Abstract
The possible effect of body condition score (BCS) on ovarian function, sexual behavior, (i.e., females and males; appetitive and consummative), estrus, ovulation, and reproductive performance was investigated in crossbred dairy goats under extensive conditions. Based on their BCS, female goats (n = 48) were divided into two experimental groups, either with a low (LG; 1.0 ± 0.2 units; n = 26) or with a high BCS (HG: 2.5 ± 0.9 units; n = 22). Bucks n = 4; cc = 2.5. Female sexual behavior (i.e., acceptance of anogenital sniffing and approach, flight, and tail wagging) was higher in the HG (p < 0.05). In addition, bucks exposed to HG goats showed more approach and mounting attempts (p < 0.01). There were no significant differences (p > 0.05) between the groups in either the follicular diameter or size. Interestingly, although follicular volume, pregnancy rate, kidding rate, and total number of kids born alive were higher in the HG goats (p < 0.05), there were no significant differences (p < 0.05) in fetal mortality and prolificacy between the HG and LG goats. In addition, the response variables of female ovulation, total number of corpus luteum, ovulation rate, corpus luteum diameter, and luteal tissue volume were more favorable in the HG goats (p < 0.05). Our research results demonstrate that a higher BCS improves not only the reproductive responses, but also sexual behavior (i.e., female and male). Larger ovarian structures, higher ovulation rates, higher pregnancy and kidding rates, and higher prolificacy were observed in the HG goats managed under a marginal semiarid extensive production system.
1. Introduction
The Comarca Lagunera (26° N) is a semi-arid, agro-ecological region in northern Mexico with more than 390,000 crossbred dairy goats [1]; this region is the leader in goat milk production in Mexico [2]. In these latitudes, significant variations in the live weight (LW) and body condition score (BCS) are observed throughout the year due to drastic variations in the availability of native vegetation and crop residues. Goats exhibit seasonal dependence in reproduction and production (i.e., milk and meat-kid) due to their endogenous circannual rhythm [3]. In addition to photoperiods, other factors, such as breed, socio-sexual cues, and nutritional status act as modulators of the reproductive cycle. Female goats have an anestrous period from March to May, and a reproductive period from August to February, with a reproductive transition period from June to July respectively [4,5]. To counteract this handicap, the male effect has been used to induce and synchronize goat sexual activity, but the estrus response to the male socio-sexual stimulus may be affected by the female BCS at the time of breeding in these anestrous goats. Goats with a poor BCS may respond to the buck’ stimulus, but this response is delayed and is less than in goats with an appropriate BCS [6,7].
In goats, energy reserves have been defined as a determining factor that can modify, in a positive fashion, not only the duration of the reproductive season and the estrous cycle characteristics, but also the ovulation rate in crossbred goats at this latitude (26° N) [8]. In fact, under scarcity of forage, goats with a low BCS exposed to a 7d feed supplementation period improved not only the proportion of estrus and ovulating females, but also their ovulation rates [7]. Additionally, higher rates of embryonic implantation and conception have been registered in goats submitted to nutritional supplementation [9]. The last in line with increases in the conception rate, fecundity, and twinning rates [10].
Additionally, high BCS bucks exert a greater number of sexual behaviors, such as lateral approaches, anogenital sniffing, mounting attempts, self-marking, flehmen, and mounting with intromission [11]. Also, and quite interesting, larger female goats depicting a higher hierarchy within the herd and positively related to BCS show an augmented estrus latency, as well as increased sexual behaviors regarding the subordinated goats [12]. Based on the findings obtained at this latitude and with similar goat genotypes, we hypothesize that on one side, bucks exposed to high BCS-females exert more frequent sexual behaviors, and positively influence the diameter and volume of some ovarian structures aligned with augmented reproductive outcomes regarding low-BCS goats. While the benefits of an improved body condition upon reproductive outcomes has been extensively addressed, there is a lack of information merging the effects of body condition upon three fundamental aspects of global reproductive fitness: (1) the male sexual behavior when exposed to either low or high body-conditioned females, and how these divergently body-conditioned goats behaviorally respond to such possible male stimulus, (2) the potential strength of the male effect upon ovarian function by quantifying the size of different ovarian structures linked to their function, and (3) tracking how such male-to-female interactions in divergently body-conditioned goats may affect such ovarian structures and functions not only in the first trimester of pregnancy, but also upon the total reproductive outcomes observed during the peripartum and postpartum stages (i.e., litter size). Such a multifaceted approach and the escorted generated information are currently underreported in the scientific literature. If such possible behavioral, physiologic, and metabolic scenarios are interconnected, such information is central to understand how such interconnectivity would affect the number of kids born alive (i.e., litter size), a biological variable that strongly influences not only the adult physiology, but also the economic return of goat keepers and their families. This study aims to understand such a research question.
2. Materials and Methods
2.1. General
All experimental procedures, methods, and handling of experimental units used in this study complied with the international [13] and national [14] standards for the ethical use, care, and welfare of animals in research, with the institutional approval reference number UAAAN-UL-21-425502002-2850.
2.2. Description of the Location and Environmental Conditions
The study was conducted under the conditions of 25°47′ NL, 103°21′ WL, and an altitude of 1111 m. Rainfall occurs from June to September, respectively, with an annual average of 230 mm. The photoperiod variations are from 13:41 h of light during the summer solstice and from 10:19 h during the winter solstice, respectively. This region has a dry climate with an average annual temperature of 22.3 °C, with a maximum of 42 °C in summer down to 0 °C in winter, respectively [15]. All the experimental animals were managed under extensive conditions foraging the native rangeland, mainly consisting of grasses and bushes and eventually crop residues [16]. All goats were subcutaneously dewormed two months before the study (Ivermectin 1%, Baymec, Bayer®, Mexico City, Mexico) and received vitamins A (500,000 IU), D3 (75,000), and E (50 mg) (Vigantol: ADE + Selenium®, Zapopan Jalisco Mexico, Mexico).
2.3. Animals and Their Management
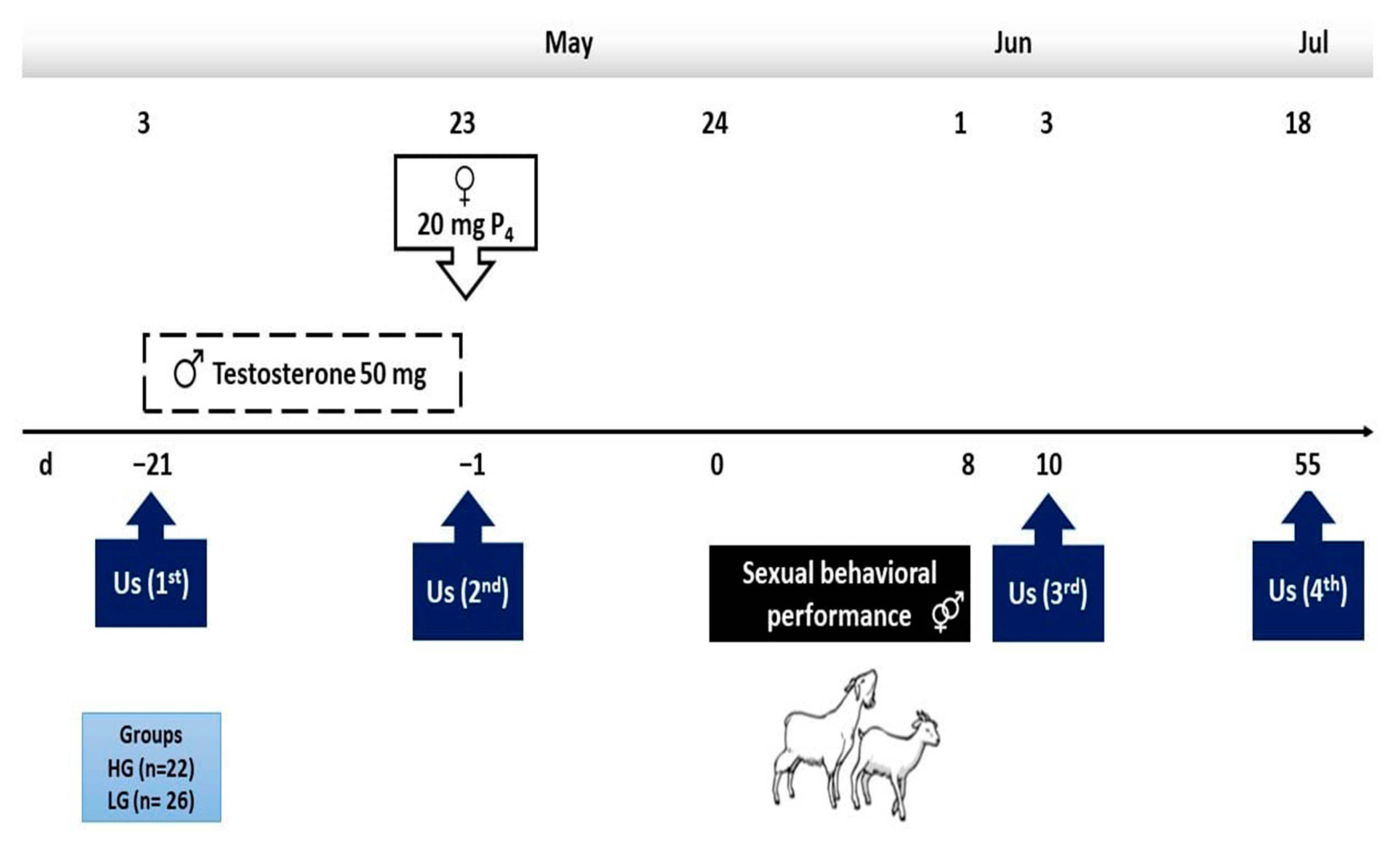
From a herd of 153 mixed-bred goats (i.e., Granadina, Murciana, Malagueña × Alpine, Saanen, and Anglo-Nubian sires) [17], 48 multiparous females with explicit divergent BCS (i.e., high or low), were selected. Thereafter, throughout the use of transrectal ultrasonography, both the non-pregnant and anovulatory status were twice confirmed (i.e., 21 days apart; Aloka SSD 500 Tokyo, Japan; 7.5 MHz transducer). All the goats grazed on rangeland from 10:00 a.m. to 8:00 p.m., respectively, and were penned at night in partially roofed pens with free access to water and mineral salts. Goats stayed in the rangeland for approximately 7 h, walking almost 10 to 12 km d−1, with occasional access to crop residues. The main activities carried out along with the experimental period are depicted in Figure 1.
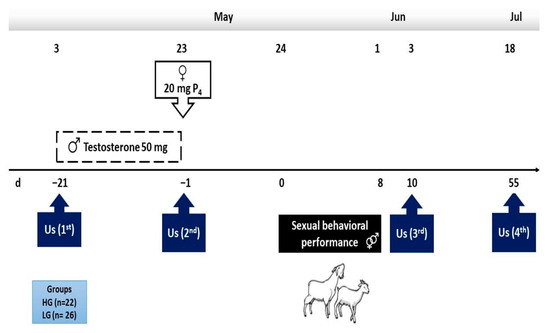
Figure 1.
Schematic representation of the experimental protocol in two experimental groups at either high (HG, n = 22) or low (LG, n = 26) body condition score in mixed-breed goats in northern Mexico under a marginal semiarid production system.
2.4. Treatments and Experimental Groups
2.4.1. Females
As mentioned, two divergently BCS groups were formed: low (LG; 1.0 ± 0.2 units; n = 26) and high (HG; 2.5 ± 0.9 units; n = 22) considering a BCS scale of 1 (emaciated) to 4 (obese), respectively, as previously proposed [18]. On day 1 of the experimental period, one dose of progesterone (20 mg, i.m.) was administered to each female in both experimental groups (Progestelas “E”, Lab Aranda, Mexico City, Mexico) to inhibit the possible presence of short cycles (Figure 1).
2.4.2. Males
Healthy adult mixed-bred bucks (n = 4; BCS = 2.5 units) of known libido and fertility were used to mate the goats during the experimental breeding. Twenty-one days prior to mating, the bucks were separated from the females, and received 50 mg i.m. of testosterone (Testosterone 50, Lab. Brovel®, Mexico City, Mexico) every third day for three weeks (i.e., 3–23 May), as proposed by Luna-Orozco et al. [19]. At d0 (day zero; 24 May) one male within the experimental group (i.e., HG or LG) was introduced at nighttime to each experimental group and remained there overnight. Thereafter, both bucks within the treatment remained in the pens while the females were conducted to the rangeland during the day (Figure 1).
2.5. Response Variables
2.5.1. Sexual Behaviors of Males and Females
The sexual behavioral performance of both females and males were registered daily (i.e., 7:00–7:30 am and 8:30–9:00 pm, respectively) for 8 d using an ethogram. To avoid bias, bucks within treatments were exchanged daily on alternate days; the number of exerted sexual behaviors by both genders were registered (i.e., male-to-female and female-to-male, respectively). Pens between treatments were separated 15 m apart and surrounded by black plastic (i.e., 1.8 m high) to avoid any possible visual contact between females’ experimental groups. The sexual behavioral variables of bucks, either appetitive or consummatory were considered, such as anogenital sniffing, and approaches (i.e., appetitive), as well as mounting attempts and mounting with ejaculation (i.e., consummatory). Regarding the female sexual behavior, the response variables involved flight, approach to the male, and tail wagging. A goat was considered in heat when it remained motionless at the time of copulation [20].
2.5.2. Estrus Response, Follicular Growth, and Ovulation
Throughout the 8 d sex behavioral test, the number of goats presenting estrus was quantified (a.m. and p.m.). Furthermore the interval to estrus (introduction of the males to time of estrus), duration of estrus (h), and the estrus-ovulation interval (h), were all registered. Twenty-four hours before d0, a transrectal ultrasonography was performed to confirm the anovulatory status; both ovaries and ovarian structures were scanned as referred by Ginther and Kot [21]. All follicles ≥2.0 mm was recorded. A goat was considered to have ovulated when the largest follicles recorded the previous day had disappeared. In addition, follicles were categorized as small (2–3.4 mm), medium (3.5–4.9 mm), and large (≥5 mm), respectively. Additionally, the proportion of ovulating goats and the ovulatory rate were determined by categorizing the type of ovulation. The diameter of each follicle was determined using the following formula: d = r (2), where r = (L/2 + A/2)/2, where L = length, and A = width. Additionally, the luteal tissue volume was calculated using the following formula: V = 4/3 × π × r3 [22].
2.5.3. Gestation, Births, and Prolificacy
Pregnancy was diagnosed 45 d after ovulation using transrectal ultrasonography. In addition, the kidding rate (i.e., goats that gave birth), prolificacy (i.e., number of kids born alive), and pregnancy rate were all also determined.
2.6. Statistical Analyzes
The response variables follicular and corpus luteum number and their diameter, follicular and luteal volume, the hour intervals between the introduction of the bucks and the onset of estrus, as well as from estrus to ovulation, along with estrus duration, ovulation occurrence relative to the estrus onset, ovulation rate, number of kids born alive, and prolificacy were all evaluated with a student’s t-test. The number of the male sexual behaviors, either appetitive and consummatory, and those exerted by females between experimental groups, the proportion of estrus females, ovulating females, ovulation rates (simple, double, triple, and quadruple), pregnancy rate at d45, fetal mortality rate, and goats kidding were compared with a chi-square test. Since all the response variables measured in females and males were individually quantified, the animal within treatment was considered as the experimental unit. All analyzes were performed with the SPSS-statistical package (ver. 24). While the most conservative SE was presented, the threshold to declare statistical significance was set at 0.05.
3. Results
The research outcomes obtained from the study support our working hypothesis as higher BCS goats denoted an increased number of sexual behaviors either in the male-to-female and (or) the female-to-male contact, concomitant to increases in the estrus and ovulatory response. Moreover, HG-goats showed not only an augmented ovulatory response, but larger ovarian structures, an increased ovulation rate, and better general reproductive outcomes (i.e., pregnancy rate, kidding rate, and prolificacy).
3.1. Male-to-Female and Female-to-Male Sexual Behaviors
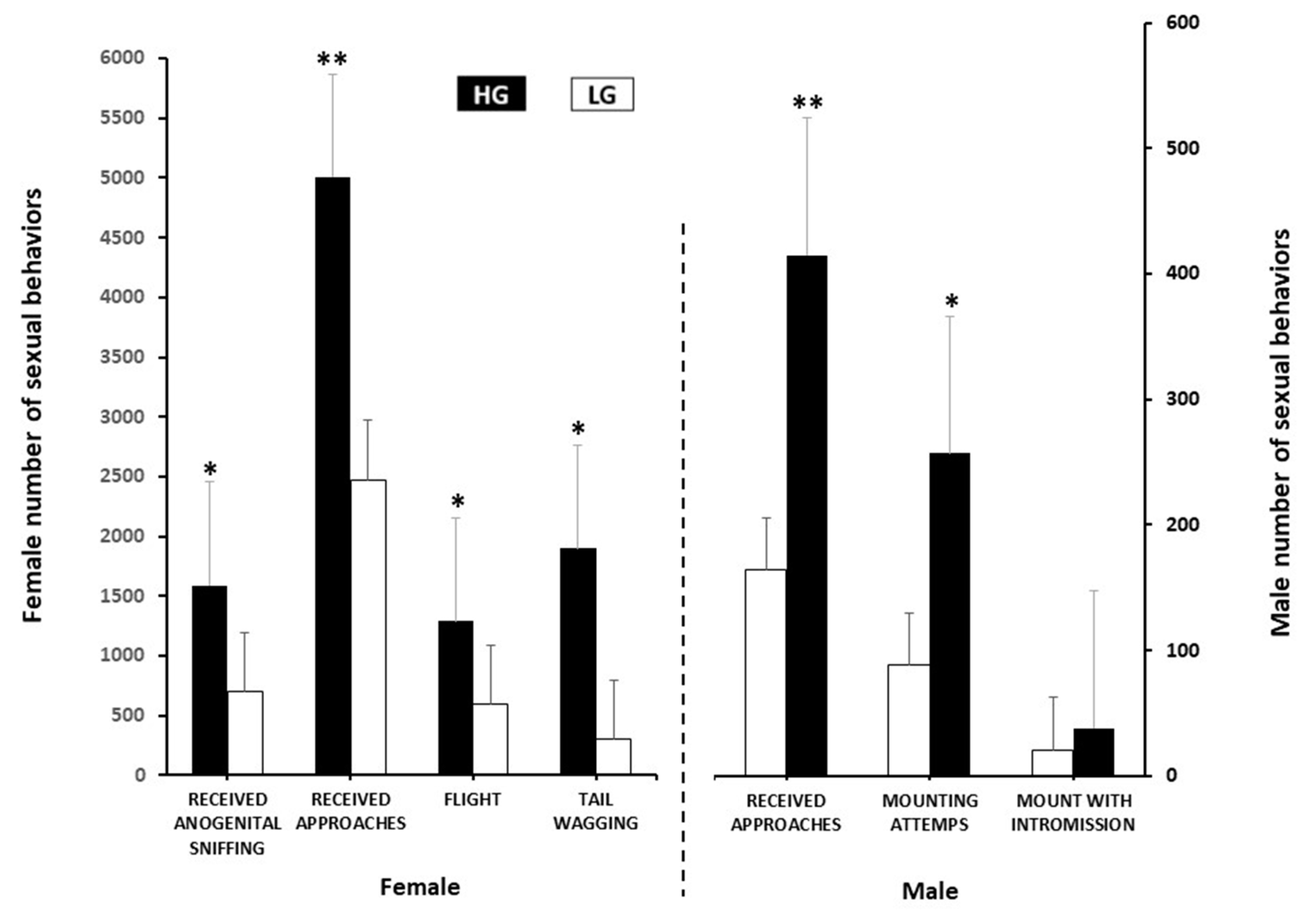
Those goats accepting anogenital sniffing, and showing flight, and tail wagging differed (p < 0.05) between groups, favoring to the HG group. The same was true regarding the male-to-female sexual interaction; both approaches to females and mounting attempts from bucks (p < 0.01) were higher in the bucks exposed to the HG goats. Regarding the variables estrus response and mounts with intromission no significant differences occurred (p > 0.05) between the groups (Figure 2).
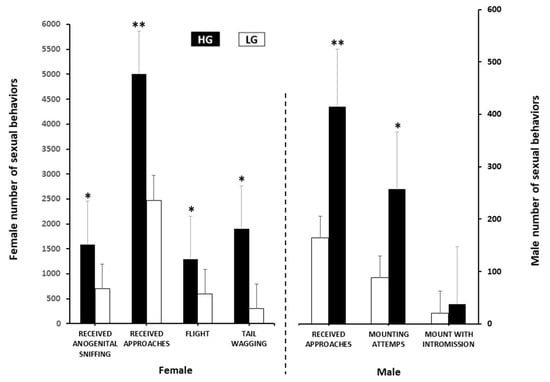
Figure 2.
Female and male sexual behaviors (number ± SEM) in two experimental groups at either high (HG, n = 22) or low (LG, n = 26) body condition score in mixed-breed goats in northern Mexico under a marginal semiarid production system. * Indicates difference between groups: (p ≤ 0.05); ** (p ≤ 0.01).
3.2. Ovarian Structures
The diameter of follicles did not differ (p > 0.05) between groups, with an average of 4.9 ± 0.3 mm. The same was true regarding the follicular size (p > 0.05), with averages (mm) of 24.5 ± 0.05, 10 ± 0.05, and 38.5 ± 0.2 units for small, medium, and large follicles, respectively (Table 1).

Table 1.
Means ± standard error of the mean (SEM) regarding follicular characteristics in two experimental groups at either high (HG n = 22) or low (LG n = 26) body condition score in mixed-breed goats in northern Mexico under a marginal semiarid production system.
3.3. Females Ovulating, Estrus Performance, Ovulation Rate, and Luteal Structures
Regarding the proportion of females ovulating, statistically significant differences emerged from not only in this variable (p < 0.05; Table 2) (i.e., almost two-fold), but also in the corpus luteum number (i.e., more than two-fold), ovulation rate (i.e., almost three-fold), corpus luteum diameter (i.e., more than two-fold) and the luteal tissue volume (i.e., almost three-fold). A statistical trend in the percentage of females presenting estrus occurred (p = 0.08). The interval to estrus, estrus latency, estrus-to-ovulation interval, and the proportion of short cycles, did not significantly differ (p > 0.05) between the HG and LG groups. The same was true (p > 0.05) regarding the type of ovulation; either single, double, triple, and quadruple ovulation (Table 2). Interestingly, however, when we added the double, triple and quadruple ovulations, which were referred as multiples, and then compared with the single ovulation (i.e., single vs. multiple) between these groups, a trend (p = 0.06) for higher values occurred in the HG-goats.

Table 2.
Least squares mean ± standard error for estrus response, and luteogenesis, in two experimental groups at either high (HG, n = 22) or low (LG, n = 26) body condition score in mixed-breed goats in northern Mexico under a marginal semiarid production system.
3.4. Pregnant Females, Fetal Mortality Rate, Goats Kidding and Prolificacy
Finally, and in a quite interesting fashion, while no significant differences (p > 0.05) in fetal losses and prolificacy occurred between both groups, both the number and percentage of pregnant females at d45, and goats kidding, as well as the total number of kids born alive significantly differed (p < 0.05) between experimental groups, favoring to the HG-goats (Table 3).

Table 3.
Frequency and mean ± standard error of several reproductive outcomes at kidding in two experimental groups at either high (HG n = 22) or low (LG n = 26) body condition score in mixed-breed goats in northern Mexico under a marginal semiarid production system.
4. Discussion
Our working hypothesis stated that bucks exposed to high BCS-females (i.e., HG goats) exert more frequent sexual behaviors, and that such an increased BCS also positively influences the diameter and the volume of some ovarian structures aligned with augmented reproductive outcomes (i.e., kidding rate) regarding low-BCS goats. Based on the observed general research outcomes, such a working hypothesis can not be rejected.
4.1. Male and Female Sexual Behaviors
Females receiving anogenital sniffing and showing flight and tail wagging favored to the HG goats. Additionally, the number of male sexual behaviors were higher when bucks were exposed to the HG goats. These data confirm that the female-BCS is a key factor influencing the performance of the sexual behavior not only of the better BCS-goats, but also in those bucks exposed to the HG-goats. The remarkable difference in the number of sexual behaviors of both females and males indicates that the higher BCS in females leads to a greater display of appetitive and consummatory sexual behaviors by the males, as well as a greater proceptivity and receptivity in the females [23]. Interestingly, the “shape” of the female body has shown to influence the sexual behavior of males, as well as the context of the interaction [24], with the proceptive behavior being defined as the most influential cue in the female-to-male interaction. In females from most mammalian species, an increase in motor activity has been related to estrus. Sexual feedback consists of three main phases: attraction, proceptivity, and receptivity [23]. The latter can be measured by counting the number of mounts or mount attempts that the female receives from the buck [23,25]. Regarding the estrus response, no significant differences between experimental groups were observed. In this respect, Mellado et al. mentioned that goats managed under rangeland conditions, and even when at low body energy reserves, they responded to the buck stimulus, presenting estrus and ovulation [26].
4.2. Ovarian Structures
No significant differences regarding the follicular size and diameter occurred between the experimental animals, irrespective of the size classification (i.e., small, medium, and large; Table 1). The latter agree with Widiyono et al. when evaluating the influence of the BCS in three sized-ovarian follicles goat groups [27]. In our study, the highest follicular volume occurred in the HG group (Table 1), in line with Widiyono, who reported that an increase in the number and development of ovarian follicles can be expected in high BCS-females.
Larger ovulation rates are essential for higher prolificacy in herds irrespective of the production systems [28]. In our latitude (26° NL), the nutritional status is a limiting factor in the reproductive outcomes of goats. In this study, the variables associated with this parameter were higher in the HG goats, meaning it can be inferred that body energy reserves directly influence the reproductive fitness. The last, agreeing with other studies where the ovulation rate was greater in females with a higher BCS than their counterparts [8,29]. Regarding estrus behavior, a statistical trend in the percentage of estrus’ females (p = 0.08) occurred. However, the response variables interval to estrus, duration of estrus, estrus-to-ovulation interval, and the proportion of short cycles did not significantly differ between the groups. The latter is in agreement with Mellado [6], and De Santiago-Miramontes et al., who reported that even goats with a poor BCS “responded” to the buck’s stimulus, although such a response was delayed and lower than in high BCS-goats [7].
4.3. Females Ovulating, Luteal Structures (i.e., Number and Diameter), and Prolificacy
As mentioned previously, differences occurred in the proportion of females ovulating, the total number of corpus luteum, the ovulation rate, the corpus luteum diameter, and the volume of luteal tissue, favoring to the HG-goats. Interestingly, all these response variables are closely related to the embryo implantation process, which requires a synergy between these ovarian structures, the uterus, and the conceptus [30]. In fact, the interactions among them notably influence the constant secretion of progesterone by the corpus luteum [31]. The last enhances the success of pregnancy during the first weeks of pregnancy [32]. These results could explain why twice as many females were diagnosed pregnant at day 45, a 42% superiority of pregnant goats in the HG group, and that this group registered three-fold as many kids born alive. The last differed from the findings reported by Mellado and Pastor [33], probably because the goats in our study did cover the threshold feeding requirements in the last third of gestation, which coincided with the rainy season and therefore with a more and better biomass availability. As for the greater pregnancy rate, kidding rate, and a greater total number of kids born alive in the HG-goats, Kenyon et al. documented the positive relationship between an adequate BCS with respect to fertility, pregnancy rate, and delivery rate [34]. Regarding the pregnancy rate, our results are consistent with those reported by Molina et al. in La Mancha goats [35], by Atti et al. in Barbarine sheep [36], as well as in Merino sheep [37]. Furthermore, Kenyon et al. reported that Romney ewes with a BCS of 2.0 were more likely to get pregnant than ewes with a lower BCS [38].
Additionally, Hussain et al. reported that a low BCS causes decreased fertility in females [39]. According to Mellado et al., the kidding rates of goats with a low BCS (i.e., three (range 1–7)) were about 20% lower than goats with a BCS higher than four [40]. Goats with a higher BCS, in addition to their larger size, stimulate increased sexual behavior in bucks, and are positively related with a higher ovulation rate and a larger luteal diameter and volume [12]. Such a scenario can promote increased progesterone production [31] and should contribute to the maintenance of pregnancy and a greater number of live-born kids. However, in our latitudes, reproductive management was carried out in the dry season due to the convenience of a higher price in the sale of kids, and therefore, it is necessary to consider a strategy of nutritional supplementation in both males and females to cover the demanding characteristic needs of the reproductive process. The last would counteract the significant nutrient deficiency that affects the embryonic implantation process during this critical period of the pregnancy stage.
5. Conclusions
Our data confirm that body reserves positively affected the sexual behavior not only in bucks exposed to the HG-goats, but also a better sexual performance in the HG-goats, as well as on the size of the ovarian structures and several reproductive outcomes. Certainly, a higher BCS improved the sexual interaction between females and males, as well as both the estrus and ovulatory responses. Additionally, better reproductive outcomes, such as the pregnancy rate, kidding rate, and an augmented number of the kids born alive occurred in the HG group. Therefore, our results indicate that BCS is central in this marginal goat production system to guarantee reproductive success. Additionally, BCS is a useful tool to define as to whether nutritional supplementation to goats on rangeland is required. The latter would have positive repercussions not only on the reproductive fitness, but also a higher income of goat farmers and their families, especially under marginal production systems.
Author Contributions
Conceptualization, A.D.S.-M., M.M. and C.A.M.-H.; Data curation, C.A.M.-H., A.D.S.-M. and A.S.-A.; Formal analysis, C.A.M.-H. and A.D.S.-M.; Funding acquisition, J.M.F.-S.; Investigation, F.G.V.-D., A.D.S.-M., A.S.-A. and J.A.B.-A.; Methodology, A.D.S.-M., A.S.-A. and M.M.; Project administration, F.G.V.-D., J.M.F.-S. and F.A.-R.; Resources, F.G.V.-D., J.A.B.-A., J.M.F.-S. and F.A.-R.; Software, F.A.-R.; Supervision, A.D.S.-M.; Validation, M.M.; Writing—original draft, C.A.M.-H., A.D.S.-M. and J.A.B.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
All of the experimental procedures were completed in accordance with the recommendations for ethical use, care, and welfare of animals in research at global (USA) and national (Mexico) levels, and they were institutionally authorized by UAAAN; the animal study protocol was authorized by the Institutional Review Board, with approval reference number: UAAAN-UL-21-425502002-2850.
Informed Consent Statement
Not applicable.
Data Availability Statement
None of the data were deposited in an official repository, yet, information can be made available upon request.
Acknowledgments
The authors thank Horacio Hernández and his family for providing their herd to carry out this study, and also Abril Ramírez, Javier Valencia, and Julia Morales for their technical assistance. We also acknowledge CONAHCYT Mexico for the scholarship awarded to Alejandro Santos.
Conflicts of Interest
The authors declare that there are no conflicts of interest that could be perceived as prejudicing the impartiality of the research reported in this manuscript. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Servicio de Informacion Agroalimentaria y Pesquera (SIAP)—Agrifood and Fishery Information System. Resumen Nacional. Población Ganadera, Avícola y Apícola. SAGARPA. 2021. Available online: http://www.siap.gob.mx/ganaderia (accessed on 30 April 2022).
- Navarrete-Molina, C.; Meza-Herrera, C.A.; Herrera-Machuca, M.A.; Macias-Cruz, U.; Veliz-Deras, F.G. Not All Ruminants Were Created Equal: Environmental and Socio-Economic Sustainability of Goats Under a Marginal-Extensive Production System. J. Clean. Prod. 2020, 255, 120237. [Google Scholar] [CrossRef]
- Álvarez, L.; Zarco, L. Los fenómenos de bioestimulación sexual en ovejas y cabras. Vet. México 2001, 32, 117–129. [Google Scholar]
- Duarte, M.G. Estacionalidad Reproductiva y Efecto del Fotoperiodo Sobre la Actividad Ovulatoria de las Hembras Caprinas Criollas de la Comarca Lagunera. Ph.D. Thesis, Universidad Nacional Autónoma de México, Mexico City, Mexico, 2000. [Google Scholar]
- Meza-Herrera, C.A.; Enríquez, R.Z.; González, H.S. Actividad Ovárica en Cabras Expuestas a un Fotoperíodo Natural Creciente en la Comarca Lagunera. Rev. Chapingo Ser. Zonas Áridas 2009, 8, 69–74. [Google Scholar]
- Mellado, M. Técnicas para el Manejo Reproductivo de las Cabras en Agostadero (Goat Reproductive Management Under Rangeland Conditions). Trop. Subtrop. Agroecosystems 2008, 9, 47–63. [Google Scholar]
- De Santiago-Miramontes, M.A.; Rivas-Muñoz, R.; Muñoz-Gutiérrez, M.; Malpaux, B.; Scaramuzzi, R.J.; Delgadillo, J.A. The Ovulation Rate in Anoestrous Female Goats Managed Under Grazing Conditions and Exposed to the Male Effect is Increased by Nutritional Supplementation. Anim. Reprod. Sci. 2008, 105, 409–416. [Google Scholar] [CrossRef]
- De Santiago-Miramontes, M.A.; Malpaux, B.; Delgadillo, J.A. Body Condition is Sociated with a Shorter Breeding Season and Reduced Ovulation Rate in Subtropical Goats. Anim. Reprod. Sci. 2009, 114, 175–182. [Google Scholar] [CrossRef]
- Fitz-Rodríguez, G.; De Santiago-Miramontes, M.A.; Scaramuzzi, R.J.; Malpaux, B.; Delgadillo, J.A. Nutritional Supplementation Improves Ovulation and Pregnancy Rates in Female Goats Managed Under Natural Grazing Conditions and Exposed to the Male Effect. Anim. Reprod. Sci. 2009, 116, 85–94. [Google Scholar] [CrossRef]
- Hafez, Y.H.; Khalifa, E.I.; El-Shafie, M.H.; Khalek, T.M.M.A.; Ahmed, M.E.; Shehata, E.I. Effect of Energy Flushing Premating and During Mating Season on Production and Reproduction Performance of Zaraibi Goats. Egypt. J. Sheep Goats Sci. 2011, 6, 7–14. [Google Scholar]
- Fabre-Nys, C. Le Comportement Sexuel des Caprins: Contrôle Hormonal et Facteurs Sociaux. INRA Prod. Anim. 2000, 13, 11–13. [Google Scholar] [CrossRef]
- Zuñiga-Garcia, S.; Meza-Herrera, C.A.; Mendoza-Cortina, A.; Perez-Marin, C.; Lopez-Flores, N.M.; Guillén-Muñoz, J.M.; López-Villalobos, N. Does Size Matters? Relationships among Social Dominance and Some Morphometric Traits upon Out-of-Season Reproductive Outcomes in Anestrus Dairy Goats Treated with P4+ eCG. Biology 2020, 9, 354. [Google Scholar] [CrossRef]
- Federation Animal Science Society (FASS). Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching, 3rd ed.; Federation Animal Science Society: Champaing, IL, USA, 2010; p. 177. [Google Scholar]
- National Academy of Medicine (NAM). Guide for the Care and Use of Laboratory Animals. In Co-Produced by the National Academy of Medicine-Mexico and the Association for Assessment and Accreditation of Laboratory Animal Care International, 1st ed.; Harlan: Mexico City, Mexico, 2002. [Google Scholar]
- Instituto Nacional de Estadística y Geografía (INEGI). Información Nacional por Entidad Federativa y Municipios. 2015. Available online: http://www.inegi.org.mx/sistemas/mexicocifras/default.aspx (accessed on 19 February 2022).
- Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP). Boletín Técnico. Coeficientes de Agostadero de la República Mexicana: Estados de Baja California, Sonora, Chihuahua, Zacatecas, Coahuila, Tamaulipas, Nuevo León, Durango y San Luis Potosí. SAG. México. 2010. Available online: http://www.gob.mx/inifap/archivo/documentos/coeficientesdeagostadero (accessed on 11 March 2022).
- Delgadillo, J.A.; Canedo, G.A.; Chemineau, P.; Guillaume, D.; Malpaux, B. Evidence for an annual reproductive rhythm independent of food availability in male Creole goats in subtropical northern Mexico. Theriogenology 1999, 52, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Walkden-Brown, S.W.; Restall, B.J.; Scaramuzzi, R.J.; Martin, G.B.; Blackberry, M.A. Seasonality in Male Australian Cashmere Goats: Long Term Effects of Castration and Testosterone or Oestradiol Treatment on Changes in LH, FSH and Prolactin Concentrations, and Body Growth. Small Rumin. Res. 1997, 26, 239–252. [Google Scholar] [CrossRef]
- Luna-Orozco, J.R.; Guillen-Muñoz, J.M.; De Santiago-Miramontes, M.A.; García, J.E.; Rodríguez-Martínez, R.; Meza-Herrera, C.A.; Mellado, M.; Véliz, F.G. Influence of Sexually Inactive Bucks Subjected to Long Photoperiod or Testosterone on the Induction of Estrus in Anovulatory Goats. Trop. Anim. Health Prod. 2012, 44, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Chemineau, P.; Daveau, A.; Maurice, F.; Delgadillo, J.A. Seasonality of Estrus and Ovulation is Not Modified by Subjecting Female Alpine Goats to a Tropical Photoperiod. Small Rumin. Res. 1992, 8, 299–312. [Google Scholar] [CrossRef]
- Ginther, O.; Kot, K. Follicular Dynamics During the Ovulatory Season in Goats. Theriogenology 1994, 42, 987–1001. [Google Scholar] [CrossRef]
- Alvarado-Espino, A.S.; Menchaca, A.; Meza-Herrera, C.A.; Carrillo-Moreno, D.I.; Zúñiga-García, S.; Arellano-Rodríguez, F.; Mellado, M.; Véliz, F.G. Ovarian response is not affected by the stage of seasonal anestrus or breed of goats when using a progesterone injection plus human chorionic gonadotropin-based protocol. Anim. Reprod. Sci. 2019, 204, 60–65. [Google Scholar] [CrossRef]
- Fabre-Nys, C.; Gelez, H. Sexual Behavior in Ewes and Other Domestic Ruminants. Horm. Behav. 2007, 52, 18–25. [Google Scholar] [CrossRef]
- Tilbrook, A.J.; Cameron, A.W.N.; Lindsay, D.R. The Influence of Ram Mating Preferences and Social Interaction Between Rams on The Proportion of Ewes Mated at Field Joining. Appl. Anim. Behav. Sci. 1987, 18, 173–184. [Google Scholar] [CrossRef]
- Beach, F.A. Sexual Attractivity, Proceptivity, and Receptivity in Female Mammals. Horm. Behav. 1976, 7, 105–138. [Google Scholar] [CrossRef]
- Mellado, M.; Valdez, R.; Lara, L.M.; García, J.E. Risk Factors Involved in Conception, Abortion, and Kidding Rates of Goats Under Extensive Conditions. Small Rumin. Res. 2004, 55, 191–198. [Google Scholar] [CrossRef]
- Widiyono, I.; Sarmin, S.; Yanuartono, Y. Influence of Body Condition Score on the Metabolic and Reproductive Status of Adult Female Kacang Goats. J. App. Anim. Res. 2020, 48, 201–206. [Google Scholar] [CrossRef]
- Meza-Herrera, C.A.; Cano-Villegas, O.; Flores-Hernandez, A.; Veliz-Deras, F.G.; Calderon-Leyva, G.; Guillen-Muñoz, J.M.; García de la Peña, C.; Rosales-Nieto, C.A.; Macias-Cruz, U.; Avendaño-Reyes, L. Reproductive Outcomes of Anestrous Goats Supplemented with Spineless Opuntia megacantha Salm-Dyck Protein-Enriched Cladodes and Exposed to the Male Effect. Trop. Anim. Health Prod. 2017, 49, 1511–1516. [Google Scholar] [CrossRef] [PubMed]
- Scaramuzzi, R.J.; Campbell, B.K.; Downing, J.A.; Kendall, N.R.; Khalid, M.; Muñoz-Gutiérrez, M.; Somchit, A. A Review of the Effects of Supplementary Nutrition in the Ewe on the Concentrations of Reproductive and Metabolic Hormones and the Mechanisms that Regulate Folliculogenesis and Ovulation Rate. Reprod. Nutr. Dev. 2006, 46, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Meza-Herrera, C.A.; Santamaría-Estrada, C.E.; Flores-Hernández, A.; Cano-Villegas, O.; De la Peña, C.G.; Macias-Cruz, U.; Calderón-Leyva, G.; Ángel-García, O.; Mellado, M.; Carrillo-Moreno, D.; et al. The Opuntia Effect Upon the Out-Of-Season Embryo Implantation Rate in Goats: Corpus Luteal Number, Corpus Luteal Diameter and Serum Progesterone Concentrations. Livest. Sci. 2019, 228, 201–206. [Google Scholar] [CrossRef]
- Rostami, B.; Hajizadeh, R.; Shahir, M.H.; Aliyari, D. The Effect of Post-mating hCG or Progesterone Administration on Reproductive Performance of Afshari× Booroola-Merino Crossbred Ewes. Trop. Anim. Health Prod. 2017, 49, 245–250. [Google Scholar] [CrossRef]
- Brooks, K.; Burns, G.; Spencer, T.E. Conceptus elongation in ruminants: Roles of progesterone, prostaglandin, interferon tau and cortisol. J. Anim. Sci. Biotechnol. 2014, 16, 50–53. [Google Scholar] [CrossRef]
- Mellado, M.; Pastor, F.J. Aborto no Infeccioso en Caprinos. Cienc. Anim. Bras. 2006, 7, 167–175. [Google Scholar]
- Kenyon, P.R.; Maloney, S.K.; Blache, D. Review of Sheep Body Condition Score in Relation to Production Characteristics. N. Z. J. Agric. Res. 2014, 57, 38–64. [Google Scholar] [CrossRef]
- Molina, A.; Gallego, L.; Torres, A.; Vergara, H. Effect of mating season and level of body reserves on fertility and prolificacy of Manchega ewes. Small Rumin. Res. 1994, 14, 209–217. [Google Scholar] [CrossRef]
- Atti, N.; Thériez, M.; Abdennebi, L. Relationship Between Ewe Body Condition at Mating and Reproductive Performance in the Fat-Tailed Barbarine Breed. Anim. Res. 2001, 50, 135–144. [Google Scholar] [CrossRef]
- Kleemann, D.O.; Walker, S.K. Fertility in South Australian Commercial Merino Flocks: Sources of Reproductive Wastage. Theriogenology 2005, 63, 2075–2088. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, P.R.; Morel, P.C.H.; Morris, S.T. The Effect of Individual Liveweight and Condition Scores of Ewes at Mating on Reproductive and Scanning Performance. N. Z. Vet. J. 2004, 52, 230–235. [Google Scholar] [CrossRef]
- Hussain, Q.; Havrevoll, Ø.; Eik, L.O.; Ropstad, E. Efectos de la ingesta de energía sobre la glucosa plasmática, los ácidos grasos no esterificados y la concentración de acetoacetato en cabras preñadas. Investig. Sobre Pequeños Ruminates 1996, 21, 89–96. [Google Scholar]
- Mellado, M.; Cantú, L.; Suárez, J.E. Effects of Body Condition, Length of Breeding Period, Buck: Doe Ratio, and Month of Breeding on Kidding Rates in Goats Under Extensive Conditions in Arid Zones of Mexico. Small Rumin. Res. 1996, 23, 29–35. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).