Photosynthetic Efficiency and Antioxidative Response of Soybean Exposed to Selective Herbicides: A Field Study
Abstract
1. Introduction
2. Materials and Methods
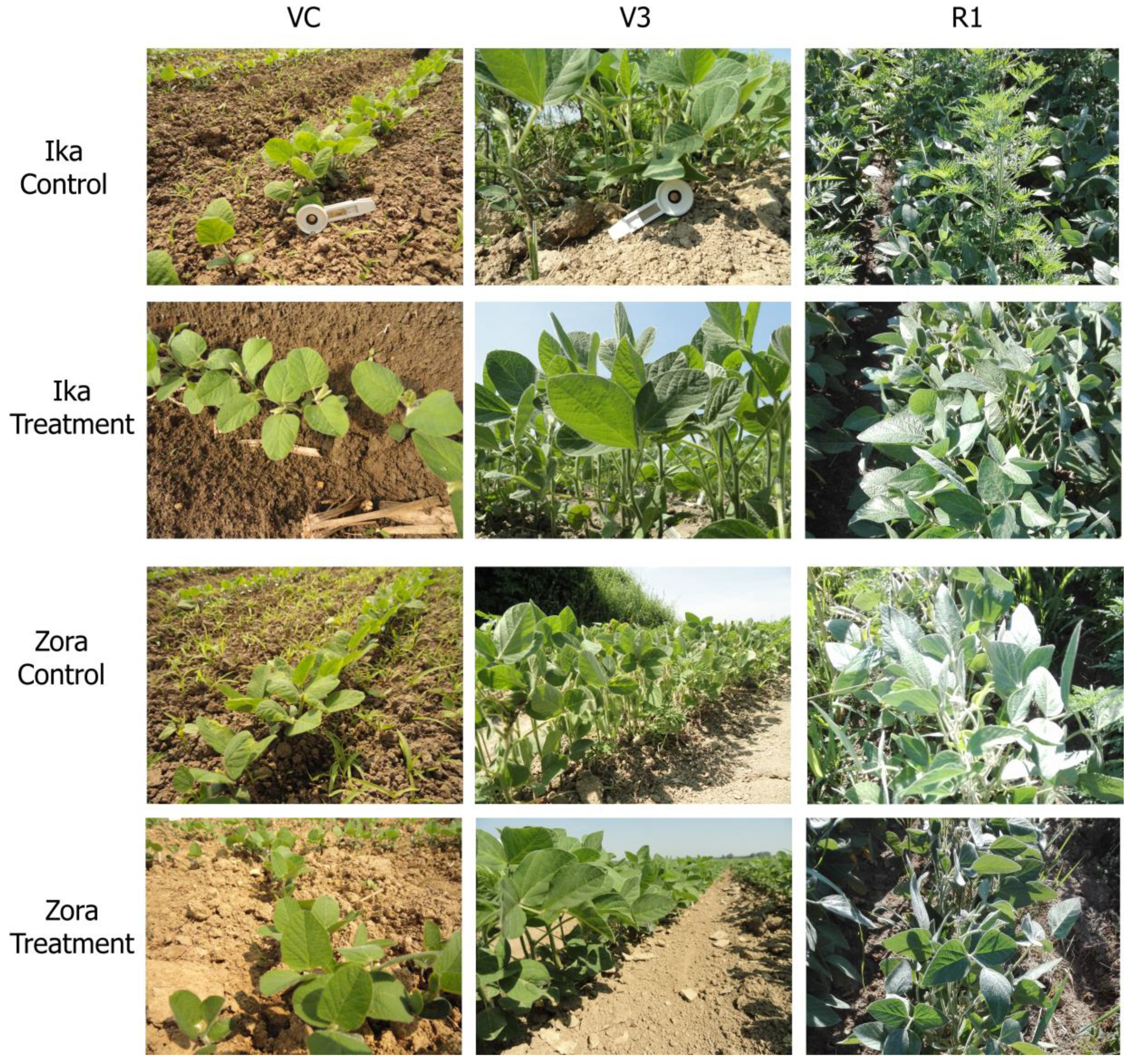
2.1. Plant Meterial
2.2. Herbicide Treatments
2.3. Biochemical Analyses
2.4. Photosynthetic Pigments Determination
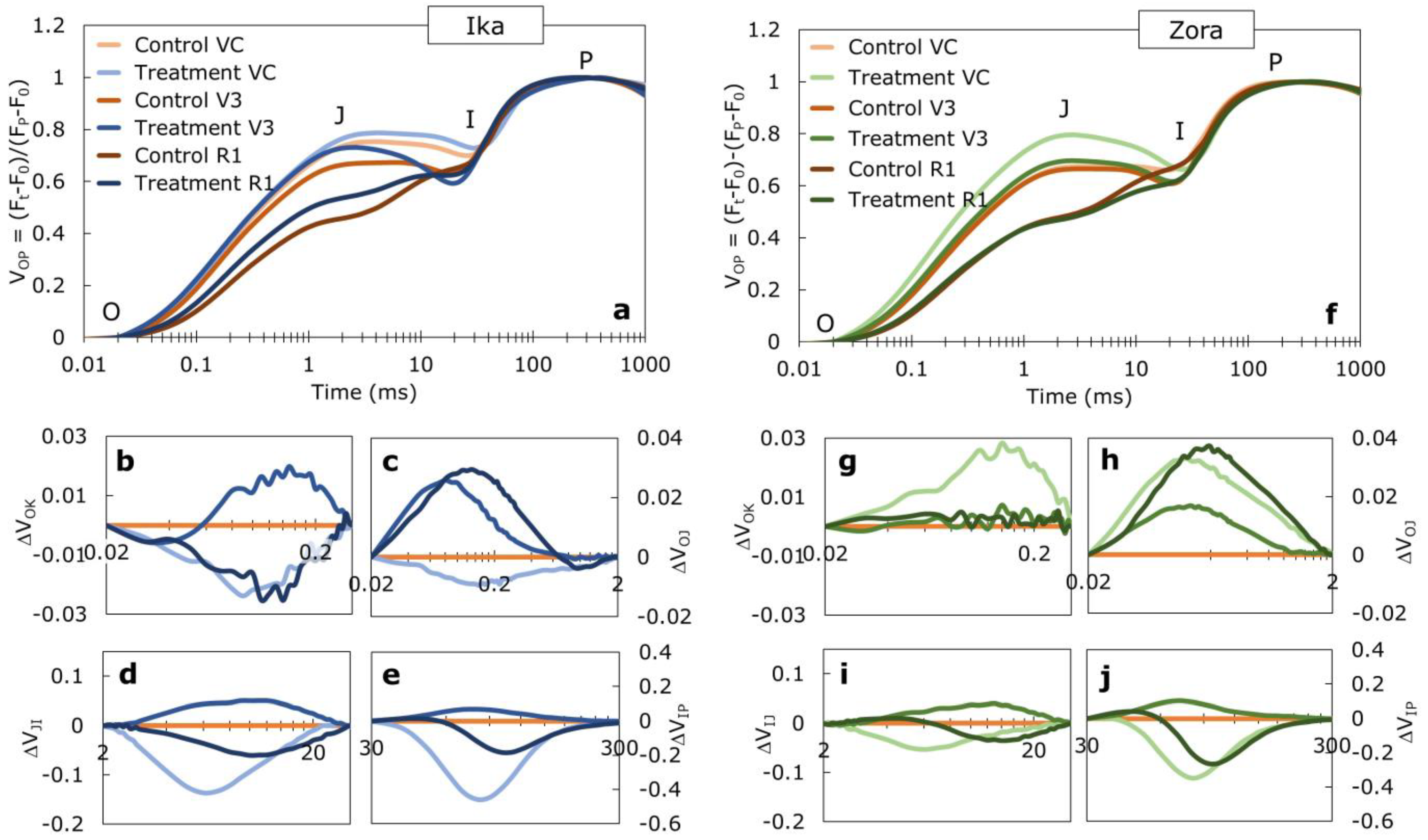
2.5. Chlorophyll a Fluorescence Measurements
2.6. Statistical Analysis
3. Results
3.1. Antioxidative Response after Herbicide Treatments
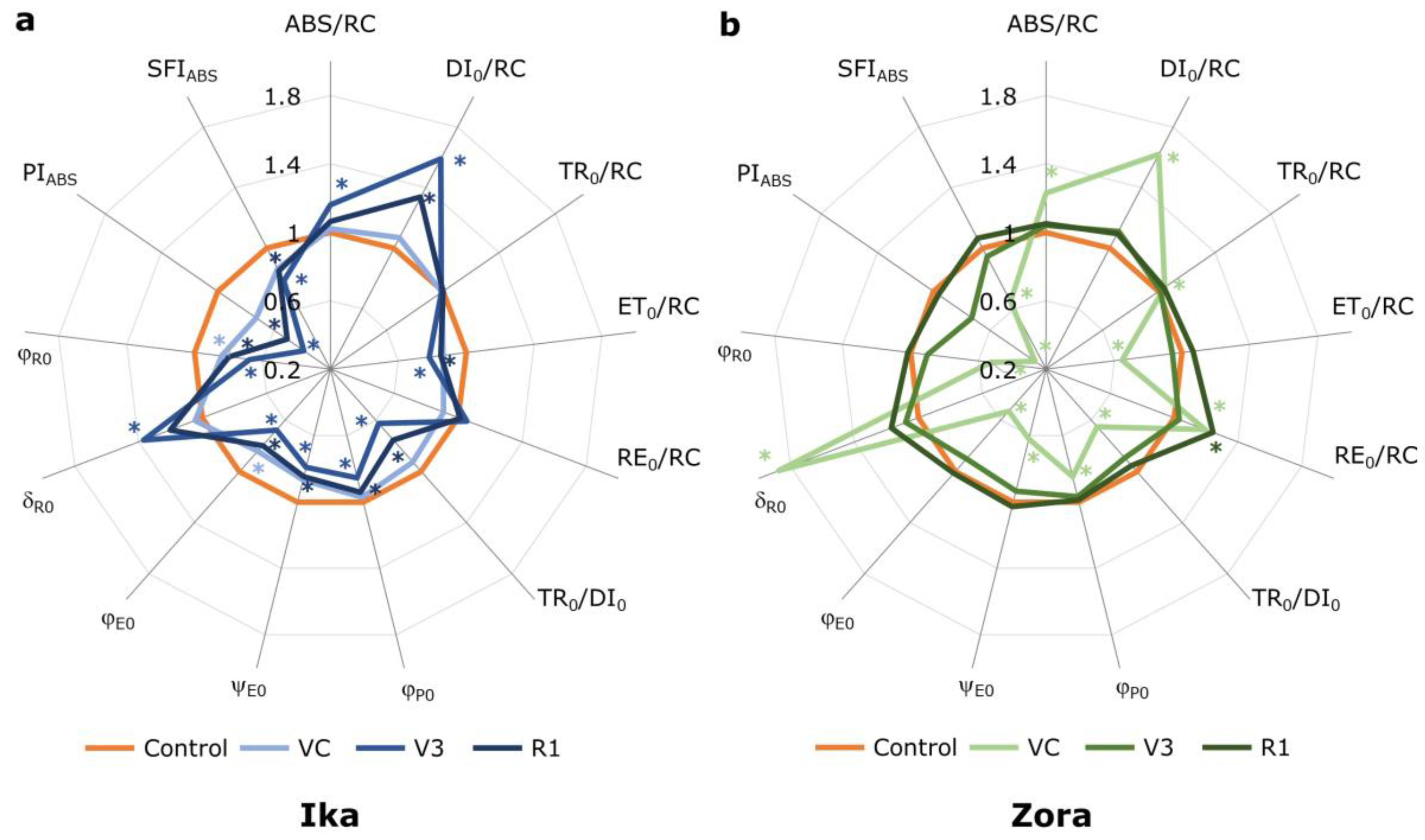
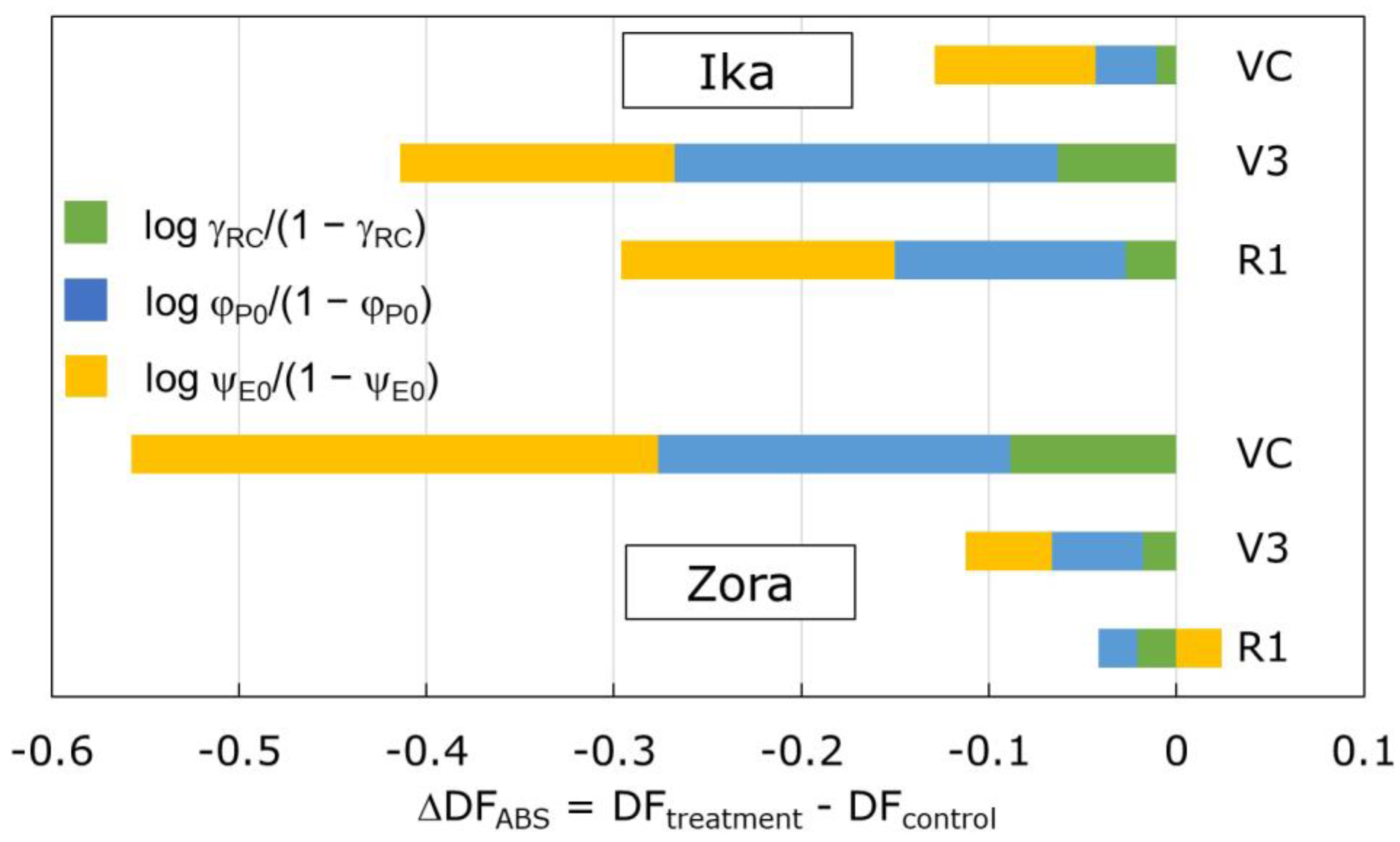
3.2. Effect of Herbicide Treatment on PSII Functioning
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pagano, M.C.; Miransari, M. The Importance of Soybean Production Worldwide. In Abiotic and Biotic Stresses in Soybean Production; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–26. [Google Scholar]
- Shea, Z.; Singer, W.M.; Zhang, B. Soybean Production, Versatility, and Improvement. In Legume Crops-Prospects, Production and Uses; IntechOpen: London, UK, 2020; pp. 29–50. [Google Scholar]
- Adamič, S.; Leskovšek, R. Soybean (Glycine max (L.) Merr.) Growth, Yield, and Nodulation in the Early Transition Period from Conventional Tillage to Conservation and No-Tillage Systems. Agronomy 2021, 11, 2477. [Google Scholar] [CrossRef]
- Sobko, O.; Stahl, A.; Hahn, V.; Zikeli, S.; Claupein, W.; Gruber, S. Environmental Effects on Soybean (Glycine max (L.) Merr) Production in Central and South Germany. Agronomy 2020, 10, 1847. [Google Scholar] [CrossRef]
- Fehr, W.; Caviness, C. Stages of Soybean Development. In Special Report No. 80; Coperative Extension Service Agriculture and Home Economics Experiment Station, Iowa State University: Ames, IA, USA, 1977. [Google Scholar]
- Kim, E.; Hwang, S.; Lee, I. SoyNet: A Database of Co-Functional Networks for Soybean Glycine Max. Nucleic Acids Res. 2017, 45, D1082–D1089. [Google Scholar] [CrossRef]
- Purcell, L.C.; Salmeron, M.; Ashlock, L. Soybean Growth and Development. Ark. Soybean Prod. Handb. 2014, 197, 1–8. [Google Scholar]
- dos Santos Ferreira, A.; Freitas, D.M.; da Silva, G.G.; Pistori, H.; Folhes, M.T. Weed Detection in Soybean Crops Using ConvNets. Comput. Electron. Agric. 2017, 143, 314–324. [Google Scholar] [CrossRef]
- Nandula, V.K. Herbicide Resistance Traits in Maize and Soybean: Current Status and Future Outlook. Plants 2019, 8, 337. [Google Scholar] [CrossRef]
- Duke, S.O. Overview of Herbicide Mechanisms of Action. Environ. Health Perspect. 1990, 87, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Fedtke, C. Approaches to and Definitions of the Mechanisms of Action of Herbicides. In Biochemistry and Physiology of Herbicide Action; Springer: Berlin/Heidelberg, Germany, 1982; pp. 1–14. [Google Scholar]
- Hassannejad, S.; Lotfi, R.; Ghafarbi, S.P.; Oukarroum, A.; Abbasi, A.; Kalaji, H.M.; Rastogi, A. Early Identification of Herbicide Modes of Action by the Use of Chlorophyll Fluorescence Measurements. Plants 2020, 9, 529. [Google Scholar] [CrossRef] [PubMed]
- Dayan, F.E.; Zaccaro, M.L.d.M. Chlorophyll Fluorescence as a Marker for Herbicide Mechanisms of Action. Pestic. Biochem. Physiol. 2012, 102, 189–197. [Google Scholar] [CrossRef]
- Manna, M.; Achary, V.M.M. ROS Signaling and Its Role in Plants. In Sensory Biology of Plants; Springer: Singapore, 2019. [Google Scholar]
- Volkov, A.G.; Mwesigwa, J. Electrochemistry of Soybean: Effects of Uncouplers, Pollutants, and Pesticides. J. Electroanal. Chem. 2001, 496, 153–157. [Google Scholar] [CrossRef]
- Das, P.K.; Bagchi, S.N. Bentazone and Bromoxynil Induce H+ and H2O2 Accumulation, and Inhibit Photosynthetic O2 Evolution in Synechococcous elongatus PCC7942. Pestic. Biochem. Physiol. 2010, 97, 256–261. [Google Scholar] [CrossRef]
- Gage, K.L.; Krausz, R.F.; Walters, S.A. Emerging Challenges for Weed Management in Herbicide−Resistant Crops. Agriculture 2019, 9, 180. [Google Scholar] [CrossRef]
- Goltsev, V.; Kalaji, H.; Paunov, M.; Bąba, W.; Horaczek, T.; Mojski, J.; Kociel, H.; Allakhverdiev, S. Variable Chlorophyll Fluorescence and Its Use for Assessing Physiological Condition of Plant Photosynthetic Apparatus. Russ. J. Plant Physiol. 2016, 63, 869–893. [Google Scholar] [CrossRef]
- Rastogi, A.; Kovar, M.; He, X.; Zivcak, M.; Kataria, S.; Kalaji, H.; Skalicky, M.; Ibrahimova, U.; Hussain, S.; Mbarki, S. JIP-Test as a Tool to Identify Salinity Tolerance in Sweet Sorghum Genotypes. Photosynthetica 2020, 58, 333–343. [Google Scholar] [CrossRef]
- Swoczyna, T.; Kalaji, H.M.; Bussotti, F.; Mojski, J.; Pollastrini, M. Environmental Stress−What Can We Learn from Chlorophyll a Fluorescence Analysis in Woody Plants? A Review. Front. Plant Sci. 2022, 13, 1048582. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, U. Pulse-Amplitude-Modulation (PAM) Fluorometry and Saturation Pulse Method: An Overview. Chlorophyll A Fluoresc. A Signat. Photosynth. 2004, 19, 279–319. [Google Scholar]
- Khalid, M.; Raza, M.; Yu, H.; Sun, F.; Zhang, Y.; Lu, F.; Si, L.; Iqbal, N.; Khan, I.; Fu, F. Effect of Shade Treatments on Morphology, Photosynthetic and Chlorophyll Fluorescence Characteristics of Soybeans (Glycine max L. Merr.). Appl. Ecol. Environ. Res. 2019, 17. [Google Scholar] [CrossRef]
- Sun, L.; Xu, H.; Hao, H.; An, S.; Lu, C.; Wu, R.; Su, W. Effects of Bensulfuron-Methyl Residue on Photosynthesis and Chlorophyll Fluorescence in Leaves of Cucumber Seedlings. PLoS ONE 2019, 14, e0215486. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Boulahia, K.; Carol, P.; Planchais, S.; Abrous-Belbachir, O. Phaseolus vulgaris L. Seedlings Exposed to Prometryn Herbicide Contaminated Soil Trigger an Oxidative Stress Response. J. Agric. Food Chem. 2016, 64, 3150–3160. [Google Scholar]
- Caverzan, A.; Piasecki, C.; Chavarria, G.; Stewart, C.N., Jr.; Vargas, L. Defenses against ROS in Crops and Weeds: The Effects of Interference and Herbicides. Int. J. Mol. Sci. 2019, 20, 1086. [Google Scholar] [CrossRef] [PubMed]
- Piasecki, C.; Rizzardi, M.; Schons, J.; Caverzan, A.; Chavarria, G. Does the Interference of GR® Volunteer Corn Alters Stress Metabolism on Soybean? Planta Daninha 2018, 36, e018171955. [Google Scholar] [CrossRef]
- Song, J.-S.; Chung, J.-H.; Lee, K.J.; Kwon, J.; Kim, J.-W.; Im, J.-H.; Kim, D.-S. Herbicide-Based Weed Management for Soybean Production in the Far Eastern Region of Russia. Agronomy 2020, 10, 1823. [Google Scholar] [CrossRef]
- Zhang, J.J.; Lu, Y.C.; Zhang, J.J.; Tan, L.R.; Yang, H. Accumulation and Toxicological Response of Atrazine in Rice Crops. Ecotoxicol. Environ. Saf. 2014, 102, 105–112. [Google Scholar] [CrossRef]
- Jiang, L.; Yang, H. Prometryne-Induced Oxidative Stress and Impact on Antioxidant Enzymes in Wheat. Ecotoxicol. Environ. Saf. 2009, 72, 1687–1693. [Google Scholar] [CrossRef]
- Halliwell, B. Free Radicals and Other Reactive Species in Disease. In e LS; Wiley: Hoboken, NJ, USA, 2001; pp. 1–7. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Ali, L.; Jo, H.; Song, J.T.; Lee, J.-D. The Prospect of Bentazone−Tolerant Soybean for Conventional Cultivation. Agronomy 2020, 10, 1650. [Google Scholar] [CrossRef]
- Barić, K.; Ostojić, Z. Possibilities of Weed Control in Soybean. Agron. Glas. 2000, 63, 71–84. [Google Scholar]
- Zhu, J.; Patzoldt, W.L.; Radwan, O.; Tranel, P.J.; Clough, S.J. Effects of Photosystem−II−interfering Herbicides Atrazine and Bentazon on the Soybean Transcriptome. Plant Genome 2009, 2, 191–205. [Google Scholar] [CrossRef]
- Aebi, H. [13] Catalase in Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen Peroxide Is Scavenged by Ascorbate−Specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Verma, S.; Dubey, R. Lead Toxicity Induces Lipid Peroxidation and Alters the Activities of Antioxidant Enzymes in Growing Rice Plants. Plant Sci. 2003, 164, 645–655. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Choudhuri, M.A. Implications of Hydrogen Peroxide—Ascorbate System on Membrane Permeability of Water Stressed Vigna Sedlings. New Phytol. 1985, 99, 355–360. [Google Scholar] [CrossRef]
- Lichtenthaler, H. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Strasser, R.J.; Tsimilli−Michael, M.; Srivastava, A. Analysis of the Chlorophyll a Fluorescence Transient. In Chlorophyll a Fluorescence; Springer: Berlin/Heidelberg, Germany, 2004; pp. 321–362. [Google Scholar]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The Fluorescence Transient as a Tool to Characterize and Screen Photosynthetic Samples. Probing Photosynth. Mech. Regul. Adapt. 2000, 25, 445–483. [Google Scholar]
- van Heerden, P.D.; Tsimilli-Michael, M.; Krüger, G.H.; Strasser, R.J. Dark Chilling Effects on Soybean Genotypes during Vegetative Development: Parallel Studies of CO2 Assimilation, Chlorophyll a Fluorescence Kinetics O−J−I−P and Nitrogen Fixation. Physiol. Plant. 2003, 117, 476–491. [Google Scholar] [CrossRef]
- Yusuf, M.A.; Kumar, D.; Rajwanshi, R.; Strasser, R.J.; Tsimilli-Michael, M.; Sarin, N.B. Overexpression of γ−Tocopherol Methyl Transferase Gene in Transgenic Brassica juncea Plants Alleviates Abiotic Stress: Physiological and Chlorophyll a Fluorescence Measurements. Biochim. Biophys. Acta (BBA)-Bioenerg. 2010, 1797, 1428–1438. [Google Scholar] [CrossRef]
- Bowman, H.D.; Hydrick, H.T.; Bond, J.A.; Allen, T.W. Response of Soybean (Glycine max (L.) Merr.) and Weed Control with Postemergence Herbicides and Combinations of Cytokinin Mixtures. Agronomy 2022, 12, 3086. [Google Scholar] [CrossRef]
- Da Silva, A.F.; Galon, L.; Aspiazu, I.; Alves, E.; Concenco, G.; Ramos Junior, E.U.; Ribeiro Roch, P.R. Weed Management in the Soybean Crop. In Soybean−Pest Resistance; El-Shemy, H., Ed.; InTech: Rijeka, Croatia, 2013; Volume 13, pp. 85–112. [Google Scholar]
- Roncatto, E.; Barroso, A.A.M.; Novello, B.D.; Gonçalves, R.; Jarek, T.; Yung, M. Residual Herbicides and Cover Crops Interactions for Soybean Weed Control. JAS 2022, 14, 47. [Google Scholar] [CrossRef]
- Mehdizadeh, M.; Mushtaq, W.; Siddiqui, S.A.; Ayadi, S.; Kaur, P.; Yeboah, S.; Mazraedoost, S.; AL-Taey, D.K.; Tampubolon, K. Herbicide Residues in Agroecosystems: Fate, Detection, and Effect on Non-Target Plants. Rev. Agric. Sci. 2021, 9, 157–167. [Google Scholar] [CrossRef]
- Brown, H.M.; Brattsten, L.B.; Lilly, D.E.; Hanna, P.J. Metabolic Pathways and Residue Levels of Thifensulfuron Methyl in Soybeans. J. Agric. Food Chem. 1993, 41, 1724–1730. [Google Scholar] [CrossRef]
- Lamberth, C. Small Ring Chemistry in Crop Protection. Tetrahedron 2019, 75, 4365–4383. [Google Scholar] [CrossRef]
- Zhao, N.; Yan, Y.; Du, L.; Zhang, X.; Liu, W.; Wang, J. Unravelling the Effect of Two Herbicide Resistance Mutations on Acetolactate Synthase Kinetics and Growth Traits. J. Exp. Bot. 2020, 71, 3535–3542. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Liu, W.; Zhang, Y.; Liu, K.K. Action Mechanisms of Acetolactate Synthase-Inhibiting Herbicides. Pestic. Biochem. Physiol. 2007, 89, 89–96. [Google Scholar] [CrossRef]
- Hernández-Mesa, M.; García-Campaña, A.M. Determination of Sulfonylurea Pesticide Residues in Edible Seeds Used as Nutraceuticals by QuEChERS in Combination with Ultra-High-Performance Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. A 2020, 1617, 460831. [Google Scholar] [CrossRef]
- Priess, G.L.; Norsworthy, J.K.; Roberts, T.L.; Gbur, E.E. Impact of Postemergence Herbicides on Soybean Injury and Canopy Formation. Weed Technol. 2020, 34, 727–734. [Google Scholar] [CrossRef]
- Sancar, B.Ç.; Akhan, M.; Öztürk, M.; Ergün, Ö. Pesticide Residues in Vegetables and Fruits from Istanbul by LC-MS/MS. Harran Tarım Ve Gıda Bilim. Derg. 2022, 26, 303–315. [Google Scholar] [CrossRef]
- Shahid, E.; Khan, D.; Qaisrani, M.; Noman, M.; Rani, A.; Ali, S. Effect of Pesticide Residues on Agriculture Crops. J. Toxicol. Pharmaceut. Sci 2021, 5, 18–23. [Google Scholar]
- Markulj Kulundžić, A.; Josipović, A.; Matoša Kočar, M.; Viljevac Vuletić, M.; Antunović Dunić, J.; Varga, I.; Cesar, V.; Sudarić, A.; Lepeduš, H. Physiological Insights on Soybean Response to Drought. Agric. Water Manag. 2022, 268, 107620. [Google Scholar] [CrossRef]
- Rutherford, A.W.; Krieger-Liszkay, A. Herbicide-Induced Oxidative Stress in Photosystem II. Trends Biochem. Sci. 2001, 26, 648–653. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Račková, L.; Paganová, V.; Swoczyna, T.; Rusinowski, S.; Sitko, K. Can Chlorophyll a Fluorescence Parameters Be Used as Bio-−Indicators to Distinguish between Drought and Salinity Stress in Tilia cordata Mill? Environ. Exp. Bot. 2018, 152, 149–157. [Google Scholar] [CrossRef]
- Antunović Dunić, J.; Mlinarić, S.; Pavlović, I.; Lepeduš, H.; Salopek-Sondi, B. Comparative Analysis of Primary Photosynthetic Reactions Assessed by OJIP Kinetics in Three Brassica Crops after Drought and Recovery. Appl. Sci. 2023, 13, 3078. [Google Scholar] [CrossRef]
- Begović, L.; Galić, V.; Abičić, I.; Lončarić, Z.; Lalić, A.; Mlinarić, S. Implications of Intra−Seasonal Climate Variations on Chlorophyll a Fluorescence and Biomass in Winter Barley Breeding Program. Photosynthetica 2020, 58, 995–1008. [Google Scholar] [CrossRef]
- Mlinarić, S.; Begović, L.; Tripić, N.; Piškor, A.; Cesar, V. Evaluation of Light−Dependent Photosynthetic Reactions in Reynoutria japonica Houtt. Leaves Grown at Different Light Conditions. Front. Plant Sci. 2021, 12, 1515. [Google Scholar] [CrossRef] [PubMed]
- Mlinarić, S.; Cesar, V.; Lepeduš, H. Antioxidative Response and Photosynthetic Regulatory Mechanisms in Common Fig Leaves after Short−term Chilling Stress. Ann. Appl. Biol. 2021, 178, 315–327. [Google Scholar] [CrossRef]
- Šrajer Gajdošik, M.; Vicić, A.; Gvozdić, V.; Galić, V.; Begović, L.; Mlinarić, S. Effect of Prolonged Photoperiod on Light-Dependent Photosynthetic Reactions in Cannabis. Int. J. Mol. Sci. 2022, 23, 9702. [Google Scholar] [CrossRef] [PubMed]
- Krüger, G.; De Villiers, M.; Strauss, A.; De Beer, M.; Van Heerden, P.; Maldonado, R.; Strasser, R. Inhibition of Photosystem II Activities in Soybean (Glycine max) Genotypes Differing in Chilling Sensitivity. S. Afr. J. Bot. 2014, 95, 85–96. [Google Scholar] [CrossRef]
- Gururani, M.A.; Venkatesh, J.; Ganesan, M.; Strasser, R.J.; Han, Y.; Kim, J.-I.; Lee, H.-Y.; Song, P.-S. In vivo Assessment of Cold Tolerance through Chlorophyll a Fluorescence in Transgenic Zoysiagrass Expressing Mutant Phytochrome A. PLoS ONE 2015, 10, e0127200. [Google Scholar] [CrossRef]
- Dąbrowski, P.; Baczewska-Dąbrowska, A.H.; Kalaji, H.M.; Goltsev, V.; Paunov, M.; Rapacz, M.; Wójcik-Jagła, M.; Pawluśkiewicz, B.; Bąba, W.; Brestic, M. Exploration of Chlorophyll a Fluorescence and Plant Gas Exchange Parameters as Indicators of Drought Tolerance in Perennial Ryegrass. Sensors 2019, 19, 2736. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a Fluorescence as a Tool to Monitor Physiological Status of Plants under Abiotic Stress Conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef]
- Zagorchev, L.; Atanasova, A.; Albanova, I.; Traianova, A.; Mladenov, P.; Kouzmanova, M.; Goltsev, V.; Kalaji, H.M.; Teofanova, D. Functional Characterization of the Photosynthetic Machinery in Smicronix galls on the Parasitic Plant Cuscuta campestris by JIP-Test. Cells 2021, 10, 1399. [Google Scholar] [CrossRef]
- Zagorchev, L.; Traianova, A.; Teofanova, D.; Li, J.; Kouzmanova, M.; Goltsev, V. Special Issue in Honour of Prof. Reto J. Strasser–Influence of Cuscuta campestris Yunck. on the Photosynthetic Activity of Ipomoea tricolor Cav.−In vivo Chlorophyll a Fluorescence Assessment. Photosynthetica 2020, 58, 422–432. [Google Scholar] [CrossRef]
- Gonçalves, J.F.; Santos, U.M., Jr.; Nina, A.R., Jr.; Chevreuil, L.R. Energetic Flux and Performance Index in Copaiba (Copaifera multijuga Hayne) and Mahogany (Swietenia macrophylla King) Seedlings Grown under Two Irradiance Environments. Braz. J. Plant Physiol. 2007, 19, 171–184. [Google Scholar] [CrossRef]
- De Sousa, C.P.; De Farias, M.E.; Schock, A.A.; Bacarin, M.A. Photosynthesis of Soybean under the Action of a Photosystem II−Inhibiting Herbicide. Acta Physiol. Plant. 2014, 36, 3051–3062. [Google Scholar] [CrossRef]
- Yordanov, I.; Goltsev, V.; Stefanov, D.; Chernev, P.; Zaharieva, I.; Kirova, M.; Gecheva, V.; Strasser, R.J. Preservation of Photosynthetic Electron Transport from Senescence-Induced Inactivation in Primary Leaves after Decapitation and Defoliation of Bean Plants. J. Plant Physiol. 2008, 165, 1954–1963. [Google Scholar] [CrossRef]
- Bhattacharjee, S. The Language of Reactive Oxygen Species Signaling in Plants. J. Bot. 2012, 2012, 985298. [Google Scholar] [CrossRef]
- Pandey, S.; Fartyal, D.; Agarwal, A.; Shukla, T.; James, D.; Kaul, T.; Negi, Y.K.; Arora, S.; Reddy, M.K. Abiotic Stress Tolerance in Plants: Myriad Roles of Ascorbate Peroxidase. Front. Plant Sci. 2017, 8, 581. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G. Herbicides and Its Role in Induction of Oxidative Stress—A Review. Int. J. Environ. Agric. Biotechnol. 2019, 4, 995–1004. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Oukarroum, A.; Alexandrov, V.; Kouzmanova, M.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Allakhverdiev, S.I.; Goltsev, V. Identification of Nutrient Deficiency in Maize and Tomato Plants by in vivo Chlorophyll a Fluorescence Measurements. Plant Physiol. Biochem. 2014, 81, 16–25. [Google Scholar] [CrossRef]
- Bradshaw, L.D.; Barrett, M.; Poneleit, C.G. Physiological Basis for Differential Bentazon Susceptibility Among Corn (Zea Mays) Inbreds. Weed Sci. 1992, 40, 522–527. [Google Scholar] [CrossRef]
- Hugie, J.A.; Bollero, G.A.; Tranel, P.J.; Riechers, D.E. Defining the Rate Requirements for Synergism Between Mesotrione and Atrazine in Redroot Pigweed (Amaranthus retroflexus). Weed Sci. 2008, 56, 265–270. [Google Scholar] [CrossRef]
- Duke, S.O. (Ed.) Weed Physiology. Volume II, Herbicide Physiology; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2018; ISBN 978-1-351-07773-6. [Google Scholar]
- Riechers, D.E.; Kreuz, K.; Zhang, Q. Detoxification without Intoxication: Herbicide Safeners Activate Plant Defense Gene Expression. Plant Physiol. 2010, 153, 3–13. [Google Scholar] [CrossRef] [PubMed]

| Quantum Efficiencies and Flux Ratios | |
| ABS/RC | Effective antenna size of an active reaction center (RC). Expresses the total number of photons absorbed by Chl molecules of all RC divided by the total number of active RCs |
| ET0/RC | Electron transport per active RC |
| TR0/RC | Maximal trapping rate of PSII. Describes the maximal rate by which an excitation is trapped by the RC |
| DI0/RC | Effective dissipation per active RC |
| RE0/RC | Electron flux reducing end electron acceptors at the PSI acceptor side per RC |
| TR0/DI0 | Flux ration trapping per dissipation |
| φP0 = TR0/ABS | Maximum quantum yield of primary photochemistry, the probability that an absorbed photon will be trapped by the PSII RC and will reduce one QA |
| ψE0 = ET0/TR0 | Probability that an absorbed photon will enter the electron transport chain, electron transport efficiency |
| φE0 = ET0/ABS | Quantum yield for electron transport |
| δR0 = RE0−ET0 | Probability that an electron is transported from reduced PQ to the electron acceptor side of PSI |
| φR0 = RE0/ABS | Quantum yield of electron transport from QA− to the PSI end electron acceptors |
| Performance Index and Driving Forces | |
| SFI | Structure–function index on absorption basis; (RC/ABS) × φP0 × ψE0 |
| PIABS | Performance index (potential) for energy conservation from photons absorbed by PSII to the reduction in intersystem electron acceptors; [γRC/(1 − γRC)][ φP0/(1 − φP0)][ψE0/(1 − ψE0)] |
| DFABS = log PIABS | Total driving forces for photosynthesis of the observed system, created by summing up the partial driving forces for each of the several bifurcations |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Begović, L.; Jurišić, N.; Šrajer Gajdošik, M.; Mikuška, A.; Mlinarić, S. Photosynthetic Efficiency and Antioxidative Response of Soybean Exposed to Selective Herbicides: A Field Study. Agriculture 2023, 13, 1385. https://doi.org/10.3390/agriculture13071385
Begović L, Jurišić N, Šrajer Gajdošik M, Mikuška A, Mlinarić S. Photosynthetic Efficiency and Antioxidative Response of Soybean Exposed to Selective Herbicides: A Field Study. Agriculture. 2023; 13(7):1385. https://doi.org/10.3390/agriculture13071385
Chicago/Turabian StyleBegović, Lidija, Nikola Jurišić, Martina Šrajer Gajdošik, Alma Mikuška, and Selma Mlinarić. 2023. "Photosynthetic Efficiency and Antioxidative Response of Soybean Exposed to Selective Herbicides: A Field Study" Agriculture 13, no. 7: 1385. https://doi.org/10.3390/agriculture13071385
APA StyleBegović, L., Jurišić, N., Šrajer Gajdošik, M., Mikuška, A., & Mlinarić, S. (2023). Photosynthetic Efficiency and Antioxidative Response of Soybean Exposed to Selective Herbicides: A Field Study. Agriculture, 13(7), 1385. https://doi.org/10.3390/agriculture13071385







