Evaluation of Antiphytoviral and Antibacterial Activity of Essential Oil and Hydrosol Extracts from Five Veronica Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Extracts—Review Protocol
2.2. Antiphytoviral Activity Assay
2.3. Antibacterial Activity
2.3.1. Microbial Strains and Culture Conditions
2.3.2. Broth Microdilution Testing
2.4. Statistical Analysis
3. Results and Discussion
3.1. Free Volatile Compounds of Five Veronica Species
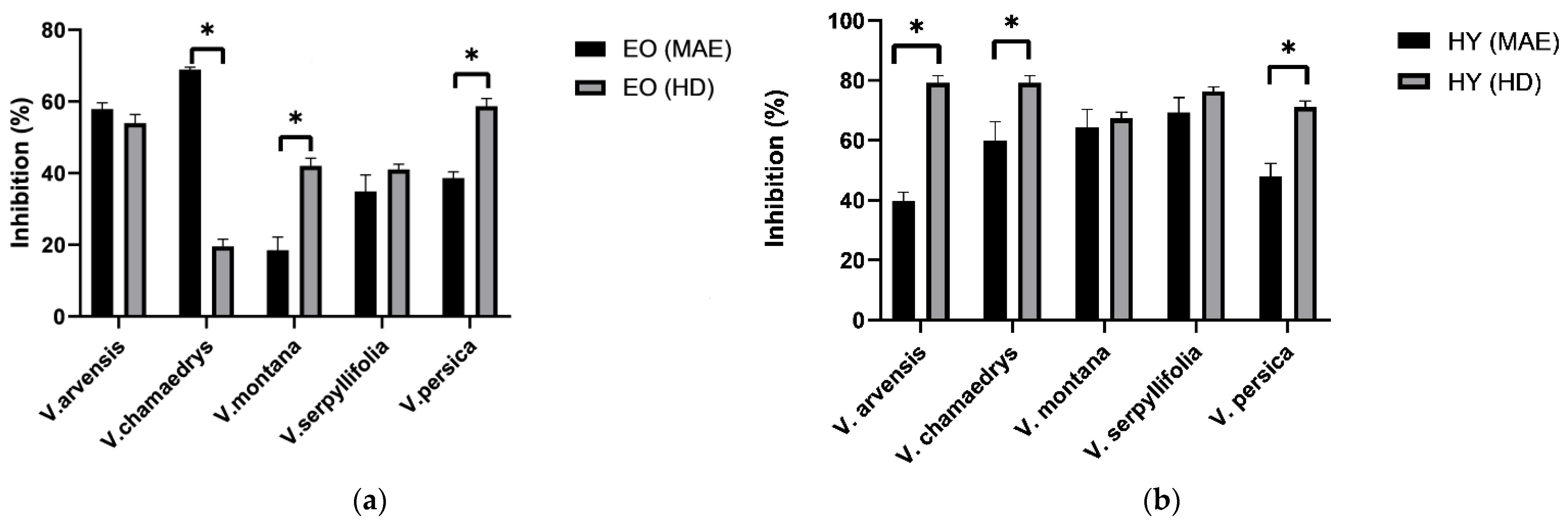
3.2. Antiphytoviral Activity of Veronica Species
3.3. Antibacterial Activity of Five Veronica Species
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ngegba, P.M.; Cui, G.; Khalid, M.Z.; Zhong, G. Use of Botanical Pesticides in Agriculture as an Alternative to Synthetic Pesticides. Agriculture 2022, 12, 600. [Google Scholar] [CrossRef]
- Souto, A.L.; Sylvestre, M.; Tölke, E.D.; Tavares, J.F.; Barbosa-Filho, J.M.; Cebrián-Torrejón, G. Plant-Derived Pesticides as an Alternative to Pest Management and Sustainable Agricultural Production: Prospects, Applications and Challenges. Molecules 2021, 26, 4835. [Google Scholar] [CrossRef] [PubMed]
- Koma, S. Plants as Potential Sources of Pesticidal Agents: A Review. In Pesticides—Advances in Chemical and Botanical Pesticides; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Ali, A.D.; Ior, L.D.; Dogo, G.A.; Joshua, J.I.; Gushit, J.S. Ethnobotanical Survey of Plants Used as Biopesticides by Indigenous People of Plateau State, Nigeria. Diversity 2022, 14, 851. [Google Scholar] [CrossRef]
- Albach, D.C.; Martínez-Ortega, M.M.; Fischer, M.A.; Chase, M.W. A New Classification of the Tribe Veroniceae-Problems and a Possible Solution. Taxon 2004, 53, 429–452. [Google Scholar] [CrossRef]
- Walters, S.M.; Webb, D.A. Veronica L. In Flora Europaea; Tutin, T.G., Heywood, V.H., Burges, N.A., Moore, D.M., Valentine, D.H., Walters, S.M., Webb, D.A., Eds.; Cambridge University Press: Cambridge, UK, 1972; Volume 3, pp. 242–251. ISBN 052108489X. [Google Scholar]
- Xue, H.; Chen, K.-X.; Zhang, L.-Q.; Li, Y.-M. Review of the Ethnopharmacology, Phytochemistry, and Pharmacology of the Genus Veronica. Am. J. Chin. Med. 2019, 47, 1193–1221. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Shetty, M.S.; Anil Kumar, N.V.; Živković, J.; Calina, D.; Docea, A.O.; Emamzadeh-Yazdi, S.; Kılıç, C.S.; Goloshvili, T.; Nicola, S.; et al. Veronica Plants—Drifting from Farm to Traditional Healing, Food Application, and Phytopharmacology. Molecules 2019, 24, 2454. [Google Scholar] [CrossRef]
- Kostadinova, E.P.; Alipieva, K.I.; Kokubun, T.; Taskova, R.M.; Handjieva, N.V. Phenylethanoids, Iridoids and a Spirostanol Saponin from Veronica turrilliana. Phytochemistry 2007, 68, 1321–1326. [Google Scholar] [CrossRef]
- Mocan, A.; Vodnar, D.C.; Vlase, L.; Crișan, O.; Gheldiu, A.M.; Crișan, G. Phytochemical Characterization of Veronica officinalis L., V. teucrium L. and V. orchidea Crantz from Romania and Their Antioxidant and Antimicrobial Properties. Int. J. Mol. Sci. 2015, 16, 21109–21127. [Google Scholar] [CrossRef]
- Jun, Y.; Lee, S.M.; Ju, H.K.; Lee, H.J.; Choi, H.K.; Jo, G.S.; Kim, Y.S. Comparison of the Profile and Composition of Volatiles in Coniferous Needles According to Extraction Methods. Molecules 2016, 21, 363. [Google Scholar] [CrossRef]
- Kovaleva, A.; Osmachko, A.; Ilina, T.; Goryacha, O.; Omelyanchik, L.; Grytsyk, A.; Koshovyi, O. Chemical Composition of Essential Oils from Flowers of Veronica longifolia L., Veronica incana L. and Veronica spicata L. Sci. Rise Pharm. Sci. 2022, 38, 69–79. [Google Scholar] [CrossRef]
- Nazlić, M.; Kremer, D.; Akrap, K.; Topić, S.; Vuletić, N.; Dunkić, V. Extraction, Composition and Comparisons–Free Volatile Compounds from Hydrosols of Nine Veronica Taxa. Horticulturae 2023, 9, 16. [Google Scholar] [CrossRef]
- Dunkić, V.; Nazlić, M.; Ruščić, M.; Vuko, E.; Akrap, K.; Topić, S.; Milović, M.; Vuletić, N.; Puizina, J.; Jurišić Grubešić, R.; et al. Hydrodistillation and Microwave Extraction of Volatile Compounds: Comparing Data for Twenty-One Veronica Species from Different Habitats. Plants 2022, 11, 902. [Google Scholar] [CrossRef] [PubMed]
- Nazlić, M.; Akrap, K.; Kremer, D.; Dunkić, V. Hydrosols of Veronica Species—Natural Source of Free Volatile Compounds with Potential Pharmacological Interest. Pharmaceuticals 2022, 15, 1378. [Google Scholar] [CrossRef]
- Aćimović, M.; Tešević, V.; Smiljanić, K.; Cvetković, M.; Stanković, J.; Kiprovski, B.; Sikora, V. Hydrolates: By-Products of Essential Oil Distillation: Chemical Composition, Biological Activity and Potential Uses. Adv. Technol. 2020, 9, 54–70. [Google Scholar] [CrossRef]
- Ilieva, Y.; Dimitrova, L.; Georgieva, A.; Vilhelmova-Ilieva, N.; Zaharieva, M.M.; Kokanova-Nedialkova, Z.; Dobreva, A.; Nedialkov, P.; Kussovski, V.; Kroumov, A.D.; et al. In Vitro Study of the Biological Potential of Wastewater Obtained after the Distillation of Four Bulgarian Oil-Bearing Roses. Plants 2022, 11, 1073. [Google Scholar] [CrossRef] [PubMed]
- Raveau, R.; Fontaine, J.; Lounès-Hadj Sahraoui, A. Essential Oils as Potential Alternative Biocontrol Products against Plant Pathogens and Weeds: A Review. Foods 2020, 9, 365. [Google Scholar] [CrossRef]
- Stenberg, J.A.; Sundh, I.; Becher, P.G.; Björkman, C.; Dubey, M.; Egan, P.A.; Friberg, H.; Gil, J.F.; Jensen, D.F.; Jonsson, M.; et al. When Is It Biological Control? A Framework of Definitions, Mechanisms, and Classifications. J. Pest. Sci. 2021, 94, 665–676. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Dachbrodt-Saaydeh, S.; Kudsk, P.; Messéan, A. Toward a Reduced Reliance on Conventional Pesticides in European Agriculture. Plant Dis. 2016, 100, 10–24. [Google Scholar] [CrossRef]
- Vuko, E.; Rusak, G.; Dunkic, V.; Kremer, D.; Kosalec, I.; Rada, B.; Bezic, N. Inhibition of Satellite RNA Associated Cucumber Mosaic Virus Infection by Essential Oil of Micromeria croatica (Pers.) Schott. Molecules 2019, 24, 1342. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Oluwaseun Ademiluyi, A.; et al. Biological Activities of Essential Oils: From Plant Chemoecology to Traditional Healing Systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef]
- Wani, A.R.; Yadav, K.; Khursheed, A.; Rather, M.A. An Updated and Comprehensive Review of the Antiviral Potential of Essential Oils and Their Chemical Constituents with Special Focus on Their Mechanism of Action against Various Influenza and Coronaviruses. Microb. Pathog. 2021, 152, 104620. [Google Scholar] [CrossRef] [PubMed]
- Mieres-Castro, D.; Ahmar, S.; Shabbir, R.; Mora-Poblete, F. Antiviral Activities of Eucalyptus Essential Oils: Their Effectiveness as Therapeutic Targets against Human Viruses. Pharmaceuticals 2021, 14, 1210. [Google Scholar] [CrossRef] [PubMed]
- Beeby, E.; Magalhães, M.; Poças, J.; Collins, T.; Lemos, M.F.L.; Barros, L.; Ferreira, I.C.F.R.; Cabral, C.; Pires, I.M. Secondary Metabolites (Essential Oils) from Sand-Dune Plants Induce Cytotoxic Effects in Cancer Cells. J. Ethnopharmacol. 2020, 258, 112803. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Isman, M.B.; Tak, J.H. Insecticidal Activity of 28 Essential Oils and a Commercial Product Containing Cinnamomum cassia Bark Essential Oil against Sitophilus zeamais Motschulsky. Insects 2020, 11, 474. [Google Scholar] [CrossRef] [PubMed]
- Cazella, L.N.; Glamoclija, J.; Soković, M.; Gonçalves, J.E.; Linde, G.A.; Colauto, N.B.; Gazim, Z.C. Antimicrobial Activity of Essential Oil of Baccharis dracunculifolia DC (Asteraceae) Aerial Parts at Flowering Period. Front. Plant. Sci. 2019, 10, 27. [Google Scholar] [CrossRef]
- Vuko, E.; Radman, S.; Jerković, I.; Kamenjarin, J.; Vrkić, I.; Fredotović, Ž. A Plant Worthy of Further Study—Volatile and Non-Volatile Compounds of Portenschlagiella ramosissima (Port.) Tutin and Its Biological Activity. Pharmaceuticals 2022, 15, 1454. [Google Scholar] [CrossRef]
- Bishop, C.D. Antiviral Activity of the Essential Oil of Melaleuca alternifolia (Maiden Amp; Betche) Cheel (Tea Tree) against Tobacco Mosaic Virus. J. Essent. Oil Res. 1995, 7, 641–644. [Google Scholar] [CrossRef]
- Pandey, A.K.; Kumar, P.; Singh, P.; Tripathi, N.N.; Bajpai, V.K. Essential Oils: Sources of Antimicrobials and Food Preservatives. Front. Microbiol. 2017, 7, 2161. [Google Scholar] [CrossRef]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- World Health Organization. Foodborne Disease Burden Epidemiology Reference Group. In WHO Estimates of the Global Burden of Foodborne Diseases; World Health Organization: Geneva, Switzerland, 2015; ISBN 9789241565165. [Google Scholar]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Kattula, D.; Burkert, F. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Vuko, E.; Dunkić, V.; Maravić, A.; Ruščić, M.; Nazlić, M.; Radan, M.; Ljubenkov, I.; Soldo, B.; Fredotović, Ž. Not Only a Weed Plant—Biological Activities of Essential Oil and Hydrosol of Dittrichia viscosa (L.) Greuter. Plants 2021, 10, 1837. [Google Scholar] [CrossRef]
- Fredotović, Ž.; Puizina, J.; Nazlić, M.; Maravić, A.; Ljubenkov, I.; Soldo, B.; Vuko, E.; Bajić, D. Phytochemical Characterization and Screening of Antioxidant, Antimicrobial and Antiproliferative Properties of Allium × Cornutum Clementi and Two Varieties of Allium cepa L. Peel Extracts. Plants 2021, 10, 832. [Google Scholar] [CrossRef] [PubMed]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 12.0. Available online: http://www.eucast.org (accessed on 13 June 2023).
- Maravić, A.; Rončević, T.; Krce, L.; Ilić, N.; Galić, B.; Čulić Čikeš, V.; Carev, I. Halogenated Boroxine Dipotassium Trioxohydroxytetrafluorotriborate K2[B3O3F4OH] Inhibits Emerging Multidrug-Resistant and β-Lactamase-Producing Opportunistic Pathogens. Drug Dev. Ind. Pharm. 2019, 45, 1770–1776. [Google Scholar] [CrossRef] [PubMed]
- Popović, M.; Maravić, A.; Čulić, V.Č.; Đulović, A.; Burčul, F.; Blažević, I. Biological Effects of Glucosinolate Degradation Products from Horseradish: A Horse That Wins the Race. Biomolecules 2020, 10, 343. [Google Scholar] [CrossRef] [PubMed]
- Shukurova, M.K.; Asikin, Y.; Chen, Y.; Kusano, M.; Watanabe, K.N. Profiling of Volatile Organic Compounds in Wild Indigenous Medicinal Ginger (Zingiber barbatum Wall.) from Myanmar. Metabolites 2020, 10, 248. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Lao, Y.; Pan, Y.; Chen, Y.; Zhao, H.; Gong, L.; Xie, N.; Mo, C.H. Synergistic Antimicrobial Effectiveness of Plant Essential Oil and Its Application in Seafood Preservation: A Review. Molecules 2021, 26, 307. [Google Scholar] [CrossRef] [PubMed]
- Delazar, A.; Nahar, L.; Hamedeyazdan, S.; Sarker, S.D. Microwave-Assisted Extraction in Natural Products Isolation. Methods Mol. Biol. 2012, 864, 89–115. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Han, Z.; Xu, Y.; Yao, L. In Vitro and In Vivo Anti-Tobacco Mosaic Virus Activities of Essential Oils and Individual Compounds. J. Microbiol. Biotechnol. 2013, 23, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Othman, B.A.; Shoman, S.A. Antiphytoviral Activity of the Plectranthus tenuiflorus on Some Important Viruses. Int. J. Agric. Biol. 2004, 6, 844–849. [Google Scholar]
- Taglienti, A.; Donati, L.; Ferretti, L.; Tomassoli, L.; Sapienza, F.; Sabatino, M.; Di Massimo, G.; Fiorentino, S.; Vecchiarelli, V.; Nota, P.; et al. In Vivo Antiphytoviral Activity of Essential Oils and Hydrosols From Origanum vulgare, Thymus vulgaris, and Rosmarinus officinalis to Control Zucchini Yellow Mosaic Virus and Tomato Leaf Curl New Delhi Virus in Cucurbita pepo L. Front. Microbiol. 2022, 13, 840893. [Google Scholar] [CrossRef]
- Soosaar, J.L.M.; Burch-Smith, T.M.; Dinesh-Kumar, S.P. Mechanisms of Plant Resistance to Viruses. Nat. Rev. Microbiol. 2005, 3, 789–798. [Google Scholar] [CrossRef]
- Kim, D.S.; Hwang, B.K. An Important Role of the Pepper Phenylalanine Ammonia-Lyase Gene (PAL1) in Salicylic Acid-Dependent Signalling of the Defence Response to Microbial Pathogens. J. Exp. Bot. 2014, 65, 2295–2306. [Google Scholar] [CrossRef] [PubMed]
- Abdelkhalek, A.; Al-Askar, A.A.; Hafez, E. Differential Induction and Suppression of the Potato Innate Immune System in Response to Alfalfa Mosaic Virus Infection. Physiol. Mol. Plant. Pathol. 2020, 110, 101485. [Google Scholar] [CrossRef]
- Berka-Zougali, B.; Ferhat, M.A.; Hassani, A.; Chemat, F.; Allaf, K.S. Comparative Study of Essential Oils Extracted from Algerian Myrtus communis L. Leaves Using Microwaves and Hydrodistillation. Int. J. Mol. Sci. 2012, 13, 4673–4695. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, F.; Liu, J.; Zou, Y.; Chen, X. A Comparison of Volatile Fractions Obtained from Lonicera macranthoides via Different Extraction Processes: Ultrasound, Microwave, Soxhlet Extraction, Hydrodistillation, and Cold Maceration. Integr. Med. Res. 2015, 4, 171–177. [Google Scholar] [CrossRef]
- Dunkić, V.; Kosalec, I.; Kosir, I.; Potocnik, T.; Cerenak, A.; Koncic, M.; Vitali, D.; Muller, I.; Kopricanec, M.; Bezic, N.; et al. Antioxidant and Antimicrobial Properties of Veronica spicata L. (Plantaginaceae). Curr. Drug Targets 2015, 16, 1660–1670. [Google Scholar] [CrossRef]
- Stojković, D.S.; Živković, J.; Soković, M.; Glamočlija, J.; Ferreira, I.C.F.R.; Janković, T.; Maksimović, Z. Antibacterial Activity of Veronica montana L. Extract and of Protocatechuic Acid Incorporated in a Food System. Food Chem. Toxicol. 2013, 55, 209–213. [Google Scholar] [CrossRef]
- Hassan, A.; Ullah, H.; Bonomo, M.G. Antibacterial and Antifungal Activities of the Medicinal Plant Veronica biloba. J. Chem. 2019, 2019, 5264943. [Google Scholar] [CrossRef]
- Živković, J.; Barreira, J.C.M.; Stojković, D.; Ćebović, T.; Santos-Buelga, C.; Maksimović, Z.; Ferreira, I.C.F.R. Phenolic Profile, Antibacterial, Antimutagenic and Antitumour Evaluation of Veronica urticifolia Jacq. J. Funct. Foods 2014, 9, 192–201. [Google Scholar] [CrossRef]
- Li, X.Z.; Nikaido, H. Efflux-Mediated Drug Resistance in Bacteria: An Update. Drugs 2009, 69, 1555–1623. [Google Scholar] [CrossRef]
- Lambert, P.A. Cellular Impermeability and Uptake of Biocides and Antibiotics in Gram-Positive Bacteria and Mycobacteria. J. Appl. Microbiol. 2002, 92, 46S–54S. [Google Scholar] [CrossRef]
- Vogl, S.; Picker, P.; Mihaly-Bison, J.; Fakhrudin, N.; Atanasov, A.G.; Heiss, E.H.; Wawrosch, C.; Reznicek, G.; Dirsch, V.M.; Saukel, J.; et al. Ethnopharmacological in Vitro Studies on Austria’s Folk Medicine—An Unexplored Lore in Vitro Anti-Inflammatory Activities of 71 Austrian Traditional Herbal Drugs. J. Ethnopharmacol. 2013, 149, 750–771. [Google Scholar] [CrossRef]
- Harput, U.Ş.; Genç, Y.; Khan, N.; Saracoglu, I. Radical Scavenging Effects of Different Veronica Species. Rec. Nat. Prod. 2011, 5, 100–107. [Google Scholar]
- Datta, A.; Burall, L. Current Trends in Foodborne Human Listeriosis. Food Saf. 2018, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ki, V.; Rotstein, C. Bacterial Skin and Soft Tissue Infections in Adults: A Review of Their Epidemiology, Pathogenesis, Diagnosis, Treatment and Site of Care. Can. J. Infect. Dis. Med. Microbiol. 2008, 19, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Chambers, H.F.; DeLeo, F.R. Waves of Resistance: Staphylococcus Aureus in the Antibiotic Era. Nat. Rev. Microbiol. 2009, 7, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Ali, E.S.; Uddin, S.J.; Shaw, S.; Islam, M.A.; Ahmed, M.I.; Chandra Shill, M.; Karmakar, U.K.; Yarla, N.S.; Khan, I.N.; et al. Phytol: A Review of Biomedical Activities. FCT 2018, 121, 82–94. [Google Scholar] [CrossRef]
- Montanari, R.M.; Barbosa, L.C.; Demuner, A.J.; Silva, C.J.; Carvalho, L.S.; Andrade, N.J. Chemical Composition and Antibacterial Activity of Essential Oils from Verbenaceae Species: Alternative Sources of (E)-Caryophyllene and Germacrene-D. Quim. Nova 2011, 34, 1550–1555. [Google Scholar] [CrossRef]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial Activity of Terpenes and Terpenoids Present in Essential Oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef]
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A. A Comprehensive Review of the Antibacterial, Antifungal and Antiviral Potential of Essential Oils and Their Chemical Constituents against Drug-Resistant Microbial Pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef]
- Ouattara, B.; Simard, R.E.; Holley, R.A.; Piette, G.J.-P.; Bégin, A. Antibacterial Activity of Selected Fatty Acids and Essential Oils against Six Meat Spoilage Organisms. Int. J. Food Microbiol. 1997, 37, 155–162. [Google Scholar] [CrossRef]
- Oz, M.; Lozon, Y.; Sultan, A.; Yang, K.H.S.; Galadari, S. Effects of Monoterpenes on Ion Channels of Excitable Cells. Pharmacol. Ther. 2015, 152, 83–97. [Google Scholar] [CrossRef] [PubMed]
| Taxa | Locality | Latitude | Longitude | Altitude a.s.l. (m) | Voucher No. |
|---|---|---|---|---|---|
| V. arvensis | Hvar Island | 43°10′42.3″ N | 16°36′43.6″ E | 38 | CROVeS-12-2021 |
| V. chamaedrys | Radoboj | 46°09′49.4″ N | 15°55′36.1″ E | 260 | CROVeS-13-2021 |
| V. montana | Papuk Mt | 45°30′38.1″ N | 17°39′57.2″ E | 761 | CROVeS-15-2021 |
| V. serpyllifolia | Zagreb | 45°49′40.3″ N | 15°58′59.5″ E | 192 | CROVeS-20-2021 |
| V. persica | Samoborsko gorje | 45°49′41.6″ N | 15°40′32.9″ E | 301 | CROVeS-18-2021 |
| Species | Isolation Method | EO Fraction | HY Fraction | ||
|---|---|---|---|---|---|
| Stock Conc. | Max. Tested Conc. | Stock Conc. | Max. Tested Conc. | ||
| V. arvensis | HD | 58.03 | 17.43 | 8.16 | 2.45 |
| V. arvensis | MAE | 56.45 | 16.95 | 6.21 | 1.86 |
| V. chamaedrys | HD | 81.6 | 24.5 | 5.03 | 1.51 |
| V. chamaedrys | MAE | 67.9 | 20.39 | 9.7 | 2.91 |
| V. montana | MAE | 75.3 | 22.61 | 7.39 | 2.22 |
| V. montana | HD | 114.45 | 34.37 | 6.17 | 1.85 |
| V. serpyllifolia | MAE | 65.18 | 19.57 | 5.9 | 1.77 |
| V. serpyllifolia | HD | 90.88 | 27.29 | 8.31 | 2.49 |
| V. persica | MAE | 75.18 | 22.55 | 6.32 | 1.89 |
| V. persica | HD | 142.83 | 42.89 | 6.11 | 1.83 |
| Species | (E)-Caryophyllene | Caryophyllene Oxide | Hexadecanoic Acid | Hexahydrofarnesyl Acetone | Phytol | Pentacosane | |
|---|---|---|---|---|---|---|---|
| V. arvensis | HD | 6.21 | 14.11 | 3.17 | 6.35 | 7.54 | 0.71 |
| V. arvensis | MAE | 3.25 | 7.11 | 17.42 | 17.55 | 22.57 | - |
| V. chamaedrys | HD | 2.43 | 6.25 | 5.73 | 10.82 | 31.66 | 0.56 |
| V. chamaedrys | MAE | 1.05 | 1.22 | 15.83 | 16.69 | 18.88 | 8.36 |
| V. montana | HD | 0.13 | 7.28 | 9.24 | 6.86 | 18.53 | 10.47 |
| V. montana | MAE | 0.44 | 2.61 | 5.81 | 9.17 | 37.03 | 14.90 |
| V. serpyllifolia | HD | 2.11 | 4.19 | 12.28 | 7.92 | 39.79 | 0.98 |
| V. serpyllifolia | MAE | 6.83 | 14.74 | 7.71 | 6.54 | 18.72 | 0.18 |
| V. persica | HD | 9.29 | 10.11 | 7.35 | 10.31 | 20.21 | - |
| V. persica | MAE | 2.62 | 3.14 | 5.31 | 18.47 | 23.71 | 5.27 |
| Species | Linalool | (E)-Caryophyllene | Caryophyllene Oxide | α-Muurolol | Benzene Acetaldehyde | (E)-β-Damascenone | β-Ionone | |
|---|---|---|---|---|---|---|---|---|
| V. arvensis | HD | - | 1.63 | 7.92 | 9.76 | 13.52 | 11.21 | 8.94 |
| V. arvensis | MAE | 7.53 | - | 13.66 | 2.02 | 10.32 | 8.85 | 13.71 |
| V. chamaedrys | HD | 1.03 | 2.04 | 21.11 | 23.16 | 8.64 | 5.01 | 9.37 |
| V. chamaedrys | MAE | 3.15 | 3.31 | 18.16 | 22.45 | 5.43 | 3.35 | 7.16 |
| V. montana | HD | 5.45 | 5.82 | 4.88 | - | 25.33 | 4.43 | 10.55 |
| V. montana | MAE | 4.64 | 6.24 | 8.14 | - | 19.52 | 36.04 | - |
| V. serpyllifolia | HD | 3.52 | 5.12 | 37.03 | 1.24 | 16.44 | 3.73 | 5.32 |
| V. serpyllifolia | MAE | 4.84 | 5.27 | 18.83 | 10.36 | 4.33 | 4.95 | 11.46 |
| V. persica | HD | - | 1.44 | 22.73 | 7.21 | 11.03 | 9.32 | 11.73 |
| V. persica | MAE | 6.68 | 3.72 | 10.17 | - | 20.05 | 2.05 | 16.49 |
| Bacterial Species | Strain Origin | MIC/MBC of EO-Derived FVC (mg/mL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| V. persica | V. montana | V. serpyllifolia | V. chamaedrys | V. arvensis | |||||||
| MAE | HD | MAE | HD | MAE | HD | HD | MAE | HD | MAE | ||
| Gram-positive bacteria | |||||||||||
| Staphylococcus aureus | ATCC 29213 | 22.55/ >22.55 | 42.89/ 42.89 | 22.61/ >22.61 | 17.18/ 17.18 | 4.89/ 9.78 | 6.82/ 13.64 | 24.5/ 24.5 | 20.39/ 20.39 | 8.71/ 8.71 | 16.95/ >16.95 |
| Staphylococcus aureus | Clinical/ MRSA | 22.5/ >22.5 | 42.89/ >42.89 | 22.61/ >22.61 | 17.18/ 17.18 | 9.78/ 9.78 | 13.64/ 13.64 | 24.5/ >24.5 | 20.39/ 20.39 | 8.71/ 8.71 | 16.95/ >16.95 |
| Staphylococcus epidermidis | Human | 22.55/ >22.55 | 42.89/ >42.89 | >22.61/ >22.61 | 17.18/ 17.18 | 9.78/ 9.78 | 13.64/ 13.64 | 24.5/ 24.5 | 20.39/ 20.39 | 8.71/ 8.71 | 16.95/ >16.95 |
| Streptococcus pyogenes | ATCC 19615 | 5.64/ 11.28 | 5.36/ 5.36 | 2.83/ 2.83 | 8.59/ 8.59 | 0.61/ 1.22 | 1.71/ 1.71 | 6.13/ 12.25 | 10.19/ 10.19 | 4.36/ 4.36 | 4.24/ 8.47 |
| Streptococcus agalactiae | Clinical | 5.64/ 11.28 | 5.36/ 10.72 | 2.83/ 5.65 | 8.59/ 8.59 | 1.22/ 1.22 | 1.71/ 1.71 | 12.25/ 12.25 | 10.19/ 10.19 | 4.36/ 4.36 | 4.24/ 8.47 |
| Enterococcusfaecalis | ATCC 29212 | 1.41/ 5.64 | 2.68/ 10.72 | 5.65/ 22.61 | 4.29/ 4.29 | 1.22/ 1.22 | 1.71/ 1.71 | 24.5/ 24.5 | 1.28/ 5.09 | 1.08/ 2.17 | 2.12/ 8.47 |
| Listeriamonocytogenes | ATCC 19111 | 1.41/ 2.82 | 2.68/ 21.45 | 2.83/ 11.31 | 2.15/ 2.15 | 1.22/ 1.22 | 0.43/ 0.85 | 6.13/ 24.5 | 0.32/ 1.28 | 2.17/ 4.36 | 0.53/ 2.12 |
| Bacillus cereus | Food | 5.64/ 11.28 | 10.72/ 21.45 | 11.31/ >22.61 | 4.29/ 4.29 | 2.45/ 2.45 | 1.71/ 1.71 | 12.25/ 24.50 | 20.39/ 20.39 | 4.36/ 4.36 | 8.47/ 8.47 |
| Gram-negative bacteria | |||||||||||
| Escherichia coli | ATCC 25922 | >22.55/ >22.55 | >42.89/ >42.89 | >34.37/ >34.37 | >34.37/ >34.37 | 19.57/ 19.57 | 27.29/ >27.29 | >24.50/ >24.50 | >20.39/ >20.39 | 17.43/ 17.43 | >16.95/ >16.95 |
| Acinetobacter baumannii | ATCC 19606 | >22.55/ >22.55 | >42.89/ >42.89 | >34.37/ >34.37 | >34.37/ >34.37 | 19.57/ 19.57 | 27.29/ >27.29 | >24.50/ >24.50 | >20.39/ >20.39 | 17.43/ >17.43 | >16.95/ >16.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazlić, M.; Dunkić, V.; Dželalija, M.; Maravić, A.; Mandić, M.; Srečec, S.; Vrca, I.; Vuko, E.; Kremer, D. Evaluation of Antiphytoviral and Antibacterial Activity of Essential Oil and Hydrosol Extracts from Five Veronica Species. Agriculture 2023, 13, 1517. https://doi.org/10.3390/agriculture13081517
Nazlić M, Dunkić V, Dželalija M, Maravić A, Mandić M, Srečec S, Vrca I, Vuko E, Kremer D. Evaluation of Antiphytoviral and Antibacterial Activity of Essential Oil and Hydrosol Extracts from Five Veronica Species. Agriculture. 2023; 13(8):1517. https://doi.org/10.3390/agriculture13081517
Chicago/Turabian StyleNazlić, Marija, Valerija Dunkić, Mia Dželalija, Ana Maravić, Mihaela Mandić, Siniša Srečec, Ivana Vrca, Elma Vuko, and Dario Kremer. 2023. "Evaluation of Antiphytoviral and Antibacterial Activity of Essential Oil and Hydrosol Extracts from Five Veronica Species" Agriculture 13, no. 8: 1517. https://doi.org/10.3390/agriculture13081517
APA StyleNazlić, M., Dunkić, V., Dželalija, M., Maravić, A., Mandić, M., Srečec, S., Vrca, I., Vuko, E., & Kremer, D. (2023). Evaluation of Antiphytoviral and Antibacterial Activity of Essential Oil and Hydrosol Extracts from Five Veronica Species. Agriculture, 13(8), 1517. https://doi.org/10.3390/agriculture13081517











