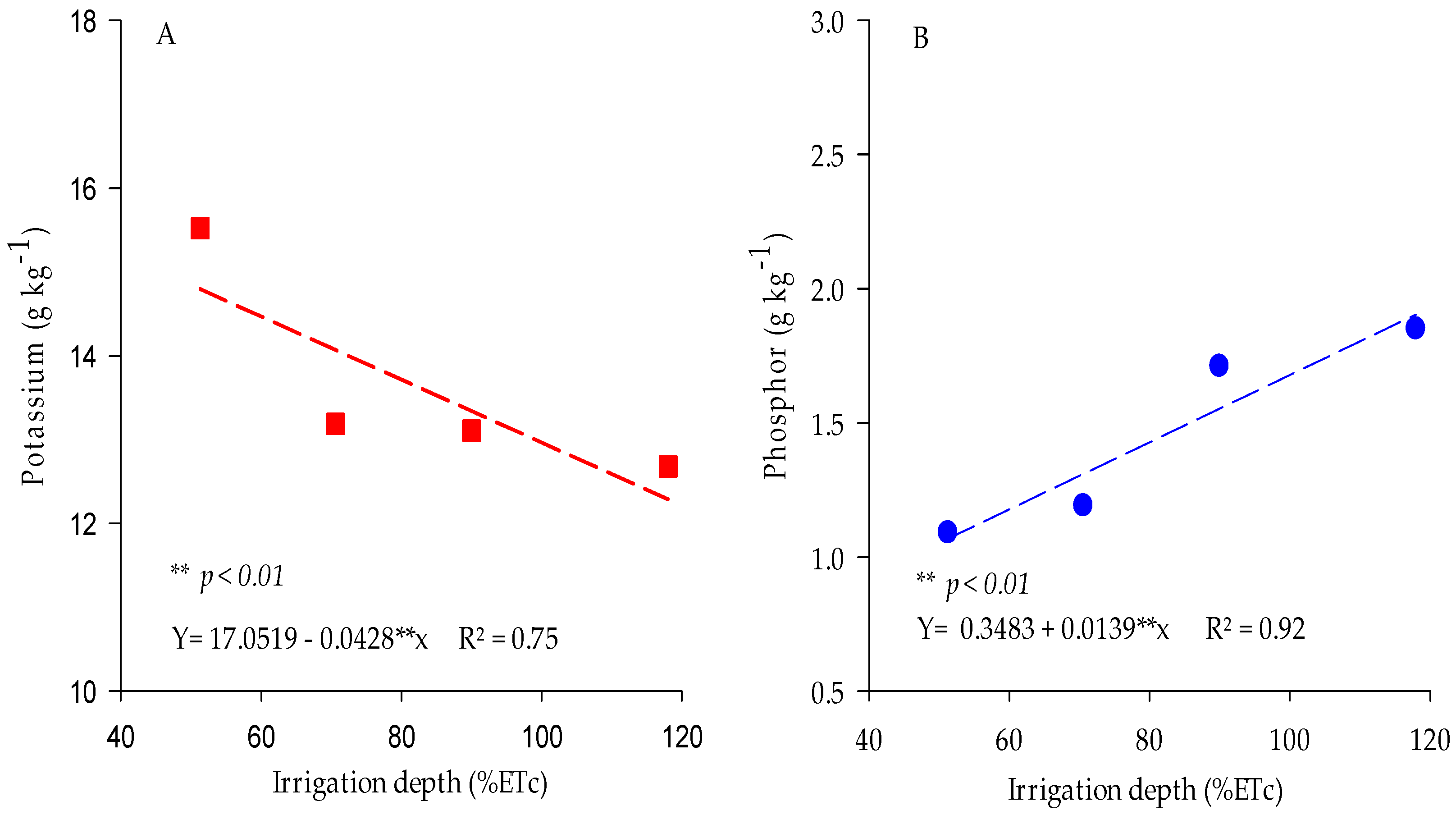
3.1.1. Potassium and Phosphorus
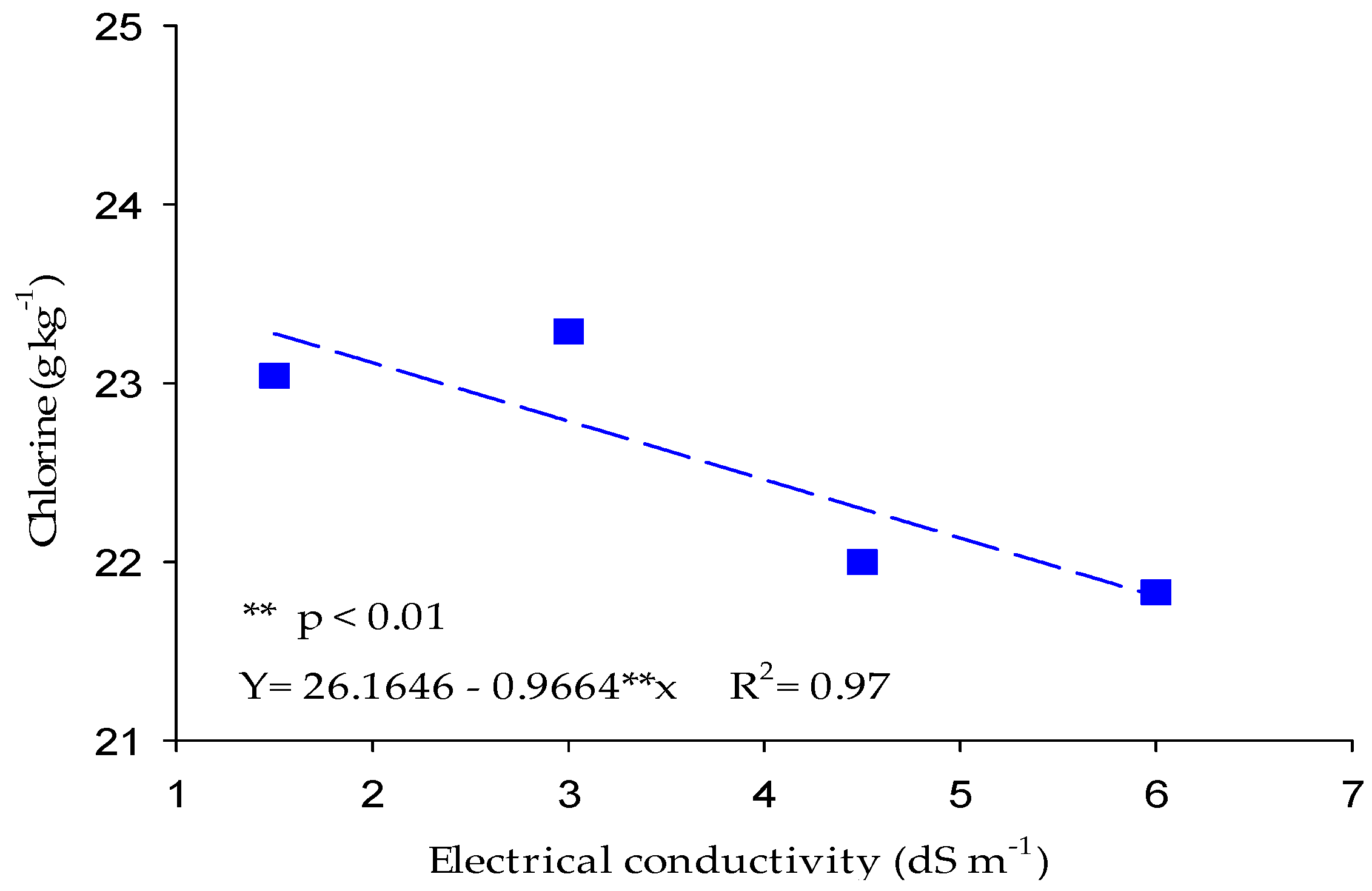
3.1.2. Chlorine
3.2. Production Variables
3.3. Pearson Correlation Matrix
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Impa, S.M.; Perumal, R.; Bean, S.R.; John Sunoj, V.S.; Jagadish, S.V.K. Water Deficit and Heat Stress Induced Alterations in Grain Physico-Chemical Characteristics and Micronutrient Composition in Field Grown Grain Sorghum. J. Cereal Sci. 2019, 86, 124–131. [Google Scholar] [CrossRef]
- Kirchner, J.H.; Robaina, A.D.; Peiter, M.X.; Torres, R.R.; Mezzomo, W.; Ben, L.H.B.; Pimenta, B.D.; Pereira, A.C. Funções de Produção e Eficiência No Uso Da Água Em Sorgo Forrageiro Irrigado. Rev. Bras. Ciências Agrárias—Braz. J. Agric. Sci. 2019, 14, e5646. [Google Scholar] [CrossRef]
- Barros, B.G.D.F.; de Freitas, A.D.S.; Tabosa, J.N.; de Lyra, M.D.C.C.P.; Mergulhão, A.C.D.E.S.; da Silva, A.F.; Oliveira, W.D.S.; Fernandes-Júnior, P.I.; Sampaio, E.V.D.S.B. Biological Nitrogen Fixation in Field-Grown Sorghum under Different Edaphoclimatic Conditions Is Confirmed by N Isotopic Signatures. Nutr. Cycl. Agroecosyst. 2020, 117, 93–101. [Google Scholar] [CrossRef]
- Tabosa, A.R.M.; Lima, L.E.; França, J.G.E.; Carvalho, E.X. Cadernos Do Semiárido Riquezas & Oportunidades /Conselho Regional de Engenharia e Agronomia de Pernambuco. In Histórico e Importância do Sorgo; CREA-PE: Recife, Brazil, 2020; pp. 1–84. [Google Scholar]
- França, I.S.; de Sales Silva, J.C.; de Lima, P.Q. A Importância Do Sorgo Na Pecuária Bovina Leiteira No Brasil. Nutr. Rev. Eletrônica 2017, 14, 4964–4969. [Google Scholar]
- Costa, A.R.F.C.; De Medeiros, J.F. Água Salina Como Alternativa Para Irrigação de Sorgo Para Geração de Energia No Nordeste Brasileiro. Water Resour. Irrig. Manag. 2017, 2017, 169–177. [Google Scholar]
- Araya, A.; Kisekka, I.; Gowda, P.H.; Prasad, P.V.V. Grain Sorghum Production Functions under Different Irrigation Capacities. Agric. Water Manag. 2018, 203, 261–271. [Google Scholar] [CrossRef]
- Klocke, N.L.; Currie, R.S.; Tomsicek, D.J.; Koehn, J.W. Sorghum Yield Response to Deficit Irrigation. Trans. ASABE 2012, 55, 947–955. [Google Scholar] [CrossRef]
- Kaplan, M.; Kara, K.; Unlukara, A.; Kale, H.; Buyukkilic Beyzi, S.; Varol, I.S.; Kizilsimsek, M.; Kamalak, A. Water Deficit and Nitrogen Affects Yield and Feed Value of Sorghum Sudangrass Silage. Agric. Water Manag. 2019, 218, 30–36. [Google Scholar] [CrossRef]
- Mansour, M.M.F.; Emam, M.M.; Salama, K.H.A.; Morsy, A.A. Sorghum under Saline Conditions: Responses, Tolerance Mechanisms, and Management Strategies. Planta 2021, 254, 24. [Google Scholar] [CrossRef]
- Silva, J.L.D.A.; de Medeiros, J.F.; Alves, S.S.V.; Oliveira, F.D.A.D.; Junior, M.J.D.S.; Nascimento, I.B.D. Uso de Águas Salinas Como Alternativa Na Irrigação e Produção de Forragem No Semiárido Nordestino. Rev. Bras. Eng. Agrícola Ambient. 2014, 18, 66–72. [Google Scholar] [CrossRef]
- Silva, J.J.F.; Migliorini, R.B. Caracterização das águas subterrâneas do aquífero furnas na região sul do estado de mato grosso. Geociências 2014, 2, 261–277. [Google Scholar]
- Schossler, T.R.; Machado, D.M.; Zuffo, A.M.; Andrade, F.R.; Piauilino, A.C. Salinidade: Efeitos na fisiologia e na nutrição mineral de plantas. Enciclopédia Biosf. 2012, 8, 1563–1578. [Google Scholar]
- Ayers, R.S.; Westcot, D. Salinity Problems. In Water Quality for Agriculture; Irrigation and Drainage, Paper 29; FAO: Roma, Italy, 1985. [Google Scholar]
- Kenneth, K.T.; Neeltje, C.K. Agricultural Drainage Water Management in Arid and Semi-Arid Areas; Irrigation and Drainage, Paper 61; FAO: Roma, Italy, 2022. [Google Scholar]
- Farooq, M.; Hussain, M.; Wakeel, A.; Siddique, K.H.M. Salt Stress in Maize: Effects, Resistance Mechanisms, and Management. A Review. Agron. Sustain. Dev. 2015, 35, 461–481. [Google Scholar] [CrossRef]
- El Sabagh, A.; Hossain, A.; Islam, M.S.; Barutcular, C.; Hussain, S.; Hasanuzzaman, M.; Akram, T.; Mubeen, M.; Nasim, W.; Fahad, S.; et al. Drought and Salinity Stresses in Barley: Consequences and Mitigation Strategies. Aust. J. Crop. Sci. 2019, 13, 810–820. [Google Scholar] [CrossRef]
- Tari, I.; Laskay, G.; Takács, Z.; Poór, P. Response of Sorghum to Abiotic Stresses: A Review. J. Agron. Crop. Sci. 2013, 199, 264–274. [Google Scholar] [CrossRef]
- Almodares, A.; Hadi, M.R.; Kholdebarin, B.; Samedani, B.; Kharazian, Z.A. The Response of Sweet Sorghum Cultivars to Salt Stress and Accumulation of Na+, CI and K+ Ions in Relation to Salinity. J. Environ. Biol. 2014, 35, 733–739. [Google Scholar]
- Yan, K.; Xu, H.; Cao, W.; Chen, X. Salt Priming Improved Salt Tolerance in Sweet Sorghum by Enhancing Osmotic Resistance and Reducing Root Na+ Uptake. Acta Physiol. Plant. 2015, 37, 203. [Google Scholar] [CrossRef]
- Shakeri, E.; Emam, Y.; Pessarakli, M.; Tabatabaei, S.A. Biochemical Traits Associated with Growing Sorghum Genotypes with Saline Water in the Field. J. Plant Nutr. 2020, 43, 1136–1153. [Google Scholar] [CrossRef]
- Calone, R.; Sanoubar, R.; Lambertini, C.; Speranza, M.; Vittori Antisari, L.; Vianello, G.; Barbanti, L. Salt Tolerance and Na Allocation in Sorghum Bicolor under Variable Soil and Water Salinity. Plants 2020, 9, 561. [Google Scholar] [CrossRef]
- Joardar, J.; Razir, S.; Islam, M.; Kobir, M. Salinity Impacts on Experimental Fodder Sorghum Production. SAARC J. Agric. 2018, 16, 145–155. [Google Scholar] [CrossRef]
- De Lacerda, C.F.; Michael, H.; De Morais, M.; Prisco, J.T.; Gomes, E. Interação Entre Salinidade e Fósforo Em Plantas de Sorgo Forrageiro. Rev. Ciência Agronômica 2006, 37, 258–263. [Google Scholar]
- Soares Filho, W.S.; Gheyi, H.R.; Brito, M.E.B.; Nobre, R.G.; Fernandes, P.D.; Miranda, R.D.S. Melhoramento Genético e Seleção de Cultivares Tolerantes à Salinidade. In Manejo da Salinidade na Agricultura: Estudos Básicos e Aplicados; Expressão Gráfica e Editora: Fortaleza, Brazil, 2016. [Google Scholar]
- Santos, H.G.; Jacomine, P.K.T.; Anjos, L.H.C.; Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R. Sistema Brasileiro de Classificação de Solos. Embrapa Solos 2018, 353, 77. [Google Scholar]
- Ayers, R.S.; Westcot, D. Water Quality for Agriculture. A Qualidade Da Água Na Agricultura. In Estudos FAO: Irrigação e Drenagem 29—Revisado; Gheyi, H.R.; de Medeiros, J.F.; Damasceno, e.F.A.V., Translators; UFPB: Campina Grande, Brazil, 1999; p. 153. [Google Scholar]
- de A. Silva Júnior, L.G.; Gheyi, H.R.; de Medeiros, J.F. Composição Química De Águas Do Cristalino Do Nordeste Brasileiro. Rev. Bras. Eng. Agrícola Ambient. 1999, 3, 11–17. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. FAO 2006. Evapotranspiración del Cultivo—Guías Para la Determinación de Los Requerimientos de Agua de Los Cultivos; FAO: Roma, Italy, 2006; p. 298. [Google Scholar]
- Chapman, H.; Pratt, P. Methods of Analysis for Soils, Plants and Waters; University of Califomia: Riverside, CA, USA, 1961. [Google Scholar]
- Martinez, H.E.P.; Carvalho, J.G.; Souza, R.B.; Diagnose, F.; Ribeiro, A.C.; Guimarães, P.T.G.; Alvarez, V.V.H. Recomendações Para o Uso de Corretivos e Fertilizantes em Minas Gerais; UFV: Viçosa, Brazil, 1999; p. 168. [Google Scholar]
- Martineau, E.; Domec, J.C.; Bosc, A.; Denoroy, P.; Fandino, V.A.; Lavres, J.; Jordan-Meille, L. The Effects of Potassium Nutrition on Water Use in Field-Grown Maize (Zea mays L.). Environ. Exp. Bot. 2017, 134, 62–71. [Google Scholar] [CrossRef]
- Hsiao, T.C. Water Stress, Growth, and Osmotic Adjustment. Phil. Trans. R Soc. Lond. 1976, 273, 479–500. [Google Scholar]
- Turner, N.C. Adaptation to Water Deficits: A Changing Perspective. Funct. Plant Biol. 1986, 13, 175–190. [Google Scholar] [CrossRef]
- Chaves, M.M.; Pereira, J.S.; Maroco, J.; Rodrigues, M.L.; Ricardo, C.P.P.; Osório, M.L.; Carvalho, I.; Faria, T.; Pinheiro, C. How Plants Cope with Water Stress in the Field. Photosynthesis and Growth. Ann. Bot. 2002, 89, 907–916. [Google Scholar] [CrossRef]
- Leão, D.A.S.; Freire, A.L.O.; De Miranda, J.R.P. Estado Nutricional De Sorgo Cultivado Sob Estresse Hídrico E Adubação Fosfatada. Pesqui. Agropecuária Trop. 2011, 41, 74–79. [Google Scholar] [CrossRef]
- Catuchi, T.A.; Guidorizzi, F.V.C.; Guidorizi, K.A.; Barbosa, A.D.M.; Souza, G.M. Respostas Fisiológicas de Cultivares de Soja à Adubação Potássica Sob Diferentes Regimes Hídricos. Pesqui. Agropecu. Bras. 2012, 47, 519–527. [Google Scholar] [CrossRef]
- Mendes, H.S.J.; Paula, N.F.D.; Scarpinatti, E.A.; de Paula, R.C. Respostas Fisiológicas de Genótipos de Eucalyptus Grandis × E. Urophylla à Disponibilidade Hídrica e Adubação Potássica. Cern. Lavras 2013, 19, 603–611. [Google Scholar] [CrossRef]
- Silva, A.R.A.; Bezerra, F.M.L.; de Lacerda, C.F.; de S. Miranda, R.; Marques, E.C. Ion Accumulation in Young Plants of the “green Dwarf” Coconut under Water and Salt Stress. Rev. Cienc. Agron. 2018, 49, 249–258. [Google Scholar] [CrossRef]
- Kuwahara, F.A.; Souza, G.M.; Guidorizi, K.A.; Costa, C.; Meirelles, P.R.D.L. Phosphorus as a Mitigator of the Effects of Water Stress on the Growth and Photosynthetic Capacity of Tropical C4 Grasses. Acta Sci.—Agron. 2016, 38, 363–370. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Álvarez, S.; Barba-Espín, G.; Hernández, J.A.; Sánchez-Blanco, M.J. Salts and Nutrients Present in Regenerated Waters Induce Changes in Water Relations, Antioxidative Metabolism, Ion Accumulation and Restricted Ion Uptake in Myrtus communis L. Plants. Plant Physiol. Biochem. 2014, 85, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of Salinity Stress on Plants and Its Tolerance Strategies: A Review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef] [PubMed]
- de Queiroz, G.C.M.; de Medeiros, J.F.; da Silva, R.R.; da Silva Morais, F.M.; de Sousa, L.V.; de Souza, M.V.P.; da Nóbrega Santos, E.; Ferreira, F.N.; da Silva, J.M.C.; Clemente, M.I.B.; et al. Growth, Solute Accumulation, and Ion Distribution in Sweet Sorghum under Salt and Drought Stresses in a Brazilian Potiguar Semiarid Area. Agriculture 2023, 13, 803. [Google Scholar] [CrossRef]
- Marschner, P. Nutrição Mineral de Plantas Superiores de Marschner, 3rd ed.; Academic Press: Cambridge, UK, 2012. [Google Scholar]
- Lucini, L.; Borgognone, D.; Rouphael, Y.; Cardarelli, M.; Bernardi, J.; Colla, G. Mild Potassium Chloride Stress Alters the Mineral Composition, Hormone Network, and Phenolic Profile in Artichoke Leaves. Front. Plant Sci. 2016, 7, 948. [Google Scholar] [CrossRef]
- De Sousa, C.H.C.; De Lacerda, C.F.; Bezerra, F.M.L.; Filho, G.E.; Sousa, A.E.C.; Sousa, G.G. De Respostas MORfofisiológicas de Plantas de Sorgo, Feijão-de-Corda e Algodão Sob Estresse Salino. Agropecuária Técnica 2010, 31, 29–36. [Google Scholar]
- Du, T.; Kang, S.; Zhang, J.; Davies, W.J. Deficit Irrigation and Sustainable Water-Resource Strategies in Agriculture for China’s Food Security. J. Exp. Bot. 2015, 66, 2253–2269. [Google Scholar] [CrossRef]
- dos Santos, D.; Guimarães, V.F.; Klein, J.; Fioreze, S.L.; Macedo Júnior, E.K. Cultivares de Trigo Submetidas a Déficit Hídrico No Início Do Florescimento, Em Casa de Vegetação. Rev. Bras. Eng. Agrícola Ambient. 2012, 16, 836–842. [Google Scholar] [CrossRef]
- Freitas, C.A.S.D.; Silva, A.R.A.D.; Bezerra, F.M.L.; Lacerda, C.F.D.; Pereira, F.J.V.; De Sousa, G.G. Produção de Matéria Seca e Trocas Gasosas Em Cultivares de Mamoneira Sob Níveis de Irrigação. Rev. Bras. Eng. Agrícola Ambient. 2011, 15, 1168–1174. [Google Scholar] [CrossRef]
- Silva, S.M.L.D.S. Avaliação da Tolerância à Salinidade em Quatro Genótipos de Sorgo Sacarino. CNR-ISTI Tech. Rep. 2015, 3, 356–369. [Google Scholar]
- Endres, L.; De Souza, J.L.; Teodoro, I.; Marroquim, P.M.G.; Santos, C.M.; De Brito, J.E.D. Gas Exchange Alteration Caused by Water Deficit during the Bean Reproductive Stage Alterações Das Trocas Gasosas Na Fase Reprodutiva e Produtividade Do Feijão Sob Déficit Hídrico. Rev. Bras. Eng. Agrícola Ambient. 2010, 14, 11–16. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Moller, I.M.; Murphy, A. Fisiologia e Desenvolvimento Vegetal, 6th ed.; Artimed: Porto Alegre, Brazil, 2017; ISBN 978-85-8271-366-2. [Google Scholar]
- Carroll, A.D.; Moyen, C.; Van Kesteren, P.; Tooke, F.; Battey, N.H.; Brownlee, C. Ca2+, Annexins, and GTP Modulate Exocytosis from Maize Root Cap Protoplasts. Plant Cell 1998, 10, 1267–1276. [Google Scholar] [CrossRef] [PubMed]



| Layer | Soil physics | ||||||
| Sand | Silt | Clay | Soil Density | ||||
| cm | g g−1 | g cm−3 | |||||
| 0–20 | 0.780 | 0.060 | 0.160 | 1.620 | |||
| Soil chemistry | |||||||
| EC | pH | Ca2+ | Mg2+ | Na+ | K+ | P | |
| dS m −1 | cmolc dm−3 | mg dm−3 | |||||
| 0–20 | 0.07 | 8.10 | 7.70 | 0.60 | 0.10 | 0.51 | 8.60 |
| EC | Na+ | Ca2+ | Mg2+ | K+ | Cl− | SO42− | HCO3− |
|---|---|---|---|---|---|---|---|
| dS m−1 | (mmol L−1) | ||||||
| 1.5 | 5.00 | 4.00 | 1.00 | 0.12 | 8.10 | 0.15 | 7.00 |
| 3.0 | 19.00 | 4.00 | 1.50 | 0.12 | 22.10 | 0.75 | 6.90 |
| 4.5 | 28.50 | 6.00 | 2.25 | 0.12 | 35.60 | 1.40 | 6.90 |
| 6.0 | 38.00 | 8.00 | 3.00 | 0.12 | 49.10 | 2.15 | 6.80 |
| SV | DF | Statistical Significance by F-Test | |||||
|---|---|---|---|---|---|---|---|
| Na | K | Ca | Mg | P | Cl | ||
| Block | 2 | 0.110 | 0.719 | 0.028 | 0.324 | 0.320 | 0.087 |
| Salt | 3 | 0.943 | 0.227 | 0.512 | 0.167 | 0.874 | 0.001 |
| Comp. L | 1 | 0.731 | 0.334 | 0.169 | 0.042 | 0.982 | 0.000 |
| Comp. Q | 1 | 0.628 | 0.093 | 0.816 | 0.543 | 0.405 | 0.732 |
| Irrig. | 3 | 0.421 | 0.002 | 0.152 | 0.326 | 0.000 | 0.388 |
| Comp. L | 1 | 0.288 | 0.001 | 0.768 | 0.152 | 0.000 | 0.153 |
| Comp. Q | 1 | 0.689 | 0.045 | 0.494 | 0.299 | 0.847 | 0.977 |
| Salt × Irrig. | 9 | 0.262 | 0.765 | 0.054 | 0.103 | 0.243 | 0.675 |
| Residue | 30 | - | - | - | - | - | - |
| Total | 47 | - | - | - | - | - | - |
| CV (%) | 11.30 | 12.82 | 16.06 | 20.97 | 24.92 | 11.00 | |
| Average | 0.51 | 13.62 | 4.31 | 2.13 | 1.46 | 90.04 | |
| SV | DF | Statistical Significance by F-Test | |||||
|---|---|---|---|---|---|---|---|
| 76 DAP | 95 DAP | ||||||
| DMP | Yield | TDM | DMP | Yield | TDM | ||
| Block | 2 | 0.000 | 0.000 | 0.463 | 0.985 | 0.108 | 0.362 |
| Salt | 3 | 0.841 | 0.042 | 0.192 | 0.525 | 0.928 | 0.656 |
| Comp. L | 0.902 | 0.132 | 0.263 | 0.699 | 0.968 | 0.701 | |
| Comp. Q | 0.519 | 0.739 | 0.511 | 0.342 | 0.514 | 0.340 | |
| Lam | 3 | 0.345 | 0.000 | 0.000 | 0.799 | 0.000 | 0.009 |
| Comp. L | 0.106 | 0.000 | 0.000 | 0.546 | 0.000 | 0.006 | |
| Comp. Q | 0.547 | 0.212 | 0.403 | 0.422 | 0.342 | 0.342 | |
| Salt × Lam | 9 | 0.263 | 0.088 | 0.629 | 0.175 | 0.766 | 0.454 |
| Residue | 30 | - | - | - | - | - | - |
| Total | 47 | - | - | - | - | - | - |
| CV (%) | 10.68 | 15.14 | 22.29 | 13.91 | 15.31 | 21.85 | |
| Average | 30.00 | 65.88 | 19.58 | 38.00 | 83.14 | 31.74 | |
| Na | K | Ca | Mg | P | Cl | DMP76 | Yield76 | TDM76 | DMP95 | Yield95 | TDM95 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na | 1 | |||||||||||
| K | 0.53 | 1 | ||||||||||
| Ca | 0.38 | 0.29 | 1 | |||||||||
| Mg | 0.50 | 0.33 | 0.80 | 1 | ||||||||
| P | 0.34 | −0.04 | 0.34 | 0.35 | 1 | |||||||
| Cl | 0.14 | 0.25 | 0.06 | 0.17 | −0.06 | 1 | ||||||
| DMP 76 | −0.34 | −0.36 | 0.06 | −0.17 | 0.04 | −0.22 | 1 | |||||
| Yield 76 | 0.09 | −0.28 | 0.11 | 0.08 | 0.52 | 0.01 | −0.04 | 1 | ||||
| TDM 76 | −0.06 | −0.42 | 0.14 | 0.01 | 0.50 | −0.08 | 0.43 | 0.88 | 1 | |||
| DMP 95 | −0.03 | −0.06 | 0.15 | 0.09 | −0.09 | 0.11 | 0.04 | 0.13 | 0.14 | 1 | ||
| Yield 95 | −0.06 | −0.34 | 0.02 | 0.04 | 0.22 | −0.15 | −0.01 | 0.49 | 0.44 | 0.63 | 1 | |
| TDM 95 | −0.04 | −0.41 | −0.08 | −0.01 | 0.35 | −0.29 | −0.05 | 0.53 | 0.45 | 0.07 | 0.81 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, R.R.; de Medeiros, J.F.; de Queiroz, G.C.M.; de Sousa, L.V.; de Souza, M.V.P.; de Almeida Bastos do Nascimento, M.; da Silva Morais, F.M.; da Nóbrega, R.F.; Silva, L.M.e.; Ferreira, F.N.; et al. Ionic Response and Sorghum Production under Water and Saline Stress in a Semi-Arid Environment. Agriculture 2023, 13, 1127. https://doi.org/10.3390/agriculture13061127
da Silva RR, de Medeiros JF, de Queiroz GCM, de Sousa LV, de Souza MVP, de Almeida Bastos do Nascimento M, da Silva Morais FM, da Nóbrega RF, Silva LMe, Ferreira FN, et al. Ionic Response and Sorghum Production under Water and Saline Stress in a Semi-Arid Environment. Agriculture. 2023; 13(6):1127. https://doi.org/10.3390/agriculture13061127
Chicago/Turabian Styleda Silva, Rodrigo Rafael, José Francismar de Medeiros, Gabriela Carvalho Maia de Queiroz, Leonardo Vieira de Sousa, Maria Vanessa Pires de Souza, Milena de Almeida Bastos do Nascimento, Francimar Maik da Silva Morais, Renan Ferreira da Nóbrega, Lucas Melo e Silva, Fagner Nogueira Ferreira, and et al. 2023. "Ionic Response and Sorghum Production under Water and Saline Stress in a Semi-Arid Environment" Agriculture 13, no. 6: 1127. https://doi.org/10.3390/agriculture13061127
APA Styleda Silva, R. R., de Medeiros, J. F., de Queiroz, G. C. M., de Sousa, L. V., de Souza, M. V. P., de Almeida Bastos do Nascimento, M., da Silva Morais, F. M., da Nóbrega, R. F., Silva, L. M. e., Ferreira, F. N., Clemente, M. I. B., Cordeiro, C. J. X., de Castro Granjeiro, J. C., Constante, D. C., & da Silva Sá, F. V. (2023). Ionic Response and Sorghum Production under Water and Saline Stress in a Semi-Arid Environment. Agriculture, 13(6), 1127. https://doi.org/10.3390/agriculture13061127










