Vegetative Endotherapy—Advances, Perspectives, and Challenges
Abstract
:1. Introduction
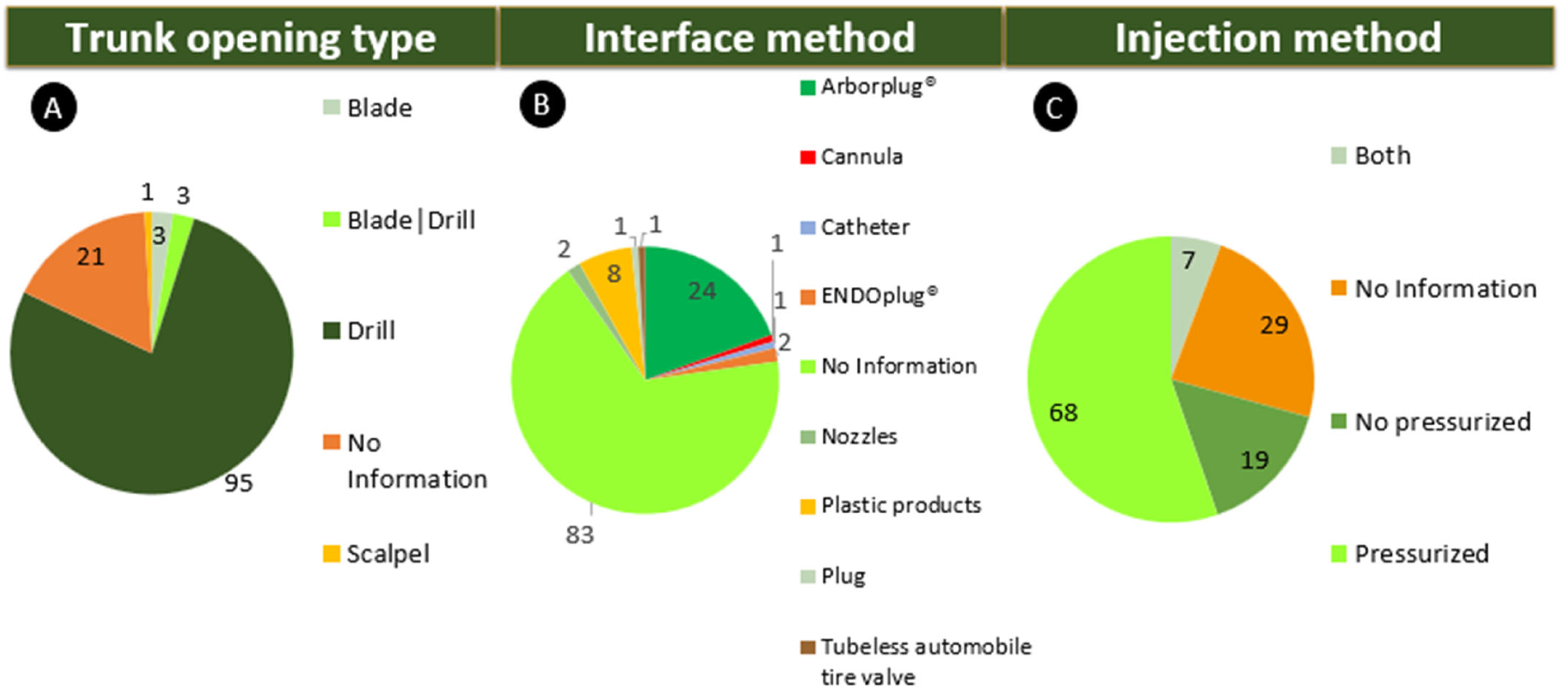
Classification of Endotherapeutic Systems
- (i)
- Trunk opening: When the trunk is opened by drilling, the contact surface increases through the exposure of the conductive sap vessels, allowing the introduction of the applied products. Blades are drill-free ports, and perforations can be round-shaped, with a screw thread or lenticular shape. The term blade was considered in this article, as proposed by Montecchio [13], as a metal instrument designed to cut and/or pierce with impact and not with rotation as with drills. In this case, the blades remain in the trunk until the end of the application [1,13,32,40,41,42].
- (ii)
- Interface methods: Some technologies use removable injectors that can leave the port (hole) open. Other technologies use a retention catheter known as a “plug”. It can be a valve system (rubber septum) that assists in pressurized applications with the presence of a self-sealing septum that prevents leakage, maintains the precise pressure in the application process, prevents product loss or waste, minimizes the injection locations, limits the wound, inhibits decomposition and/or infection and, in some cases, accelerates healing. Leaks can occur depending on the application method if a lot of pressure or large application volume is exerted. Therefore, creating tests to determine thresholds for these criteria, such as pressure and strength during the application, depth, and size of the hole on a given crop to prevent leaks, waste, or cracks in the trunk, is essential [15,27,43,44,45].
- (iii)
- Injection methods: The injection method uses applicators and can be divided into two categories:
- (A)
- Pressurized techniques can reduce treatment time because pressure accelerates the absorption of the applied products. In addition, this technique is more effective for some species of trees that are naturally slower to absorb, especially on cloudy/cold days when translocation/evaporation takes longer. One drawback of using this technology is the possibility of bubble formation (embolism) that can crack the bark and trunk, creating leaks or product rejection when applied under high pressure. Trunk water content and its hydraulic process can be non-invasively monitored through frequency sensors. Studies have shown that the injection of air and dyes can fill many vessels close to the application site with air [46,47,48]. For this reason, procedures for accurately assessing pressure for a given crop should be implemented to prevent damage related to excess pressure in the trees.
- (B)
- Nonpressurized or depressurized techniques: When the drill cuts the sap-conducting vessels, sap flow as well as water and pressure potential (potential water gradient) exerted in this affected area are stopped; therefore, the absence of an external force impairs the absorption of products in the trunk, losing translocation efficiency in the leaf evapotranspiration process [15,49]. Depending on the formulation, it may cause the product to precipitate in the ports. According to Kuhns [15], the lack of pressure slows absorption and treatment, requiring a greater volume to be applied, thereby increasing costs. It is important to emphasize that when applications leave an external device, such as a tube or container, the risk of chemical exposure and vandalism may increase as remotion or breakage of the accessory by malicious damage or intentional destruction. For these procedures, when the ports are permanently open, they also become an open wound. Successive treatments using fixed accessories can be associated with necrotic area development. Since the injured tissue is susceptible to the entry of bacteria, it is exposed to the accumulation of water, causing it to rot near this area. Still, over time, in some cases, the trunk/stem can cause severe exudation or damage to the accessory, such as material dryness and breaks inside the trunk. Therefore, leaving a plant with an open wound for endotherapeutic applications over time increases the likelihood of serious problems, such as rotting in the area around the wound and the waste of the applied product [15].
- (iv)
- Injection volume: Classification is related to the volume applied.
| Classification Parameters | Description | Commercial Examples |
|---|---|---|
| Trunk opening | Drills: Most technologies available on the market use perforations with 4 to 10 mm drill bits. This definition is associated with the structure and type of stem of the crop. Drilling above these dimensions is not recommended as it causes major injuries to the trunk. | Arborjet®, Fertinyect®, Arboprof®, Chemjet®, ENDOplant®, ENDOkit Manual® |
| Blades: Opening the trunk without using drills. The technologies that use blades reduce the impact of disruption of vascular tissues, as the sharp spirals of the drill bits do when cutting the tissues during the insertion and removal of the drill. Because it does not form a space for absorbing the applied product, strong pressure is needed to introduce them. This can generate structural damage to the trunk. Depending on the species and climate/season, it may take a long time to introduce products. | Bite®, Arborsytem® | |
| Interface method | Plugs: These represent an important communication between the tree vascular system and product application equipment. When installed, the plugs are stuck/fixed in the bark and/or in very close points and serve as an access point for the application of the product. There are some models of plugs with different diameters on the market. | Biodegradable such as Arborbiokaps® and Medicap, or permanent ENDOterapia Vegetal™ and Arborjet®. |
| Injection method |
| |
| Fertinyect®, Mauget®, Chemjet® | |
| Intus®, Arboprof® | |
| Bite®, Arborsystem® Arborjet® ENDOterapia Vegetal™ | |
| Vita Caule®, SOS Palm® | |
| Medi-ject®, Xyllakill | |
| Injectionvolume | Macroinfusion/Macroinjection: Corresponds to systems where volumes greater than 15 mL are applied to each port. | |
| Microinfusion/Microinjection: This is equivalent to applications with a volume of less than 15 mL at each port point | ||
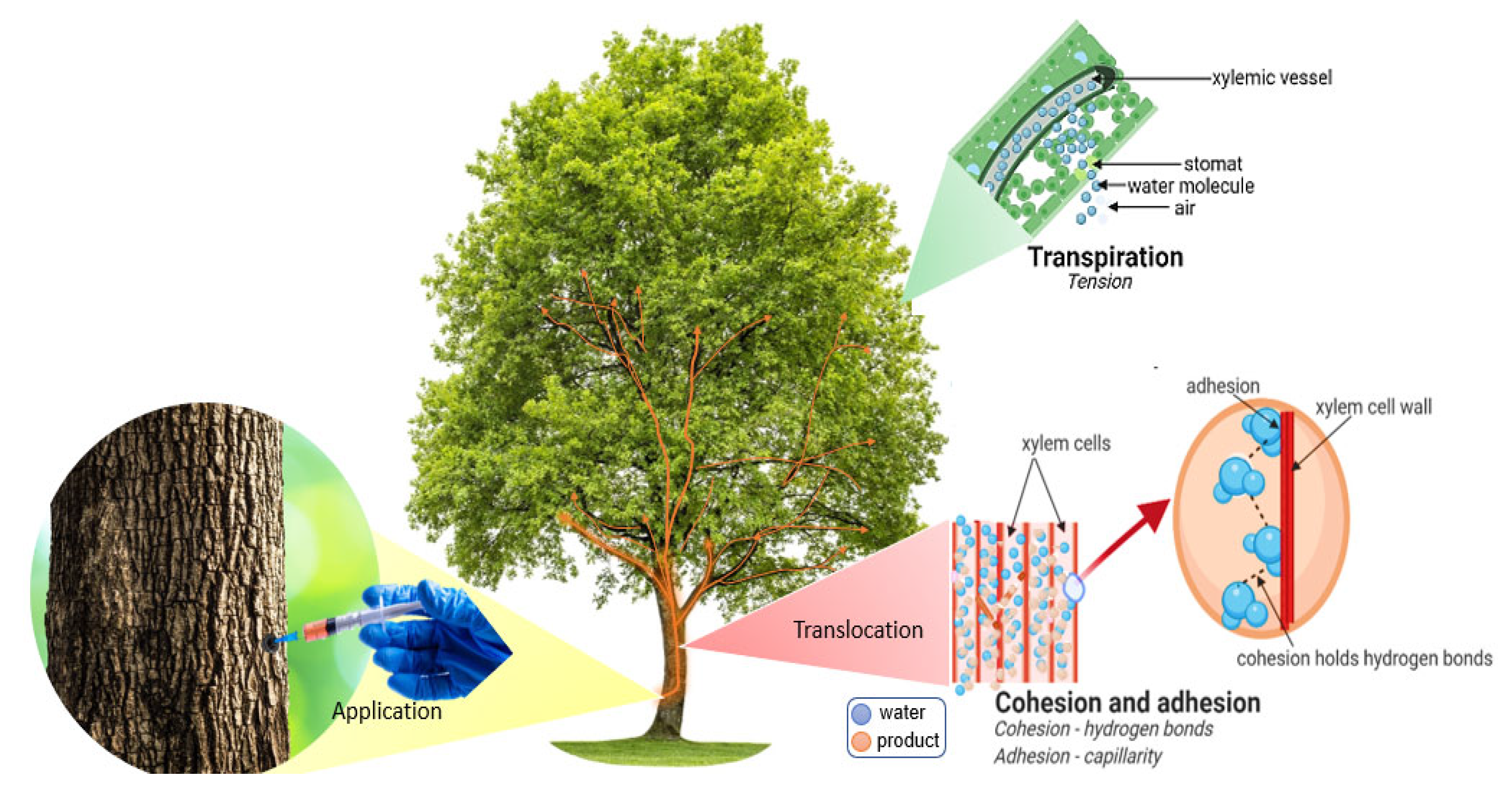
2. Physiology and Plant Morphology
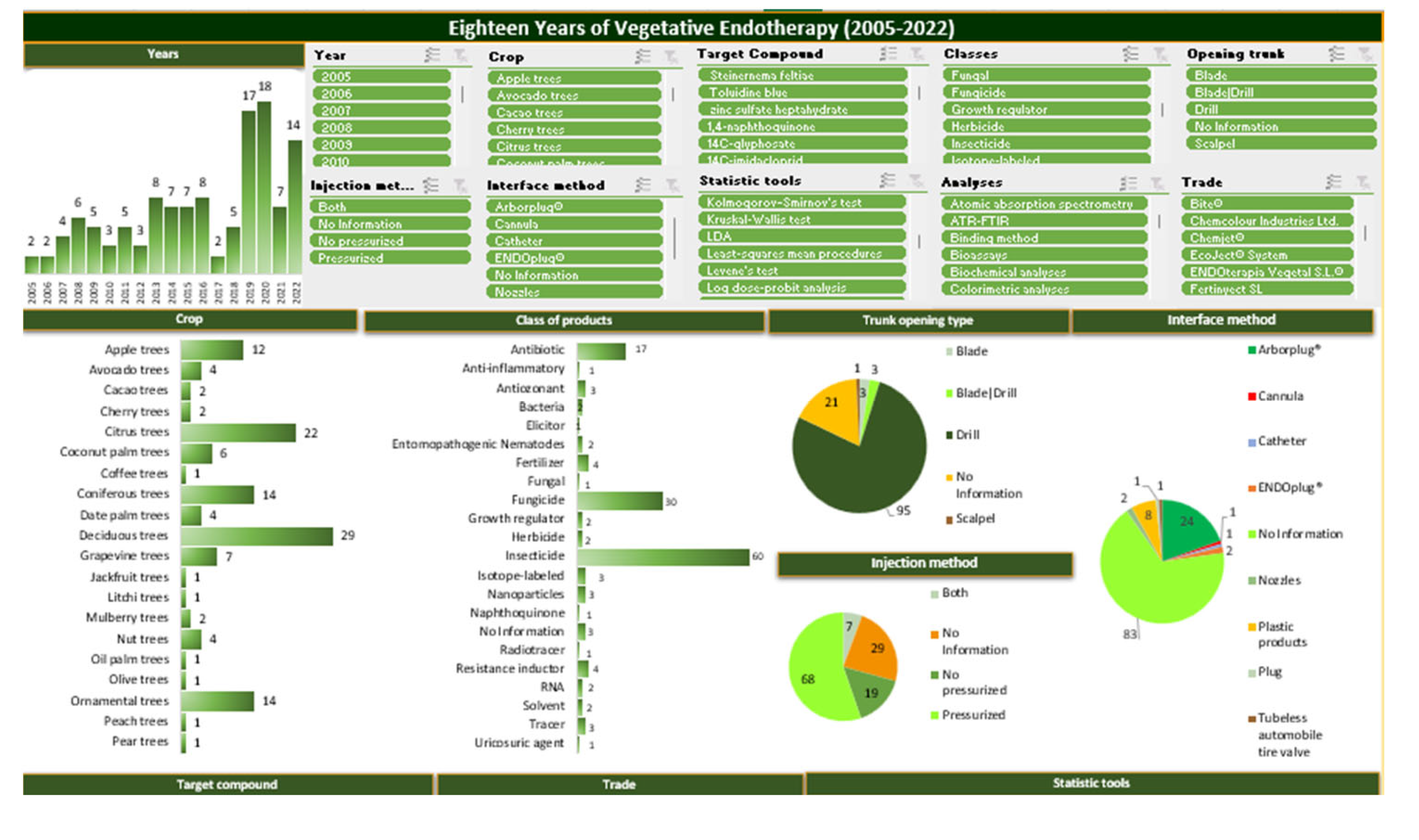
3. Endotherapy in the Last 18 Years
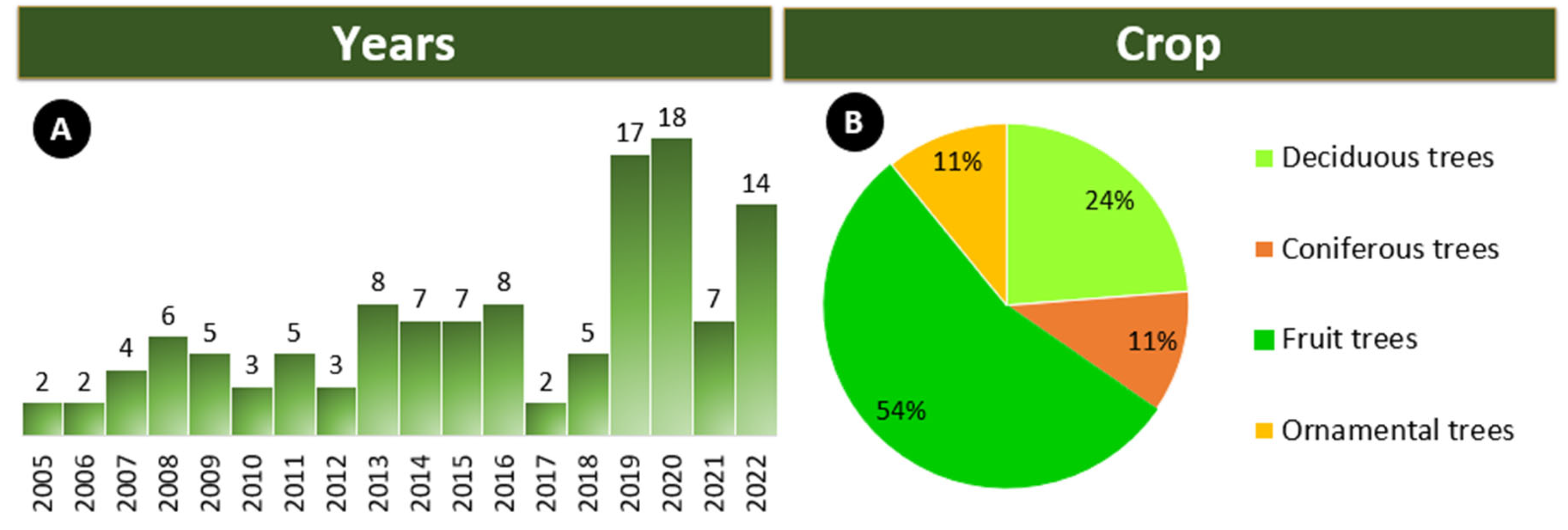
3.1. Crops
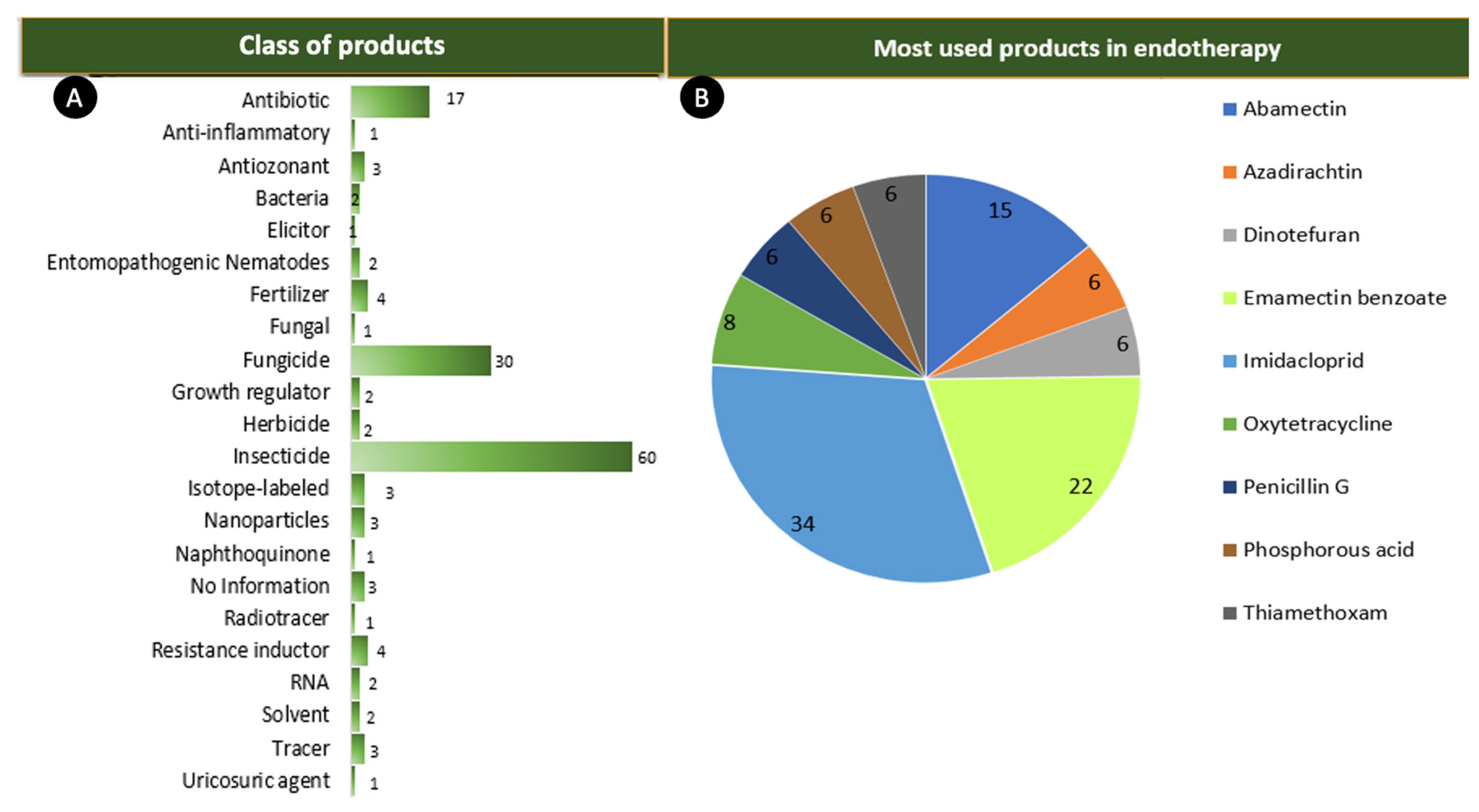
3.2. Products Used in Endotherapeutic Treatment
4. Evaluation of Different Endotherapeutic Treatments
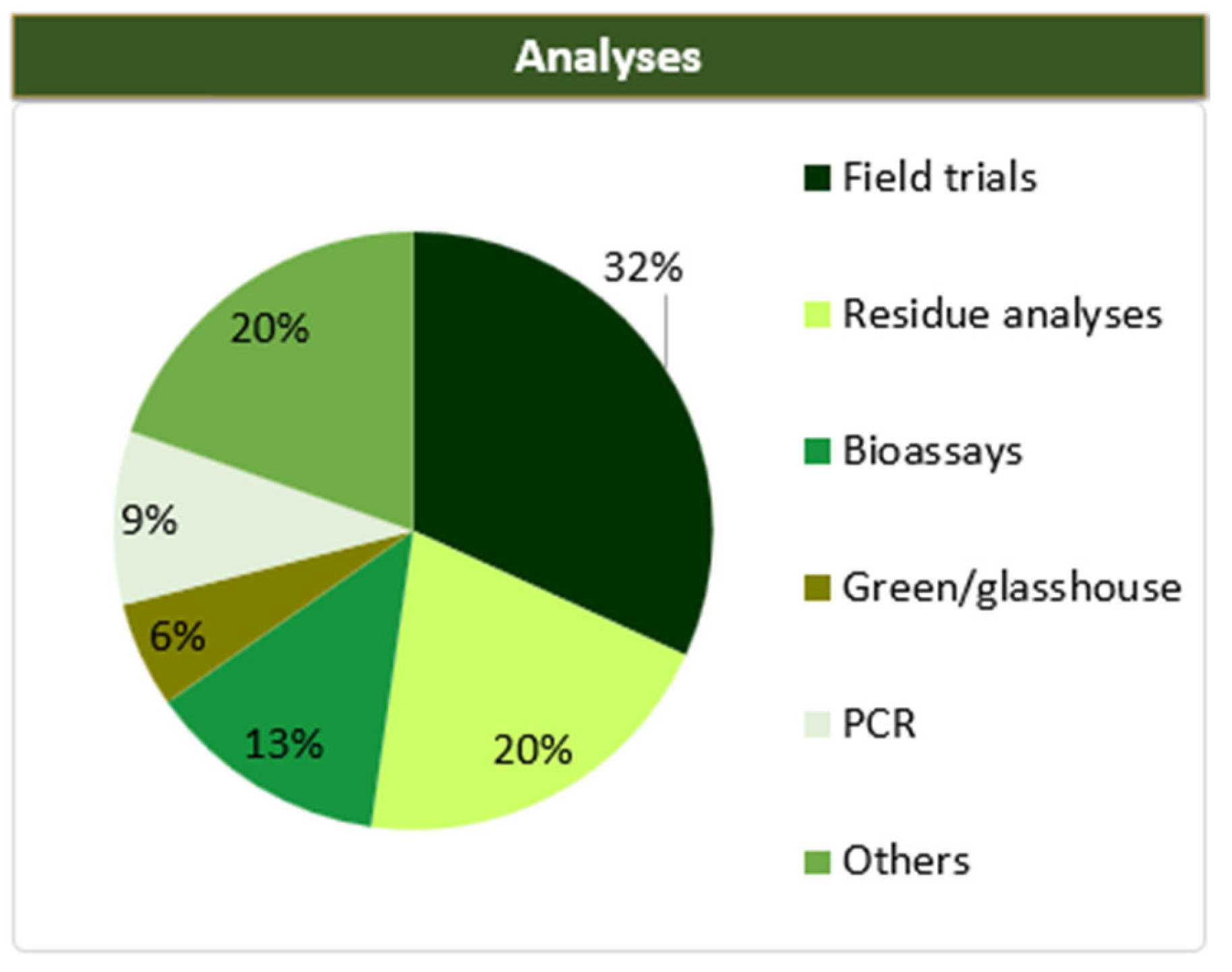
5. Analysis after Endotherapeutic Applications
6. Challenges and Advances
- (1)
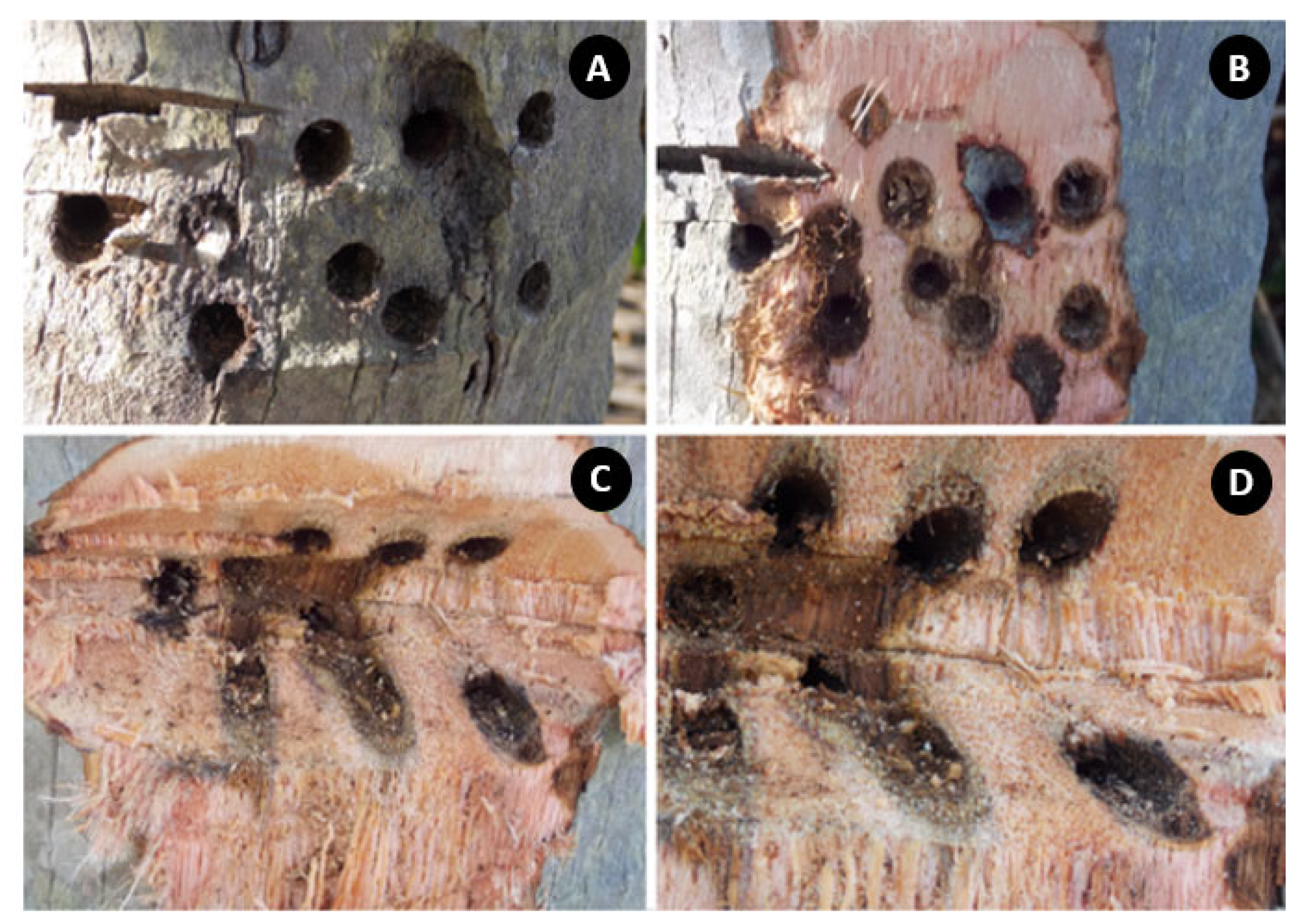
- Development of new technologies and tools to open ports that are less invasive in the tree trunks, especially for palm tree stem (Figure 7 and Figure 8). Some technologies, such as the blades mentioned in this review, are manual and are not practical for hardwood and may have difficulties introducing products such as Bite Infusion® for coconut palm trees.
- (2)
- (3)
- Nonpressurized injection methods that use a pipe or catheter attached to the trunk can expose treatments to risks in cases of accidents and vandalism. Trunks with deep, inclined holes are more susceptible to fungi, microorganisms, and rot trunks, as sap and rainwater tend to accumulate (Figure 11).
- (4)
- Do not leave ports exposed and apply pastes or healing products to prevent the proliferation of microorganisms/pests.
- (5)
- Develop new formulations focused on endotherapeutic use using products that replace synthetic pesticides and antibiotics in the control of pests and diseases with natural products such as essential oils with antimicrobial/antibacterial content [153,154] and less harmful products that reach the target and/or that make it less attractive to pest attack. Since specific formulations for endotherapy are extremely limited, there is an untapped market to be explored with new bioformulations. This may represent one of the greatest challenges to overcome in the coming years.
- (6)
- The extensive application of pesticides and antibiotics without criteria has been the subject of many questions regarding dosage, viscosity, and concentration of the active ingredient during applications that may create resistance in pathogens. New formulations with adjuvant action could include an application for multiple pathogens and control the entire pathosystemic problem. This lack of information prevents the determination of application intervals, treatment duration, and maximum residue limit assessments for fruit trees.
- (7)
- In pressurized injection methods, depressurization of the system after the plug was installed in the tree was not approached in the articles. Removing the air from the system (plug-tree) so that the applied product competes with air for space is essential to prevent cracks in the bark and an embolism that can lead to the tree’s death. A simple mechanism could introduce products via endotherapy and prevent clogging and leakage during application.
- (8)
- The implementation of endotherapy as a trend within the NBS can contribute to the interest of more researchers for solutions inspired by efficient application techniques using less harmful products with more sustainable proposals.
7. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berger, C.; Laurent, F. Trunk Injection of Plant Protection Products to Protect Trees from Pests and Diseases. J. Crop Prot. 2019, 124, 104831. [Google Scholar] [CrossRef]
- Fidgen, J.G.; Kittelson, N.T.; Eckberg, T.; Doccola, J.; Randall, C. Emamectin Benzoate Reduces Defoliation by Choristoneura Occidentalis Freeman (Lepidoptera: Tortricidae) on Three Host Species. West. J. Appl. For. 2013, 28, 170–173. [Google Scholar] [CrossRef]
- Solla, A.; García, L.; Pérez, A.; Cordero, A.; Cubera, E.; Moreno, G. Evaluating Potassium Phosphonate Injections for the Control of Quercus ilex Decline in SW Spain: Implications of Low Soil Contamination by Phytophthora cinnamomi and Low Soil Water Content on the Effectiveness of Treatments. Phytoparasitica 2009, 37, 303–316. [Google Scholar] [CrossRef]
- Wise, J.C.; VanWoerkom, A.H.; Aćimović, S.G.; Sundin, G.W.; Cregg, B.M.; Vandervoort, C. Trunk Injection: A Discriminating Delivering System for Horticulture Crop IPM. Entomol. Ornithol. Herpetol. 2014, 3, 2. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Broschat, T.K.; Latham, W.G.; Elliott, M.L. Dynamics and Distribution of Trunk injected Phosphite in Coconut Palm. HortScience 2015, 50, 1327–1331. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Nangong, Z. Precision Trunk Injection Technology for Treatment of Huanglongbing (HLB)-Affected Citrus Trees—A Review. JPDP 2022, 129, 15–34. [Google Scholar] [CrossRef]
- Archer, L.; Crane, J.H.; Albrecht, U. Trunk Injection as a Tool to Deliver Plant Protection Materials—An Overview of Basic Principles and Practical Considerations. Horticulturae 2022, 8, 552. [Google Scholar] [CrossRef]
- Ferry, M.; Gomez, S. Assessment of Risks and Potential of Injection Techniques in Integrated Programs to Eradicate the Red Palm Weevil: Review and New Perspectives. Fruits 2014, 69, 143–157. [Google Scholar] [CrossRef]
- Byrne, F.J.; Krieger, R.I.; Doccola, J.; Morse, J.G. Seasonal Timing of Neonicotinoid and Organophosphate Trunk Injections to Optimize the Management of Avocado Thrips in California Avocado Groves. J. Crop Prot. 2014, 57, 20–26. [Google Scholar] [CrossRef]
- Coslor, C.C.; Vandervoort, C.; Wise, J.C. Control of Insect Pests Using Trunk Injection in a Newly Established Apple Orchard. Int. J. Fruit Sci. 2019, 19, 151–164. [Google Scholar] [CrossRef]
- Mota-Sanchez, D.; Cregg, B.M.; McCullough, D.G.; Poland, T.M.; Hollingworth, R.M. Distribution of Trunk-Injected 14C-Imidacloprid in Ash Trees and Effects on Emerald Ash Borer (Coleoptera: Buprestidae) Adults. J. Crop Prot. 2009, 28, 655–661. [Google Scholar] [CrossRef]
- Aćimović, S.G.; Vanwoerkom, A.H.; Reeb, P.D.; Vandervoort, C.; Garavaglia, T.; Cregg, B.M.; Wise, J.C. Spatial and Temporal Distribution of Trunk-Injected Imidacloprid in Apple Tree Canopies. Pest Manag. Sci. 2014, 70, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Montecchio, L. A Venturi Effect Can Help Cure Our Trees. JoVE J. Vis. Exp. 2013, 80, e51199. [Google Scholar] [CrossRef] [Green Version]
- Helson, B.V.; Lyons, D.B.; Wanner, K.W.; Scarr, T.A. Control of Conifer Defoliators with Neem-Based Systemic Bioinsecticides Using a Novel Injection Device. Can. Entomol. 2001, 133, 729–744. [Google Scholar] [CrossRef]
- Kuhns, M. Getting Chemicals into Trees Without Spraying. 2011, p. 1188. Available online: https://digitalcommons.usu.edu/extension_curall/1188 (accessed on 12 May 2020).
- Puttamuk, T.; Zhang, S.; Duan, Y.; Jantasorn, A.; Thaveechai, N. Effect of Chemical Treatments on “Candidatus Liberibacter Asiaticus” Infected Pomelo (Citrus Maxima). J. Crop Prot. 2014, 65, 114–121. [Google Scholar] [CrossRef]
- Potter, D.A.; Foss, L.; Baumler, R.E.; Held, D.W. Managing Eastern Tent Caterpillars Malacosoma americanum (F) on Horse Farms to Reduce Risk of Mare Reproductive Loss Syndrome. Pest Manag. Sci. 2005, 61, 3–15. [Google Scholar] [CrossRef]
- Ploetz, R.C.; Pérez-Martínez, J.M.; Evans, E.A.; Inch, S.A. Toward Fungicidal Management of Laurel Wilt of Avocado. Plant Dis. 2011, 95, 977–982. [Google Scholar] [CrossRef] [Green Version]
- Darrieutort, G.; Lecomte, P. Evaluation of a Trunk Injection Technique to Control Grapevine Wood Diseases. Phytopathol. Mediterr. 2007, 46, 50–57. [Google Scholar]
- Zhang, M.; Yang, C.; Powell, C.A.; Avery, P.B.; Wang, J.; Huang, Y.; Duan, Y. Field Evaluation of Integrated Management for Mitigating Citrus Huanglongbing in Florida. Front. Plant. Sci. 2019, 9, 1890. [Google Scholar] [CrossRef] [Green Version]
- Paoletti, E.; Manning, W.J.; Spaziani, F.; Tagliaferro, F. Gravitational Infusion of Ethylenediurea (EDU) into Trunks Protected Adult European Ash Trees (Fraxinus excelsior L.) from Foliar Ozone Injury. Environ. Pollut. 2007, 145, 869–873. [Google Scholar] [CrossRef]
- Paoletti, E.; Contran, N.; Manning, W.J.; Tagliaferro, F. Ethylenediurea (EDU) Affects the Growth of Ozone-Sensitive and Tolerant Ash (Fraxinus excelsior) Trees under Ambient O3 Conditions. Sci. World J. 2007, 7, 128–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paoletti, E.; Contran, N.; Manning, W.J.; Castagna, A.; Ranieri, A.; Tagliaferro, F. Protection of Ash (Fraxinus excelsior) Trees from Ozone Injury by Ethylenediurea (EDU): Roles of Biochemical Changes and Decreased Stomatal Conductance in Enhancement of Growth. Environ. Pollut. 2008, 155, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Costonis, A.C. Tree Injection: Perspective Macro-Injection/Micro-Injection. Arboric. J. 1981, 7, 275–277. [Google Scholar] [CrossRef]
- Sánchez-Zamora, M.A.; Fernández-Escobar, R. Uptake and Distribution of Trunk Injections in Conifers. Arboric. J. 2004, 30, 73–79. [Google Scholar] [CrossRef]
- Smith, K.T.; Lewis, P.A. Potential Concerns for Tree Wound Response from Stem Injection. In Proceedings of the Third Hemlock Wooly Adelgid Conference, Asheville, NC, USA, 1–3 February 2005; pp. 173–178. [Google Scholar]
- Tanis, S.R.; Cregg, B.M.; Mota-Sanchez, D.; McCullough, D.G.; Poland, T.M. Spatial and Temporal Distribution of Trunk-Injected 14C-Imidacloprid in Fraxinus Trees. Pest Manag. Sci. 2012, 68, 529–536. [Google Scholar] [CrossRef]
- Wise, J.C. Advances in Insect Control and Resistance Management, 1st ed.; Horowitz, A.R., Ishaaya, I., Eds.; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-31798-4. [Google Scholar]
- McCullough, D.G.; Poland, T.M.; Lewis, P.A. Lethal Trap Trees: A Potential Option for Emerald Ash Borer (Agrilus planipennis Fairmaire) Management. Pest Manag. Sci. 2016, 72, 1023–1030. [Google Scholar] [CrossRef]
- Aćimović, S.G.; Zeng, Q.; McGhee, G.C.; Sundin, G.W.; Wise, J.C. Control of Fire Blight (Erwinia amylovora) on Apple Trees with Trunk-Injected Plant Resistance Inducers and Antibiotics and Assessment of Induction of Pathogenesis-Related Protein Genes. Front. Plant Sci. 2015, 6, 16. [Google Scholar] [CrossRef] [Green Version]
- Doccola, J.J.; Wild, P.M. Tree Injection as an Alternative Method of Insecticide Application. In Insecticides—Basic and Other Applications; Soloneski, S., Ed.; InTech: Rijeka, Croatia, 2004; p. 268. [Google Scholar]
- VanWoerkom, A.H.; Aćimović, S.G.; Sundin, G.W.; Cregg, B.M.; Mota-Sanchez, D.; Vandervoort, C.; Wise, J.C. Trunk Injection: An Alternative Technique for Pesticide Delivery in Apples. J. Crop Prot. 2014, 65, 173–185. [Google Scholar] [CrossRef]
- Wheeler, C.E.; Vandervoort, C.; Wise, J.C. Organic Control of Pear Psylla in Pear with Trunk Injection. Insects 2020, 11, 650. [Google Scholar] [CrossRef]
- Chaney, W.A. Anatomy and Physiology Related to Chemical Movement in Trees. Arboric. J. 1985, 12, 85–91. [Google Scholar] [CrossRef]
- Ferreira, J.A. Desenvolvimento de Métodos Analíticos Para Determinação de Agrotóxicos Em Estipe de Coqueiro (Cocos Nucifera Linn.), Água-de-Coco e Albúmen Sólido Por UHPLC-MS/MS e Avaliação da Translocação por Endoterapia, University of Campinas. 2016. Available online: http://repositorio.unicamp.br/Acervo/Detalhe/978937 (accessed on 2 October 2020).
- Fernández-Escobar, R.; Barranco, D.; Benlloch, M. Overcoming Iron Chlorosis in Olive and Peach Trees Using a Low-Pressure Trunk-Injection Method. HortScience 2019, 28, 192–194. [Google Scholar] [CrossRef]
- Cohen-Shacham, E.; Walters, G.; Janzen, C.; Maginnis, S. Nature-Based Solutions to Address Global Societal Challenges; Cohen-Shacham, E., Walters, G., Janzen, C., Maginnis, S., Eds.; IUCN International Union for Conservation of Nature: Gland, Switzerland, 2016. [Google Scholar]
- Sowińska-Świerkosz, B.; García, J. What Are Nature-Based Solutions (NBS)? Setting Core Ideas for Concept Clarification. Nat. Based Solut. 2022, 2, 100009. [Google Scholar] [CrossRef]
- Egan, P.A.; Chikoye, D. (Eds.) Harnessing Nature-Based Solutions for Smallholder Plant Health in a Changing Climate; SLU Global: Uppsala, Sweden, 2021; ISBN 9789157698155. [Google Scholar]
- Ascunce, M.S.; Shin, K.; Huguet-Tapia, J.C.; Poudel, R.; Garrett, K.A.; van Bruggen, A.H.C.; Goss, E.M. Penicillin Trunk Injection Affects Bacterial Community Structure in Citrus Trees. Microb. Ecol. 2019, 78, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Cowles, R.S.; Montgomery, M.E.; Cheah, C.A.S.J. Activity and Residues of Imidacloprid Applied to Soil and Tree Trunks to Control Hemlock Woolly Adelgid (Hemiptera: Adelgidae) in Forests. J. Econ Entomol. 2006, 99, 1258–1267. [Google Scholar] [CrossRef] [PubMed]
- González, M.; Romero, M.Á.; Serrano, M.S.; Sánchez, M.E. Fosetyl-Aluminium Injection Controls Root Rot Disease Affecting Quercus suber in Southern Spain. Eur. J. Plant Pathol. 2020, 156, 101–109. [Google Scholar] [CrossRef]
- Dembilio, Ó.; Riba, J.M.; Gamón, M.; Jacas, J.A. Mobility and Efficacy of Abamectin and Imidacloprid against Rhynchophorus ferrugineus in Phoenix canariensis by Different Application Methods. Pest Manag. Sci. 2015, 71, 1091–1098. [Google Scholar] [CrossRef]
- Doccola, J.J.; Smitley, D.R.; Davis, T.W.; Aiken, J.J.; Wild, P.M. Tree Wound Responses Following Systemic Insecticide Trunk Injection Treatments in Green Ash (Fraxinus pennsylvanica Marsh.) as Determined by Destructive Autopsy. Arboric Urban For. 2011, 37, 6–12. [Google Scholar] [CrossRef]
- Doccola, J.J.; Ramasamy, I.; Castillo, P.; Taylor, C.; Sifleet, S. Erratum: Efficacy of Arborjet VIPER Microinjections in the Management of Hemlock Woolly Adelgid (Adelges tsugae). Arboric. J. 2005, 31, 203–206. [Google Scholar] [CrossRef]
- Hao, G.Y.; Wheller, J.W.; Holbrook, M.N.; Goldstein, G. Investigating Xylem Embolism Formation, Refilling and Water Storage in Tree Trunks Using Frequency Domain Reflectometry. J. Exp. Bot. 2013, 64, 2321–2332. [Google Scholar] [CrossRef] [Green Version]
- Kuroda, K. Mechanism of Cavitation Development in the Pine Wilt Disease. Eur. J. Plant Pathol. 1991, 21, 82–89. [Google Scholar] [CrossRef]
- Rosner, S.; Johnson, D.M.; Voggeneder, K.; Domec, J.C. The Conifer-Curve: Fast Prediction of Hydraulic Conductivity Loss and Vulnerability to Cavitation. Ann. For. Sci. 2019, 76, 82. [Google Scholar] [CrossRef] [Green Version]
- Stephano-Hornedo, J.L.; Torres-Gutiérrez, O.; Toledano-Magaña, Y.; Gradilla-Martínez, I.; Pestryakov, A.; Sánchez-González, A.; García-Ramos, J.C.; Bogdanchikova, N. ArgovitTM Silver Nanoparticles to Fight Huanglongbing Disease in Mexican Limes (Citrus Aurantifolia Swingle). RSC Adv. 2020, 10, 6146–6155. [Google Scholar] [CrossRef]
- Tyree, M.T.; Service, U.F.; Forestry, A.; Box, P.O.; Burlington, S. The Cohesion—Tension Theory of Sap Ascent: Current Controversies. J. Exp. Bot. 1997, 48, 1753–1765. [Google Scholar] [CrossRef] [Green Version]
- Taiz, L.; Zeiger, E. Plant Physiology, 3rd ed.; Sinauer Associates: Sunderland, MA, USA, 2002; ISBN 0878938230. [Google Scholar]
- Wheeler, T.D.; Stroock, A.D. The Transpiration of Water at Negative Pressures in a Synthetic Tree. Nature 2008, 455, 208–212. [Google Scholar] [CrossRef] [PubMed]
- McElrone, A.J.; Choat, B.; Gambetta, G.A.; Brodersen, C.R. Water Uptake and Transport in Vascular Plants. Nat. Sci. Educ. 2013, 4, 6. [Google Scholar] [CrossRef]
- Azoulay-shemer, T.; Palomares, A.; Bagheri, A.; Israelsson-Nordstrom, M.; Engineer, C.B. Guard Cell Photosynthesis Is Critical for Stomatal Turgor Production, yet Does Not Directly Mediate CO2-and ABA-Induced Stomatal Closing. TPJ 2015, 83, 567–581. [Google Scholar] [CrossRef] [Green Version]
- Tardieu, F. Plant Response to Environmental Conditions: Assessing Potential Production, Water Demand, and Negative Effects of Water Deficit. Front. Physiol. 2013, 4, 17. [Google Scholar] [CrossRef] [Green Version]
- Collins, C.; Fryer, M.; Grosso, A. Plant Uptake of Non-Ionic Organic Chemicals. Environ. Sci. Technol. 2006, 40, 45–52. [Google Scholar]
- Spellman, F.R. The Science of Water: Concepts and Applications, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2014; ISBN 9781482242935. [Google Scholar]
- Ferreira, J.A.; Talamine, V.; Facco, J.F.; Rizzetti, T.M.; Ferreira, J.M.S.; Oliveira, F.A.; Prestes, O.D.; Zanella, R.; Martins, M.L.; Adaime, M.B.; et al. Determination of Pesticide Residues in Coconut Tree Trunks by Modified QuEChERS Method and Ultra-High-Performance Liquid Chromatography Coupled to Triple Quadrupole Tandem Mass Spectrometry. Anal. Methods 2015, 7, 4237–4245. [Google Scholar] [CrossRef] [Green Version]
- Gardner, C.L.; da Silva, D.R.; Pagliai, F.A.; Pan, L.; Padgett-Pagliai, K.A.; Blaustein, R.A.; Merli, M.L.; Zhang, D.; Pereira, C.; Teplitski, M.; et al. Assessment of Unconventional Antimicrobial Compounds for the Control of ‘Candidatus Liberibacter asiaticus’, the Causative Agent of Citrus Greening Disease. Sci. Rep. 2020, 10, 5395. [Google Scholar] [CrossRef] [Green Version]
- Killiny, N.; Hijaz, F.; Gonzalez-blanco, P.; Jones, S.E.; Pierre, M.O.; Vincent, C.I. Effect of Adjuvants on Oxytetracycline Uptake upon Foliar Application in Citrus. Antibiotics 2020, 9, 677. [Google Scholar] [CrossRef] [PubMed]
- McVay, J.; Sun, X.; Jones, D.; Urbina, H.; Aldeek, F.; Cook, J.M.; Jeyaprakash, A.; Hodges, G.; Smith, T. Limited Persistence of Residues and Metabolites in Fruit and Juice Following Penicillin Trunk Infusion in Citrus Affected by Huanglongbing. J. Crop Prot. 2019, 125, 104753. [Google Scholar] [CrossRef]
- Li, J.; Pang, Z.; Duan, S.; Lee, D.; Kolbasov, V.G.; Wang, N. The in Planta Effective Concentration of Oxytetracycline against ‘Candidatus Liberibacter asiaticus’ for Suppression of Citrus Huanglongbing. Phytopathology 2019, 109, 2046–2054. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kolbasov, V.G.; Lee, D.; Pang, Z.; Huang, Y.; Collins, N.; Wang, N. Residue Dynamics of Streptomycin in Citrus Delivered by Foliar Spray and Trunk Injection and Effect on “Candidatus Liberibacter asiaticus” Titer. Phytopathology 2021, 111, 1095–1103. [Google Scholar] [CrossRef]
- Li, J.; Kolbasov, V.G.; Pang, Z.; Duan, S.; Lee, D.; Huang, Y.; Xu, J.; Teper, D.; Lamichhane, T.; Wang, N. Evaluation of the Control Effect of SAR Inducers against Citrus Huanglongbing Applied by Foliar Spray, Soil Drench or Trunk Injection. Phytopathol. Res. 2021, 3, 2. [Google Scholar] [CrossRef]
- Archer, L.; Qureshi, J.; Albrecht, U. Efficacy of Trunk Injected Imidacloprid and Oxytetracycline in Managing Huanglongbing and Asian Citrus Psyllid in Infected Sweet Orange (Citrus sinensis) Trees. Agriculture 2022, 12, 1592. [Google Scholar] [CrossRef]
- Hu, J.; Jiang, J.; Wang, N. Control of Citrus Huanglongbing via Trunk Injection of Plant Defense Activators and Antibiotics. Phytopathology 2018, 108, 186–195. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Wang, N. Evaluation of the Spatiotemporal Dynamics of Oxytetracycline and Its Control Effect against Citrus Huanglongbing via Trunk Injection. Phytopathology 2016, 106, 1495–1503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hijaz, F.; Nehela, Y.; Al-Rimawi, F.; Vincent, C.I.; Killiny, N. The Role of the Xylem in Oxytetracycline Translocation within Citrus Trees. Antibiotics 2020, 9, 691. [Google Scholar] [CrossRef]
- Hopkins, D.L.; Ager, K.L. Biological Control of Citrus Huanglongbing with Eb92-1, a Benign Strain of Xylella fastidiosa. Plant Dis. 2021, 105, 2914–2918. [Google Scholar] [CrossRef]
- Yao, T.; Zhou, Y.; Hu, J.; Xiao, T.; Zhou, C. Genomic Evolutionary Relationship of SWEET Genes and Their Responses to HLB Disease and Oxytetracycline Treatment in Valencia Sweet Orange. Biologia 2021, 76, 1685–1689. [Google Scholar] [CrossRef]
- Vincent, C.I.; Hijaz, F.; Pierre, M.; Killiny, N. Systemic Uptake of Oxytetracycline and Streptomycin in Huanglongbing-Affected Citrus Groves after Foliar Application and Trunk Injection. Antibiotics 2022, 11, 1092. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Ascunce, M.S.; Narouei-Khandan, H.A.; Sun, X.; Jones, D.; Kolawole, O.O.; Goss, E.M.; van Bruggen, A.H.C. Effects and Side Effects of Penicillin Injection in Huanglongbing Affected Grapefruit Trees. J. Crop Prot. 2016, 90, 106–116. [Google Scholar] [CrossRef]
- Al-Rimawi, F.; Hijaz, F.; Nehela, Y.; Batuman, O.; Killiny, N. Uptake, Translocation, and Stability of Oxytetracycline and Streptomycin in Citrus Plants. Antibiotics 2019, 8, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Killiny, N.; Gonzalez-Blanco, P.; Santos-Ortega, Y.; Al-Rimawi, F.; Levy, A.; Hijaz, F.; Albrecht, U.; Batuman, O. Tracing Penicillin Movement in Citrus Plants Using Fluorescence-Labeled Penicillin. Antibiotics 2019, 8, 262. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Ashworth, V.E.T.M.; Geitner, N.K.; Wiesner, M.R.; Ginnan, N.; Rolshausen, P.; Roper, C.; Jassby, D. Delivery, Fate, and Mobility of Silver Nanoparticles in Citrus Trees. ACS Nano 2020, 14, 2966–2981. [Google Scholar] [CrossRef]
- Ichinose, K.; Miyazi, K.; Matsuhira, K.; Yasuda, K.; Sadoyama, Y.; Tuan, D.H.; van Bang, D. Unreliable Pesticide Control of the Vector Psyllid Diaphorina Citri (Hemiptera: Psyllidae) for the Reduction of Microorganism Disease Transmission. J. Environ. Sci. Health B 2010, 45, 466–472. [Google Scholar] [CrossRef]
- Aćimović, S.G.; Cregg, B.M.; Sundin, G.W.; Wise, J.C. Comparison of Drill- and Needle-Based Tree Injection Technologies in Healing of Trunk Injection Ports on Apple Trees. Urban For. Urban Green. 2016, 19, 151–157. [Google Scholar] [CrossRef]
- Dalakouras, A.; Jarausch, W.; Buchholz, G.; Bassler, A.; Braun, M.; Manthey, T.; Krczal, G.; Wassenegger, M. Delivery of Hairpin Rnas and Small Rnas into Woody and Herbaceous Plants by Trunk Injection and Petiole Absorption. Front. Plant Sci. 2018, 9, 1253. [Google Scholar] [CrossRef] [Green Version]
- Wise, J.C.; Wise, A.G.; Rakotondravelo, M.; Vandervoort, C.; Seeve, C.; Fabbri, B. Trunk Injection Delivery of DsRNA for RNAi-Based Pest Control in Apple Trees. Pest Manag. Sci. 2022, 78, 3528–3533. [Google Scholar] [CrossRef]
- Coslor, C.C.; Vandervoort, C.; Wise, J.C. Insecticide Dose and Seasonal Timing of Trunk Injection in Apples Influence Efficacy and Residues in Nectar and Plant Parts. Pest Manag. Sci. 2019, 75, 1453–1463. [Google Scholar] [CrossRef] [PubMed]
- Aćimović, S.G.; Vanwoerkom, A.H.; Garavaglia, T.; Vandervoort, C.; Sundin, G.W.; Wise, J.C. Seasonal and Cross-Seasonal Timing of Fungicide Trunk Injections in Apple Trees to Optimize Management of Apple Scab. Plant Dis. 2016, 100, 1606–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Percival, G.C.; Boyle, S. Evaluation of Microcapsule Trunk Injections for the Control of Apple Scab and Powdery Mildew. Ann. Appl. Biol. 2005, 147, 119–127. [Google Scholar] [CrossRef]
- Abbasi, P.A.; Ali, S.; Braun, G.; Bevis, E.; Fillmore, S. Reducing Apple Scab and Frogeye or Black Rot Infections with Salicylic Acid or Its Analogue on Field-Established Apple Trees. Can. J. Plant Pathol. 2019, 41, 345–354. [Google Scholar] [CrossRef]
- Amanifar, N.; Taghavi, M.; Salehi, M. Xylella fastidiosa from Almond in Iran: Overwinter Recovery and Effects of Antibiotics. Phytopathol. Mediterr. 2016, 55, 337–345. [Google Scholar] [CrossRef]
- Gentile, S.; Valentino, D.; Tamietti, G. Control of ink disease by trunk injection of potassium phosphite. J. Plant Pathol. 2009, 91, 565–571. [Google Scholar]
- Akinsanmi, O.A.; Drenth, A. Phosphite and Metalaxyl Rejuvenate Macadamia Trees in Decline Caused by Phytophthora cinnamomi. J. Crop Prot. 2013, 53, 29–36. [Google Scholar] [CrossRef]
- Mokhtaryan, A.; Sheikhigarjan, A.; Arbab, A.; Mohammadipour, A.; Ardestanirostami, H. The Efficiency of Systemic Insecticides and Complete Fertilizer by Trunk Injection Method against Leopard Moth in Infested Walnut Trees. J. Basic Appl. Zool. 2021, 82, 55. [Google Scholar] [CrossRef]
- García-Martínez, M.M.; Campayo, A.; Moratalla-López, N.; de la Hoz, K.S.; Alonso, G.L.; Salinas, M.R. Ozonated Water Applied in Grapevines Is a New Agronomic Practice That Affects the Chemical Quality of Wines. Eur. Food Res. Technol. 2021, 247, 1869–1882. [Google Scholar] [CrossRef]
- Campayo, A.; Cebrián-Tarancón, C.; García-Martínez, M.M.; Salinas, M.R.; Alonso, G.L.; Serrano de la Hoz, K. Preliminary Studies on Endotherapy Based Application of Ozonated Water to Bobal Grapevines: Effect on Wine Quality. Molecules 2022, 27, 5155. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; Langa-Lomba, N.; González-García, V.; Casanova-Gascón, J.; Martín-Gil, J.; Santiago-Aliste, A.; Torres-Sánchez, S.; Martín-Ramos, P. Lignin–Chitosan Nanocarriers for the Delivery of Bioactive Natural Products against Wood-Decay Phytopathogens. Agronomy 2022, 12, 461. [Google Scholar] [CrossRef]
- MacHado, T.O.; Beckers, S.J.; Fischer, J.; Müller, B.; Sayer, C.; De Araújo, P.H.H.; Landfester, K.; Wurm, F.R. Bio-Based Lignin Nanocarriers Loaded with Fungicides as a Versatile Platform for Drug Delivery in Plants. Biomacromolecules 2020, 21, 2755–2763. [Google Scholar] [CrossRef] [PubMed]
- Morton, A.; García-Del-Pino, F. Field Efficacy of the Entomopathogenic Nematode Steinernema Feltiae against the Mediterranean Flat-Headed Rootborer Capnodis Tenebrionis. J. Appl. Entomol. 2008, 132, 632–637. [Google Scholar] [CrossRef]
- Byrne, F.J.; Almanzor, J.; Tellez, I.; Eskalen, A.; Grosman, D.M.; Morse, J.G. Evaluation of Trunk-Injected Emamectin Benzoate as a Potential Management Strategy for Kuroshio Shot Hole Borer in Avocado Trees. J. Crop Prot. 2020, 132, 105136. [Google Scholar] [CrossRef]
- Byrne, F.J.; Urena, A.A.; Robinson, L.J.; Krieger, R.I.; Doccola, J.; Morse, J.G. Evaluation of Neonicotinoid, Organophosphate and Avermectin Trunk Injections for the Management of Avocado Thrips in California Avocado Groves. Pest Manag. Sci. 2012, 68, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Masikane, S.L.; Novela, P.; Mohale, P.; McLeod, A. Effect of Phosphonate Application Timing and -Strategy on Phosphite Fruit and Root Residues of Avocado. J. Crop Prot. 2020, 128, 105008. [Google Scholar] [CrossRef]
- McMahon, P.J.; Purwantara, A.; Wahab, A.; Imron, M.; Lambert, S.; Keane, P.J.; Guest, D.I. Phosphonate Applied by Trunk Injection Controls Stem Canker and Decreases Phytophthora Pod Rot (Black Pod) Incidence in Cocoa in Sulawesi. Australas. Plant Pathol. 2010, 39, 170–175. [Google Scholar] [CrossRef]
- Opoku, I.Y.; Akrofi, A.Y.; Appiah, A.A. Assessment of Sanitation and Fungicide Application Directed at Cocoa Tree Trunks for the Control of Phytophthora Black Pod Infections in Pods Growing in the Canopy. Eur. J. Plant Pathol. 2007, 117, 167–175. [Google Scholar] [CrossRef]
- Martinez, H.E.P.; Poltronieri, Y.; Cecon, P.R. Supplying Zinc Salt Tablets Increased Zinc Concentration and Yield of Coffee Trees. J. Plant Nutr. 2015, 38, 1073–1082. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Ferreira, J.M.S.; Talamini, V.; Lins, P.M.P.; Farias, S.C.C.; Bottoli, C.B.G. Translocation of Pesticides in Coconut Palm by Endotherapy with the Addition of Different Adjuvants. Ciên. Nat. 2020, 42, e56. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Ferreira, J.M.S.; Talamini, V.; de Fátima Facco, J.; Rizzetti, T.M.; Prestes, O.D.; Adaime, M.B.; Zanella, R.; Bottoli, C.B.G. Determination of Pesticides in Coconut (Cocos nucifera Linn.) Water and Pulp Using Modified QuEChERS and LC–MS/MS. Food Chem. 2016, 213, 616–624. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Fassoni, A.C.; Ferreira, J.M.S.; Lins, P.M.P.; Bottoli, C.B.G. Cyproconazole Translocation in Coconut Palm Tree Using Vegetative Endotherapy: Evaluation by LC-MS/MS and Mathematical Modeling. Horticulturae 2022, 8, 1099. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Almeida, G.B.; Lins, P.M.P.; Tavares, M.M.; Farias, S.C.C.; Queiroz, S.C.N. Study of Insecticide Translocation in Coconut Palm Trees after Using Pressurized Endotherapy. Anal. Methods 2022, 14, 4851–4860. [Google Scholar] [CrossRef] [PubMed]
- Paraiba, L.C.; Ferreira, J.M.S.; Ferracini, V.L.; Ramos, S.R.R.; Cerdeira, A.L.; Assalin, M.R.; Pazianotto, R.A.A.; Santos, A.J.; Paraiba, C.C.M. Modeling Pesticide Translocation Injected by Endotherapy into the Stem of Coconut Tree (Cocos nucifera L.). Span. J. Agric. Res. 2022, 20, e1002. [Google Scholar] [CrossRef]
- Schulte, M.J.; Martin, K.; Sauerborn, J. Effects of Azadirachtin Injection in Litchi Trees (Litchi Chinensis Sonn.) on the Litchi Stink Bug (Tessaratoma Papillosa Drury) in Northern Thailand. J. Pest Sci. 2006, 79, 241–250. [Google Scholar] [CrossRef]
- Antoniou, P.P.; Markakis, E.A.; Tjamos, S.E.; Paplomatas, E.J.; Tjamos, E.C. Novel Methodologies in Screening and Selecting Olive Varieties and Root-Stocks for Resistance to Verticillium dahliae. Eur. J. Plant Pathol. 2008, 122, 549–560. [Google Scholar] [CrossRef]
- Amiri, A.; Bussey, K.E.; Riley, M.B.; Schnabel, G. Propiconazole Inhibits Armillaria tabescens in Vitro and Translocates into Peach Roots Following Trunk Infusion. Plant Dis. 2008, 92, 1293–1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mashal, M.M.; Obeidat, B.F. The Efficacy Assessment of Emamectin Benzoate Using Micro Injection System to Control Red Palm Weevil. Heliyon 2019, 5, e01833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atwa, A.A.; Hegazi, E.M. Comparative Susceptibilities of Different Life Stages of the Red Palm Weevil (Coleoptera: Curculionidae) Treated by Entomopathogenic Nematodes. J. Econ. Entomol. 2014, 107, 1339–1347. [Google Scholar] [CrossRef]
- Hesami, A.; Jafari, N.; Shahriari, M.H.; Zolfi, M. Yield and Physico-Chemical Composition of Date-Palm (Phoenix Dactylifera) as Affected by Nitrogen and Zinc Application. Commun. Soil Sci. Plant Anal. 2017, 48, 1943–1954. [Google Scholar] [CrossRef]
- Saleh, J. Yield and Chemical Composition of “Piarom” Date Palm as Affected by Levels and Methods of Iron Fertilization. Int. J. Plant Prod. 2008, 2, 207–214. [Google Scholar] [CrossRef]
- Sarto I Monteys, V.; Ribes, A.C.; Savin, I. The Invasive Longhorn Beetle Xylotrechus Chinensis, Pest of Mulberries, in Europe: Study on Its Local Spread and Efficacy of Abamectin Control. PLoS ONE 2021, 16, e0245527. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Boukouvala, M.C.; Skourti, A.; Nika, E.P.; Papadoulis, G.T. Trunk Injection with Insecticides Manages Xylotrechus chinensis (Chevrolat) (Coleoptera: Cerambycidae). Insects 2022, 13, 1106. [Google Scholar] [CrossRef]
- Chen, Y.; Flint, M.L.; Coleman, T.W.; Doccola, J.J.; Grosman, D.M.; Wood, D.L.; Seybold, S.J. Impact of the Goldspotted Oak Borer, Agrilus auroguttatus, on the Health of Coast Live Oak before and after Treatment with Two Systemic Insecticides. Pest Manag. Sci. 2015, 71, 1540–1552. [Google Scholar] [CrossRef] [PubMed]
- Giraldo-Charria, D.L.; Moreno, F.; Salazar-Uribe, J.C. Effect of Pruning, Fertilization and Pesticide Injection on Crown Dieback in Urban Trees in Colombia: Analysis of Factors Involved. Rev. Fac. Nac. Agron. Medellin 2019, 72, 8883–8895. [Google Scholar] [CrossRef]
- Contreras-Ruiz, C.; Alvarado-Rosales, D.; Cibrián-Tovar, D.; Valdovinos-Ponce, G. Chemical Control with Ethephon (2-Chloroethylphosphonic Acid) of the True Mistletoe Struthanthus interruptus (KUNTH) G. DON. Agrociencia 2018, 52, 741–755. [Google Scholar]
- Flower, C.E.; Dalton, J.E.; Knight, K.S.; Brikha, M.; Gonzalez-Meler, M.A. To Treat or Not to Treat: Diminishing Effectiveness of Emamectin Benzoate Tree Injections in Ash Trees Heavily Infested by Emerald Ash Borer. Urban For. Urban Green. 2015, 14, 790–795. [Google Scholar] [CrossRef] [Green Version]
- Kreutzweiser, D.; Thompson, D.; Grimalt, S.; Chartrand, D.; Good, K.; Scarr, T. Environmental Safety to Decomposer Invertebrates of Azadirachtin (Neem) as a Systemic Insecticide in Trees to Control Emerald Ash Borer. Ecotoxicol. Environ. Saf. 2011, 74, 1734–1741. [Google Scholar] [CrossRef]
- Dal Maso, E.; Cocking, J.; Montecchio, L. Efficacy Tests on Commercial Fungicides against Ash Dieback in Vitro and by Trunk Injection. Urban For. Urban Green. 2014, 13, 697–703. [Google Scholar] [CrossRef]
- McCullough, D.G.; Poland, T.M.; Tluczek, A.R.; Anulewicz, A.; Wieferich, J.; Siegert, N.W. Emerald Ash Borer (Coleoptera: Buprestidae) Densities over a 6-Yr Period on Untreated Trees and Trees Treated with Systemic Insecticides at 1-, 2-, and 3-Yr Intervals in a Central Michigan Forest. J. Econ. Entomol. 2019, 112, 201–212. [Google Scholar] [CrossRef]
- Romero, M.A.; González, M.; Serrano, M.S.; Sánchez, M.E. Trunk Injection of Fosetyl-Aluminium Controls the Root Disease Caused by Phytophthora cinnamomi on Quercus ilex Woodlands. Ann. Appl. Biol. 2019, 174, 313–318. [Google Scholar] [CrossRef]
- Ferracini, C.; Alma, A. How to Preserve Horse Chestnut Trees from Cameraria ohridella in the Urban Environment. J. Crop Prot. 2008, 27, 1251–1255. [Google Scholar] [CrossRef]
- Gubka, A.; Zubrik, M.; Rell, S.; Gareau, N.; Goble, T.; Nikolov, C.; Galko, J.; Vakula, J.; Kunca, A.; Dejonge, R. The Effectiveness of the Neem Product TreeAzin® in Controlling Cameraria ohridella (Lepidoptera: Gracillariidae: Lithocolletinae). Eur. J. Entomol. 2020, 117, 463–473. [Google Scholar] [CrossRef]
- Jagiełło, R.; Walczak, U.; Iszkuło, G.; Karolewski, P.; Baraniak, E.; Giertych, M.J. Impact of Cameraria ohridella on Aesculus hippocastanum Growth and Long-Term Effects of Trunk Injection with Pesticides. Int. J. Pest Manag. 2019, 65, 33–43. [Google Scholar] [CrossRef]
- Kobza, M.; Juhásová, G.; Adamčíková, K.; Onrušková, E. Bauminjektion Zur Bekämpfung Der Rosskastanien-Miniermotte, Cameraria ohridella (Lepidoptera: Gracillariidae). Gesunde Pflanz. 2011, 62, 139–143. [Google Scholar] [CrossRef]
- Ali, A.D.; Caldwell, D.L. Management of Staining and Galling Associated with Oxhorn Bucida Trees in Florida. Fla. Entomol. 2017, 100, 602–606. [Google Scholar] [CrossRef]
- Reding, M.E.; Ranger, C.M. Residue Age and Attack Pressure Influence Efficacy of Insecticide Treatments against Ambrosia Beetles (Coleoptera: Curculionidae). J. Econ. Entomol. 2018, 111, 269–276. [Google Scholar] [CrossRef]
- Ugine, T.A.; Gardescu, S.; Hajek, A.E. The Within-Season and between-Tree Distribution of Imidacloprid Trunk-Injected into Acer platanoides (Sapindales: Sapindaceae). J. Econ. Entomol. 2013, 106, 874–882. [Google Scholar] [CrossRef]
- Rolando, C.A.; Gous, S.F.; Berndt, L.A.; Bulman, L.S.; Carlson, C.A. Stem Injection of a Systemic Insecticide to Control Uraba lugens on Urban Lophostemon confertus Trees. Pest Manag. Sci. 2011, 67, 1062–1068. [Google Scholar] [CrossRef]
- Szczepaniec, A.; Creary, S.F.; Laskowski, K.L.; Nyrop, J.P.; Raupp, M.J. Neonicotinoid Insecticide Imidacloprid Causes Outbreaks of Spider Mites on Elm Trees in Urban Landscapes. PLoS ONE 2011, 6, e20018. [Google Scholar] [CrossRef] [Green Version]
- Xu, T.; Jacobsen, C.M.; Hara, A.H.; Li, J.; Li, Q.X. Efficacy of Systemic Insecticides on the Gall Wasp Quadrastichus erythrinae in Wiliwili Trees (Erythrina spp.). Pest Manag. Sci. 2009, 65, 163–169. [Google Scholar] [CrossRef]
- Chen, R.-F.; Wang, H.-H.; Wang, C.-Y. Translocation and Metabolism of Injected Glyphosate in Lead Tree (Leucaena leucocephala). Weed Sci. 2009, 57, 229–234. [Google Scholar] [CrossRef]
- Maso, E.D.; Linaldeddu, B.T.; Fanchin, G.; Faccoli, M.; Montecchio, L. The Potential for Pesticide Trunk Injections for Control of Thousand Cankers Disease of Walnut. Phytopathol. Mediterr. 2019, 58, 73–79. [Google Scholar]
- Grosman, D.M.; Fettig, C.J.; Jorgensen, C.L.; Munson, A.S. Effectiveness of Two Systemic Insecticides for Protecting Western Conifers from Mortality Due to Bark Beetle Attack. West. J. Appl. For. 2010, 25, 181–185. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, O.; Almqvist, C.; Weslien, J. Systemic Insecticide and Gibberellin Reduced Cone Damage and Increased Flowering in a Spruce Seed Orchard. J. Econ. Entomol. 2012, 105, 916–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cha, D.J.; Kim, J.; Kim, D.S. Nematicidal Activities of Three Naphthoquinones against the Pine Wood Nematode, Bursaphelenchus Xylophilus. Molecules 2019, 24, 3634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, F.; Guo, K.; Chen, A.; Chen, S.; Lin, H.; Zhou, X. Transcriptomic Profiling of Effects of Emamectin Benzoate on the Pine Wood Nematode Bursaphelenchus xylophilus. Pest Manag. Sci. 2020, 76, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Sousa, E.; Naves, P.; Vieira, M. Prevention of Pine Wilt Disease Induced by Bursaphelenchus xylophilus and Monochamus galloprovincialis by Trunk Injection of Emamectin Benzoate. Phytoparasitica 2013, 41, 143–148. [Google Scholar] [CrossRef]
- Lee, S.C.; Lee, H.R.; Kim, D.S.; Kwon, J.H.; Huh, M.J.; Park, I.K. Emamectin Benzoate 9.7% SL as a New Formulation for a Trunk-Injections against Pine Wood Nematode, Bursaphelenchus xylophilus. J. For. Res. 2020, 31, 1399–1403. [Google Scholar] [CrossRef]
- Jeon, H.W.; Park, A.R.; Sung, M.; Kim, N.; Mannaa, M.; Han, G.; Kim, J.; Koo, Y.; Seo, Y.S.; Kim, J.C. Systemic Acquired Resistance-Mediated Control of Pine Wilt Disease by Foliar Application with Methyl Salicylate. Front. Plant Sci. 2022, 12, 812414. [Google Scholar] [CrossRef]
- Di Sora, N.; Rossini, L.; Contarini, M.; Chiarot, E.; Speranza, S. Endotherapic Treatment to Control Toumeyella parvicornis Cockerell Infestations on Pinus pinea L. Pest Manag. Sci. 2022, 78, 2443–2448. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, K.; Yamane, K.; Itoh, Y. Radial Movement of Minerals in the Trunks of Standing Japanese Cedar (Cryptomeria Japonica D. Don) Trees in Summer by Tracer Analysis. Forests 2020, 11, 562. [Google Scholar] [CrossRef]
- Kuroda, K.; Yamane, K.; Itoh, Y. Cellular Level in Planta Analysis of Radial Movement of Artificially Injected Caesium in Cryptomeria Japonica Xylem. Trees 2018, 32, 1505–1517. [Google Scholar] [CrossRef]
- Eisenback, B.M.; Salom, S.M.; Kok, L.T.; Lagalante, A.F. Impacts of Trunk and Soil Injections of Low Rates of Imidacloprid on Hemlock Woolly Adelgid (Hemiptera: Adelgidae) and Eastern Hemlock (Pinales: Pinaceae) Health. J. Econ. Entomol. 2014, 107, 250–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chihaoui-Meridja, S.; Harbi, A.; Abbes, K.; Chaabane, H.; La Pergola, A.; Chermiti, B.; Suma, P. Systematicity, Persistence and Efficacy of Selected Insecticides Used in Endotherapy to Control the Red Palm Weevil Rhynchophorus ferrugineus (Olivier, 1790) on Phoenix canariensis. Phytoparasitica 2020, 48, 75–85. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, J.; Li, Y.; Li, J.; Shi, X.H. Evaluation of the Effectiveness of Insecticide Trunk Injections for Control of Latoia lepida (Cramer) in the Sweet Olive Tree Osmanthus fragrans. PeerJ 2016, 2016, e2480. [Google Scholar] [CrossRef] [Green Version]
- Bhandari, B.P.; Cheng, Z. Trunk Injection of Systemic Insecticides to Control Stem and Leaf Gall Wasps, Josephiella Species (Hymenoptera: Agaonidae), on Chinese Banyan (Rosales: Moraceae) in Hawaii. Fla Entomol. 2016, 99, 172–177. [Google Scholar] [CrossRef]
- Pavela, R.; Žabka, M.; Kalinkin, V.; Kotenev, E.; Gerus, A.; Shchenikova, A.; Chermenskaya, T. Systemic Applications of Azadirachtin in the Control of Corythucha ciliata (Say, 1832) (Hemiptera, Tingidae), a Pest of Platanus sp. Plant Protect. Sci. 2013, 49, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Reding, M.E.; Oliver, J.B.; Schultz, P.B.; Ranger, C.M.; Youssef, N.N. Ethanol Injection of Ornamental Trees Facilitates Testing Insecticide Efficacy against Ambrosia Beetles (Coleoptera: Curculionidae: Scolytinae). J. Econ. Entomol. 2013, 106, 289–298. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.H.; Che, S.C.; Qiu, L.F.; Li, G.; Shao, J.L.; Zhong, L.; Zhang, G.F.; Xu, H. Efficacy of Emamectin Benzoate Trunk Injection against the Asian Long-Horned Beetle [Anoplophora Glabripennis (Coleoptera: Cerambycidae)]. J. Econ. Entomol. 2020, 113, 340–347. [Google Scholar] [CrossRef]
- Baró, A.; Saldarelli, P.; Saponari, M.; Montesinos, E.; Montesinos, L. Nicotiana benthamiana as a Model Plant Host for Xylella fastidiosa: Control of Infections by Transient Expression and Endotherapy with a Bifunctional Peptide. Front. Plant Sci. 2022, 13, 1061463. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Kaneko, S.; Yoshimura, T. Effects of Dinotefuran Trunk Injection against the Red-Necked Longhorn Beetle Aromia Bungii (Coleoptera: Cerambycidae) in Japanese Flowering Cherry Trees. J. For. Res. 2022, 27, 460–468. [Google Scholar] [CrossRef]
- Yang, J.; Duan, G.; Li, C.; Liu, L.; Han, G.; Zhang, Y.; Wang, C. The Crosstalks Between Jasmonic Acid and Other Plant Hormone Signaling Highlight the Involvement of Jasmonic Acid as a Core Component in Plant Response to Biotic and Abiotic Stresses. Front. Plant Sci. 2019, 10, 1349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werrie, P.Y.; Burgeon, C.; Le Goff, G.J.; Hance, T.; Fauconnier, M.L. Biopesticide Trunk Injection into Apple Trees: A Proof of Concept for the Systemic Movement of Mint and Cinnamon Essential Oils. Front. Plant Sci. 2021, 12, 650132. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Barbosa, P.; Vieira, P.; Vicente, C.S.L.; Figueiredo, A.C.; Mota, M. Phytochemicals as Biopesticides against the Pinewood Nematode Bursaphelenchus xylophilus: A Review on Essential Oils and Their Volatiles. Plants 2021, 10, 2614. [Google Scholar] [CrossRef]
- Huang, C.Y.; Araujo, K.; Sánchez, J.N.; Kund, G.; Trumble, J.; Roper, C.; Godfrey, K.E.; Jin, H. A Stable Antimicrobial Peptide with Dual Functions of Treating and Preventing Citrus Huanglongbing. Proc. Natl. Acad. Sci. USA 2021, 118, e2019628118. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Queiroz, S.C.N. Multiresidue Method for Determination of Pesticides in Coconut (Cocos nucifera Linn.) Endosperm by Using GC–MS/MS and UHPLC–MS/MS Analysis. J. Food Compos. Anal. 2021, 97, 103764. [Google Scholar] [CrossRef]




| Classifications | Species | Cultivars/Scientific name | References | |
|---|---|---|---|---|
| Citrus trees | Orange trees | Citrus sinensis | [59,60,61] | |
| Citrus sinensis (L.) Osbeck on Swingle citrumelo (Citrus paradisi) Macf Duncan grapefruit x Poncirus trifoliata (L.) Raf | [62,63,64,65] | |||
| Citrus sinensis L. Osbeck | ||||
| Hamlin sweet orange on Swingle citrumelo rootstock | [60,61, 62,63,64,65,66,67,68] | |||
| Valencia orange trees | [69] | |||
| Citrus sinensis L. cv. Valencia | [70] | |||
| Hamlin sweet orange (C. x sinensis) | [71] | |||
| Grapefruit trees | Citrus paradisi Macfad | [59,61,72] | ||
| Lime trees | Citrus aurantifolia Swingle | [49,73,74] | ||
| Mexican lime and clementine mandarin cv. Nour grafted to Carrizo rootstock | [22,75] | |||
| Pomelo trees | Citrus maxima | [16] | ||
| Mandarin trees | Citrus nobilis Loureiro onto volkameriana rootstock (C. volkameriana Pasquale) | [76] | ||
| Apple trees | Malus domestica Borkhausen | [12,32,77,78] | ||
| Malus pumila Miller | [10,79] | |||
| Malus domestica Borkhausen cv. Red Delicious | [80,81] | |||
| Malus domestica Borkh. cv. Gala | [30] | |||
| Malus domestica Borkh. cv. Mac Spur | [12,81] | |||
| No specific cultivar | [82,83] | |||
| Nut trees | Almond trees | Prunus dulcis | [33] | |
| Macadamia trees | [84] | |||
| Chestnut and walnut trees | [85] | |||
| Chestnut variety Marrone di Chiusa Pesio | [86] | |||
| Persian walnut trees | Juglans regia L. | [87] | ||
| Grapevine trees | [19,69,78,88,89,90,91] | |||
| Cherry trees | [92] | |||
| Prunus cerasifera and Prunus spp. | [13] | |||
| Avocado trees | [9,93,94,95] | |||
| Cacao trees | [96,97] | |||
| Coffee trees | [98] | |||
| Coconut palm trees | PB 121 coconut hybrid | [58,99,100,101,102] | ||
| [5,103] | ||||
| Litchi trees | [104] | |||
| Olive trees | [105] | |||
| Peach trees | [106] | |||
| Date palm trees | [107,108,109,110] | |||
| Pear trees | Pyrus communis L., var. Bartlett | [33] | ||
| Mulberry trees | [111,112] | |||
| Classifications | Species | Cultivars/Scientific Name | References |
|---|---|---|---|
| Deciduous trees | Ash | Fraxinus spp. | [29,115,116,117] |
| White ash | Fraxinus americana | [11,27] | |
| Common ash | Fraxinus excelsior | [21,22,23,118] | |
| Green ash | Fraxinus pennsylvanica Marsh. | [11,27,44,119] | |
| Poplar and Ficus | Populus, Ficus | [115] | |
| Coast live oak | Quercus agrifolia | [113] | |
| Cork oak | Quercus suber L. | [42] | |
| OakEnglish oakPlane treesLondon plane | Quercus spp.; Quercus robur; Platanus spp.; Platanus acerifolia | [13] | |
| Holm oak | Quercus ilex | [3,120] | |
| Pedunculate oak | Quercus robur L. | [82] | |
| Horse chestnut | Aesculus hippocastanum L. | [13,121,122,123,124] | |
| Black olive | Bucida buceras L. | [125,126] | |
| Paper birch | Betula papyrifera Marsh. | [46] | |
| Norway maple | Acer platanoides L. | [127] | |
| Queensland Brush Box. | Lophostemon confertus | [128] | |
| Elm | Ulmus americana | [129] | |
| Wiliwili | Erythrina spp. | [130] | |
| Lead | Leucaena leucocephala | [131] | |
| Black walnut | Juglans nigra | [132] | |
| Black cherry | Prunus serotina Ehrarth | [17] | |
| Archontophoenix cunninghamiana (H.Wendl.) H.Wendl. & Drude; Bauhinia picta (Kunth) DC.; Caesalpinia pluviosa DC.; Eriobotrya japonica (Thunb.) Lindl.; Ficus benjamina L; Fraxinus chinensis Roxb.; Handroanthus chrysanthus (Jacq.) S. O. Grose; Jacaranda mimosifolia D. Don; Lafoensia punicifolia DC.; Lagerstroemia speciosa (L.) Pers.; Pithecellobium dulce (Roxb.) Benth.; Roystonea regia (Kunth) O. F. Cook; Spathodea campanulata P. Beauv.; Terminalia catappa L.; Syzygium malaccense (L.) Merr. & L. M. Perry. | [114] | ||
| Classifications | Species | Cultivars/Scientific Name | References |
|---|---|---|---|
| Coniferous trees | Pine | Pinus massoniana | [136] |
| Pinus thunbergii | [135,138] | ||
| Pinus pinaster Aiton | [137] | ||
| Pinus ponderosa, Pinus contorta, Picea engelmannii | [133] | ||
| Pinus densiflora | [139] | ||
| Pinus pinea L. | [140] | ||
| Japanese Cedar | Cryptomeria japonica | [141,142] | |
| Eastern Hemlock | Tsuga canadensis Carrière | [143] | |
| Hemlock | Tsuga spp. | [41] | |
| Grand fir, Douglas-fir, alpine fir | [2] | ||
| Cedar of Lebanon | Cedrus libani | [13] | |
| Norway spruce | Picea abies (L.) Karst. | [134] | |
| Classifications | Species | Cultivars/Scientific Name | References |
|---|---|---|---|
| Ornamental trees | Canary Island date palm | Phoenix canariensis | [43,144] |
| Sweet olive | Osmanthus fragrans | [145] | |
| Chinese banyan | Ficus microcarpa L. | [146] | |
| Plane | Platanus × acerifolia (Aiton) Willd | [147] | |
| Magnolia virginiana L. | [148] | ||
| Willow | Salix matsudana cv. ‘Pendula’ | [149] | |
| Palm tree | [13] | ||
| Tobacco Nicotiana benthamiana | [150] | ||
| Japanese cherry trees | [151] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, J.A.; Esparraguera, L.B.; Queiroz, S.C.N.; Bottoli, C.B.G. Vegetative Endotherapy—Advances, Perspectives, and Challenges. Agriculture 2023, 13, 1465. https://doi.org/10.3390/agriculture13071465
Ferreira JA, Esparraguera LB, Queiroz SCN, Bottoli CBG. Vegetative Endotherapy—Advances, Perspectives, and Challenges. Agriculture. 2023; 13(7):1465. https://doi.org/10.3390/agriculture13071465
Chicago/Turabian StyleFerreira, Jordana Alves, Llorenç Baronat Esparraguera, Sonia Claudia Nascimento Queiroz, and Carla Beatriz Grespan Bottoli. 2023. "Vegetative Endotherapy—Advances, Perspectives, and Challenges" Agriculture 13, no. 7: 1465. https://doi.org/10.3390/agriculture13071465







