Flowering Phenology of Olive Cultivars in Two Climate Zones with Contrasting Temperatures (Subtropical and Mediterranean)
Abstract
1. Introduction
2. Materials and Methods
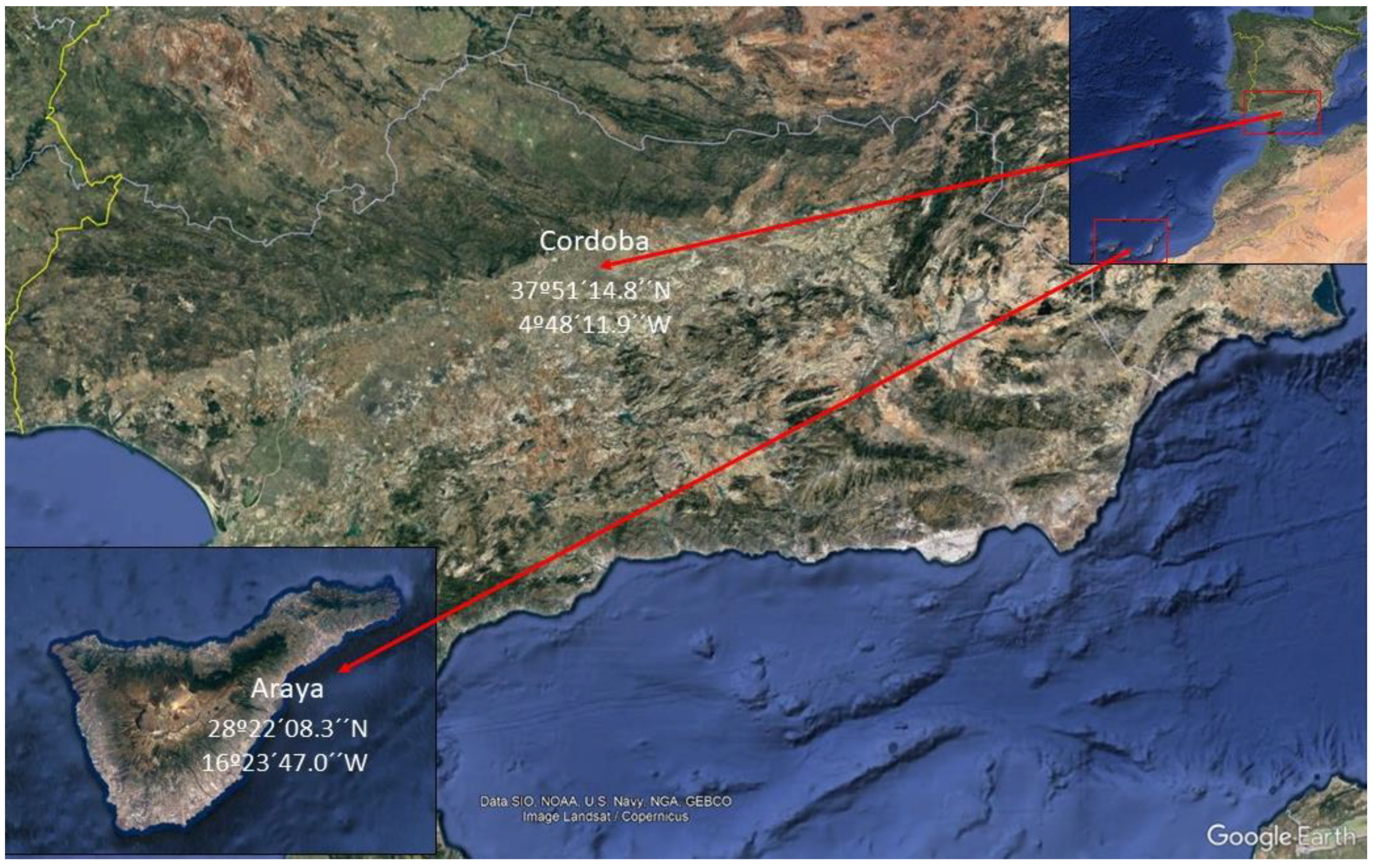
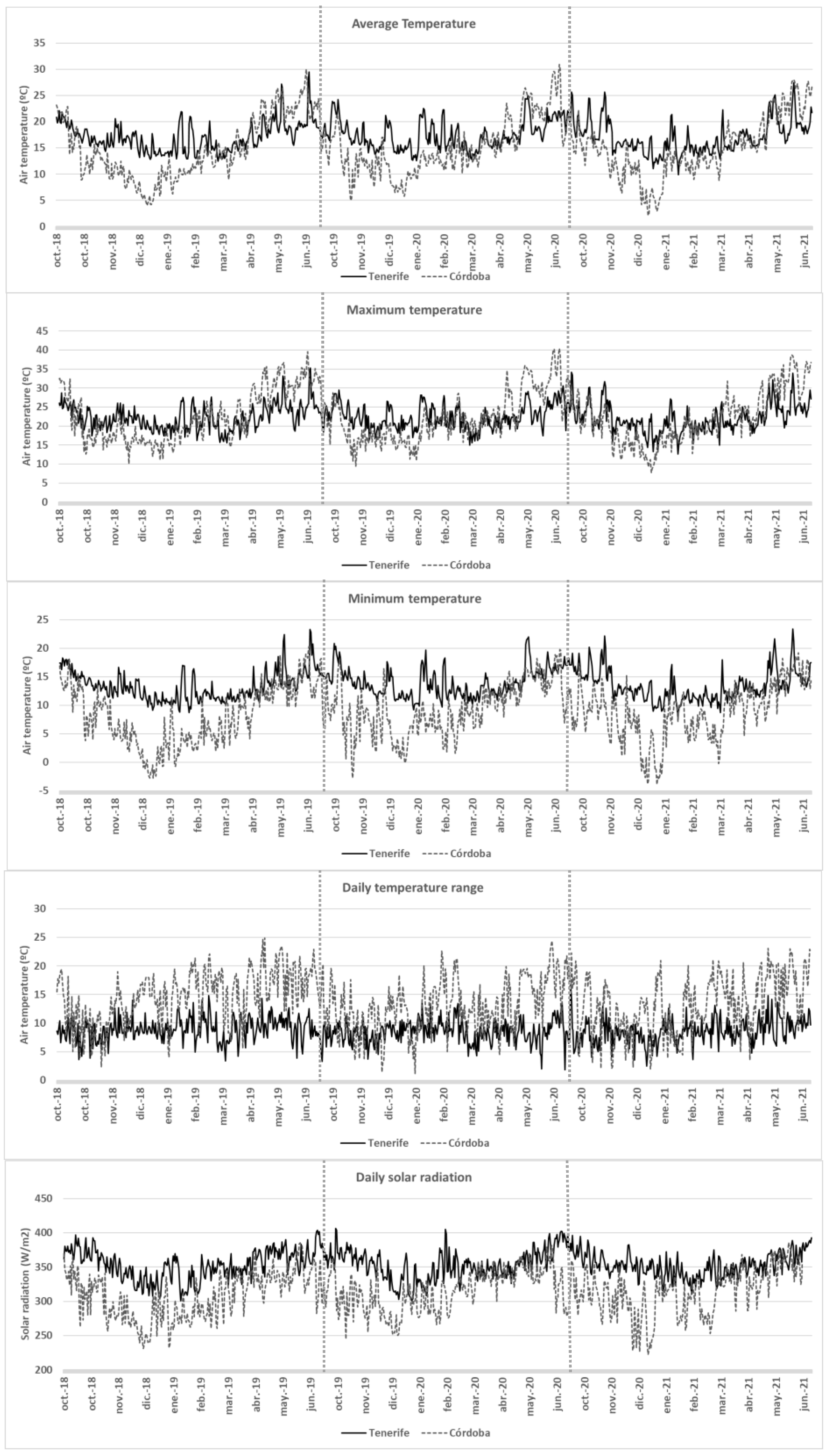
2.1. Plant Material and Location
2.2. Flowering Phenology
- Length of flowering period (FP): 61 days being the earliest stage to 68 days being the most common stage.
- Length of full bloom period (FBP): Number of days from first time stage 61 appears as most common to last time for stage 65 (full bloom, at least 50% of flowers open) appears as most common.
- Full bloom date (FBD): Average Julian date of the start and end of the FBP.
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Belaj, A.; Ninot, A.; Gómez-Gálvez, F.J.; El Riachy, M.; Gurbuz-Veral, M.; Torres, M.; Lazaj, A.; Klepo, T.; Paz, S.; Ugarte, J.; et al. Utility of EST-SNP Markers for Improving Management and Use of Olive Genetic Resources: A Case Study at the Worldwide Olive Germplasm Bank of Córdoba. Plants 2022, 11, 921. [Google Scholar] [CrossRef]
- Beltrán, G.; Bucheli, M.E.; Aguilera, M.P.; Belaj, A.; Jimenez, A. Squalene in Virgin Olive Oil: An Olive Varietal Screening. Eur. J. Lipid Sci. Technol. 2015, 118, 1250–1253. [Google Scholar] [CrossRef]
- Gómez-Gálvez, F.J.; Pérez-Mohedano, D.; de la Rosa-Navarro, R.; Belaj, A. High-Throughput Analysis of the Canopy Traits in the Worldwide Olive Germplasm Bank of Córdoba Using Very High-Resolution Imagery Acquired from Unmanned Aerial Vehicle (UAV). Sci. Hortic. 2021, 278, 109851. [Google Scholar] [CrossRef]
- Belaj, A.; del Carmen Dominguez-Garcia, M.; Gustavo Atienza, S.; Martin Urdiroz, N.; de la Rosa, R.R.; Satovic, Z.; Martin, A.; Kilian, A.; Trujillo, I.; Valpuesta, V.; et al. Developing a Core Collection of Olive (Olea Europaea L.) Based on Molecular Markers (DArTs, SSRs, SNPs) and Agronomic Traits. Tree Genet. Genomes 2012, 8, 365–378. [Google Scholar] [CrossRef]
- Lopez-Escudero, F.J.; Mercado-Blanco, J. Verticillium Wilt of Olive: A Case Study to Implement an Integrated Strategy to Control a Soil-Borne Pathogen. Plant Soil 2011, 344, 1–50. [Google Scholar] [CrossRef]
- Lorite, I.J.; Cabezas, J.; Ruiz-Ramos, M.; de la Rosa, R.; Soriano, M.; León, L.; Santos, C.; Gabaldón-Leal, C. Enhancing the Sustainability of Mediterranean Olive Groves through Adaptation Measures to Climate Change Using Modelling and Response Surfaces. Agric. For. Meteorol. 2022, 313, 108742. [Google Scholar] [CrossRef]
- Gabaldón-Leal, C.; Ruiz-Ramos, M.; de la Rosa, R.; León, L.; Belaj, A.; Rodríguez, A.; Santos, C.; Lorite, I.J.J. Impact of Changes in Mean and Extreme Temperatures Caused by Climate Change on Olive Flowering in Southern Spain. Int. J. Climatol. 2017, 37, 940–957. [Google Scholar] [CrossRef]
- De Melo-Abreu, J.P.; Barranco, D.; Cordeiro, A.M.; Tous, J.; Rogado, B.M.; Villalobos, F.J.; Demeloabreu, J. Modelling Olive Flowering Date Using Chilling for Dormancy Release and Thermal Time. Agric. For. Meteorol. 2004, 125, 117–127. [Google Scholar] [CrossRef]
- Luedeling, E. Climate Change Impacts on Winter Chill for Temperate Fruit and Nut Production: A Review. Sci. Hortic. 2012, 144, 218–229. [Google Scholar] [CrossRef]
- Fraga, H.; Pinto, J.G.; Viola, F.; Santos, J.A. Climate Change Projections for Olive Yields in the Mediterranean Basin. Int. J. Climatol. 2020, 40, 769–781. [Google Scholar] [CrossRef]
- Mairech, H.; López-Bernal, Á.; Moriondo, M.; Dibari, C.; Regni, L.; Proietti, P.; Villalobos, F.J.; Testi, L. Is New Olive Farming Sustainable? A Spatial Comparison of Productive and Environmental Performances between Traditional and New Olive Orchards with the Model OliveCan. Agric. Syst. 2020, 181, 102816. [Google Scholar] [CrossRef]
- Moriondo, M.; Trombi, G.; Ferrise, R.; Brandani, G.; Dibari, C.; Ammann, C.M.; Lippi, M.M.; Bindi, M. Olive Trees as Bio-Indicators of Climate Evolution in the Mediterranean Basin. Glob. Ecol. Biogeogr. 2013, 22, 818–833. [Google Scholar] [CrossRef]
- Abou-Saaid, O.; El Yaacoubi, A.; Moukhli, A.; El Bakkali, A.; Oulbi, S.; Delalande, M.; Farrera, I.; Kelner, J.-J.; Lochon-Menseau, S.; El Modafar, C.; et al. Statistical Approach to Assess Chill and Heat Requirements of Olive Tree Based on Flowering Date and Temperatures Data: Towards Selection of Adapted Cultivars to Global Warming. Agronomy 2022, 12, 2975. [Google Scholar] [CrossRef]
- Navas-Lopez, J.F.; León, L.; Rapoport, H.F.; Moreno-Alías, I.; Lorite, I.J.; de la Rosa, R. Genotype, Environment and Their Interaction Effects on Olive Tree Flowering Phenology and Flower Quality. Euphytica 2019, 215, 184. [Google Scholar] [CrossRef]
- Belaj, A.; De la Rosa, R.; León, L.; Gabaldón-Leal, C.; Santos, C.; Porras, R.; De la Cruz-Blanco, M.; Lorite, I.J. Phenological Diversity in a World Olive Germplasm Bank: Potential Use for Breeding Programs and Climate Change Studies. Span. J. Agric. Res. 2020, 18, e0701. [Google Scholar] [CrossRef]
- Benlloch-González, M.; Sánchez-lucas, R.; Benlloch, M.; Fernández-escobar, R. An Approach to Global Warming Effects on Flowering and Fruit Set of Olive Trees Growing under Field Conditions. Sci. Hortic. 2018, 240, 405–410. [Google Scholar] [CrossRef]
- Medina-Alonso, M.G.; Navas, J.F.; Cabezas, J.M.; Weiland, C.M.; Ríos-Mesa, D.; Lorite, I.J.; León, L.; de la Rosa, R. Differences on Flowering Phenology under Mediterranean and Subtropical Environments for Two Representative Olive Cultivars. Environ. Exp. Bot. 2020, 180, 104239. [Google Scholar] [CrossRef]
- Belaj, A.; de la Rosa, R.; Lorite, I.J.; Mariotti, R.; Cultrera, N.G.M.; Beuzón, C.R.; González-Plaza, J.J.; Muñoz-Mérida, A.; Trelles, O.; Baldoni, L. Usefulness of a New Large Set of High Throughput EST-SNP Markers as a Tool for Olive Germplasm Collection Management. Front. Plant Sci. 2018, 9, 1320. [Google Scholar] [CrossRef]
- Leon, L.; Beltran, G.; Aguilera, M.P.; Rallo, L.; Barranco, D.; De la Rosa, R. Oil Composition of Advanced Selections from an Olive Breeding Program. Eur. J. Lipid Sci. Technol. 2011, 113, 870–875. [Google Scholar] [CrossRef]
- Sanz-Cortés, F.; Martínez-Calvo, J.; Badenes, M.L.; Bleiholder, H.; Hack, H.; Llácer, G.; Meier, U. Phenological Growth Stages of Olive Trees (Olea Europaea). Ann. Appl. Biol. 2002, 140, 151–157. [Google Scholar] [CrossRef]
- Vuletin Selak, G.; Cuevas, J.; Goreta Ban, S.; Pinillos, V.; Dumicic, G.; Perica, S. The Effect of Temperature on the Duration of the Effective Pollination Period in “Oblica” Olive (Olea Europaea) Cultivar. Ann. Appl. Biol. 2014, 164, 85–94. [Google Scholar] [CrossRef]
- Hector, A.; Von Felten, S.; Schmid, B. Analysis of Variance with Unbalanced Data: An Update for Ecology & Evolution. J. Anim. Ecol. 2010, 79, 308–316. [Google Scholar] [CrossRef]
- Aybar, V.E.; De Melo-Abreu, J.P.; Searles, P.S.; Matias, A.C.; Del Río, C.; Caballero, J.M.; Rousseaux, M.C. Evaluation of Olive Flowering at Low Latitude Sites in Argentina Using a Chilling Requirement Model. Span. J. Agric. Res. 2015, 13, e0901. [Google Scholar] [CrossRef]
- Kaniewski, D.; Marriner, N.; Morhange, C.; Khater, C.; Terral, J.-F.; Besnard, G.; Otto, T.; Luce, F.; Couillebault, Q.; Tsitsou, L.; et al. Climate Change Threatens Olive Oil Production in the Levant. Nat. Plants 2023, 9, 219–227. [Google Scholar] [CrossRef]
- Rubio-Valdés, G.; Cabello, D.; Rapoport, H.F.; Rallo, L. Olive Bud Dormancy Release Dynamics and Validation of Using Cuttings to Determine Chilling Requirement. Plants 2022, 11, 3461. [Google Scholar] [CrossRef]
- Hamze, L.M.; Trentacoste, E.R.; Searles, P.S.; Rousseaux, M.C. Spring Reproductive and Vegetative Phenology of Olive (Olea Europaea L.) Cultivars at Different Air Temperatures along a Latitudinal-Altitudinal Gradient in Argentina. Sci. Hortic. 2022, 304, 111327. [Google Scholar] [CrossRef]
- Aguilera, F.; Ruiz Valenzuela, L. Study of the Floral Phenology of Olea Europaea L. in Jaén Province (SE Spain) and Its Relation with Pollen Emission. Aerobiologia 2009, 25, 217–225. [Google Scholar] [CrossRef]
- Rapoport, H.F. The Reproductive Biology of the Olive Tree and Its Relationship to Extreme Environmental Conditions. Acta Hortic. 2014, 1057, 41–50. [Google Scholar]
- Aguilera, F.; Orlandi, F.; Ruiz-valenzuela, L.; Msallem, M.; Fornaciari, M. Analysis and Interpretation of Long Temporal Trends in Cumulative Temperatures and Olive Reproductive Features Using a Seasonal Trend Decomposition Procedure. Agric. For. Meteorol. 2015, 203, 208–216. [Google Scholar] [CrossRef]
- Malik, N.S.A.; Bradford, J.M. Inhibition of Flowering in “Arbequina” Olives from Chilling at Lower Temperatures. J. Food Agric. Environ. 2009, 7, 429–431. [Google Scholar]
- Luedeling, E.; Zhang, M.; McGranahan, G.; Leslie, C. Validation of Winter Chill Models Using Historic Records of Walnut Phenology. Agric. For. Meteorol. 2009, 149, 1854–1864. [Google Scholar] [CrossRef]
- Serrano, A.; Rodríguez-Jurado, D.; Román, B.; Bejarano-Alcázar, J.; De la Rosa, R.; León, L. Verticillium Wilt Evaluation of Olive Breeding Selections Under Semi-Controlled Conditions. Plant Dis. 2021, 105, 1781–1790. [Google Scholar] [CrossRef]
- Rallo, L.; Barranco, D.; de la Rosa, R.; León, L. ‘Chiquitita’ Olive. HortScience 2008, 43, 529–531. [Google Scholar] [CrossRef]
- Meza, F.; Darbyshire, R.; Farrell, A.; Lakso, A.; Lawson, J.; Meinke, H.; Nelson, G.; Stockle, C. Assessing Temperature-Based Adaptation Limits to Climate Change of Temperate Perennial Fruit Crops. Glob. Change Biol. 2023, 29, 2557–2571. [Google Scholar] [CrossRef]
- Castillo-Llanque, F.J.; Rapoport, H.F.; Baumann Samanez, H. Irrigation Withholding Effects on Olive Reproductive Bud Development for Conditions with Insufficient Winter Chilling. Acta Hortic. 2014, 1057, 113–119. [Google Scholar]
- Torres, M.; Pierantozzi, P.; Searles, P.; Rousseaux, M.C.; García-Inza, G.; Miserere, A.; Bodoira, R.; Contreras, C.; Maestri, D. Olive Cultivation in the Southern Hemisphere: Flowering, Water Requirements and Oil Quality Responses to New Crop Environments. Front. Plant Sci. 2017, 8, 1830. [Google Scholar] [CrossRef]
- Chuine, I.; Bonhomme, M.; Legave, J.-M.; García de Cortázar-Atauri, I.; Charrier, G.; Lacointe, A.; Améglio, T. Can Phenological Models Predict Tree Phenology Accurately in the Future? The Unrevealed Hurdle of Endodormancy Break. Glob. Change Biol. 2016, 22, 3444–3460. [Google Scholar] [CrossRef]
- López-Bernal, Á.; García-Tejera, O.; Testi, L.; Orgaz, F.; Villalobos, F.J. Studying and Modelling Winter Dormancy in Olive Trees. Agric. For. Meteorol. 2020, 280, 107776. [Google Scholar] [CrossRef]


| FP | FBP | FBD | |
|---|---|---|---|
| Cultivar | 2.5 | 1.1 | 8.0 |
| Environment | 52.5 | 8.3 | 54.4 |
| Cultivar × Environment | 11.8 | 12.1 | 16.1 |
| Error | 33.2 | 78.6 | 21.6 |
| CO-19 | CO-20 | CO-21 | TF-19 | TF-20 | TF-21 | Average | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arbequina | 25.0 | fgh | 16.7 | gh | 13.2 | gh | 79.4 | a | 32.3 | efg | 34.2 | efg | 33.5 | n.s. |
| Coratina | 12.0 | gh | 12.0 | gh | 54.0 | bcde | 42.0 | cdefg | 37.3 | defg | 31.5 | n.s. | ||
| Hojiblanca | 15.0 | gh | 10.0 | gh | 38.3 | defg | 54.3 | bcd | 36.0 | defg | 30.7 | n.s. | ||
| Koroneiki | 20.0 | gh | 15.6 | gh | 10.2 | gh | 62.6 | bc | 26.7 | fg | 35.6 | defg | 28.5 | n.s. |
| Martina | 9.0 | gh | 13.5 | gh | 16.2 | gh | 66.1 | b | 33.3 | efg | 36.7 | defg | 29.1 | n.s. |
| Picholine | 13.3 | gh | 10.0 | gh | 42.3 | cdefg | 23.0 | fgh | 38.7 | defg | 25.5 | n.s. | ||
| Picual | 16.0 | gh | 15.2 | gh | 8.0 | h | 44.8 | cdef | 52.5 | cde | 28.5 | fg | 27.5 | n.s. |
| Average | 17.5 | c | 14.5 | c | 11.4 | c | 55.4 | a | 37.7 | b | 35.3 | b | ||
| CO-19 | CO-20 | CO-21 | TF-19 | TF-20 | TF-21 | Average | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arbequina | 14.8 | n.s. | 4.5 | n.s. | 7.8 | n.s. | 8.9 | n.s. | 6.9 | n.s. | 10.0 | n.s. | 8.8 | n.s. |
| Coratina | 5.7 | n.s. | 8.0 | n.s. | 27.0 | n.s. | 9.5 | n.s. | 9.0 | n.s. | 11.8 | n.s. | ||
| Hojiblanca | 8.0 | n.s. | 6.2 | n.s. | 5.7 | n.s. | 10.0 | n.s. | 9.3 | n.s. | 7.8 | n.s. | ||
| Koroneiki | 8.0 | n.s. | 6.6 | n.s. | 5.6 | n.s. | 13.2 | n.s. | 10.0 | n.s. | 6.8 | n.s. | 8.4 | n.s. |
| Martina | 3.0 | n.s. | 3.8 | n.s. | 9.2 | n.s. | 10.3 | n.s. | 8.8 | n.s. | 10.3 | n.s. | 7.6 | n.s. |
| Picholine | 6.0 | n.s. | 7.0 | n.s. | 18.0 | n.s. | 9.5 | n.s. | 6.7 | n.s. | 9.4 | n.s. | ||
| Picual | 8.0 | n.s. | 6.5 | n.s. | 4.5 | n.s. | 9.7 | n.s. | 11.5 | n.s. | 6.5 | n.s. | 7.8 | n.s. |
| Average | 8.4 | ab | 5.9 | C | 6.9 | bc | 13.3 | a | 9.5 | ab | 8.4 | ab | ||
| CO-19 | CO-20 | CO-21 | TF-19 | TF-20 | TF-21 | Average | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arbequina | 111.4 | cdef | 109.9 | cdefg | 110.1 | cdefg | 89.4 | h | 62.0 | i | 94.5 | gh | 96.2 | c |
| Coratina | 113.2 | cdef | 110.0 | cdefg | 127.5 | abc | 96.8 | efgh | 96.5 | fgh | 108.8 | a | ||
| Hojiblanca | 115.7 | bcde | 110.9 | cdef | 119.5 | bcd | 88.7 | h | 97.3 | efgh | 106.4 | a | ||
| Koroneiki | 118.0 | bcde | 116.1 | bcde | 111.2 | cdef | 135.8 | a | 60.5 | i | 95.2 | fgh | 106.1 | ab |
| Martina | 125.5 | abcd | 110.4 | cdefg | 107.8 | defg | 114.5 | cde | 61.1 | i | 95.4 | fgh | 102.4 | bc |
| Picholine | 114.7 | bcde | 110.5 | cdefg | 133.0 | ab | 59.3 | i | 94.7 | fgh | 102.4 | bc | ||
| Picual | 118.0 | bcde | 117.1 | bcde | 112.1 | cdef | 117.7 | bcde | 97.3 | efgh | 95.5 | fgh | 109.6 | a |
| Average | 118.2 | c | 113.9 | c | 110.4 | c | 119.6 | c | 75.1 | a | 95.6 | b | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina-Alonso, M.G.; Cabezas, J.M.; Ríos-Mesa, D.; Lorite, I.J.; León, L.; de la Rosa, R. Flowering Phenology of Olive Cultivars in Two Climate Zones with Contrasting Temperatures (Subtropical and Mediterranean). Agriculture 2023, 13, 1312. https://doi.org/10.3390/agriculture13071312
Medina-Alonso MG, Cabezas JM, Ríos-Mesa D, Lorite IJ, León L, de la Rosa R. Flowering Phenology of Olive Cultivars in Two Climate Zones with Contrasting Temperatures (Subtropical and Mediterranean). Agriculture. 2023; 13(7):1312. https://doi.org/10.3390/agriculture13071312
Chicago/Turabian StyleMedina-Alonso, María G., Jose M. Cabezas, Domingo Ríos-Mesa, Ignacio J. Lorite, Lorenzo León, and Raúl de la Rosa. 2023. "Flowering Phenology of Olive Cultivars in Two Climate Zones with Contrasting Temperatures (Subtropical and Mediterranean)" Agriculture 13, no. 7: 1312. https://doi.org/10.3390/agriculture13071312
APA StyleMedina-Alonso, M. G., Cabezas, J. M., Ríos-Mesa, D., Lorite, I. J., León, L., & de la Rosa, R. (2023). Flowering Phenology of Olive Cultivars in Two Climate Zones with Contrasting Temperatures (Subtropical and Mediterranean). Agriculture, 13(7), 1312. https://doi.org/10.3390/agriculture13071312









