Efficacy of Different Fungicide Spraying Techniques on the Infestation with Kabatiella zeae and Formation of Fusarium Mycotoxins in Forage Maize
Abstract
1. Introduction
2. Materials and Methods
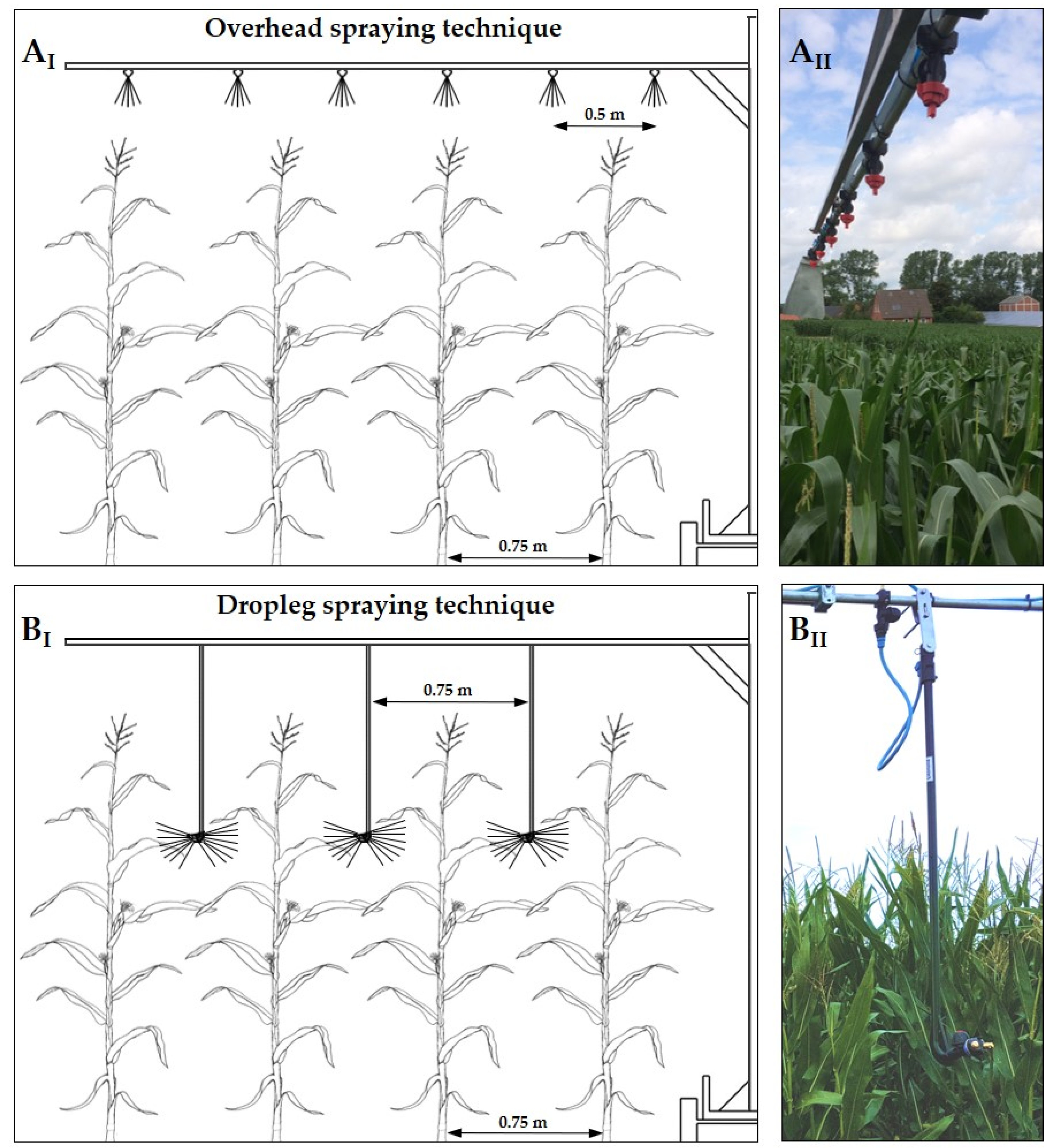
2.1. Experimental Sites and Fungicide Spraying Treatments
2.2. Disease Assessment of Kabatiella zeae
2.3. Harvest, Yield Assessment and Sample Preparation
2.4. Analysis of Mycotoxins
2.5. Statistical Analysis
3. Results
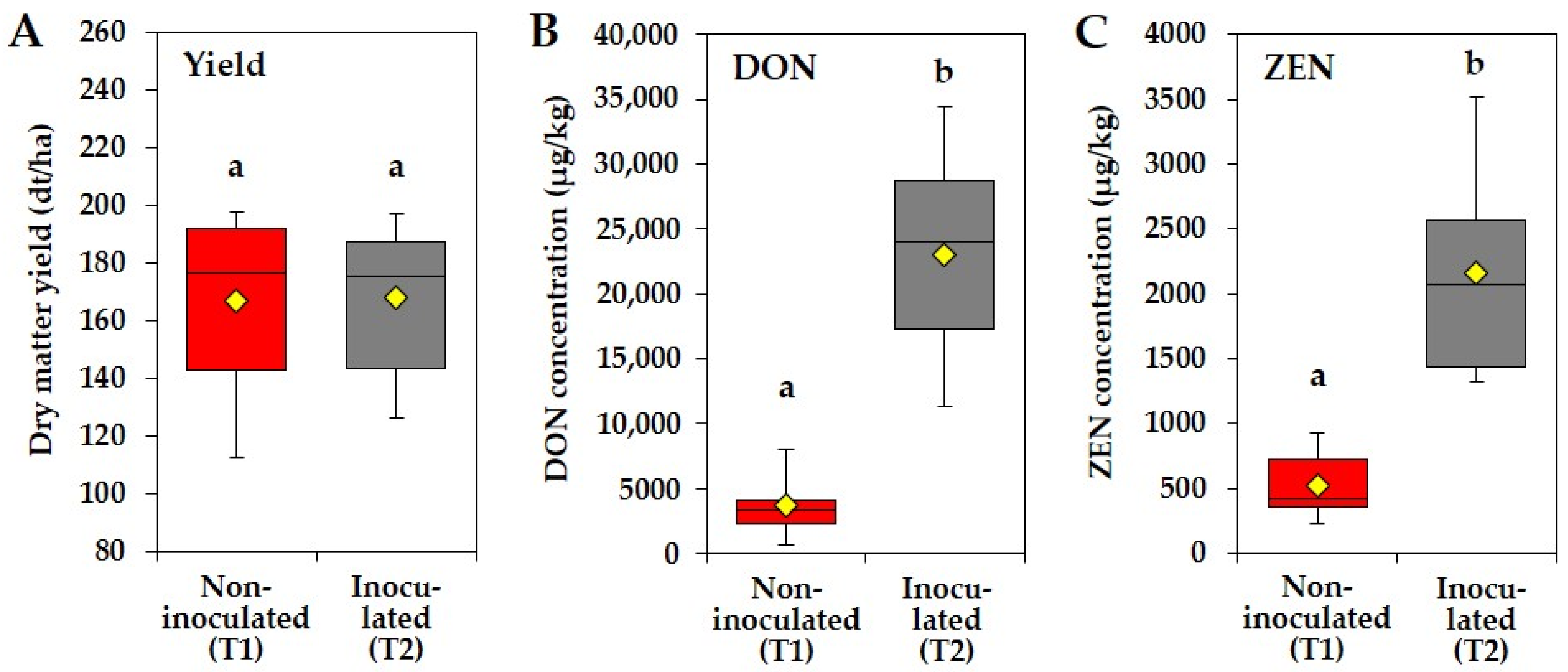
3.1. Effect of Artificial Fusarium Inoculations of Maize Main Ears on Dry Matter Yield, DON and ZEN Concentrations
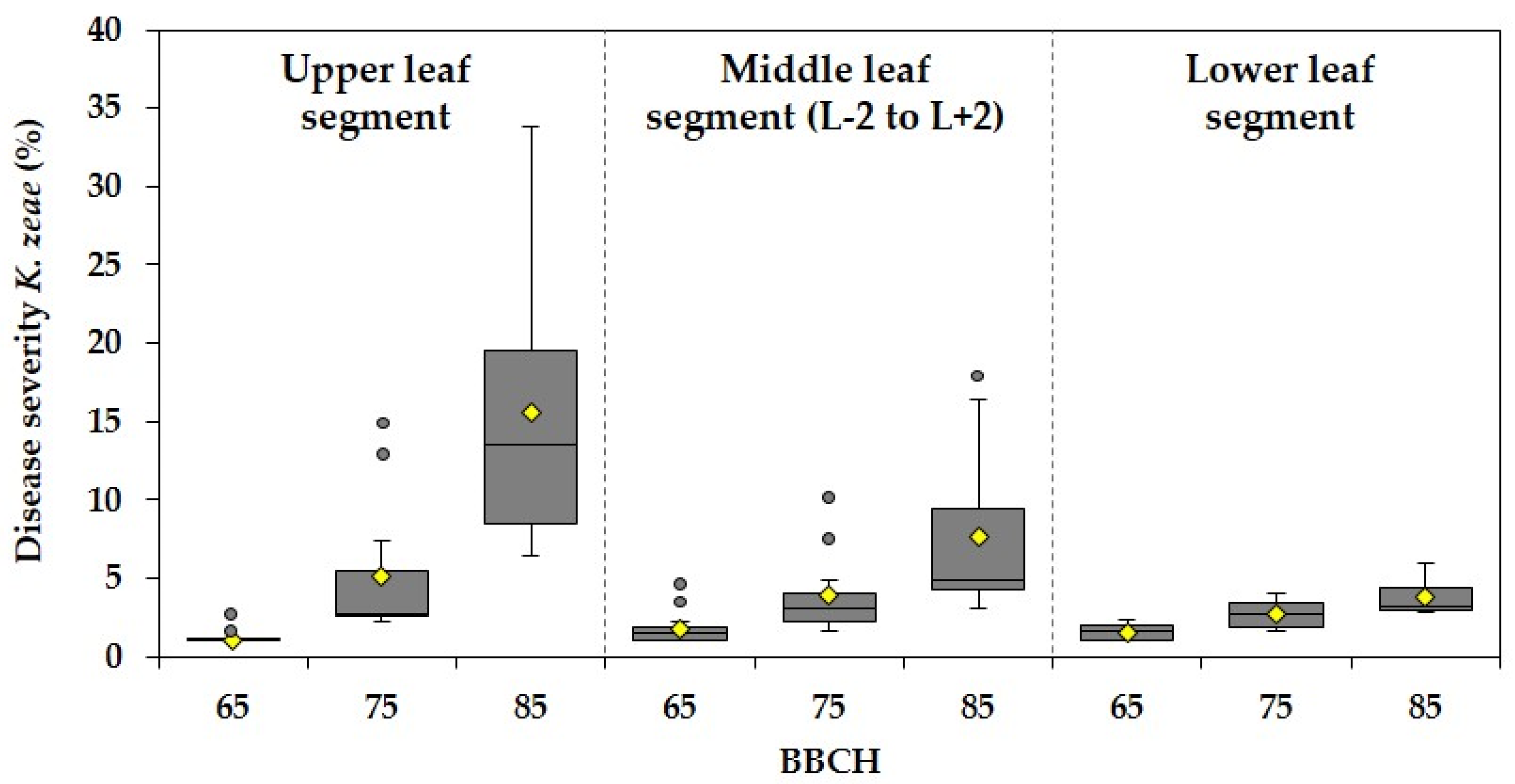
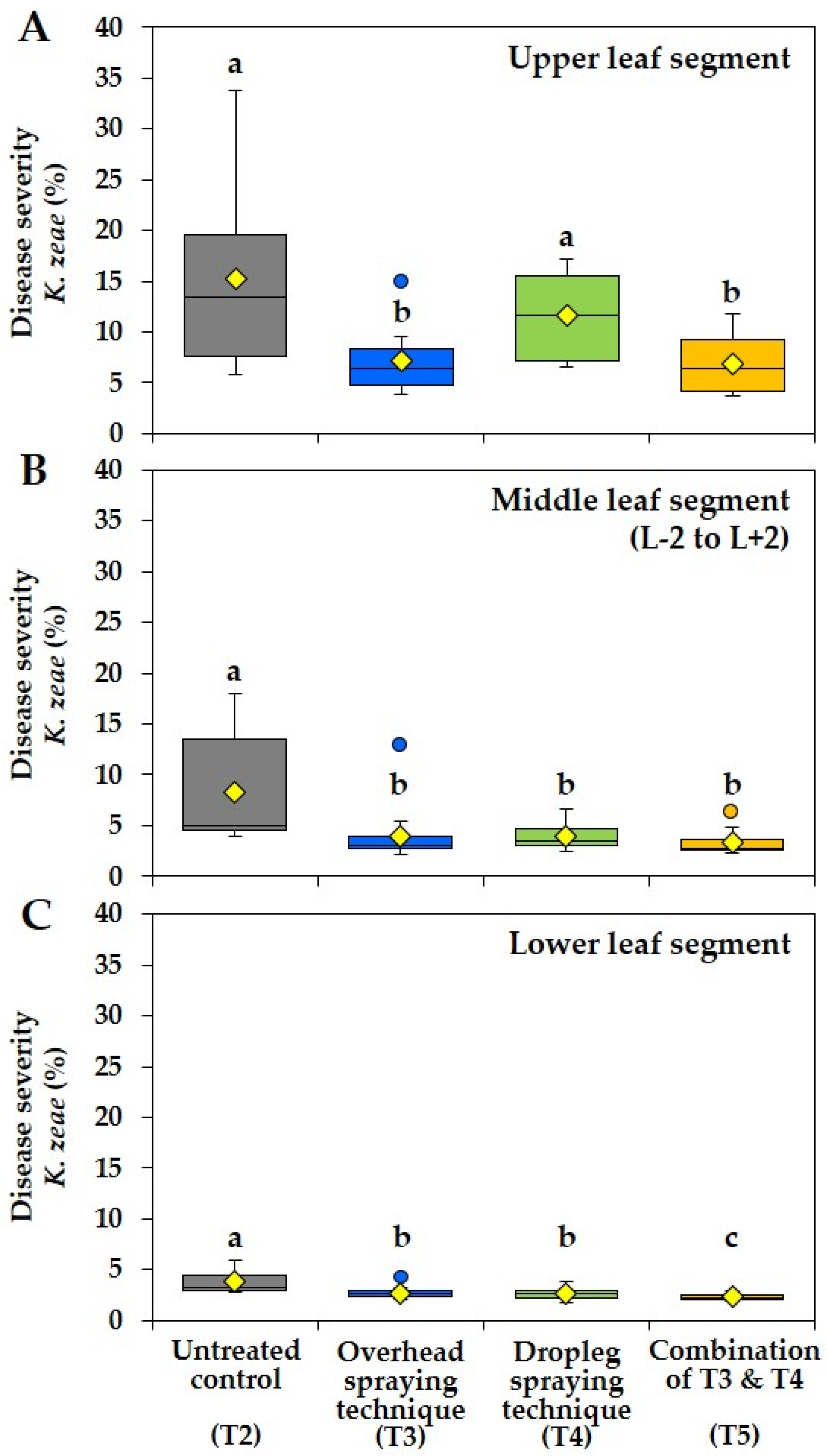
3.2. Effect of Different Fungicide Spraying Techniques on Disease Severities of Kabatiella zeae
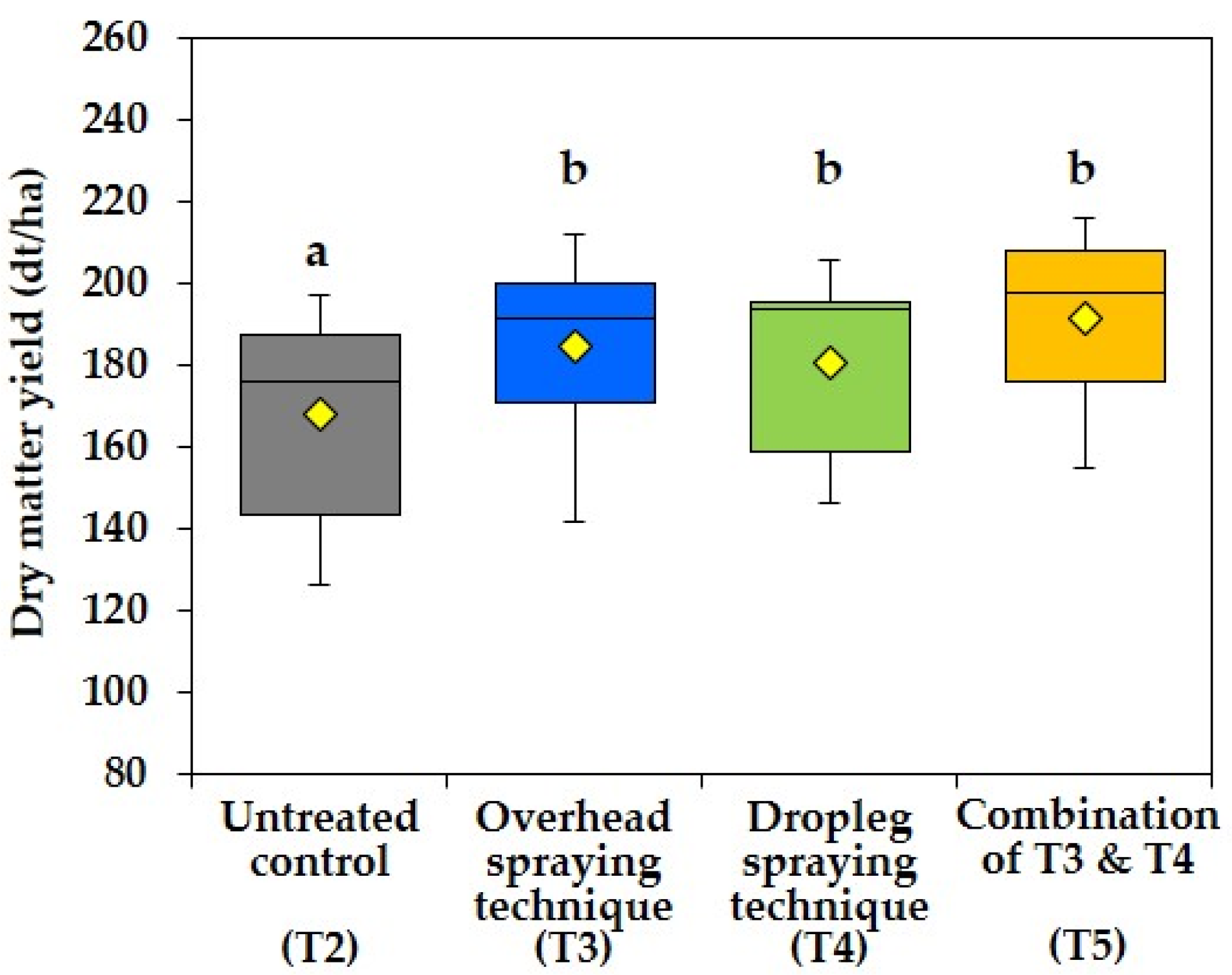
3.3. Effect of Different Fungicide Spraying Techniques on Dry Matter Yield
3.4. Effect of Different Fungicide Spraying Techniques on DON and ZEN Concentrations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poole, N.F.; Arnaudin, M.E. The role of fungicides for effective disease management in cereal crops. Can. J. Plant Pathol. 2014, 36, 1–11. [Google Scholar] [CrossRef]
- Jalli, M.; Kaseva, J.; Andersson, B.; Ficke, A.; Nistrup-Jørgensen, L.; Ronis, A.; Kaukoranta, T.; Ørum, J.-E.; Djurle, A. Yield increases due to fungicide control of leaf blotch diseases in wheat and barley as a basis for IPM decision-making in the Nordic-Baltic region. Eur. J. Plant Pathol. 2020, 158, 315–333. [Google Scholar] [CrossRef]
- Jørgensen, L.N.; Heick, T.M. Azole use in agriculture, horticulture, and wood preservation—Is it indispensable? Front. Cell. Infect. Microbiol. 2021, 11, 730297. [Google Scholar] [CrossRef] [PubMed]
- Różewicz, M.; Wyzińska, M.; Grabiński, J. The most important fungal diseases of cereals—Problems and possible solutions. Agronomy 2021, 11, 714. [Google Scholar] [CrossRef]
- Willocquet, L.; Meza, W.R.; Dumont, B.; Klocke, B.; Feike, T.; Kersebaum, K.C.; Meriggi, P.; Rossi, V.; Ficke, A.; Djurle, A.; et al. An outlook on wheat health in Europe from a network of field experiments. Crop Prot. 2021, 139, 105335. [Google Scholar] [CrossRef]
- Matthews, G.; Bateman, R.; Miller, P. Pesticide Application Methods, 4th ed.; Wiley & Blackwell: Hoboken, NJ, USA, 2014; ISBN 9781118351307. [Google Scholar]
- Bayat, A.; Bolat, A.; Soysal, A. Performance of different types of spraying methods in second crop maize production. J. Agric. Machin. Sci. 2011, 7, 53–59. [Google Scholar]
- Taube, F.; Vogeler, I.; Kluß, C.; Herrmann, A.; Hasler, M.; Rath, J.; Loges, R.; Malisch, C.S. Yield progress in forage maize in NW Europe—Breeding progress or climate change effects? Front. Plant Sci. 2020, 11, 1214. [Google Scholar] [CrossRef]
- Menechini, W.; Maggi, M.F.; Jadoski, S.O.; Leite, C.D.; Camicia, R.d.M. Aerial and ground application of fungicide in corn second crop on diseases control. Eng. Agric. 2017, 37, 116–127. [Google Scholar] [CrossRef]
- Rueegg, J.; Eder, R.; Anderau, V. Improved application techniques: Ways to higher efficacy of fungicides and insecticides in field grown vegetables. Outlooks Pest. Manag. 2006, 17, 80–84. [Google Scholar] [CrossRef]
- Brandes, M. Einsatzmöglichkeiten der Dropleg-Technik in Gemüse- und Ackerbaukulturen. J. Kult. 2021, 73, 309–315. [Google Scholar] [CrossRef]
- Hausmann, J.; Brandes, M.; Heimbach, U. Effects of dropleg application technique during flowering of oilseed rape on insect pests. Crop Prot. 2019, 126, 104917. [Google Scholar] [CrossRef]
- Hausmann, J.; Heimbach, U.; Rostás, M.; Brandes, M. The effect of insecticide application by dropleg sprayers on pollen beetle parasitism in oilseed rape. BioControl 2021, 66, 765–777. [Google Scholar] [CrossRef]
- Arny, D.C.; Smalley, E.B.; Ullstrup, A.J.; Worf, G.L.; Ahrens, R.W. Eyespot of maize, a disease new to North America. Phytopathology 1971, 61, 54–57. [Google Scholar] [CrossRef]
- Schneider, R.; Krüger, W. Kabatiella zeae Narita et Hiratsuka als Erreger einer Blattfleckenkrankheit an Mais in Deutschland. J. Phytopathol. 1972, 74, 238–248. [Google Scholar] [CrossRef]
- Reifschneider, F.J.B.; Arny, D.C. Yield loss of maize caused by Kabatiella zeae. Phytopathology 1983, 73, 607–609. [Google Scholar] [CrossRef]
- Flett, B.; Nowell, D. First report of maize eyespot caused by Aureobasidium zeae in South Africa. Afr. Plant Prot. 1995, 1, 49–50. [Google Scholar]
- Pronczuk, M.; Bojanowski, J.; Warzecha, R. Effect of leaf infection by Kabatiella zeae on stalk rot prevalence and grain yield of maize hybrids. J. Phytopathol. 2004, 152, 410–415. [Google Scholar] [CrossRef]
- Jørgensen, L.N. Significant yield increases from control of leaf diseases in maize—An overlooked problem?! Outlooks Pest. Manag. 2012, 23, 162–165. [Google Scholar] [CrossRef]
- Chen, N.; Xiao, S.; Sun, J.; He, L.; Liu, M.; Gao, W.; Xu, J.; Wang, H.; Huang, S.; Xue, C. Virulence and molecular diversity in the Kabatiella zeae population causing maize eyespot in China. Plant Dis. 2020, 104, 3197–3206. [Google Scholar] [CrossRef]
- Dingley, J.M. ‘Eye spot’ disease of maize in New Zealand. N. Z. J. Agric. Res. 1973, 16, 325–328. [Google Scholar] [CrossRef]
- Chinchilla, C.M. Eyespot (Kabatiella zeae Narita and Hiratsuka) disease progression curves in ten maize hybrids. Turrialba 1987, 37, 37–43. [Google Scholar]
- Algermissen, C. Überregionale Studien zum Einfluss von Witterungsparametern auf die Epidemiologie und Schadensdynamik von Maispathogenen (Kabatiella zeae, Exserohilum turcicum, Fusarium spp.) und deren Bekämpfung durch Integrierte Pflanzenschutzmaßnahmen. Ph.D. Thesis, Christian-Albrechts-University, Kiel, Germany, 27 January 2016. [Google Scholar]
- Oldenburg, E.; Höppner, F.; Ellner, F.; Weinert, J. Fusarium diseases of maize associated with mycotoxin contamination of agricultural products intended to be used for food and feed. Mycotoxin Res. 2017, 33, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Ferrigo, D.; Raiola, A.; Causin, R. Fusarium toxins in cereals: Occurrence, legislation, factors promoting the appearance and their management. Molecules 2016, 21, 627. [Google Scholar] [CrossRef]
- Leite, M.; Freitas, A.; Silva, A.S.; Barbosa, J.; Ramos, F. Maize food chain and mycotoxins: A review on occurrence studies. Trends Food Sci. Technol. 2021, 115, 307–331. [Google Scholar] [CrossRef]
- Munkvold, G.P. Epidemiology of Fusarium diseases and their mycotoxins in maize ears. Eur. J. Plant Pathol. 2003, 109, 705–713. [Google Scholar] [CrossRef]
- Oldenburg, E.; Ellner, F. Distribution of disease symptoms and mycotoxins in maize ears infected by Fusarium culmorum and Fusarium graminearum. Mycotoxin Res. 2015, 31, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Deutscher Wetterdienst. Klimareport Schleswig-Holstein: Fakten bis zur Gegenwart—Erwartungen für die Zukunft. Available online: https://www.dwd.de/DE/leistungen/klimareport_sh/download_report_2017.pdf?__blob=publicationFile&v=5 (accessed on 7 February 2023).
- Statistisches Amt für Hamburg und Schleswig-Holstein. Die Bodennutzung in Schleswig-Holstein. 2017. Available online: http://epub.sub.uni-hamburg.de/epub/volltexte/2018/78253/pdf/C_I_1_j_17_SH_e.pdf (accessed on 7 February 2023).
- Reid, L.M.; Hamilton, R.I.; Mather, D.E. Screening Maize for Resistance to Giberella Ear Rot; Technical Bulletin SE; Research Branch Agriculture and Agri-Food Canada: Ottawa, ON, Canada, 1996.
- Birr, T. Überregionales Monitoring zur Epidemie- und Schadensdynamik von Fusariumerregern sowie Strategien zur Befalls- und Risikominimierung der Mykotoxinbelastung in der Weizen- und Maiskultur Schleswig-Holsteins (2008–2012). Ph.D. Thesis, Christian-Albrechts-University, Kiel, Germany, 6 November 2013. [Google Scholar]
- Lancashire, P.D.; Bleiholder, H.; van den Boom, T.; Langelüddeke, P.; Stauss, R.; Weber, E.; Witzenberger, A. A uniform decimal code for growth stages of crops and weeds. Ann. Appl. Biol. 1991, 119, 561–601. [Google Scholar] [CrossRef]
- European and Mediterranean Plant Protection Organization. PP 1/272 (2) Foliar diseases on maize. EPPO Bull. 2016, 46, 388–391. [Google Scholar] [CrossRef]
- Sulyok, M.; Berthiller, F.; Krska, R.; Schuhmacher, R. Development and validation of a liquid chromatography/tandem mass spectrometric method for the determination of 39 mycotoxins in wheat and maize. Rapid Commun. Mass Spectrom. 2006, 20, 2649–2659. [Google Scholar] [CrossRef]
- R Core Team. R: A language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria). Available online: https://www.R-project.org/ (accessed on 22 June 2020).
- Carroll, R.J.; Ruppert, D. Transformation and Weighting in Regression; CRC Press: London, UK, 1988; ISBN 9781351407267. [Google Scholar]
- Pinheiro, J.C.; Bates, D.M. Mixed-Effects Models in S and S-PLUS; Springer: New York, NY, USA, 2000; ISBN 9780387227474. [Google Scholar]
- Nakagawa, S.; Schielzeth, H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 2013, 4, 133–142. [Google Scholar] [CrossRef]
- Bretz, F.; Hothorn, T.; Westfall, P.H. Multiple Comparisons Using R; Chapman & Hall/CRC Press: Boca Raton, FL, USA, 2011; ISBN 9781584885740. [Google Scholar]
- Lehoczki-Krsjak, S.; Varga, M.; Szabó-Hevér, Á.; Mesterházy, Á. Translocation and degradation of tebuconazole and prothioconazole in wheat following fungicide treatment at flowering. Pest Manag. Sci. 2013, 69, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Oliver, R.P.; Hewitt, H.G. Fungicides in Crop Protection, 2nd ed.; CABI: Wallingford, UK, 2014; ISBN 9781780641669. [Google Scholar]
- Carmona, M.; Sautua, F.; Pérez-Hérnandez, O.; Reis, E.M. Role of fungicide applications on the integrated management of wheat stripe rust. Front. Plant Sci. 2020, 11, 733. [Google Scholar] [CrossRef] [PubMed]
- Nuyttens, D.; Baetens, K.; de Schampheleire, M.; Sonck, B. Effect of nozzle type, size and pressure on spray droplet characteristics. Biosyst. Eng. 2007, 97, 333–345. [Google Scholar] [CrossRef]
- Ettle, T.; Schwarz, F.J. Effect of maize variety harvested at different maturity stages on feeding value and performance of dairy cows. Anim. Res. 2003, 52, 337–349. [Google Scholar] [CrossRef]
- Horsfall, J.G.; Dimond, A.E. Interactions of tissue sugar, growth substances and disease susceptibility. Z. Pflanzenk. Pflanzen. 1957, 64, 415–421. [Google Scholar]
- Daub, M.E.; Ehrenshaft, M. The photoactivated Cercospora toxin cercosporin: Contributions to plant disease and fundamental biology. Annu. Rev. Phytopathol. 2000, 38, 461–490. [Google Scholar] [CrossRef]
- Daub, M.E.; Herrero, S.; Chung, K.-R. Photoactivated perylenequinone toxins in fungal pathogenesis of plants. FEMS Microbiol. Lett. 2005, 252, 197–206. [Google Scholar] [CrossRef]
- Pestka, J.J. Deoxynivalenol: Toxicity, mechanisms and animal health risks. Anim. Feed Sci. Technol. 2007, 137, 283–298. [Google Scholar] [CrossRef]
- Metzler, M.; Pfeiffer, E.; Hildebrand, A. Zearalenone and its metabolites as endocrine disrupting chemicals. World Mycotoxin J. 2010, 3, 385–401. [Google Scholar] [CrossRef]
- Bryła, M.; Waśkiewicz, A.; Ksieniewicz-Woźniak, E.; Szymczyk, K.; Jędrzejczak, R. Modified Fusarium ycotoxmins in cereals and their products—Metabolism, occurrence, and toxicity: An updated review. Molecules 2018, 23, 963. [Google Scholar] [CrossRef]
- Freire, L.; Sant’Ana, A.S. Modified mycotoxins: An updated review on their formation, detection, occurrence, and toxic effects. Food Chem. Toxicol. 2018, 111, 189–205. [Google Scholar] [CrossRef] [PubMed]
- Logrieco, A.; Mulè, G.; Moretti, A.; Bottalico, A. Toxigenic Fusarium species and mycotoxins associated with maize ear rot in Europe. Eur. J. Plant Pathol. 2002, 108, 597–609. [Google Scholar] [CrossRef]
- European Commision. Commission Regulation (EC) No 1126/2007 of 28 September 2007 amending Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards Fusarium toxins in maize and maize products. Off. J. Eur. Union 2007, L255, 14–17. [Google Scholar]
- European Commission. Commission Recommendation (2006/576/EC) of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT- 2 and fumonisins in products intended for animal feeding. Off. J. Eur. Union 2006, L229, 7–9. [Google Scholar]
- Oldenburg, E.; Ellner, F. Fusarium mycotoxins in forage maize—Detection and evaluation. Mycotoxin Res. 2005, 21, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Birr, T.; Jensen, T.; Preußke, N.; Sönnichsen, F.D.; de Boevre, M.; de Saeger, S.; Hasler, M.; Verreet, J.-A.; Klink, H. Occurrence of Fusarium mycotoxins and their modified forms in forage maize cultivars. Toxins 2021, 13, 110. [Google Scholar] [CrossRef]
- Vandicke, J.; de Visschere, K.; Ameye, M.; Croubels, S.; de Saeger, S.; Audenaert, K.; Haesaert, G. Multi-mycotoxin contamination of maize silages in Flanders, Belgium: Monitoring mycotoxin levels from seed to feed. Toxins 2021, 13, 202. [Google Scholar] [CrossRef]
- Weaver, A.C.; Weaver, D.M.; Adams, N.; Yiannikouris, A. Co-occurrence of 35 mycotoxins: A seven-year survey of corn grain and corn silage in the United States. Toxins 2021, 13, 516. [Google Scholar] [CrossRef]
- Goertz, A.; Zuehlke, S.; Spiteller, M.; Steiner, U.; Dehne, H.W.; Waalwijk, C.; De Vries, I.; Oerke, E.C. Fusarium species and mycotoxin profiles on commercial maize hybrids in Germany. Eur. J. Plant Pathol. 2010, 128, 101–111. [Google Scholar] [CrossRef]
- Dorn, B.; Forrer, H.R.; Jenny, E.; Wettstein, F.E.; Bucheli, T.D.; Vogelgsang, S. Fusarium species complex and mycotoxins in grain maize from maize hybrid trials and from grower’s fields. J. Appl. Microbiol. 2011, 111, 693–706. [Google Scholar] [CrossRef]
- De Boevre, M.; Landschoot, S.; Audenaert, K.; Maene, P.; Di Mavungu, D.; Eeckhout, M.; Haesaert, G.; De Saeger, S. Occurrence and within field variability of Fusarium mycotoxins and their masked forms in maize crops in Belgium. World Mycotoxin J. 2014, 7, 91–102. [Google Scholar] [CrossRef]
- Oldenburg, E.; Höppner, F.; Weinert, J. Distribution of deoxynivalenol in Fusarium-infected forage maize. Mycotoxin Res. 2005, 21, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Schollenberger, M.; Müller, H.-M.; Ernst, K.; Sondermann, S.; Liebscher, M.; Schlecker, C.; Wischer, G.; Drochner, W.; Hartung, K.; Piepho, H.-P. Occurrence and distribution of 13 trichothecene toxins in naturally contaminated maize plants in Germany. Toxins 2012, 4, 778–787. [Google Scholar] [CrossRef] [PubMed]



| Parameter | Location | |||
|---|---|---|---|---|
| Barkhorn | Hemdingen | Hohenschulen | ||
| Coordinates 1 | x | 1,073,958 | 1,091,656 | 1,110,278 |
| y | 7,211,748 | 7,122,399 | 7,229,975 | |
| Previous crop | Forage maize | Forage maize | Forage maize | |
| Soil cultivation | Reduced tillage | Plough | Reduced tillage | |
| Planting date | 24 April | 3 May | 2 May | |
| Harvest date | 17 October | 16 October | 9 October | |
| Parameter | Treatment | ||
|---|---|---|---|
| T3—Overhead Spraying | T4—Dropleg Spraying (Sub-Canopy) | T5—Combination of T3 and T4 | |
| Spray volume (L/ha) | 400 | 400 | 200 1,2; 200 1,3 |
| Amount of Prosaro® (L/ha) | 1.0 | 1.0 | 0.5 1,2; 0.5 1,3 |
| Nozzle | IDK 120-03 | 2 × FT 1.5-408 4 | IDK 120-02 1,2; 2 × FT 0.75-348 1,3,4 |
| Nozzle spray angle (°) | 120 | 140 | 120 1,2; 140 1,3 |
| Nozzle spacing (m) | 0.50 | 0.75 | 0.50 1,2; 0.75 1,3 |
| Spray pressure (bar) | 2.1 | 2.0 | 2.1 1,2; 2.1 1,3 |
| Nozzle output (L/min) | 1.0 | 1.0 (2 × 0.5) 4 | 0.67 1,2; 0.5 (2 × 0.25) 1,3,4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Birr, T.; Tillessen, A.; Verreet, J.-A.; Hasler, M.; Klink, H. Efficacy of Different Fungicide Spraying Techniques on the Infestation with Kabatiella zeae and Formation of Fusarium Mycotoxins in Forage Maize. Agriculture 2023, 13, 1269. https://doi.org/10.3390/agriculture13061269
Birr T, Tillessen A, Verreet J-A, Hasler M, Klink H. Efficacy of Different Fungicide Spraying Techniques on the Infestation with Kabatiella zeae and Formation of Fusarium Mycotoxins in Forage Maize. Agriculture. 2023; 13(6):1269. https://doi.org/10.3390/agriculture13061269
Chicago/Turabian StyleBirr, Tim, Andreas Tillessen, Joseph-Alexander Verreet, Mario Hasler, and Holger Klink. 2023. "Efficacy of Different Fungicide Spraying Techniques on the Infestation with Kabatiella zeae and Formation of Fusarium Mycotoxins in Forage Maize" Agriculture 13, no. 6: 1269. https://doi.org/10.3390/agriculture13061269
APA StyleBirr, T., Tillessen, A., Verreet, J.-A., Hasler, M., & Klink, H. (2023). Efficacy of Different Fungicide Spraying Techniques on the Infestation with Kabatiella zeae and Formation of Fusarium Mycotoxins in Forage Maize. Agriculture, 13(6), 1269. https://doi.org/10.3390/agriculture13061269






