Effects of Altitude and Continuous Cropping on Arbuscular Mycorrhizal Fungi Community in Siraitia grosvenorii Rhizosphere
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Sampling and Processing
2.3. Determination of Physicochemical Properties of Rhizosphere Soil
2.4. Determination of AMF Colonization and Spore Density
2.5. Determination of AMF Molecular Diversity in S. grosvenorii Rhizosphere
3. Data Processing
3.1. Sequencing Data Processing
3.2. Statistical Analysis
4. Results
4.1. Physicochemical Properties of Rhizosphere Soil of S. grosvenorii
4.2. AMF Colonization, and AMF Spore Density of S. grosvenorii
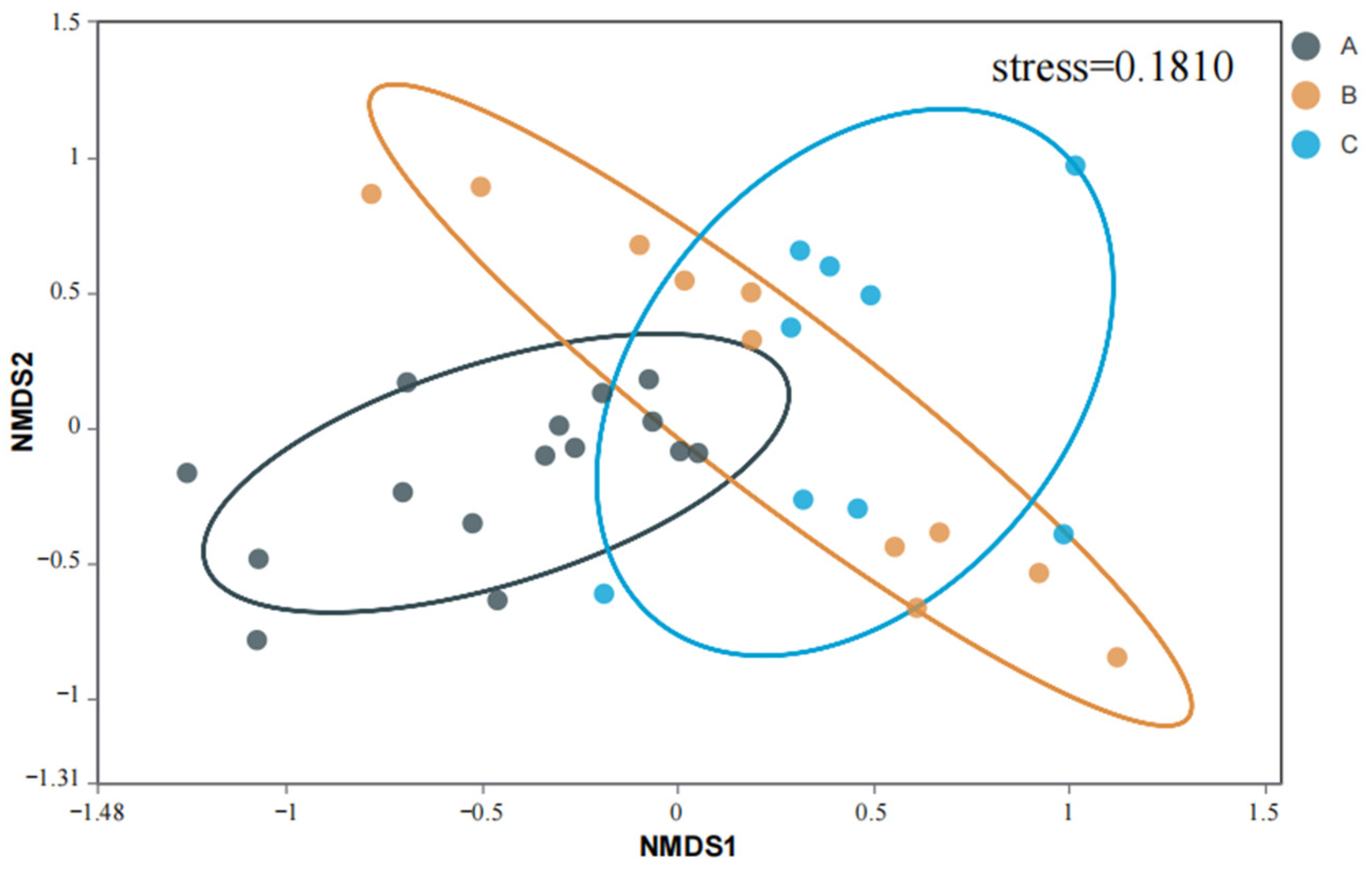
4.3. AMF Community Composition in Rhizosphere Soil of S. grosvenorii
4.4. AMF Diversity in Rhizosphere Soil of S. grosvenorii
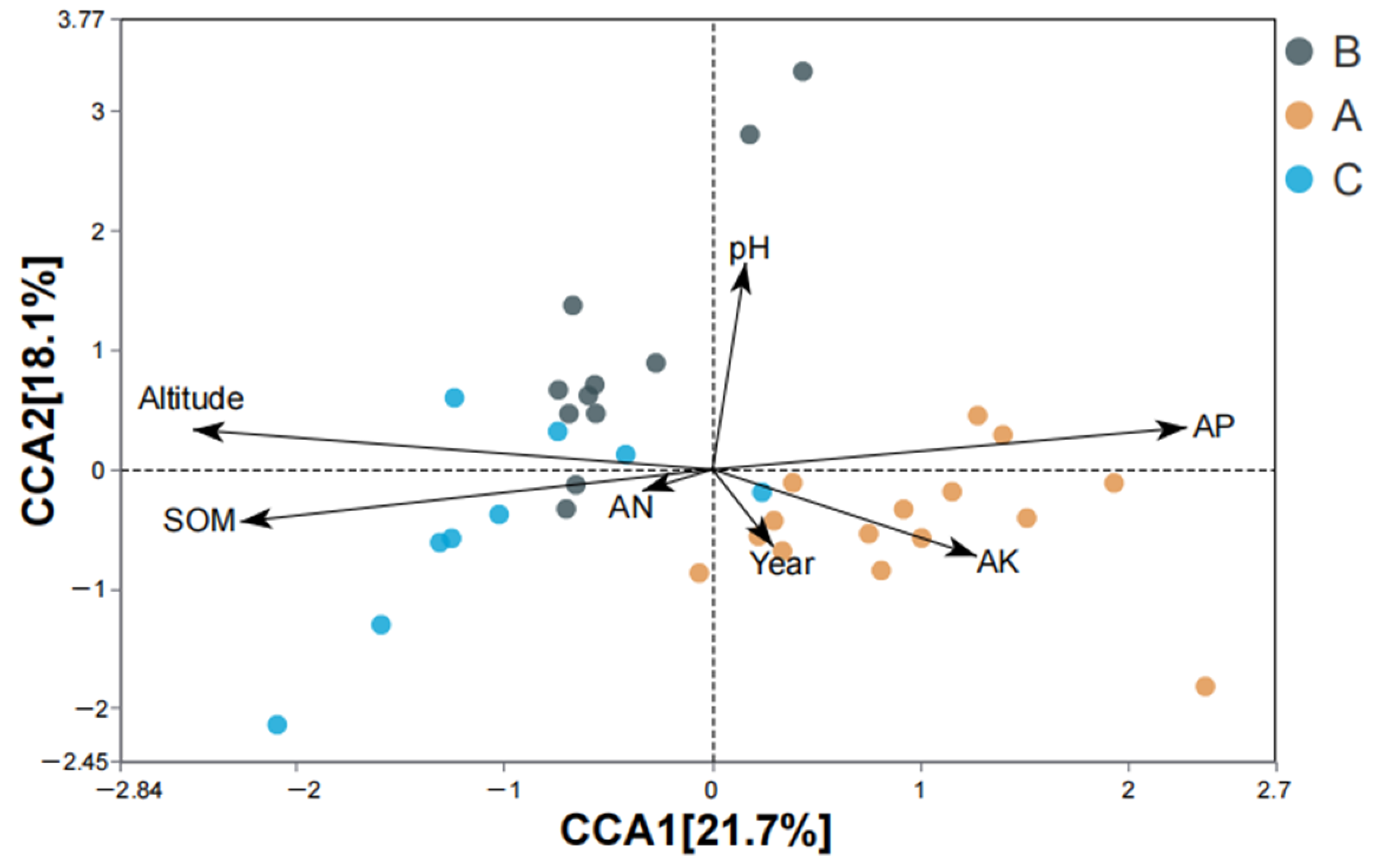
4.5. Effects of Environmental Factors on AMF Community Composition of S. grosvenorii
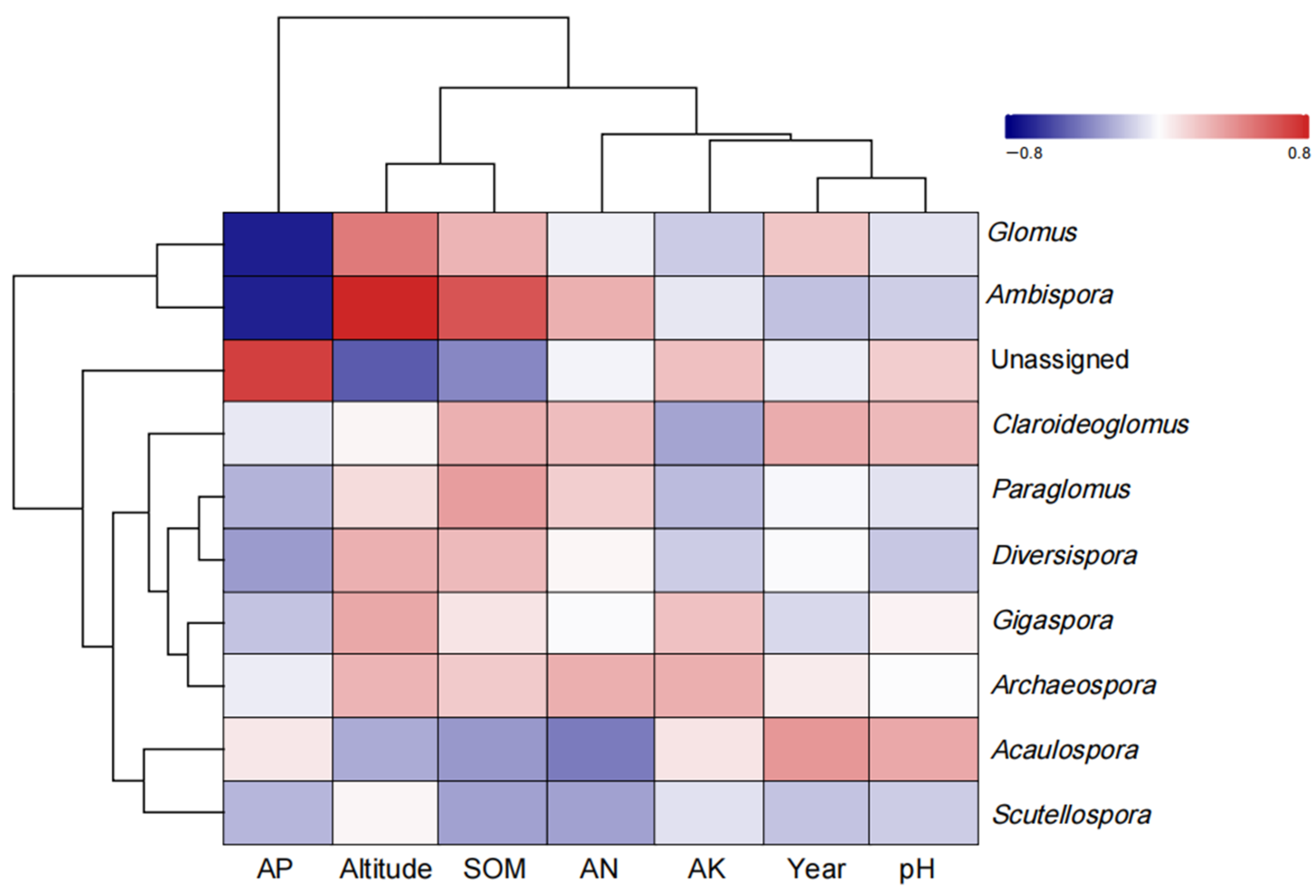
4.6. Relationships between Environmental Factors and AMF Parameters of S. grosvenorii
5. Discussion
5.1. Medicinal Plant Mycorrhizal Fungi State
5.2. AMF Community Composition in Rhizosphere Soil of S. grosvenorii
5.3. Effects of Altitude on AMF Community Composition in S. grosvenorii Roots
5.4. Effects of Continuous Cropping Years on AMF Community in S. grosvenorii
5.5. Effects of Soil Factors on AMF of S. grosvenorii
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, L.; Zhang, Z.; Zhou, L. Advances in the studies on symbiotic arbuscular mycorrhizal fungi of traditional Chinese medicinal plants. Biocell 2022, 46, 2559–2573. [Google Scholar] [CrossRef]
- Wang, B.; Qiu, Y.L. Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 2006, 16, 299–363. [Google Scholar] [CrossRef] [PubMed]
- Hartoyo, B.; Trisilawati, O. Diversity of arbuscular mycorrhiza fungi (AMF) in the rhizosphere of sugarcane. Conference Series. IOP Conference Series: Earth and Environmental Science. IOP Publ. 2021, 653, 012066. [Google Scholar] [CrossRef]
- Mbodj, D.; Effa-Effa, B.; Kane, A.; Manneh, B.; Gantet, P.; Laplaze, L.; Diedhiou, A.G.; Grondin, A. Arbuscular mycorrhizal symbiosis in rice: Establishment, environmental control and impact on plant growth and resistance to abiotic stresses. Rhizosphere 2018, 8, 12–26. [Google Scholar] [CrossRef]
- Wang, X.F.; Zhang, X.; Zhao, R.H.; Yu, J.; Gu, W.; Li, R.; Cao, G.H.; He, S. Effect and mechanism of arbuscular mycorrhizal fungi in herbs. Chin. J. Exp. Tradit. Med. Formulae 2020, 26, 217–226. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.L. Contribution of arbuscular mycorrhizal fungi to N uptake by plants. J. Shanxi Datong Univ. (Nat. Sci. Ed.) 2008, 24, 75–78. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=YBSF200806029&DbName=CJFQ2008 (accessed on 13 April 2023).
- Drigo, B.; Pijl, A.S.; Duyts, H.; Kielak, A.M.; Gamper, H.A.; Houtekamer, M.J.; Boschker, H.T.S.; Bodelier, P.L.E.; Whiteley, A.S.; van Veen, J.A.; et al. Shifting carbon flow from roots into associated microbial communities in response to elevated atmospheric CO2. Proc. Natl. Acad. Sci. USA 2010, 107, 10938–10942. [Google Scholar] [CrossRef]
- Krüger, M.; Teste, F.P.; Laliberté, E.; Lambers, H.; Coghlan, M.; Zemunik, G.; Bunce, M. The rise and fall of arbuscular mycorrhizal fungal diversity during ecosystem retrogression. Mol. Ecol. 2015, 24, 4912–4930. [Google Scholar] [CrossRef]
- Montiel-Rozas, M.D.; López-García, Á.; Kjøller, R.; Madejón, E.; Rosendahl, S. Organic amendments increase phylogenetic diversity of arbuscular mycorrhizal fungi in acid soil contaminated by trace elements. Mycorrhiza 2016, 26, 575–585. [Google Scholar] [CrossRef]
- Svenningsen, N.B.; Watts-Williams, S.J.; Joner, E.J.; Battini, F.; Efthymiou, A.; Cruz-Paredes, C.; Nybroe, O.; Jakobsen, I. Suppression of the activity of arbuscular mycorrhizal fungi by the soil microbiota. ISME J. 2018, 12, 1296–1307. [Google Scholar] [CrossRef] [Green Version]
- Coutinho, E.S.; Fernandes, G.W.; Berbara, R.L.L.; Valério, H.M.; Goto, B.T. Variation of arbuscular mycorrhizal fungal communities along an altitudinal gradient in rupestrian grasslands in Brazil. Mycorrhiza 2015, 25, 627–638. [Google Scholar] [CrossRef]
- Peng, Y.L.; Cai, X.B. Changes of arbuscular mycorrhizal fungal community in an alpine grassland altitudinal gradient. Acta Ecol. Sin. 2015, 35, 7475–7484. [Google Scholar] [CrossRef]
- Jiang, S.T.; Hu, X.X.; Kang, Y.L.; Xie, C.Y.; An, X.R.; Dong, C.X.; Xu, Y.C.; Shen, Q.R. Arbuscular mycorrhizal fungal communities in the rhizospheric soil of litchi and mango orchards as affected by geographic distance, soil properties and manure input. Appl. Soil Ecol. 2020, 152, 103593. [Google Scholar] [CrossRef]
- Ma, L.; Ma, K.; Yang, G.L.; Niu, H.X.; Dai, X.H. Effects of continuous potato cropping on the diversity of soil microorganisms. Chin. J. Eco-Agric. 2015, 23, 589–596. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Xu, G.P.; Zhou, L.W.; Teng, Q.M.; He, W.; Zhang, Z.F.; Zhang, D.N. Analysis the nutrient compositions of Siraitia grosvenorii in the hilly region at north Guangxi. Hubei Agric. Sci. 2020, 59, 151–154. [Google Scholar] [CrossRef]
- Yi, Q.Z.; Liang, X.R.; You, Y.M.; Wei, Z.X. Occurrence and control of root-knot nematode disease on Momordica grosvenori swingle. J. Guangxi Agric. 2010, 25, 24–25+36. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=GXLB201005011&DbName=CJFQ2010 (accessed on 24 May 2023).
- Zhang, D.N.; Zhou, L.W.; Duan, C.Y.; Chen, X.X.; He, W.; Teng, Q.M.; Sun, Y.J.; Zhang, Z.F.; Xu, G.P. Contents and potential ecological risk assessment of heavy metals in soil and Siraitia grosvenorii at the hilly region in Longsheng County—Taking Baozeng Village as an example. Guihaia 2021, 41, 243–250. [Google Scholar] [CrossRef]
- Liu, S.Y. Study on the Response Mechanism of Lycium barbarum Quality to Rhizosphere Microbial Diversity. Master’s Thesis, Shihezi University, Shihezi, China, 2020. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=1022828395.nh&DbName=CMFD2023 (accessed on 10 May 2023).
- Wang, Y.S.; Zhang, S.B.; Zhang, M.Q. Arbuscular Mycorrhizal Fungi Resources and Germplasm Resources in China; China Agriculture Press: Beijing, China, 2012; pp. 150–176. [Google Scholar]
- Sheng, H.J.; Tian, L.Y.; Jiang, X.; Wang, X.L. Discussion on determination of available nitrogen in the greenhouse soil with alkali hydrolysis diffusion method. Res. Explor. Lab. 2022, 41, 5–7+35. [Google Scholar] [CrossRef]
- Bao, S.D. Analysis of Soil Agro-Chemistry, 3rd ed.; China Agriculture Press: Beijing, China, 2000; pp. 25–109. [Google Scholar]
- Lumini, E.; Orgiazzi, A.; Borriello, R.; Bonfante, P.; Bianciotto, V. Disclosing arbuscular mycorrhizal fungal biodiversity in soil through a land-use gradient using a Pyrosequencing approach. Environ. Microbiol. 2010, 12, 2165–2179. [Google Scholar] [CrossRef]
- Jiang, P. The Resources and Species Diversity Research on AM Fungi Occurring in Medicinal Plants of South Fujian. Master’s Thesis, Huaqiao University, Quanzhou, China, 2012. Available online: https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CMFD201301&filename=1013001147.nh (accessed on 20 April 2023).
- Alimi, A.A.; Ezeokoli, O.T.; Adeleke, R.; Moteetee, A. Arbuscular mycorrhizal fungal communities colonising the roots of indigenous legumes of South Africa as revealed by high-throughput DNA metabarcoding. Rhizosphere 2021, 19, 100405. [Google Scholar] [CrossRef]
- Shang, K.; Shi, L.; Li, H.B.; Yao, L.M.; Zhou, G.R.; Jiang, L. Diversity of arbuscular mycorrhizal fungi in different heights of Fanjingshan Mountain. J. Northeast. For. Univ. 2020, 48, 76–80. [Google Scholar] [CrossRef]
- Chagnon, P.-L.; Bradley, R.L.; Maherali, H.; Klironomos, J.N. A trait-based framework to understand life history of mycorrhizal fungi. Trends Plant Sci. 2013, 18, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.Y.; Sun, L.Y.; Song, F.B.; Yang, X.Q.; Zhang, M.J.; Li, S.X.; Zhu, X.C. Research advances in species diversity of arbuscular mycorrhizal fungi in terrestrial agro-ecosystem. Ying Yong Sheng Tai Xue Bao 2019, 30, 3971–3979. [Google Scholar] [CrossRef]
- Ji, C.H.; Zhang, S.B.; Gai, J.P.; Bai, D.S.; Li, X.L.; Feng, G. Arbuscular mycorrhizal fungal diversity in arid zones in northwestern China. Biodivers. Sci. 2007, 15, 77–83. [Google Scholar] [CrossRef]
- Zhao, Z.W.; Li, X.W.; Wang, G.H.; Cheng, L.Z.; Sha, T.; Yang, L.; Ren, L.C. AM fungi in the tropical rain forest of Xishuangbanna. Mycosystema 2001, 20, 316–323. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=JWXT200103005&DbName=CJFQ2001 (accessed on 1 May 2023).
- Panwar, J.; Tarafdar, J.C. Arbuscular mycorrhizal fungal dynamics under Mitragyna parvifolia (Roxb.) Korth. in Thar Desert. Appl. Soil Ecol. 2006, 34, 200–208. [Google Scholar] [CrossRef]
- Wilson, H.; Johnson, B.R.; Bohannan, B.; Pfeifer-Meister, L.; Mueller, R.; Bridgham, S.D. Experimental warming decreases arbuscular mycorrhizal fungal colonization in prairie plants along a Mediterranean climate gradient. PeerJ 2016, 4, e2083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L. Diversity of Arbuscular Mycorrhizal Fungi in Root Systems and Inter-Root Soils of Wine Grapes at the Eastern of the Helan Mountains and Their Correlation with Soil Factors. Master’s Thesis, Ningxia University, Yinchuan, China, 2022. Available online: https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CMFD202301&filename=1022060066.nh (accessed on 5 May 2023).
- Cardoso, I.M.; Boddington, C.; Janssen, B.H.; Oenema, O.; Kuyper, T.W. Distribution of mycorrhizal fungal spores in soils under agroforestry and monocultural coffee systems in Brazil. Agrofor. Syst. 2003, 58, 33–43. [Google Scholar] [CrossRef]
- Abdelhalim, T.S.; Finckh, M.R.; Babiker, A.G.; Oehl, F. Species composition and diversity of arbuscular mycorrhizal fungi in White Nile state, Central Sudan. Arch. Agron. Soil Sci. 2014, 60, 377–391. [Google Scholar] [CrossRef]
- Li, Y.D. Species Diversity and Ecological Distribution of Arbuscular Mycorrhizal Fungi in Extremely Dry Desert Plants. Master’s Thesis, Hebei University, Baoding, China, 2020. [Google Scholar] [CrossRef]
- Jie, W.G.; Liu, X.R.; Cai, B.Y. Diversity of rhizosphere soil arbuscular mycorrhizal fungi in various soybean cultivars under different continuous cropping regimes. PLoS ONE 2013, 8, e72898. [Google Scholar] [CrossRef]
- Wang, W.B.; He, X.L.; Zhao, L.L.; Wang, C.X.; Zeng, X.D.; Wang, L. Spatial distribution characteristics of arbuscular mycorrhizal fungi of Artemisia ordosica in desert areas of Northwest China. J. Fungal Res. 2020, 18, 20–30. [Google Scholar] [CrossRef]
- Zhao, H.; Li, X.Z.; Zhang, Z.M.; Zhao, Y.; Yang, J.T.; Zhu, Y.W. Species diversity and drivers of arbuscular mycorrhizal fungal communities in a semi-arid mountain in China. PeerJ 2017, 5, e4155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.J.; Chen, Y.L. Mycorrhizology; Science Press: Beijing, China, 2007; pp. 10–390. [Google Scholar]
- Vargas Gil, S.; Meriles, J.; Conforto, C.; Basanta, M.; Radl, V.; Hagn, A.; Schloter, M.; March, G.J. Response of soil microbial communities to different management practices in surface soils of a soybean agroecosystem in Argentina. Eur. J. Soil Biol. 2011, 47, 55–60. [Google Scholar] [CrossRef]
- Li, D.; Wen, L.; Zhang, W.; Yang, L.; Xiao, K.; Chen, H.; Wang, K. Afforestation effects on soil organic carbon and nitrogen pools modulated by lithology. For. Ecol. Manag. 2017, 400, 85–92. [Google Scholar] [CrossRef]
- Chen, Y.L.; Mai, Z.T.; Luo, J.; Chen, W.Y.; Cai, K.L. Arbuscular mycorrhizae fungi and its application in plant restoration of lime stone areas. Trop. For. 2017, 45, 25–28. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=RDLY201704010&DbName=CJFQ2017 (accessed on 10 May 2023).
- Kiers, E.T.; Duhamel, M.; Beesetty, Y.; Mensah, J.A.; Franken, O.; Verbruggen, E.; Fellbaum, C.R.; Kowalchuk, G.A.; Hart, M.M.; Bago, A.; et al. Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science 2011, 333, 880–882. [Google Scholar] [CrossRef] [Green Version]
- Feng, H.Y.; Feng, G.; Wang, J.G.; Li, X.L. Regulation of P status in host plant on alkaline phosphatase (ALP) activity in intraradical hyphae and development of extraradical hyphae of AM fungi. Mycosystema 2003, 22, 589–598. [Google Scholar] [CrossRef]
- Li, X.L. Influence of elevation and land use types on diversity and community composition of arbuscular mycorrhizal fungi in southeast Tibet. Ph.D. Thesis, China Agricultural University, Beijing, China, 2015. Available online: https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CDFDLAST2015&filename=1015584336.nh (accessed on 10 May 2023).
- Ma, J.; He, X.L.; Jiang, Z.M.; Wang, L.Y. Influence of soil factors on arbuscular mycorrhizal fungal infection of Salvia miltiorrhiza. Acta Agric. Boreali-Occident. Sin. 2009, 18, 194–198. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=XBNX200905043&DbName=CJFQ2009 (accessed on 12 May 2023).
- Dumbrell, A.J.; Nelson, M.; Helgason, T.; Dytham, C.; Fitter, A.H. Relative roles of niche and neutral processes in structuring a soil microbial community. ISME J. 2010, 4, 337–345. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.J.; Li, X.L. Arbuscular Mycorrhiza and Its Application; Science Press: Beijing, China, 2000; pp. 1–224. [Google Scholar]
- Xu, T.L.; Veresoglou, S.D.; Chen, Y.L.; Rillig, M.C.; Xiang, D.; Ondřej, D.; Hao, Z.P.; Liu, L.; Deng, Y.; Hu, Y.J.; et al. Plant community, geographic distance and abiotic factors play different roles in predicting AMF biogeography at the regional scale in northern China. Environ. Microbiol. Rep. 2016, 8, 1048–1057. [Google Scholar] [CrossRef]
- Shi, R.J.; Yang, M.; Guo, D.Q.; Pan, X.J.; Ding, B.; Qi, J.S.; Zhou, N. Effect of arbuscular mycorrhizal fungi inoculation on physical and chemical properties of rhizosphere soil of Paris polyphylla var. yunnanensis. Chin. J. Exp. Tradit. Med. Formulae 2020, 22, 77–85. [Google Scholar] [CrossRef]
- Rillig, M.C.; Wright, S.F.; Shaw, M.R.; Field, C.B. Artificial climate warming positively affects arbuscular mycorrhizae but decreases soil aggregate water stability in an annual grassland. Oikos 2002, 97, 52–58. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.F.; Wen, Z.M.; Qu, F.; Liu, Q.Q.; Xing, X.S.; Luo, H.; Li, M.Q.; Chen, H.; Liu, B. Diversity of soil arbuscular mycorrhizal fungi under Cunninghamia lanceolata plantation planted in successive rotations. J. Fujian Agric. For. Univ. (Nat. Sci. Ed.) 2022, 51, 510–516. [Google Scholar] [CrossRef]
- Zhang, S.R.; He, X.L.; Xu, H.B.; Liu, C.M.; Niu, K. Correlation study of AM and DSE fungi and soil factors in the rhizosphere of Ammopiptanthus mongolicus. Acta Bot. Boreali-Occident. Sin. 2013, 33, 1891–1897. [Google Scholar]
- Liu, C.M.; He, X.L.; Chen, Y.Y.; Wang, X.Q.; Jiang, Q. Spatiotemporal distribution of arbuscular mycorrhizal fungi in the rhizosphere of Ammopiptanthus mongolicus. J. Hebei Univ. Nat. Sci. Ed. 2015, 35, 278–288. [Google Scholar]
- Ouyang, R.P.; Yao, Q.Z.; Shi, J.T.; Liang, X.X. Correlation analysis of root symbiotic fungi of Artemisia argyi and soil physical and chemical factors. J. North. Agric. 2021, 49, 49–55. [Google Scholar] [CrossRef]
- Shang, X.J.; Zhang, F.M.; Li, S.; Hou, R. Spatial distribution of dark septate endophytes, arbuscular mycorrhiza fungi and ericoid mycorrhiza fungi in cultivated blueberries from Guizhou Province, Southwest China. Mycosystema 2021, 40, 2752–2770. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |




| Sampling Site | Sample Code | Altitude (m) | Continuous Cropping Years (a) | Number of Samples | pH | SOM (g kg−1) | AN (g kg−1) | AP (mg kg−1) | AK (mg kg−1) |
|---|---|---|---|---|---|---|---|---|---|
| Shangtang | A1 | 248 | 1 | 5 | 4.52 ± 0.3 | 34 ± 7 | 0.41 ± 0.14 | 605 ± 134 | 657 ± 94 |
| Jinjie | A2 | 259 | 2 | 5 | 5.01 ± 0.5 | 36 ± 6 | 0.35 ± 0.04 | 596 ± 207 | 971 ± 436 |
| Shangtang | A5 | 248 | 5 | 5 | 5.29 ± 0.2 | 40 ± 3 | 0.30 ± 0.03 | 359 ± 267 | 280 ± 154 |
| Baozeng | B1 | 513 | 1 | 5 | 5.42 ± 0.6 | 47 ± 12 | 0.28 ± 0.04 | 353 ± 249 | 325 ± 120 |
| B2 | 561 | 2 | 3 | 4.64 ± 0.2 | 48 ± 11 | 0.30 ± 0.03 | 233 ± 89 | 393 ± 138 | |
| B3 | 513 | 3 | 3 | 4.99 ± 0.5 | 60 ± 1 | 0.42 ± 0.02 | 373 ± 47 | 122 ± 42 | |
| C1 | 762 | 1 | 5 | 4.79 ± 0.3 | 87 ± 10 | 0.47 ± 0.06 | 193 ± 72 | 376 ± 91 | |
| Diling | C2 | 763 | 2 | 5 | 5.20 ± 0.4 | 74 ± 9 | 0.91 ± 1.01 | 207 ± 106 | 1013 ± 723 |
| Order | Family | Genus | Species |
|---|---|---|---|
| Archaeosporales | Ambisporaceae | Ambispora | A. leptoticha |
| Archaeosporaceae | Archaeospora | Ar. Other1 | |
| Diversisporales | Acaulosporaceae | Acaulospora | Ac. Acau10, Acaulospora sp. |
| Diversisporaceae | Diversispora | Diversispora sp. | |
| Gigasporaceae | Gigaspora | Gi. decipiens | |
| Scutellospora | S. heterogama | ||
| Glomerales | Claroideoglomeraceae | Claroideoglomus | C. Douhan9, C.GlBb12, C.lamellosum, C.ORVIN_GLO4, C. Torrecillas12b_Glo_G5 |
| Glomeraceae | Glomus | G.Alguacil09b_Glo_G3, G.caledonium, G. clarum, G.Glo3b, G. Glo49, G.MO_G17, G.MO_G18, G.MO_G40, G.ORVIN_GLO1E, G.ORVIN_GLO3B, G.ORVIN_GLO3D, G.ORVIN_GLO3E, G. Torrecillas12b_Glo_G13, G. viscosum, G. Wirsel_OTU16, G. Yamato09_E | |
| Paraglomerales | Paraglomeraceae | Paraglomus | P. Alguacil12a_Para_1, P. Alguacil12b_ACA1 |
| Factor | r | p-Value |
|---|---|---|
| Year | −0.12 | 0.959 |
| Altitude | 0.29 ** | 0.001 |
| pH | 0.07 | 0.16 |
| SOM | 0.19 * | 0.01 |
| AN | −0.01 | 0.572 |
| AP | 0.29 ** | 0.001 |
| AK | 0.09 | 0.142 |
| Year | Altitude | pH | SOM | AN | AP | AK | |
|---|---|---|---|---|---|---|---|
| AMF colonization | 0.084 | 0.454 ** | 0.317 | 0.289 | 0.004 | −0.455 ** | −0.013 |
| Spore density | −0.065 | −0.358 * | −0.314 | −0.371 * | 0.137 | 0.353 * | 0.231 |
| Shannon | 0.145 | −0.051 | 0.381 * | 0.116 | 0.141 | 0.222 | −0.145 |
| Chao1 | 0.206 | −0.189 | 0.346 * | 0.003 | −0.022 | 0.125 | −0.266 |
| Simpson | −0.103 | 0.007 | −0.292 | −0.11 | −0.212 | −0.192 | −0.011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, L.; Zhang, Z.; Zhou, L.; Huang, K. Effects of Altitude and Continuous Cropping on Arbuscular Mycorrhizal Fungi Community in Siraitia grosvenorii Rhizosphere. Agriculture 2023, 13, 1548. https://doi.org/10.3390/agriculture13081548
Yu L, Zhang Z, Zhou L, Huang K. Effects of Altitude and Continuous Cropping on Arbuscular Mycorrhizal Fungi Community in Siraitia grosvenorii Rhizosphere. Agriculture. 2023; 13(8):1548. https://doi.org/10.3390/agriculture13081548
Chicago/Turabian StyleYu, Limin, Zhongfeng Zhang, Longwu Zhou, and Kechao Huang. 2023. "Effects of Altitude and Continuous Cropping on Arbuscular Mycorrhizal Fungi Community in Siraitia grosvenorii Rhizosphere" Agriculture 13, no. 8: 1548. https://doi.org/10.3390/agriculture13081548
APA StyleYu, L., Zhang, Z., Zhou, L., & Huang, K. (2023). Effects of Altitude and Continuous Cropping on Arbuscular Mycorrhizal Fungi Community in Siraitia grosvenorii Rhizosphere. Agriculture, 13(8), 1548. https://doi.org/10.3390/agriculture13081548







