Abstract
During climate change, various unparalleled perils to agricultural systems have been observed worldwide. The detrimental impacts of heavy metal toxicity (HMs) lead to a considerable decrease in crop productivity and yield, thereby putting the agricultural system at risk and exerting a significant impact on food production. This has sparked significant worry regarding the achievement of the sustainable development goals (SDGs) pertaining to ensuring food and nutritional security for the constantly growing global population. In the current study, we have endeavored to reveal the significance of salicylic acid (SA) under arsenic (As) stress conditions in rice (Oryza sativa) plants. Being a toxic metalloid, As has adverse effects on the efficiency of photosynthesis and the assimilation of nitrogen (N) and sulphur (S) growth, and also causes alterations in defense systems and ethylene biosynthesis. The study revealed that the positive influence of SA in promoting nutrient metabolism, photosynthesis and growth under As stress was the result of its interplay with ethylene biosynthesis and the enhanced capacity of defense systems to reduce oxidative stress-mediated cellular injuries and cell deaths. In conclusion, SA can be considered a crucial physiological criterion for the development of As-tolerant rice plants.
1. Introduction
Trace amounts of heavy metals (HMs) are necessary for the optimal growth of plants; nevertheless, an excessive concentration can negatively impact regular plant metabolic and developmental processes [1]. The arsenic (As) toxicity has been recognized for a long time, and it has recently gained more significance due to its chronic and widespread impact on human health [2]. Contaminated water is the principal origin of As exposure. Rice (Oryza sativa) is especially proficient in As accumulation, and detrimental levels of As in rice grains have been documented [3]. Since rice is a traded commodity, it serves as a gateway for As to enter the food chain in areas where there is no As contamination. The primary inorganic As forms present in the surroundings are arsenite [As(III)], arsenate [As(V)] and organic As forms such as monomethylarsonic acid (MMA) and dimethylarsinic acid (DMA) [4]. As(III) is deemed more harmful than all other organic and inorganic As forms due to its interaction with thiol groups and the potential for mobility in paddy soil, leading to reduced plant growth and development [5]. Extensive research has been carried out on the phytotoxic effects of As, revealing that As-induced stress has irreversible effects on cellular growth, electrophysiological integrity, and nutrient metabolism, ultimately leading to altered metabolic pathways and programmed cell death (PCD) that coincides with untimely senescence [6,7]. The overabundance of reactive oxygen species (ROS) is generated in chloroplasts, mitochondria, and peroxisomes under As stress, resulting in lipid peroxidation and damage to biomolecules [8]. Upon being exposed to As, multiple pathways for signal transduction are activated to improve As tolerance, which help to maintain redox homeostasis in plants [9]. The physiological processes of plant growth and development are significantly affected by exposure to As, which can be attributed to the altered metabolism of nitrogen [10]. When plants experience stress, the ability to absorb and utilize nitrogen (N) is hindered, creating a challenge for various physiological processes. N assimilation is a crucial process that has a significant impact on photosynthesis and proline metabolism, as N plays an essential role as a macronutrient and a component of several vital compounds in plants [11]. The assimilation of N affects plant growth, productivity, and their ability to withstand abiotic stresses [12]. As stress can affect the uptake and assimilation of N, leading to a decrease in the net rate of nitrate (NO3−) uptake and nitrate reductase (NR) activity, as observed in many plant species [10,13]. Sulfur (S) is another essential element that plays a crucial role in plants by enhancing their ability to withstand abiotic stress [14]. This essential mineral nutrient is present in amino acids such as cysteine and methionine, as well as in co-enzymes, secondary metabolites, and antioxidants like reduced glutathione (GSH). Enzymes such as adenosine triphosphate sulfurylase (ATP-S) and serine acetyl-transferase (SAT) regulate the S metabolism, ensuring the effective assimilation of S into organic compounds [15]. Moreover, non-enzymatic antioxidants such as GSH also contribute significantly to enhancing plant tolerance to As stress [16].
When reflecting on the current circumstances, utilizing plant growth regulators (PGRs) to manipulate and characterize their effects in As-laden environments appears to be a viable method for uncovering new As-induced protective mechanisms and enhancing plant resilience [17]. Therefore, by evaluating the growth and physiological and metabolic changes in plants subjected to both optimal and stressful conditions after the application of PGRs, it is possible to decipher and regulate plant systems biology, ultimately improving the resilience and adaptability of plants under stressful environments. Salicylic acid (SA), a key regulator of redox signaling and plant immunity, has been observed in the realm of plant stress management and sustainable agro-ecology and food systems [18]. Bano et al. [19] revealed that SA application in response to As stress modulates the activities of antioxidant enzymes involved in the N metabolic pathways and impacts the signaling of the NO3− uptake system. SA is recommended for improving the capacity of plants to withstand abiotic stress by promoting growth and enhancing photosynthetic efficiency [20]. It also triggers various defense mechanisms, including both enzymatic and non-enzymatic antioxidants. Moreover, SA is believed to be involved in regulating S metabolism, which can be crucial in enhancing abiotic stress tolerance [21].
The efficacy of SA (0.5 mM in maize, Zea mays and rice, and 0.1 mM in wheat, Triticum aestivum) has been demonstrated in augmenting stress response, enhancing the overall growth and physiology, and conferring resistance to oxidative stress [20,22,23]. However, its potential in regulating ethylene biosynthesis, defense systems, ion homeostasis, N and S assimilation, and growth of rice plants under As stress conditions remains largely unexplored. Therefore, the current study aims to establish the significance of SA in the amelioration of As-induced toxicity by modulating ethylene-mediated alterations in N and S assimilation, photosynthesis, growth, and defense systems. This research will enable plant specialists to gain a comprehensive insight into the mechanisms employed by SA to detoxify As or to regulate As homeostasis in rice or potentially other cereals cultivated in HMs-polluted soil.
2. Materials and Methods
2.1. Plant Material, Growth Conditions, and Experimental Design
Healthy seeds of rice (Oryza sativa cv. IR64) were surface sterilized using 0.01% sodium hypochlorite solution, and then washed several times with double-distilled water. Surface sterilized seeds were sown at a depth of 2 cm in plastic pots with 25 cm diameter and 19 cm depth. After germination, four healthy plants of nearly equal size were kept in each pot. The study was conducted in the Net House of the Department of Botany, Jamia Hamdard, India, which was naturally illuminated with light and having day/night temperatures equal to 28 °C/24 °C (±3 °C). The experiment was carried out to investigate the effect of SA (0.5 mM) [24] applied through the foliage at 15 days after sowing (DAS) in the presence (150 μM; source sodium arsenate, Na2HAsO4; 15 DAS) or absence of As stress [22]. The four set of treatments were: untreated (control) plants, As-treated plants, plants treated with SA, and plants treated with SA under As stress were maintained, with a completely randomized block design (CRD) having three replicates (n = 3). Plants were irrigated every alternate day with Hoagland’s nutrient solution, and prepared according to the protocol given by Hoagland and Arnon [25]. Analyses were carried out at 30 DAS.
2.2. Determination of As Content by Atomic Absorption Spectrophotometer
In order to ascertain the As content, the plant tissues were subjected to an oven-drying process at 80 °C for a period of 72 h. The desiccated samples obtained from the shoot and roots (weighing 0.1 g) were pulverized and processed separately by treating them with an acidic solution at 80 °C for a duration of 48 h. As per the methodology described in Khan et al. [23], the acid mixture comprising HNO3:HClO4 (5:1 v/v) was quantified using an atomic absorption spectrophotometer (ZEEnit 65, Germany).
2.3. Determination of Oxidative Stress Indicators
H2O2 content was determined following the procedure of Okuda et al. [26]. Details are given in File S1 (Methods).
Lipid peroxidation levels in leaves were determined following the protocol of Dhindsa et al. [27] by estimating the content of thiobarbituric acid reactive substance (TBARS). Detailed procedure is given in File S1 (Methods).
Methylglyoxal content (MG) was determined following the method of Wild et al. [28]. Detailed procedure is given in File S1 (Methods).
2.4. Localization of Oxidative Stress Indicators
H2O2 in the root segments was localized by histochemical staining according to Thordal-Christensen et al. [29]. Root tissues measuring 1–2 cm in length were immersed in a Petri dish containing 12.5 µM of 2, 7 dichlorodihydrofluorescein diacetate (H2DCFDA) for 15 min, followed by rinsing three times with double-distilled water. The stained samples were placed on a glass slide and observed under a confocal microscope, using 488 nm excitation and 525 nm emission spectra.
The assessment of lipid peroxidation in the leaf tissues was conducted through histochemical staining with a slight modification in the method of Srivastava et al. [30], using Schiff’s reagent. The detection of aldehydes originating from lipid peroxidation was observed after 30 min of staining, presenting a pink-red color. After staining, the roots were washed with a sulphite solution composed of 0.5% (w/v) K2S2O5 in 0.05 M HCl and subsequently immersed in the same solution for 10 min to preserve the stained color.
2.5. Cell Viability
Cell viability visualization in root tips was carried out following the method of Alamri et al. [31]. Root tips collected from different treatments were washed thoroughly to remove the debris materials, and carefully excised using a sharp blade. Following staining with propidium iodide (25 µM), the root tips were rinsed with a potassium-phosphate buffer (pH = 7.0) and observed within the 568–610 nm range using a confocal laser scanning microscope installed in the Centre for Transgenic Plant Development (CTPD), Department of Biotechnology, Jamia Hamdard.
2.6. Determination of Assay of Antioxidant Enzyme Activities
Fresh leaf tissues were crushed using a chilled mortar and pestle with an extraction solution comprising 0.05% (v/v) Triton X-100 and 1% (w/v) polyvinylpyrrolidone (PVP) in potassium–phosphate buffer (100 mM, pH 7.0). The crushed material was spun at 15,000× g for 20 min at 4 °C. The supernatant obtained after centrifugation was utilized to conduct enzyme activity tests. In order to measure ascorbate peroxidase (APX; EC 1.11. 1.11) activity, the extraction solution was supplemented with 2.0 mM ascorbate.
The activity superoxide dismutase (SOD; EC1.15.1.1) was estimated according to Beyer et al. [32] by monitoring the inhibition of photochemical reduction of nitro blue tetrazolium (NBT). Details are given in the in File S1 (Methods) and Kumari et al. [33].
The activity of ascorbate peroxidase (APX; EC1.11.1.11) was measured according to the method of Nakano and Asada [34] by monitoring the decrease in absorbance of ascorbate at 290 nm. Details are given in File S1 (Methods) and Kumari et al. [33].
The activity of glutathione reductase (GR; EC1.6.4.2) was assayed following the method of Foyer and Halliwell [35] by monitoring the GSH-dependent oxidation of nicotinamide adenine dinucleotide phosphate (NADPH) at 340 nm. Details are given in File S1 (Methods).
The activity of glutathione peroxidase (GPX, EC 1.11.1.9) was estimated according to the method of Elia et al. [36]. Details are given in File S1 (Methods) and Kumari et al. [33].
Glyoxalase I (Gly I, EC 4.4.1.5) activity was determined by the method of Khan et al. [22]. Details are given in File S1 (Methods).
Glyoxalase II (Gly II, EC 3.1.2.6) activity was carried out according to the method of Principato et al. [37] by monitoring the formation of GSH at 412 nm for 1 min. Details are given in File S1 (Methods).
2.7. Determination of Proline Content and Activity of Proline Metabolizing Enzymes
The spectrophotometric determination of proline content was carried out using the ninhydrin method developed by Rena and Splittstoesser [38]. To extract free proline, 0.3 g of fresh leaf samples were homogenized in 3 mL of 3% sulphosalicylic acid and the resulting homogenate was centrifuged at 11,500× g for 12 min. The filtrate obtained from the homogenate was then reacted with 1 mL each of acid ninhydrin and glacial acetic acid for a duration of 1 h in a test tube that was placed in a water bath at 100 °C. The resulting mixture was extracted with toluene and the absorbance was measured at 520 nm using L-proline as a standard.
To determine the activity of pyrroline-5-carboxylate synthetase (P5CS, EC 2.7.2.11/1.2.2.41), γ-glutamyl kinase (GK, EC 2.7.2.11) and proline oxidase (PROX, EC 1.5.99.8), enzyme extract was prepared by homogenizing 500 mg leaf sample in 0.1 M Tris-HCl buffer (pH 7.5) at 4 °C. The homogenate was centrifuged at 30,000× g for 30 min and the supernatant was used as the crude extract enzyme preparation for P5CS activity and the pellet was collected and used as an extract for the assay of GK and PROX.
The activity of P5CS was evaluated using the method of Rena and Splittstoesser [38] with a minor alteration. The assay solution, comprising 1.0 mL, consisted of 128 µM NADH, 400 µM l-PCA, and 0.1 M NaPi buffer (pH 7.4). The reaction was initiated by adding PCA. The reduction in absorbance at 340 nm was monitored.
The activity of GK was assayed by the method of Hayzer and Leisinger [39] with slight modifications whose details are given in File S1 (Methods).
The activity of PROX was determined by adopting the method of Huang and Cavalieri [40] with slight modification. Details of the procedure are given in File S1 (Methods).
2.8. Determination of N Content and N Metabolism Related Enzymes
The determination of N was determined by using the methods of Lindner [41], respectively.
The leaf nitrate reductase activity (NR, EC 1.7.99.4) was measured in fresh leaves by using the method of Kuo et al. [42] by preparing the enzyme extract. Details of the procedure are given in File S1 (Methods).
The assay of nitrite reductase (NiR, EC 1.7.7.1) activity is based on the reduction in the content of nitrite in the reaction mixture as described by Hageman and Reed [43].
Glutamine-2-oxoglutarate amino transferase or glutamate synthase (NADH-GOGAT, EC 1.4.1.14) activity was assayed following the method of Singh and Srivastava [44]. Details of the procedure are given in File S1 (Methods).
2.9. Determination of S Content and S Metabolism-Related Enzymes
The sulfur content in leaf samples was determined following the turbidmetric method of Chesnin and Yien [45].
ATP Sulfurylase (ATP-S; EC 4.4.1.14) activity in leaves was measured by the method described in Lappartient and Touraine [46]. Details of the procedure are given in File S1 (Methods).
Serine acetyl transferase activity (SAT; EC 2.3.1.30) was measured according to the DTN method of Kredich and Tomkins [47] based on following the measurement of thionitrobenzoic acid spectrophotometrically at 412 mµ. Details of the procedure are given in File S1 (Methods).
Cysteine, methionine, and GSH content in leaves was determined following the method of Khan et al. [48]. Details of the procedure are given in File S1 (Methods).
2.10. Determination of Photosynthetic Traits and Plant Dry Mass Accumulation
Net photosynthesis (PN), stomatal conductance (gs), and intercellular CO2 concentration (Ci) were measured in fully expanded uppermost leaves of plants in each treatment using infrared gas analyzer (CID-340, Photosynthesis System, Bio-Science, USA). The measurements were performed between 11.00 and 12.00 h at light-saturating intensity and at 370 ± 5 μmol mol−1 atmospheric CO2 concentrations. Chlorophyll content was measured with the help of a SPAD chlorophyll meter (SPAD 502 DL PLUS, Spectrum Technologies).
The activity of Rubisco (EC 4.1.1.39) was determined spectrophotometrically by adopting the method of Usuda [49] by monitoring NADH oxidation at 30 °C at 340 nm during the conversion of 3-phosphoglycerate to glycerol 3-phosphate after the addition of enzyme extract to the assay medium. Details of the procedure are given in the File (methods S1).
The dry mass of whole plants was recorded after drying the sample in a hot air oven at 80 °C till constant weight.
2.11. Determination of Ethylene Evolution and ACS Activity
The ethylene evolution was estimated by using gas chromatograph (Agilent 8890 GC system, Jamia Hamdard New Delhi, India). The detailed protocol has been provided by [48] and in File S1 (Methods).
ACS activity was measured by following the method of Avni et al. [50] and Woeste et al. [51]. A comprehensive illustration of the procedures is mentioned in File S1 (Methods).
2.12. Statistical Analysis
The data were analyzed through a two-way analysis of variance (ANOVA) using R (4.2.1) statistical software (package library, agricolae). For comparison among means for significant differences, Tukey’s HSD post hoc test was performed (taking p ≤ 0.05). and data are presented as mean ± SE (n = 3). Values followed by different letters are statistically significant at p ≤ 0.05 by Tukey’s HSD post hoc test.
3. Results
3.1. Influence of SA on Oxidative Stress Markers and Cell Viability under As Stress
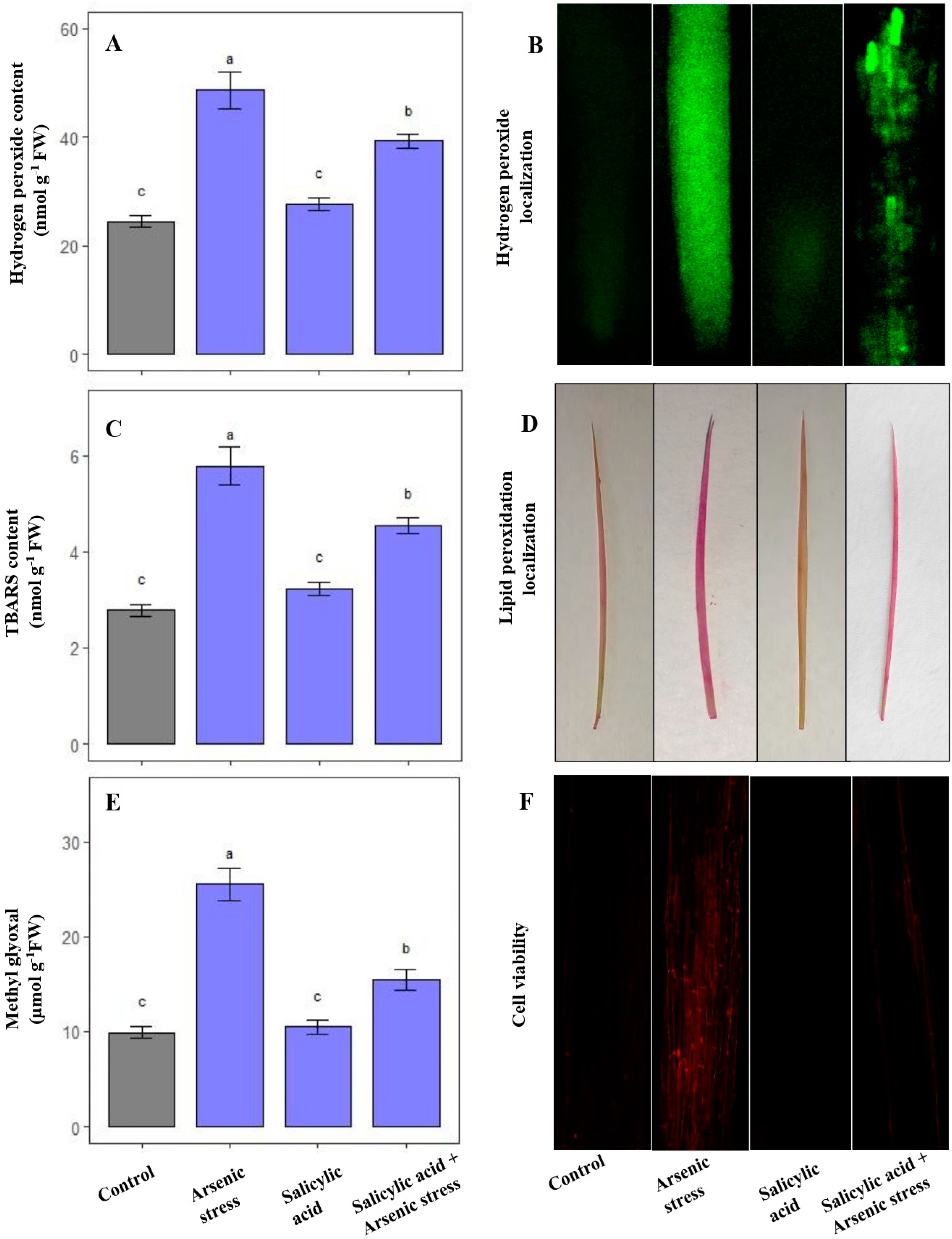
Under As stress, there was a substantial increase in the levels of oxidative stress markers including H2O2 (76%), TBARS (79%), and MG (140%) in comparison to plants grown under normal conditions. Nonetheless, the application of SA in response to As stress resulted in a significant decrease in the levels of H2O2 (19.30%), TBARS (21.5%) and MG (39.21%), as compared to As stressed plants (Figure 1A,C,E).
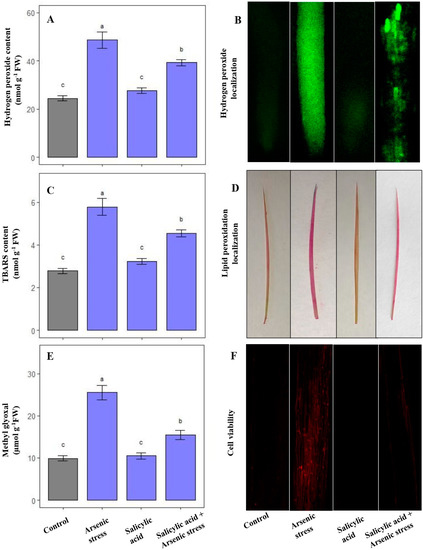
Figure 1.
Effect of salicylic acid (SA; 0.5 mM; 15DAS) on (A) H2O2 content, (B) localization of H2O2, (C) TBARS content, (D) localization of lipid peroxidation, (E) MG content, and (F) cell viability in rice (Oryza sativa) plants in the presence (150 µM) or absence of As stress at 30 DAS. Data are presented as treatments mean ± SE (n = 3). Data followed by different letters are statistically significant at p ≤ 0.05 by Tukey’s HSD post hoc test.
Visualization of H2O2 and TBARS on root and leaf tissues displayed an excess accumulation of these molecules under As stress, which was evident with the significant green and pink precipitates, respectively. Moreover, the increased accumulation of oxidative stress markers led to lowered cell viability under As stress conditions. Nevertheless, a substantial decrease in the content of H2O2 and TBARS, along with an increased number of viable cells was observed under the application of SA in As-stressed plants (Figure 1B,D,F).
3.2. Influence of SA on Antioxidant Enzymes Activities under As Stress
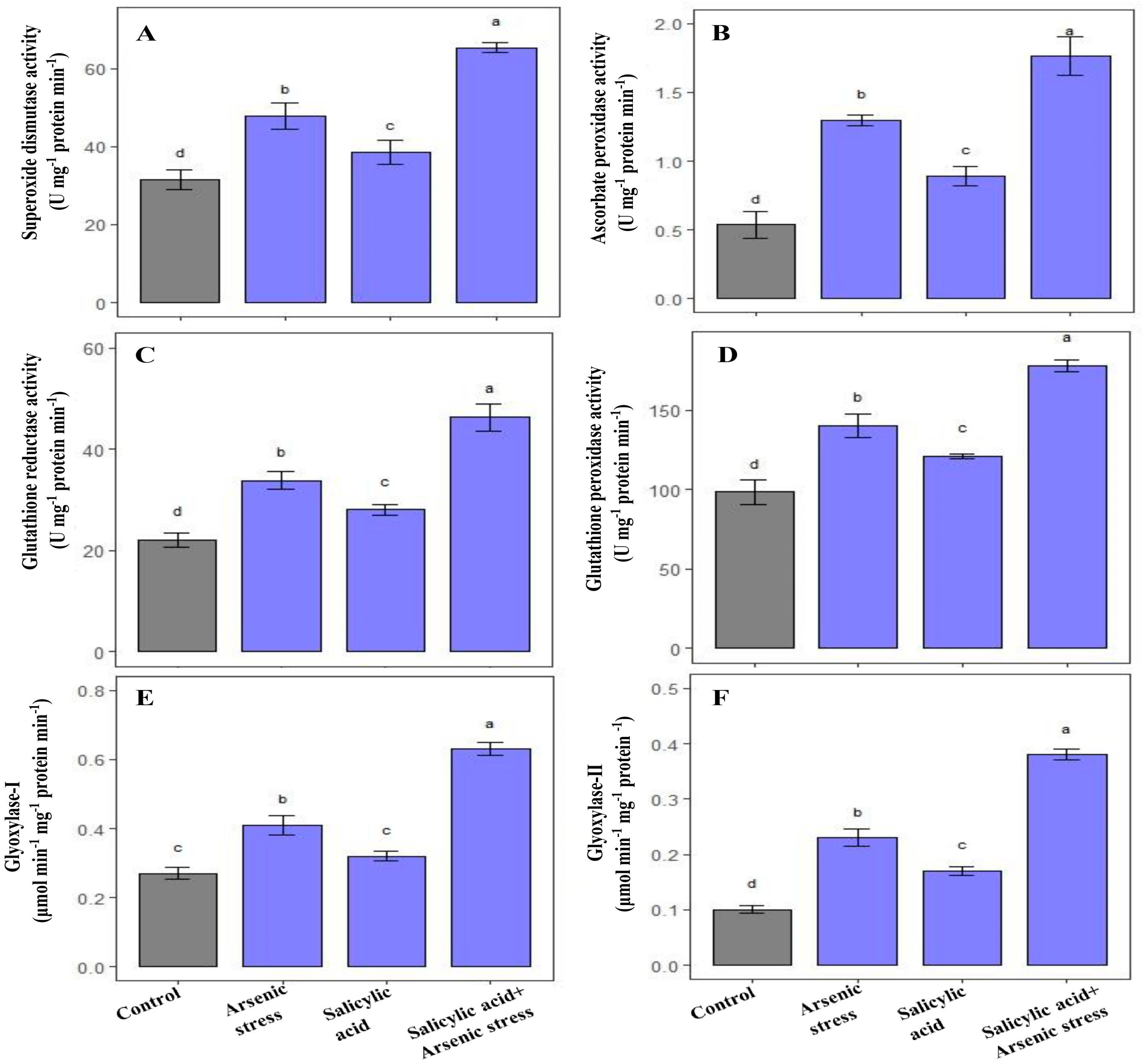
As stress enhanced the activity of SOD (52.06%), APX (105%), GPX (43%), and GR (53.46%), respectively, as compared to control-grown plants. Similarly, the application of SA in response to As stress further elevated the activities of SOD (84%), APX (140.2%), GPX (57.31%), and GR (80%), respectively, as compared to plants grown under controlled conditions (Figure 2A–D).
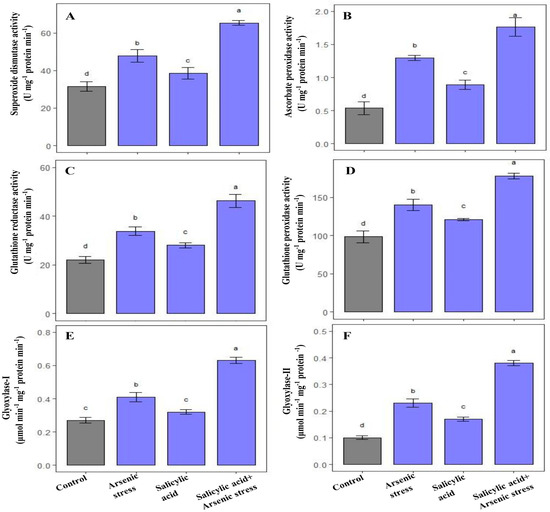
Figure 2.
Effect of salicylic acid (SA; 0.5 mM; 15DAS) on (A) SOD, (B) APX (C) GR (D) GPX (E) Gly I, and (F) Gly II activity in rice (Oryza sativa) plants in the presence (150 µM) or absence of As stress at 30 DAS. Data are presented as treatments mean ± SE (n = 3). Data followed by different letters are statistically significant at p ≤ 0.05 by Tukey’s HSD post hoc test.
Rice plants exposed to As stress showed a significant increase in the glyoxalase systems, including Gly-I (52%) and Gly-II (130%) in comparison to control plants. Similarly, supplementation of SA to As- stressed plants significantly improved the activities of Gly-I (96.30%) and Gly-II (180%) as compared to plants grown under normal conditions (Figure 2E,F).
3.3. Influence of SA on Proline Metabolism under AS Stress
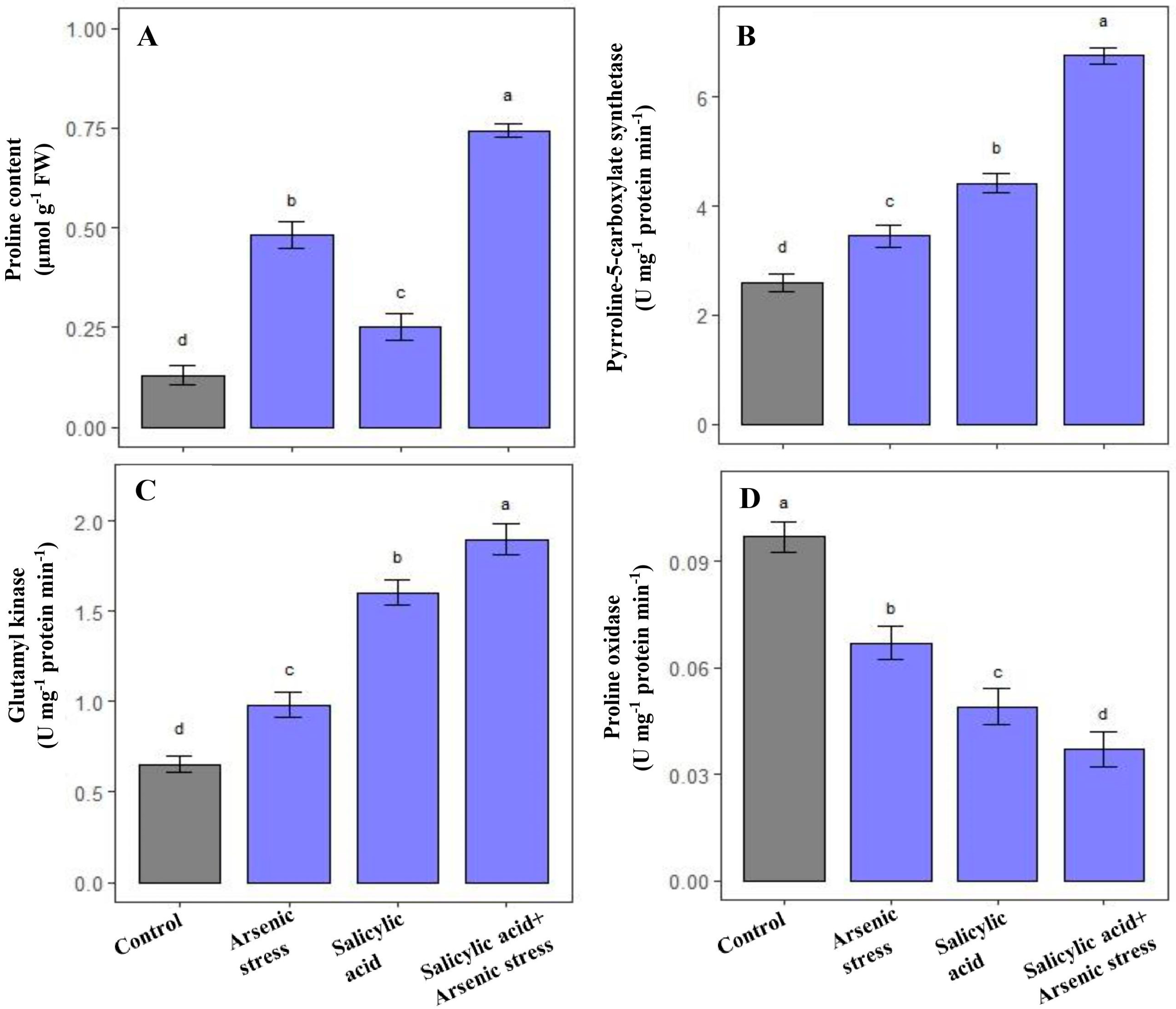
Plants grown under As stress exhibited enhanced levels of proline content along with the enzymatic activities of P5CS and GK, respectively, as compared to control plants. Further, the supplementation of SA under As stress conditions promoted the accumulation of proline (54.2%), and regulated the enzymatic activities of P5CS (91%) and GK (92%); however, PROX activity was significantly decreased by 42% as compared to As stressed rice plants (Figure 3).
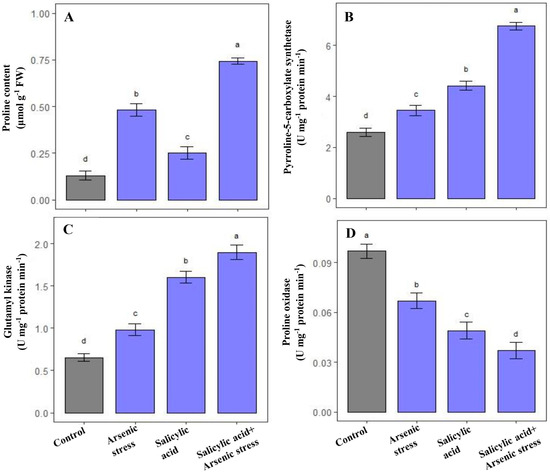
Figure 3.
Effect of salicylic acid (SA; 0.5 mM; 15DAS) on (A) proline content, (B) P5CS, (C) GK, and (D) proline oxidase activity in rice (Oryza sativa) plants in the presence (150 µM) or absence of As stress at 30 DAS. Data are presented as treatments mean ± SE (n = 3). Data followed by different letters are statistically significant at p ≤ 0.05 by Tukey’s HSD post hoc test.
3.4. Influence of SA on Plant Dry Mass and Nitrogen Metabolism under As Stress
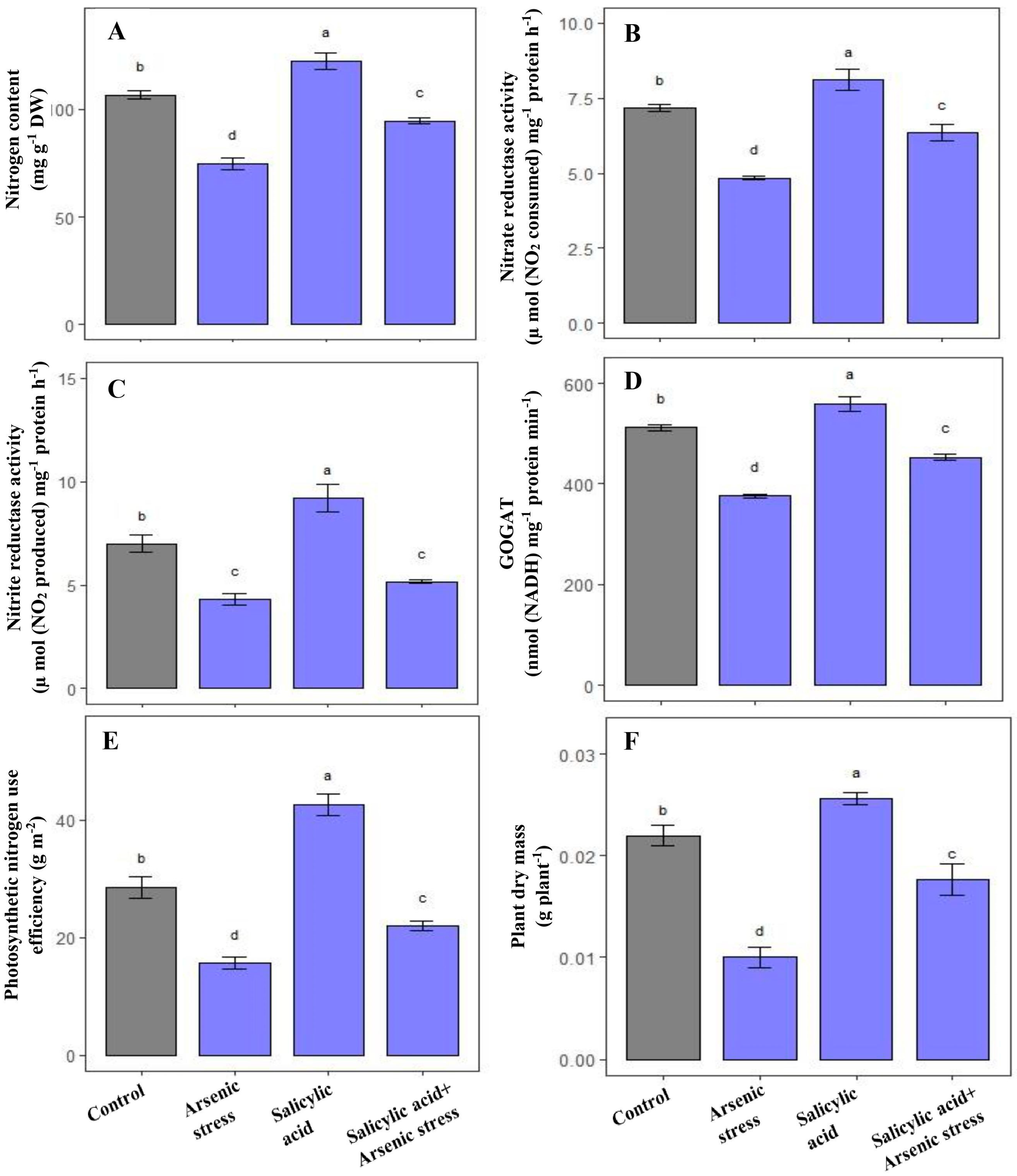
As-induced toxicity leads to a substantial decrease in N content by 33%, and NR, NiR and GOGAT activities by 39%, 29.23%, and 26.43%, respectively as compared to control plants. However, SA supplementation under As stress showed a remarkable increase in N content (63%) and further elevated the activities of NR (20.23%), NiR (27%) and GOGAT (21%), respectively, in comparison to As-treated plants (Figure 4A–D).
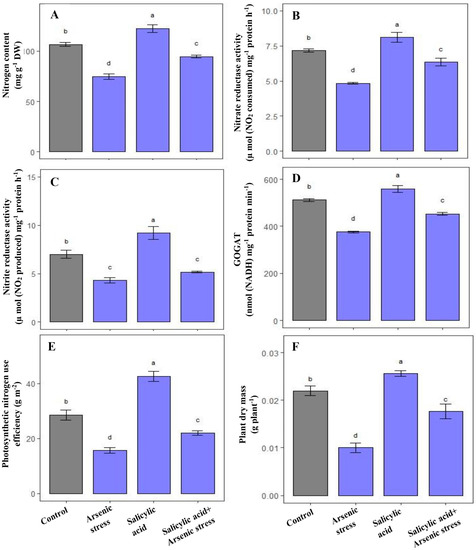
Figure 4.
Effect of salicylic acid (SA; 0.5 mM; 15DAS) on (A) N content, (B) NR activity, (C) NiR activity, (D) GOGAT activity, (E) pNUE, and (F) plant dry mass in rice (Oryza sativa) plants in the presence (150 µM) or absence of As stress at 30 DAS. Data are presented as treatments mean ± SE (n = 3). Data followed by different letters are statistically significant at p ≤ 0.05 by Tukey’s HSD post hoc test.
Plants exposed to As stress presented a marked decrease in plant dry mass (51.23%) and pNUE (43%) in comparison to control plants. Alongside this, the application of SA under As stress exhibited a prominent increase in plant dry mass (70.02%) and pNUE (35%) in comparison to As-treated plants (Figure 4E,F).
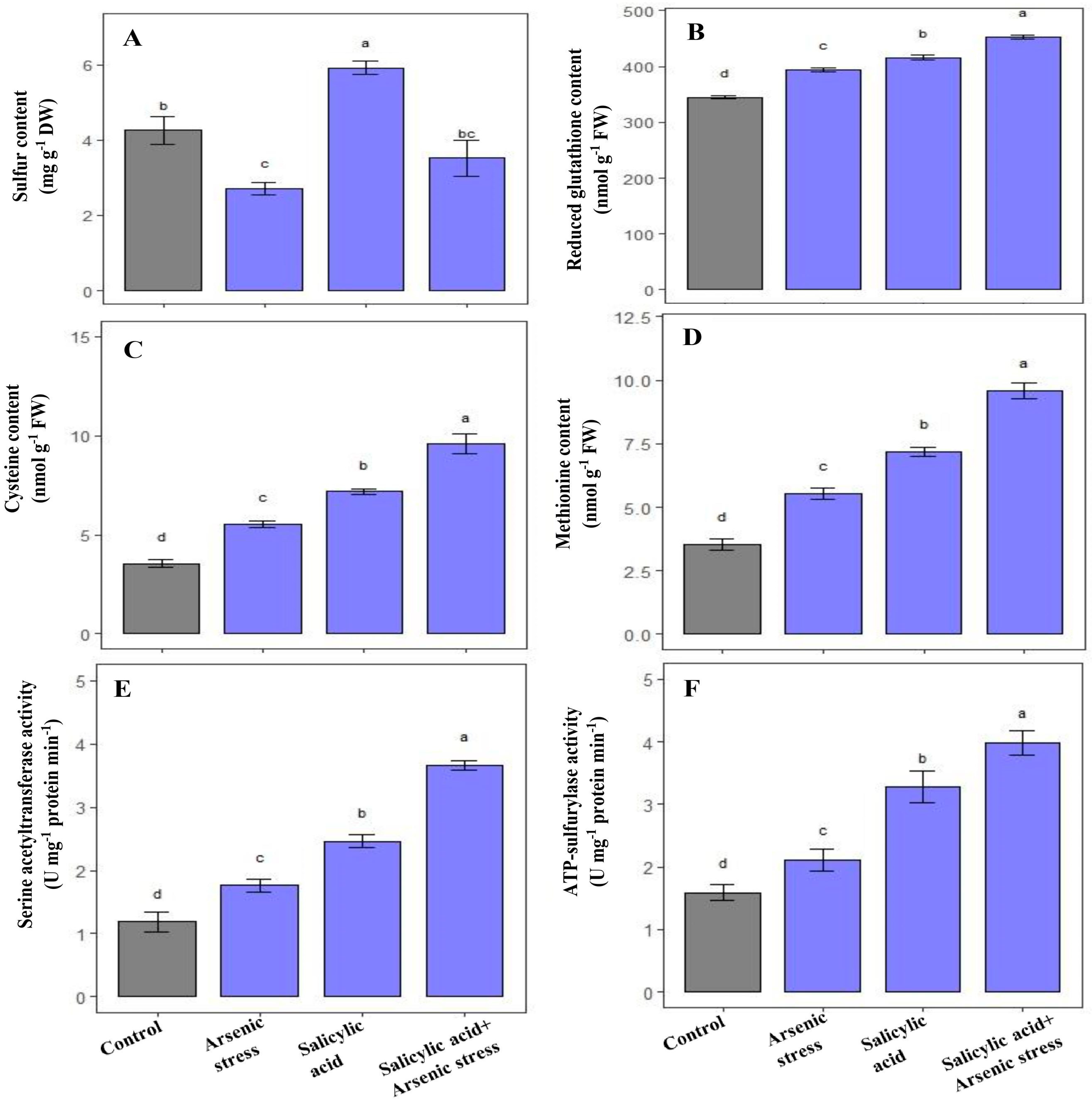
3.5. Influence of SA on Sulfur Metabolism under As Stress
During As stress, S content significantly decreased, whereas cysteine and methionine contents, along with GSH, SAT, and ATP-S activities were increased in rice plants as compared to control plants (Figure 5). However, the application of SA under As stress increased the content of S (30%), cysteine (62%), and methionine (67%), and further enhanced the activities of GSH (13.4%), SAT (108%), and ATP-S (89%), respectively, as compared to As-stressed plants (Figure 5).
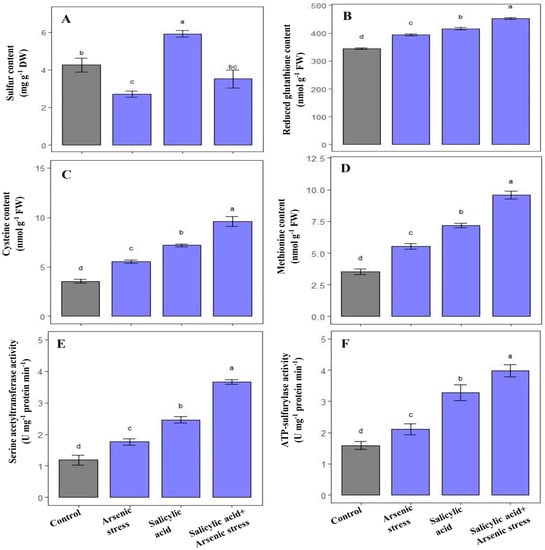
Figure 5.
Effect of salicylic acid (SA; 0.5 mM; 15DAS) on (A) S content, (B) reduced GSH content (C) Cysteine content, (D) Methionine content, (E) SAT activity, and (F) ATP-S activity in rice (Oryza sativa) plants in the presence (150 µM) or absence of As stress at 30 DAS. Data are presented as treatments mean ± SE (n = 3). Data followed by different letters are statistically significant at p ≤ 0.05 by Tukey’s HSD post hoc test.
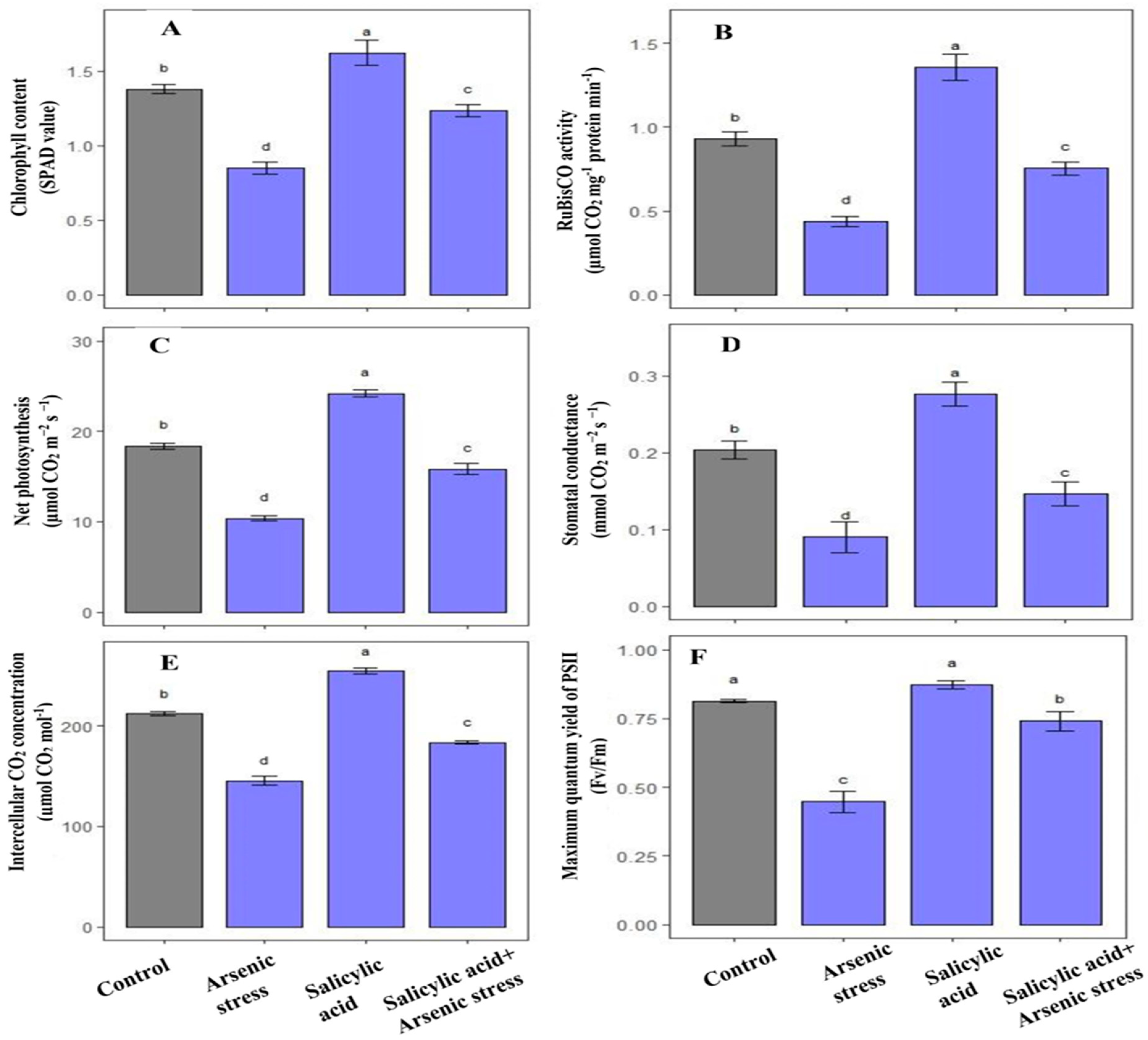
3.6. Influence of SA on Photosynthetic Attributes under As Stress
Rice plants exposed to As stress exerted a notable decrease in total chlorophyll content (38.4%) and RuBisCO (53%) activity as compared to plants grown under controlled conditions. However, the application of SA under As stress significantly increased chlorophyll content (45%) and RuBisCO (74.42%) activity as compared to As-treated plants (Figure 6).
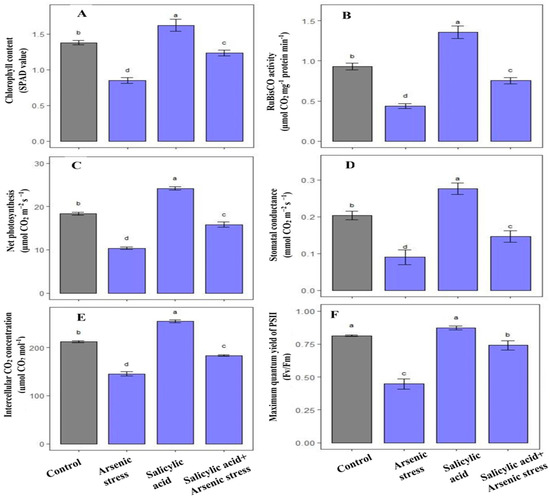
Figure 6.
Effect of salicylic acid (SA; 0.5 mM; 15DAS) on (A) total chlorophyll content, (B) RuBisCO activity, (C) net photosynthesis, (D) stomatal conductance, (E) intercellular CO2 concentration, and (F) maximum efficiency of PSII of rice (Oryza sativa) plants in the presence (150 µM) or absence of As stress at 30 DAS. Data are presented as treatments mean ± SE (n = 3). Data followed by different letters are statistically significant at p ≤ 0.05 by Tukey’s HSD post hoc test.
Gas exchange parameters including net photosynthesis, stomatal conductance, and intercellular CO2 concentration along with maximum efficiency of PSII were decreased under As stress by 43.5%, 55%, 31.43% and 25%, respectively as compared to control plants. Alongside this, the treatment of SA under As stress improved net photosynthesis (53%), stomatal conductance (62.22%), intercellular CO2 concentration (26%) along with maximum efficiency of PSII (21.31%), respectively, as compared to As-treated plants (Figure 6).
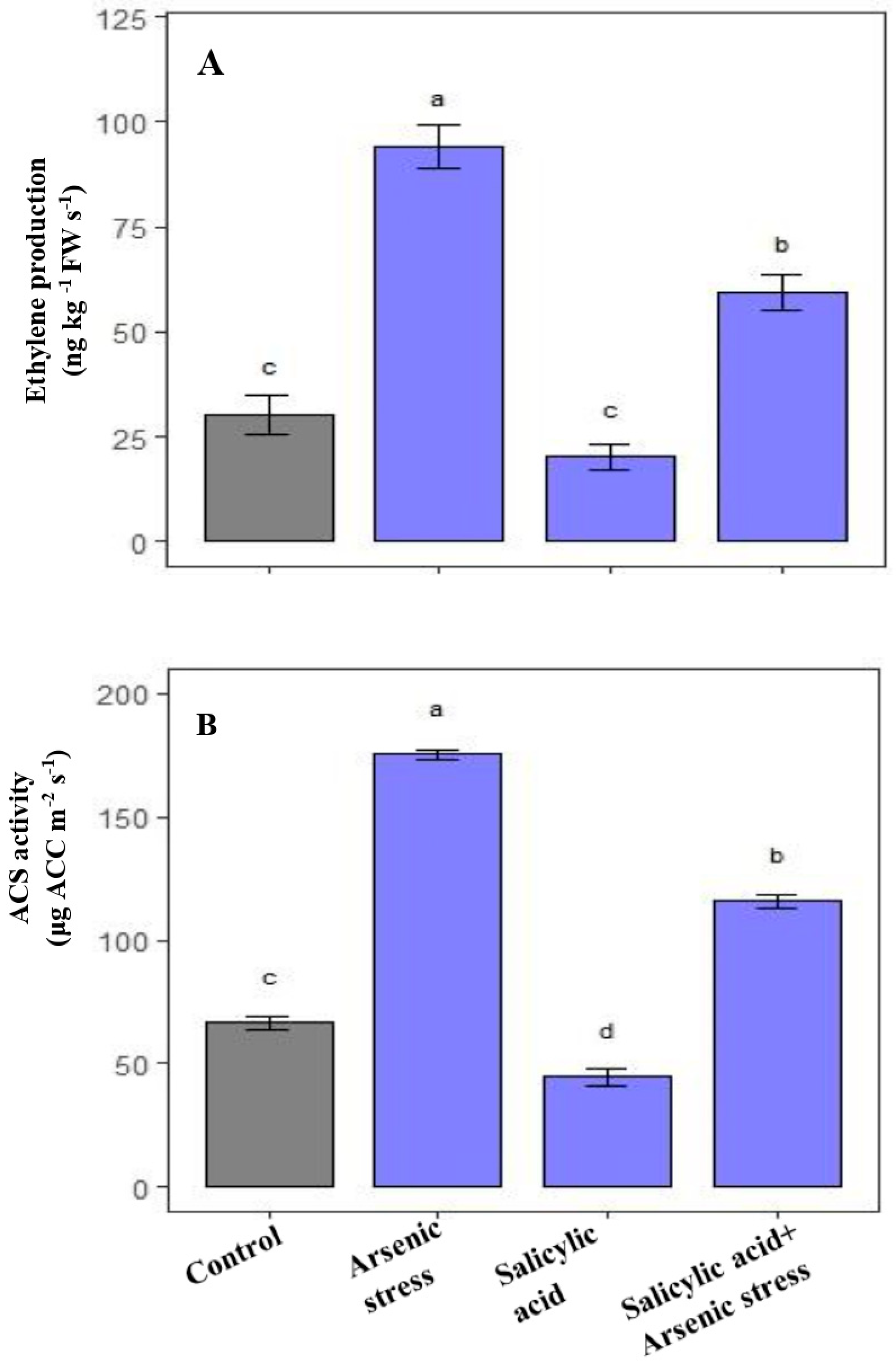
3.7. Influence of SA on Ethylene Formation and ACS Activity under As Stress
The results revealed that during As stress, the endogenous ethylene concentration along with ACS activity was significantly increased than the control plants while after supplementation of SA under As stress, the levels of endogenous ethylene along with the ACS activity decreased as compared to As-stressed plants (Figure 7).
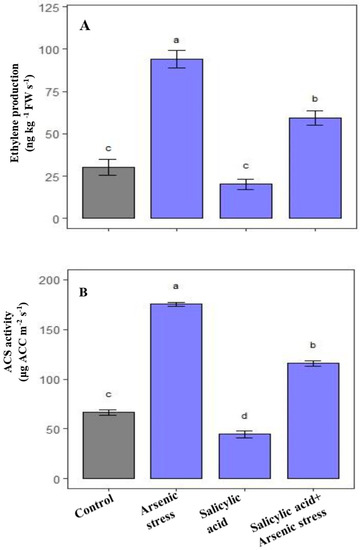
Figure 7.
Effect of salicylic acid (SA; 0.5 mM; 15DAS) on (A) Ethylene production, and (B) ACS activity of rice (Oryza sativa) plants in the presence (150 µM) or absence of As stress at 30 DAS. Data are presented as treatments mean ± SE (n = 3). Data followed by different letters are statistically significant at p ≤ 0.05 by Tukey’s HSD post hoc test.
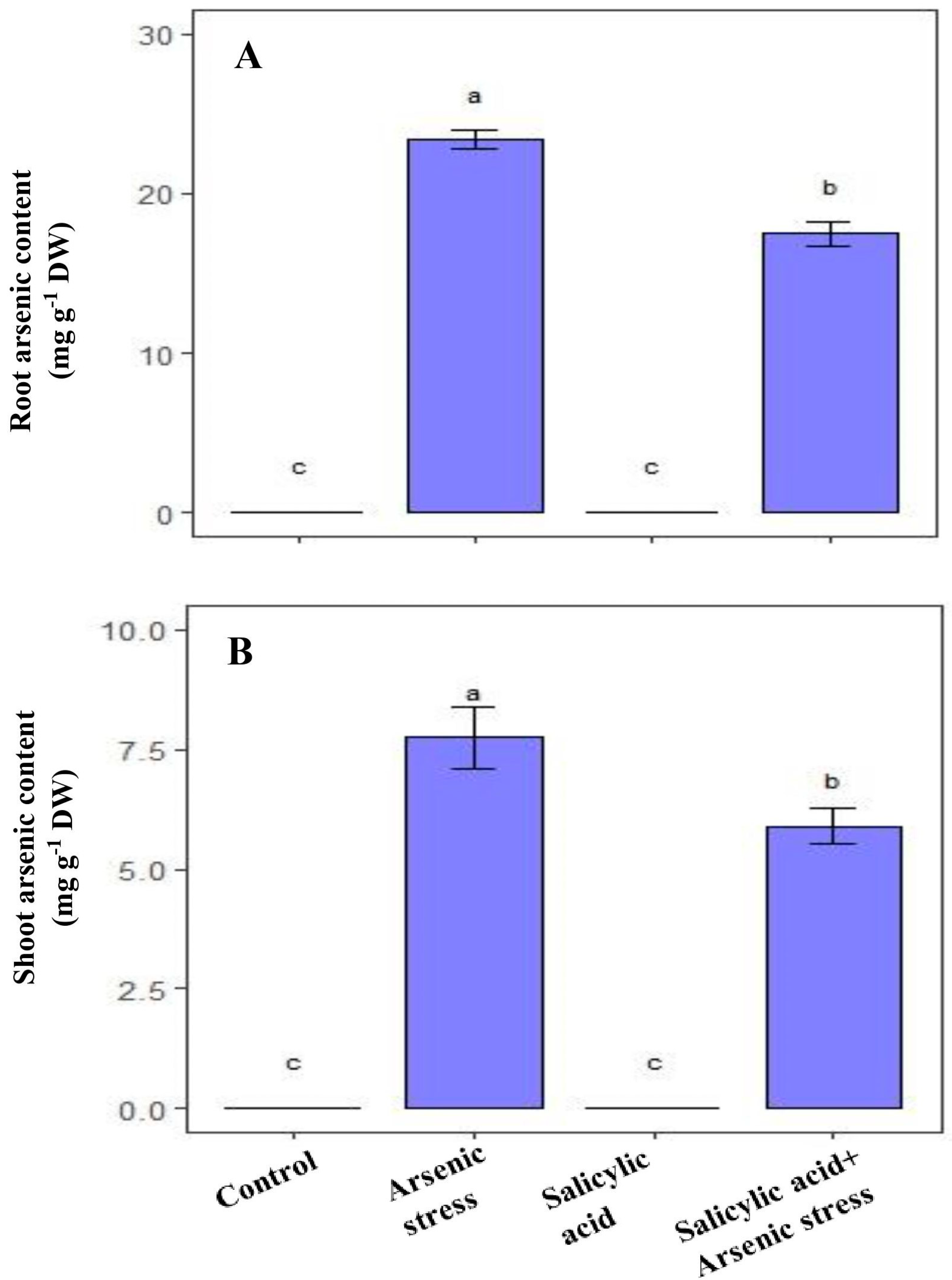
3.8. Influence of SA on As Content under As Stress
As grown rice plants showed a substantial increase in As content in both the roots (23.25 mg g−1 DW) and shoot (7.56 mg g−1 DW) as compared to the control plants. However, supplementation of SA resulted in a decrease in As accumulation in roots (17.56 mg g−1 DW) and shoot (5.73 mg g−1 DW), respectively, compared to the As-treated plants (Figure 8).
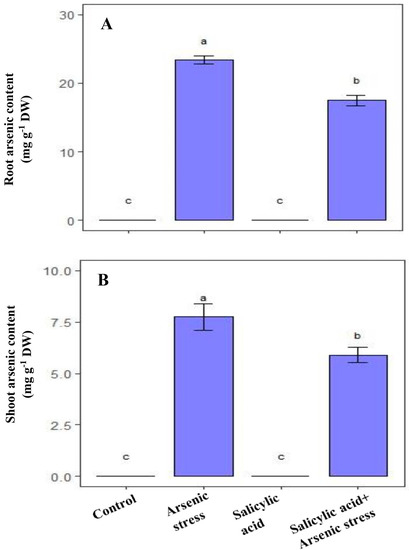
Figure 8.
Effect of salicylic acid (SA; 0.5 mM; 15DAS) on (A) root arsenic content, and (B) shoot arsenic content of rice (Oryza sativa) plants in the presence (150 µM) or absence of As stress at 30 DAS. Data are presented as treatments mean ± SE (n = 3). Data followed by different letters are statistically significant at p ≤ 0.05 by Tukey’s HSD post hoc test.
All the results are summarized in Table S1.
4. Discussion
The recent increase in climate disruption has led to a notable decline in rice yield, thereby undermining the Sustainable Development Goals (SDGs) aimed at ensuring adequate crop production in terms of both quality and quantity to cater to the burgeoning population in the future. According to the First Advance Estimate (2022-23), India’s rice production is estimated to fall 6%, while total foodgrain output is estimated to fall 4% posing a serious threat to nutrition and giving rise to concealed hunger-related health and economic challenges [52].
SA is a vital organic acid of industrial significance, which holds great importance in the production of several critical drugs, including aspirin, and is a constituent of numerous skin care products with medicinal benefits [53]. In addition to this, SA also functions as a plant growth regulator, mediating several key aspects of plant growth and inducing tolerance against abiotic stresses [1,20,48].
In light of the fluctuating environmental conditions that affect rice production, this study focused on the potential of SA to mitigate the toxicity and uptake of As in rice. Comprehensively, we have revealed that SA supplementation has a pivotal function in regulating nutrient assimilation, ethylene biosynthesis, and defense mechanisms, which ultimately lead to the reduction in As-induced cellular damage and improvements in the development and physiological traits of rice crops.
The surplus production of oxidative stress indicators, such as TBARS (a byproduct of lipid peroxidation), and H2O2 caused by As stress, leads to the initiation of cell death episodes. Therefore, these indicators serve as reliable indices and habitual stress-responsive behavior of a plant that is exposed to unfavorable environmental conditions [54]. Moreover, MG, a cytotoxin molecule, is also a hallmark trait that poses potential threats, including the inhibition of cellular growth, protein denaturation and the inactivation of antioxidant machinery during plant growth and development [55].
Through evolutionary modifications, the plant has developed distinctive characteristics that govern the activation of intricate and integral immune-activating pathways, including ROS eliminating the AsA-GSH pathway and the glyoxalase systems that detoxify MG [56]. These systems have a pivotal function in regulating redox signaling and the propagation of plant defense mechanisms, and they coordinate tolerance-associated responses to stressed environments. In this study, it was observed that As stress caused an overproduction of oxidative stress markers, resulting in a loss of cellular viability in rice plants (Figure 1). However, the supplementation of SA was found to be efficacious in limiting the invasion of As-stress-induced cellular adversities, probably by reinforcing defense-promoting metabolites and/or systemic responses. Based on the aforementioned claim, the current investigation confirmed that SA significantly induced the AsA-GSH pathway and glyoxalase systems, which was a defensive response to the constraints caused by the presence of As in rice plants. Our findings align with the report of Kaya et al. [20] who revealed that the SA application effectively enhanced the antioxidant enzyme activity and glyoxalase systems, leading to a decrease in As-induced oxidative stress indicators.
As-induced toxicity is often coupled to the increase in proline levels, an uncommon yet adaptable proteinogenic amino acid that is sensitive to sudden changes in redox homeostasis [57,58]. Proline is also closely associated with various cellular processes such as osmotic regulation, energy production in plants, nutrient assimilation, and adaptation to environmental stressors [58]. The balance between proline synthesis and breakdown is controlled by rate-limiting enzymes GK and PROX, respectively, which directly influence the amount of proline produced under optimal or stressful conditions [58]. Therefore, evaluating the activity of GK and PROX provides valuable insights into proline accumulation, which is consistent with the current findings, wherein supplementation of SA in rice plants exposed to As stress positively influenced proline levels and GK activity, while a notable reduction in the PROX activity was witnessed under As-stress conditions in SA treated rice plants (Figure 3).
Plant nutrients act as active participants in the structural and/or functional activation of enzymes that govern various physiological and metabolic processes in plants. The role of SA in buffering N metabolism has been proposed [59] and it can influence growth and stress responses by modulating carbon (C) and N metabolism [20]. In the current investigation, rice plants treated with SA in response of As stress were observed to display improved N assimilation, as evidenced by the enhanced activity of NR and NiR, along with N content. Similarly, SA assimilation was also found to be enhanced, as indicated by increased activities of ATP-S and SAT, as well as elevated levels of cysteine, methionine, and S content. To prevent the harmful effects of As stress, the incorporation of N and S is a crucial component of fundamental metabolic processes [60,61]. Cysteine functions as the connecting bridge between N and S assimilation and acts as a precursor in generating reduced glutathione (GSH) [62]. The current investigation revealed that the application of SA led to a rise in levels of cysteine and GSH. In plants, an elevated production of cysteine sustains thiol metabolism, which is imperative for As tolerance [63]. The S content in plants diminishes when subjected to As stress, which is a crucial nutrient that participates in diverse metabolic pathways and serves as a constituent of certain amino acids (such as cysteine and methionine). Khan et al. [48] put forward the idea that an upsurge in ATP-S and SAT activity results in improved S metabolism, ultimately conferring resistance to Cd-induced stress. Following the administration of SA, ATP-S and SAT activity escalated, while S content was also enhanced, highlighting the involvement of SA in S assimilation. Additionally, both As-stressed and non-stressed plants treated with SA exhibited elevated levels of Cys and methionine (Figure 5).
Photosynthesis is an important indicator of plant adaptability under abiotic stress [64,65]. Studies show that As stress can lead to an overproduction of ethylene and a hindrance to photosynthesis [66,67]. The mechanistic elucidation on the rate of chlorophyll accumulation and RuBisCO activity, being a primitive quantifiable physiological response, defines the photosynthetic capacity of a plant under optimal and stress conditions [68]. The present study has revealed a noteworthy decrease in these photosynthetic traits under As stress conditions, which could be associated with the hold back mechanisms restricting the synthesis of photosynthetic enzymes primarily RuBisCO activity and chlorophyll content in rice plants. In addition, As stress causes a reduction in gas exchange parameters and a decline in quantum yield of PSII (Figure 6). From the pooled data, it can be concluded that the presence of SA can positively impact the plant’s defense system and reduce As uptake, ultimately mitigating As-induced toxicity on photosynthesis.
Through SA application, excessive ethylene production during As stress was reduced to an optimal level, thereby resulting in the positive regulation of GSH production via the ASH-GSH cycle’s enzymatic activity. The increase in GSH production induced by SA led to a higher reduced state, which protected and enhanced a plant’s photosynthetic performance and growth in response to As stress [69]. The exogenous application of SA heightens the photosynthetic efficiency of Brassica napus (mustard) by amplifying stomatal conductance and augmenting the rate of CO2 diffusion across intercellular gaps [70]. This augmented photosynthetic rate led to an increase in the dry mass of the plants (Figure 4F). Consistent with these findings, treatment of SA through foliage was observed to promote plant physiology and growth not only in non-stressed plants, but also in plants exposed to As stress.
Thus, the current study provides a holistic approach to SA implementation promoting the stimulation of defense systems, which work together to control and strengthen the maintenance of nutrient balance, production of ethylene, and modification of characteristics related to photosynthesis and growth. The findings demonstrate that SA can be a valuable tool in minimizing the accumulation of As in rice, indicating that it has the potential to be a beneficial agricultural agent that can boost plant development, while also restricting As accumulation.
5. Conclusions
The current study indicated that the application of SA has a safeguarding function in counteracting the As-induced toxicity by augmenting the antioxidant system, proline metabolism, N–S assimilation, and protected photosynthetic and growth characteristics along with regulation of ethylene production. The modulation of ethylene production by the application of SA in response of As stress proposed that the ethylene signaling was intensified after SA supplementation. In general, SA has been demonstrated to serve as a versatile signaling molecule, not only minimizing the impact of As stress, but also enhancing the physiological efficacy of unstressed rice plants by affecting ethylene production. SA may have additional interactions with other plant hormones and As stress resilience. Thus, our findings lay out a possibility that SA supplementation could be used as a potential approach for the improvement of growth and productivity of the economically important rice crop in the As-prone areas to accelerate sustainable agriculture in the climate-changing era.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriculture13071293/s1, File S1: Methods.
Author Contributions
Conceptualization, M.I.R.K.; methodology, F.N., M.M. and B.J.; software, F.N. and M.M.; validation, M.I.R.K., M.H.S. and N.I., formal analysis, M.I.R.K.; investigation, M.I.R.K.; resources, M.I.R.K.; data curation, M.I.R.K., F.N. and M.M.; writing—original draft preparation, M.I.R.K., F.N. and M.M.; writing—review and editing, F.N. and M.I.R.K.; visualization, M.I.R.K.; supervision, M.I.R.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Researchers Supporting Project number (RSP2023R347), King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to extend their sincere appreciation to the Researchers Supporting Project number (RSP2023R347), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare that they have no known competing conflict of interest or personal relationships that could have appeared to influence the work reported in this paper.
References
- Noor, I.; Sohail, H.; Sun, J.; Nawaz, M.A.; Li, G.; Hasanuzzaman, M.; Liu, J. Heavy metal and metalloid toxicity in horticultural plants: Tolerance mechanism and remediation strategies. Chemosphere 2022, 303, 135196. [Google Scholar] [CrossRef]
- Muzaffar, S.; Khan, J.; Srivastava, R.; Gorbatyuk, M.S.; Athar, M. Mechanistic understanding of the toxic effects of arsenic and warfare arsenicals on human health and environment. Cell Biol. Toxicol. 2023, 39, 85–110. [Google Scholar] [CrossRef]
- He, Y.; Zhang, T.; Sun, Y.; Wang, X.; Cao, Q.; Fang, Z.; Chang, M.; Cai, Q.; Lou, L. Exogenous IAA alleviates arsenic toxicity to rice and reduces arsenic accumulation in rice grains. J. Plant Growth Regul. 2022, 41, 734–741. [Google Scholar] [CrossRef]
- Lee, S.G.; Kang, I.; Seo, M.N.; Lee, J.E.; Eom, S.Y.; Hwang, M.S.; Park, K.S.; Choi, B.S.; Kwon, H.J.; Hong, Y.S.; et al. Exposure levels and contributing factors of various arsenic species and their health effects on Korean adults. Arch. Environ. Contam. Toxicol. 2022, 82, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Quarles, C.D.; Sullivan, P.; Bohlim, N.; Saetveit, N. Rapid automated total arsenic and arsenic speciation by inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 2022, 37, 1240–1246. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Alamri, S.; Khan, M.N.; Corpas, F.J.; Al-Amri, A.A.; Alsubaie, Q.D.; Ali, H.M.; Kalaji, H.M.; Ahmad, P. Melatonin and calcium function synergistically to promote the resilience through ROS metabolism under arsenic-induced stress. J. Hazard. Mater. 2020, 398, 122882. [Google Scholar] [CrossRef]
- Zemanová, V.; Popov, M.; Pavlíková, D.; Kotrba, P.; Hnilička, F.; Česká, J.; Pavlík, M. Effect of arsenic stress on 5-methylcytosine, photosynthetic parameters and nutrient content in arsenic hyperaccumulator Pteris cretica (L.) var. Albo-lineata. BMC Plant Biol. 2020, 20, 130. [Google Scholar] [CrossRef]
- Hu, Y.; Li, J.; Lou, B.; Wu, R.; Wang, G.; Lu, C.; Wang, H.; Pi, J.; Xu, Y. The role of reactive oxygen species in arsenic toxicity. Biomolecules 2020, 10, 240. [Google Scholar] [CrossRef]
- Sharma, S.S.; Kumar, V.; Dietz, K.J. Emerging trends in metalloid-dependent signaling in plants. Trends Plant Sci. 2021, 26, 452–471. [Google Scholar] [CrossRef]
- Kaya, C.; Sarıoglu, A.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. The combined supplementation of melatonin and salicylic acid effectively detoxifies arsenic toxicity by modulating phytochelatins and nitrogen metabolism in pepper plants. Environ. Pollut. 2022, 297, 118727. [Google Scholar] [CrossRef]
- Praveen, A.; Pandey, A.; Gupta, M. Protective role of nitric oxide on nitrogen-thiol metabolism and amino acids profiling during arsenic exposure in Oryza sativa L. Ecotoxicology 2020, 29, 825–836. [Google Scholar] [CrossRef]
- Srivastava, S.; Pathare, V.S.; Sounderajan, S.; Suprasanna, P. Nitrogen supply influences arsenic accumulation and stress responses of rice (Oryza sativa L.) seedlings. J. Hazard. Mater. 2019, 367, 599–606. [Google Scholar] [CrossRef]
- Ghosh, S.; Saha, J.; Biswas, A.K. Interactive influence of arsenate and selenate on growth and nitrogen metabolism in wheat (Triticum aestivum L.) seedlings. Acta Physiol. Plant. 2013, 35, 1873–1885. [Google Scholar] [CrossRef]
- Srivastava, S.; Akkarakaran, J.J.; Sounderajan, S.; Shrivastava, M.; Suprasanna, P. Arsenic toxicity in rice (Oryza sativa L.) is influenced by sulfur supply: Impact on the expression of transporters and thiol metabolism. Geoderma 2016, 270, 33–42. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Hossain, M.S.; Bhuyan, M.B.; Al Mahmud, J.; Nahar, K.; Fujita, M. The role of sulfur in plant abiotic stress tolerance: Molecular interactions and defense mechanisms. In Plant Nutrients and Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Oku, H., Nahar, K., Hawrylak-Nowak, B., Eds.; Springer: Singapore, 2018; pp. 221–252. [Google Scholar]
- Jung, H.I.; Kong, M.S.; Lee, B.R.; Kim, T.H.; Chae, M.J.; Lee, E.J.; Jung, G.B.; Lee, C.H.; Sung, J.K.; Kim, Y.H. Exogenous glutathione increases arsenic translocation into shoots and alleviates arsenic-induced oxidative stress by sustaining ascorbate–glutathione homeostasis in rice seedlings. Front. Plant Sci. 2019, 10, 1089. [Google Scholar] [CrossRef]
- Nabi, A.; Naeem, M.; Aftab, T.; Khan, M.M.A.; Ahmad, P. A comprehensive review of adaptations in plants under arsenic toxicity: Physiological, metabolic and molecular interventions. Environ. Pollu. 2021, 290, 118029. [Google Scholar] [CrossRef]
- Kaya, C.; Ugurlar, F.; Ashraf, M.; Ahmad, P. Salicylic acid interacts with other plant growth regulators and signal molecules in response to stressful environments in plants. Plant Physiol. Biochem. 2023, 196, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Bano, K.; Kumar, B.; Alyemeni, M.N.; Ahmad, P. Exogenously-Sourced Salicylic Acid Imparts Resilience towards Arsenic Stress by Modulating Photosynthesis, Antioxidant Potential and Arsenic Sequestration in Brassica napus Plants. Antioxidants 2022, 11, 2010. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Corpas, F.J.; Ahmad, P. Salicylic acid-induced nitric oxide enhances arsenic toxicity tolerance in maize plants by upregulating the ascorbate-glutathione cycle and glyoxalase system. J. Hazard. Mater. 2020, 399, 123020. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.J.; Khan, N.A.; Anjum, N.A.; Masood, A.; Khan, M.I.R. Mechanistic elucidation of salicylic acid and sulphur-induced defence systems, nitrogen metabolism, photosynthetic, and growth potential of mungbean (Vigna radiata) under salt stress. J. Plant Growth Regul. 2021, 40, 1000–1016. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Jahan, B.; AlAjmi, M.F.; Rehman, M.T.; Iqbal, N.; Irfan, M.; Sehar, Z.; Khan, N.A. Crosstalk of plant growth regulators protects photosynthetic performance from arsenic damage by modulating defense systems in rice. Ecotoxicol. Environ. Saf. 2021, 222, 112535. [Google Scholar] [CrossRef] [PubMed]
- Zengin, F. Effects of exogenous salicylic acid on growth characteristics and biochemical content of wheat seeds under arsenic stress. J. Environ. Biol. 2015, 36, 249. [Google Scholar] [PubMed]
- Khan, M.I.R.; Asgher, M.; Khan, N.A. Alleviation of salt-induced photosynthesis and growth inhibition by salicylic acid involves glycinebetaine and ethylene in mungbean (Vigna radiata L.). Plant Physiol. Biochem. 2014, 80, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 32. [Google Scholar]
- Okuda, T.; Matsuda, Y.; Yamanaka, A.; Sagisaka, S. Abrupt increase in the level of hydrogen peroxide in leaves of winter wheat is caused by cold treatment. Plant Physiol. 1991, 97, 1265–1267. [Google Scholar] [CrossRef]
- Dhindsa, R.H.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence correlated with increased level of membrane permeability, lipid peroxidation and decreased level of SOD and CAT. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Wild, R.; Ooi, L.; Srikanth, V.; Münch, G. A quick, convenient and economical method for the reliable determination of methylglyoxal in millimolar concentrations: The N-acetyl-L-cysteine assay. Anal. Bioanal. Chem. 2012, 403, 2577–2581. [Google Scholar] [CrossRef]
- Thordal-Christensen, H.; Zhang, Z.; Wei, Y.; Collinge, D.B. Subcellular localization of H2O2 in plants, H2O2 accumulation in papillae and hypersensitive response during barley powdery mildew interaction. Plant J. 1997, 11, 187–1194. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Pandey, P.; Rajpoot, R.; Rani, A.; Dubey, R.S. Cadmium and lead interactive effects on oxidative stress and antioxidative responses in rice seedlings. Protoplasma 2014, 251, 1047–1065. [Google Scholar] [CrossRef]
- Alamri, S.; Kushwaha, B.K.; Singh, V.P.; Siddiqui, M.H.; Al-Amri, A.A.; Alsubaie, Q.D.; Ali, H.M. Ascorbate and glutathione independently alleviate arsenate toxicity in brinjal but both require endogenous nitric oxide. Physiol. Plant. 2021, 173, 276–286. [Google Scholar] [CrossRef]
- Beyer, W.F.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Kumari, S.; Nazir, F.; Jain, K.; Khan, M.I.R. GABA and Potassium Modulates Defence Systems, Assimilation of Nitrogen and Carbon, and Yield Traits Under Salt Stress in Wheat. J. Plant Growth Regul. 2023, 1–20. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiolgy 1981, 22, 867–880. [Google Scholar]
- Foyer, C.H.; Halliwell, B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Elia, A.C.; Galarini, R.; Taticchi, M.I.; Dörr, A.J.M.; Mantilacci, L. Antioxidant responses and bioaccumulation in Ictalurus melas under mercury exposure. Ecotoxicol. Environ. Saf. 2003, 55, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Principato, G.B.; Rosi, G.; Talesa, V.; Giovanni, E.; Uotila, L. Purification and characterization of two forms of glyoxalase II from the liver and brain of Wistar rats. Biochim. Biophys. Acta (BBA)-Protein Struct. Mol. Enzymol. 1987, 911, 349–355. [Google Scholar] [CrossRef]
- Rena, A.B.; Splittstoesser, W.E. Proline dehydrogenase and pyrroline-5-carboxylate reductase from pumpkin cotyledons. Phytochemistry 1975, 14, 657–661. [Google Scholar] [CrossRef]
- Hayzer, D.J.; Leisinger, T.H. The gene-enzyme relationships of proline biosynthesis in Escherichia coli. Microbiology 1980, 118, 287–293. [Google Scholar] [CrossRef]
- Huang, A.H.; Cavalieri, A.J. Proline oxidase and water stress-induced proline accumulation in spinach leaves. Plant Physiol. 1979, 63, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Lindner, R.C. Rapid analytical methods for some of the more common inorganic constituents of plant tissues. Plant Physiol. 1994, 19, 76–89. [Google Scholar] [CrossRef]
- Kuo, T.M.; Warner, R.L.; Kleinhofs, A. In vitro stability of nitrate reductase from barley leaves. Phytochemistry 1982, 21, 531–533. [Google Scholar] [CrossRef]
- Hageman, R.H.; Reed, A.J. Nitrate reductase from higher plants. Methods Enzymol. 1980, 69, 270–280. [Google Scholar]
- Singh, R.P.; Srivastava, H.S. Increase in glutamate synthase (NADH) activity in maize seedlings in response to nitrate and ammonium nitrogen. Physiol. Plant. 1986, 66, 413–416. [Google Scholar] [CrossRef]
- Chesnin, L.; Yien, C.H. Turbidimetric determination of available sulfates. Soil Sci. Soc. Am. J. 1951, 15, 149–151. [Google Scholar] [CrossRef]
- Lappartient, A.G.; Touraine, B. Demand-driven control of root ATP sulfurylase activity and SO42− uptake in intact canola (the role of phloem-translocated glutathione). Plant Physiol. 1996, 111, 147–157. [Google Scholar] [CrossRef]
- Kredich, N.M.; Tomkins, G.M. The enzymic synthesis of L-cysteine in Escherichia coli and Salmonella typhimurium. J. Biol. Chem. 1966, 241, 4955–4965. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.R.; Nazir, F.; Asgher, M.; Per, T.S.; Khan, N.A. Selenium and sulfur influence ethylene formation and alleviate cadmium-induced oxidative stress by improving proline and glutathione production in wheat. J. Plant Physiol. 2015, 173, 9–18. [Google Scholar] [CrossRef]
- Usuda, H. The activation state of ribulose 1, 5-bisphosphate carboxylase in maize leaves in dark and light. Plant Cell Physiol. 1985, 26, 1455–1463. [Google Scholar]
- Avni, A.; Bailey, B.A.; Mattoo, A.K.; Anderson, J.D. Induction of ethylene biosynthesis in Nicotiana tabacum by a Trichoderma viride xylanase is correlated to the accumulation of 1-aminocyclopropane-1-carboxylic acid (ACC) synthase and ACC oxidase transcripts. Plant Physiol. 1994, 106, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Woeste, K.E.; Ye, C.; Kieber, J.J. Two Arabidopsis mutants that overproduce ethylene are affected in the posttranscriptional regulation of 1-aminocyclopropane-1-carboxylic acid synthase. Plant Physiol. 1999, 119, 521–530. [Google Scholar] [CrossRef]
- Gupta, R.; Mishra, A. Climate change induced impact and uncertainty of rice yield of agro-ecological zones of India. Agric. Syst. 2019, 173, 1–11. [Google Scholar] [CrossRef]
- Sambyal, K.; Singh, R.V. Production of salicylic acid; a potent pharmaceutically active agent and its future prospects. Crit. Rev. Biotechnol. 2021, 41, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Shri, M.; Kumar, S.; Chakrabarty, D.; Trivedi, P.K.; Mallick, S.; Misra, P.; Shukla, D.; Mishra, S.; Srivastava, S.; Tripathi, R.D.; et al. Effect of arsenic on growth, oxidative stress, and antioxidant system in rice seedlings. Ecotoxicol. Environ. Saf. 2009, 72, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.G. Methylglyoxal and glyoxalase system in plants: Old players, new concepts. Bot. Rev. 2016, 82, 183–203. [Google Scholar] [CrossRef]
- Hossain, M.A.; Hoque, T.S.; Zaid, A.; Wani, S.H.; Mostofa, M.G.; Henry, R. Targeting the Ascorbate-Glutathione Pathway and the Glyoxalase Pathway for Genetic Engineering of Abiotic Stress-Tolerance in Rice. In Molecular Breeding for Rice Abiotic Stress Tolerance and Nutritional Quality; Hossain, M., Hassan, L., Iftekharuddaula, K.M., Kumar, A., Henry, R., Eds.; Wiley-Blackwell: Oxford, UK, 2021; pp. 398–427. [Google Scholar] [CrossRef]
- Singh, M.; Singh, V.P.; Dubey, G.; Prasad, S.M. Exogenous proline application ameliorates toxic effects of arsenate in Solanum melongena L. seedlings. Ecotoxicol. Environ. Saf. 2015, 117, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Szepesi, Á.; Szőllősi, R. Mechanism of proline biosynthesis and role of proline metabolism enzymes under environmental stress in plants. In Plant Metabolites and Regulation under Environmental Stress; Ahmad, P., Ahanger, M.A., Singh, V.P., Tripathi, D.K., Alam, P., Alyemeni, M.N., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 337–353. [Google Scholar] [CrossRef]
- Hayat, Q.; Hayat, S.; Alyemeni, M.N.; Ahmad, A. Salicylic acid mediated changes in growth, photosynthesis, nitrogen metabolism and antioxidant defense system in Cicer arietinum L. Plant Soil Environ. 2012, 58, 417–423. [Google Scholar] [CrossRef]
- Dixit, G.; Singh, A.P.; Kumar, A.; Dwivedi, S.; Deeba, F.; Kumar, S.; Suman, S.; Adhikari, B.; Shukla, Y.; Trivedi, P.K.; et al. Sulfur alleviates arsenic toxicity by reducing its accumulation and modulating proteome, amino acids and thiol metabolism in rice leaves. Sci. Rep. 2015, 5, 16205. [Google Scholar] [CrossRef]
- Bano, K.; Kumar, B.; Alyemeni, M.N.; Ahmad, P. Protective mechanisms of sulfur against arsenic phytotoxicity in Brassica napus by regulating thiol biosynthesis, sulfur-assimilation, photosynthesis, and antioxidant response. Plant Physiol. Biochem. 2022, 188, 1–11. [Google Scholar] [CrossRef]
- Hesse, H.; Nikiforova, V.; Gakière, B.; Hoefgen, R. Molecular analysis and control of cysteine biosynthesis: Integration of nitrogen and sulphur metabolism. J. Exp. Bot. 2004, 55, 1283–1292. [Google Scholar] [CrossRef]
- Singh, V.P.; Singh, S.; Kumar, J.; Prasad, S.M. Investigating the roles of ascorbate-glutathione cycle and thiol metabolism in arsenate tolerance in ridged Luffa seedlings. Protoplasma 2015, 252, 1217–1229. [Google Scholar] [CrossRef]
- Muhammad, I.; Shalmani, A.; Ali, M.; Yang, Q.H.; Ahmad, H.; Li, F.B. Mechanisms regulating the dynamics of photosynthesis under abiotic stresses. Front. Plant Sci. 2021, 11, 615942. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Singh Sidhu, G.P.; Bali, A.S.; Handa, N.; Kapoor, D.; Yadav, P.; Khanna, K.; et al. Photosynthetic response of plants under different abiotic stresses: A review. J. Plant Growth Regul. 2020, 39, 509–531. [Google Scholar] [CrossRef]
- Singh, N.; Gaddam, S.R.; Singh, D.; Trivedi, P.K. Regulation of arsenic stress response by ethylene biosynthesis and signaling in Arabidopsis thaliana. Environ. Exp. Bot. 2021, 185, 104408. [Google Scholar] [CrossRef]
- Srivastava, S.; Sharma, Y.K. Altered growth, photosynthetic machinery and induced oxidative stress in spinach in response to arsenic stress. J. Plant Physiol. Pathol. 2013, 1, 1–4. [Google Scholar] [CrossRef]
- Stitt, M.; Schulze, D. Does Rubisco control the rate of photosynthesis and plant growth? An exercise in molecular ecophysiology. Plant Cell Environ. 1994, 17, 465–487. [Google Scholar] [CrossRef]
- Saidi, I.; Yousfi, N.; Borgi, M.A. Salicylic acid improves the antioxidant ability against arsenic-induced oxidative stress in sunflower (Helianthus annuus) seedling. J. Plant Nutr. 2017, 40, 2326–2335. [Google Scholar] [CrossRef]
- Nazar, R.; Umar, S.; Khan, N.A. Exogenous salicylic acid improves photosynthesis and growth through increase in ascorbate-glutathione metabolism and S assimilation in mustard under salt stress. Plant Signal. Behav. 2015, 10, e1003751. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).