Genome-Wide Association Studies of Salt-Tolerance-Related Traits in Rice at the Seedling Stage Using InDel Markers Developed by the Genome Re-Sequencing of Japonica Rice Accessions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Seedling Salt Tolerance Identification of 182 japonica Rice Accessions
2.3. Whole-Genome Re-Sequencing and Identification of InDel Candidates
2.4. InDel Loci Screening and Primer Design
2.5. DNA Extraction, PCR Amplification, and Product Detection
2.6. Genetic Diversity, Population Structure, and Linkage Disequilibrium Analysis
2.7. GWAS of Salt-Tolerance-Related Traits in japonica Rice at the Seedling Stage
2.8. Data Analysis
3. Results
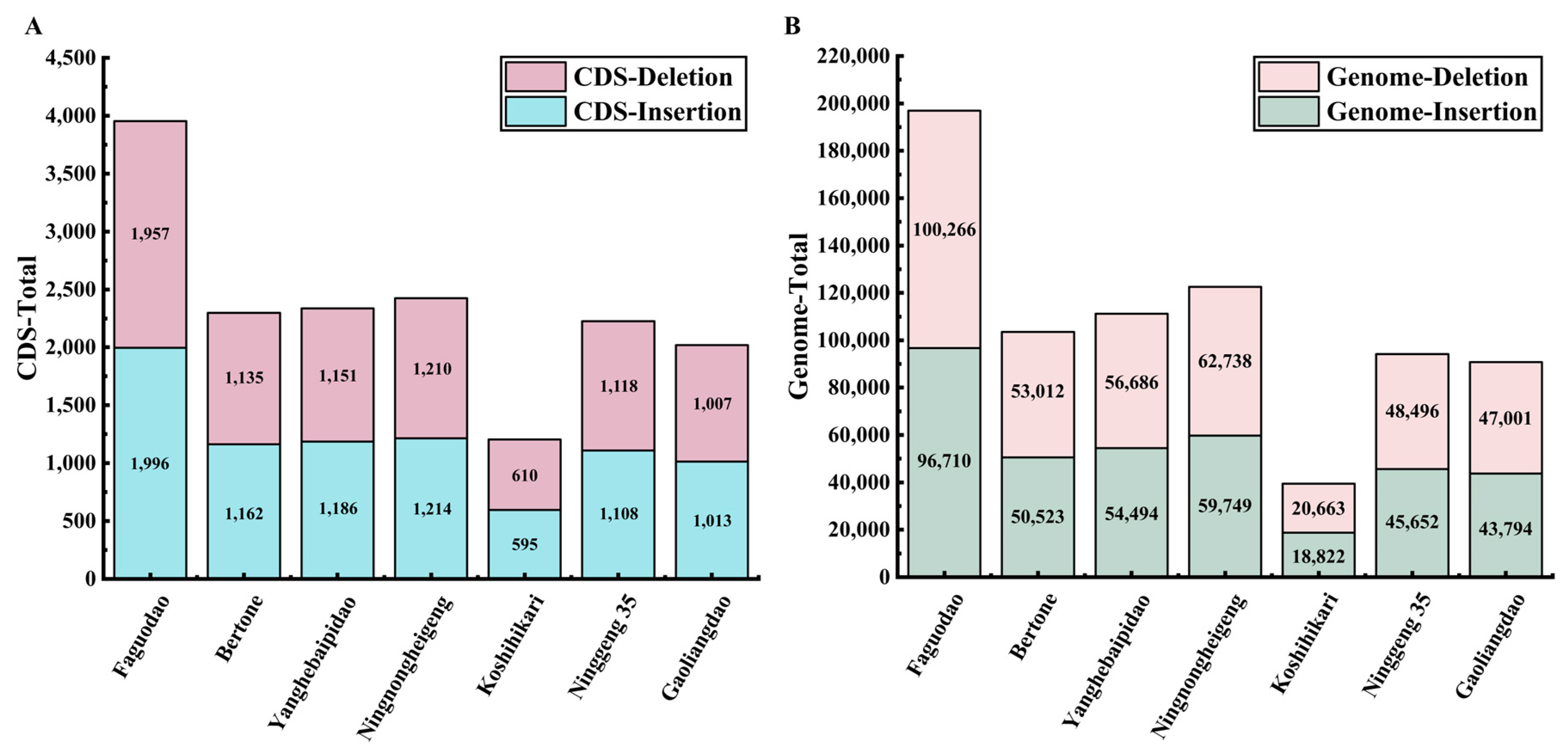
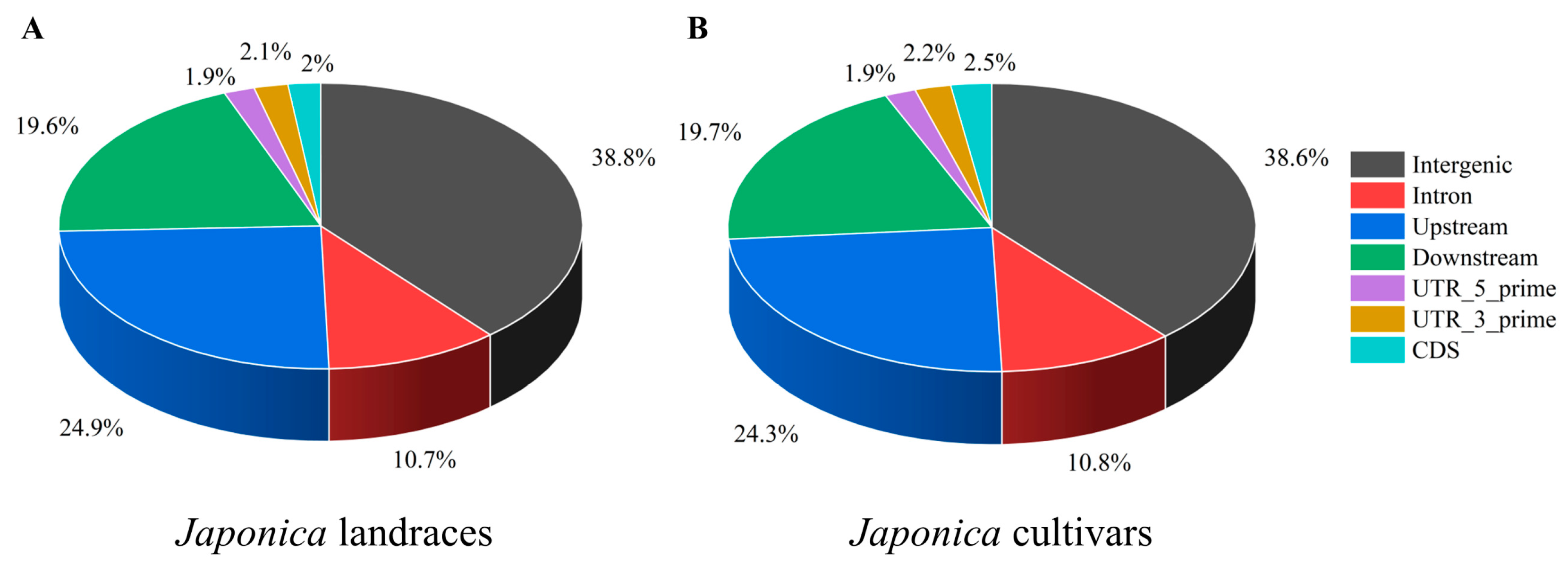
3.1. Distribution Characteristics of InDel Loci in japonica Rice Accessions
3.2. Distribution of InDels on Chromosomes of Different Types of japonica Rice Accessions
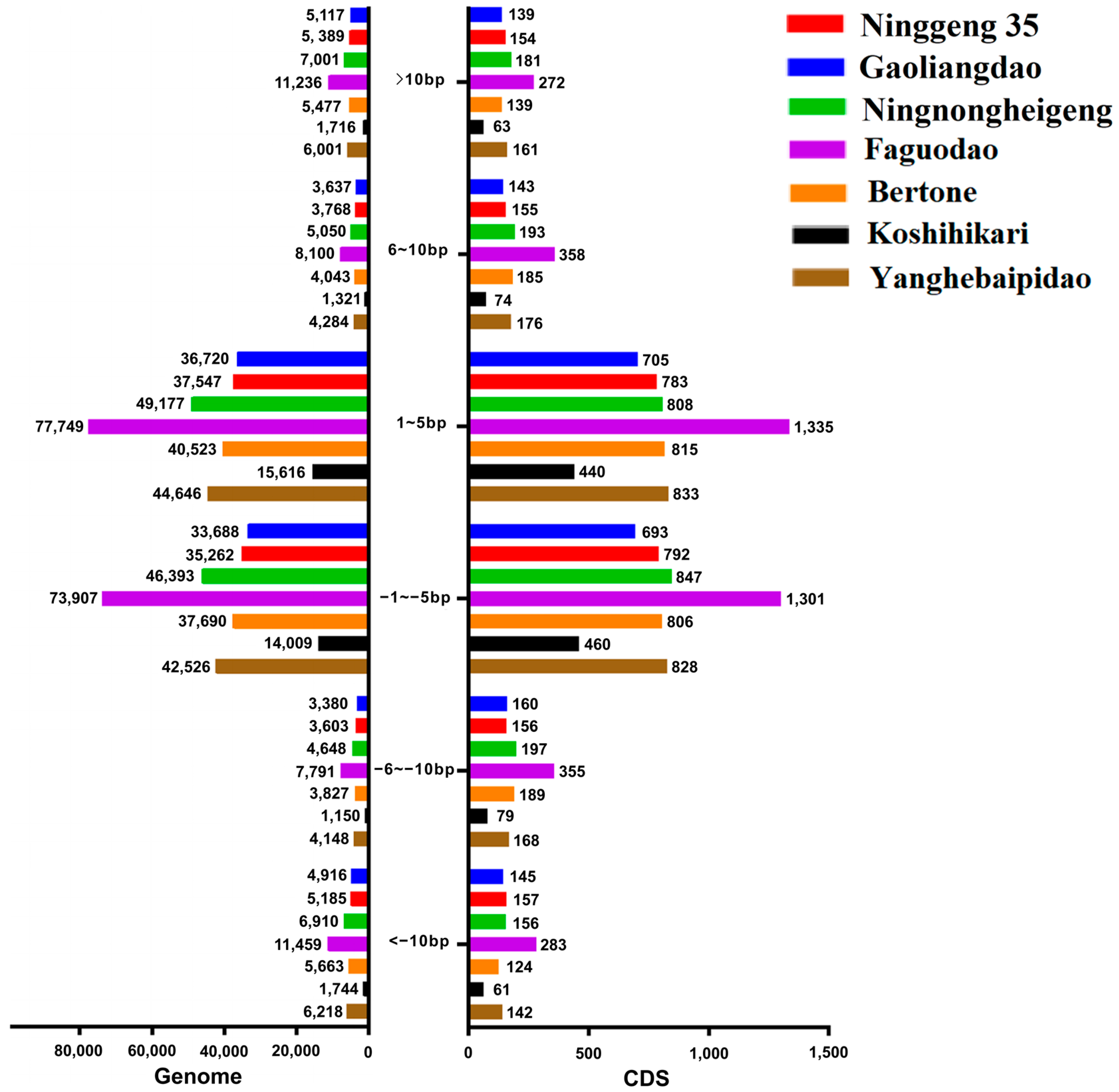
3.3. InDel Length Analysis of Seven Re-Sequenced japonica Rice Accessions
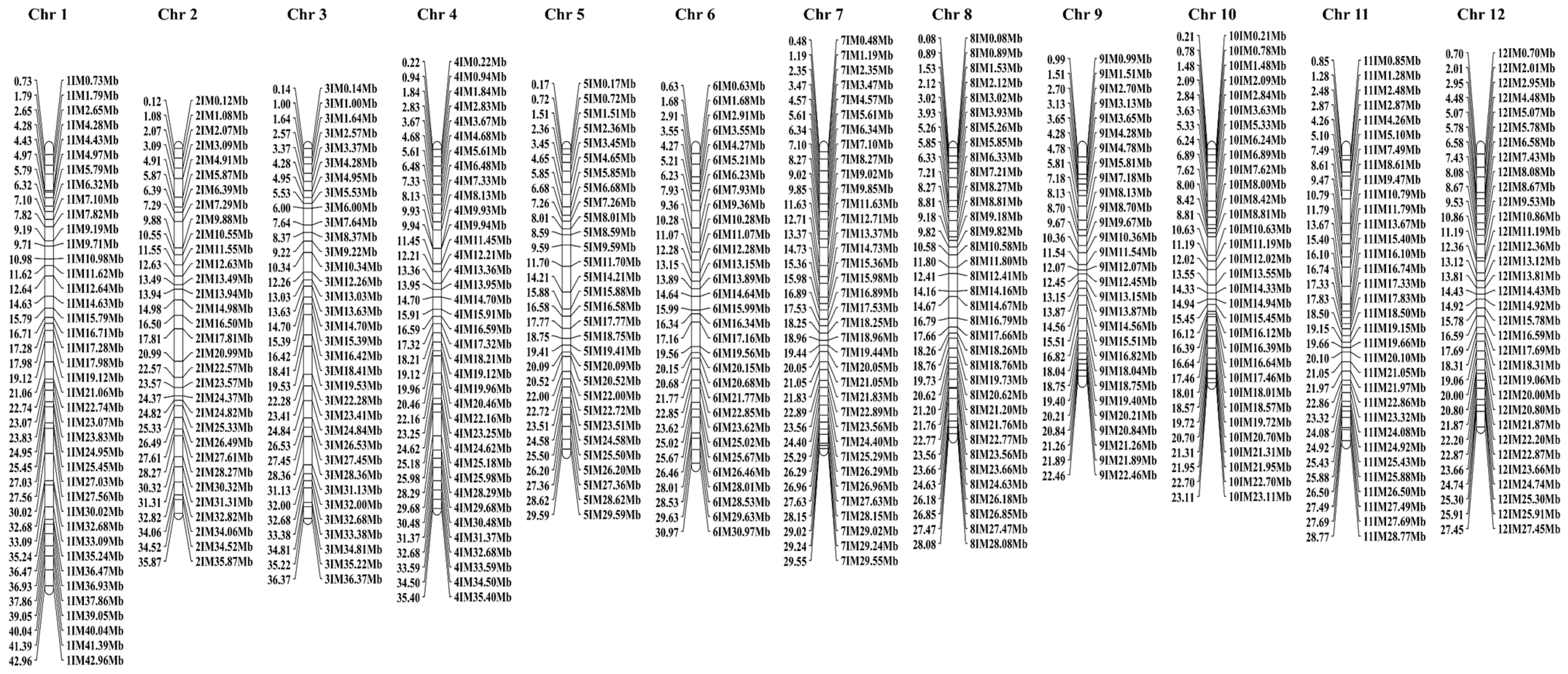
3.4. Development and Screening of Polymorphic InDel Markers
3.5. Salt-Tolerance-Related Traits of 182 japonica Rice Accessions at the Seedling Stage
3.6. Correlation of Salt-Tolerance-Related Traits in 182 japonica Rice Accessions at the Seedling Stage
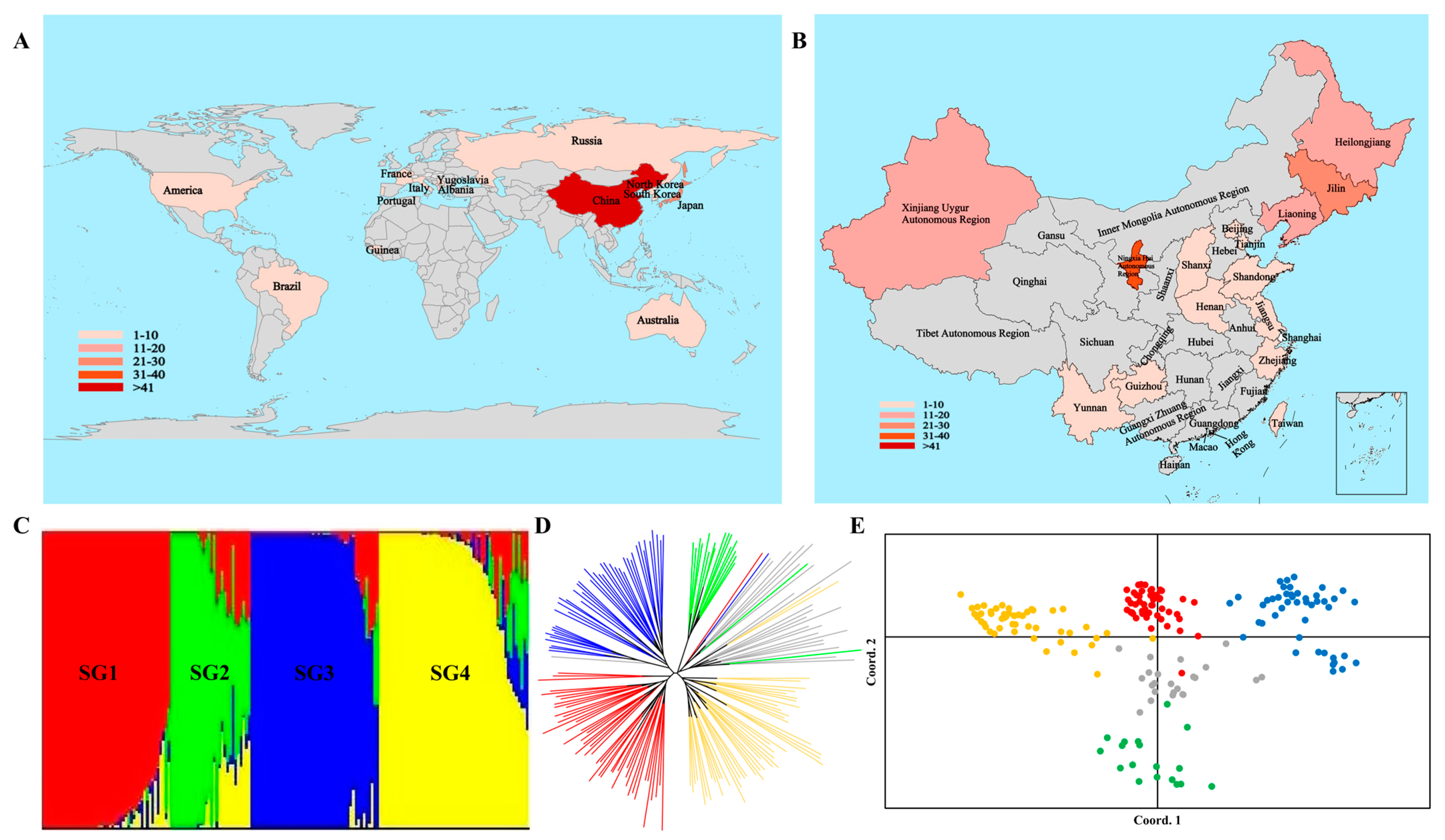
3.7. InDel Marker Genotyping and Genetic Diversity of 182 japonica Rice Accessions
3.8. GWAS of Salt-Tolerance-Related Traits in Rice Seedlings
3.8.1. Population Structure Analysis
3.8.2. GWAS of 12 Salt-Tolerance-Related Traits
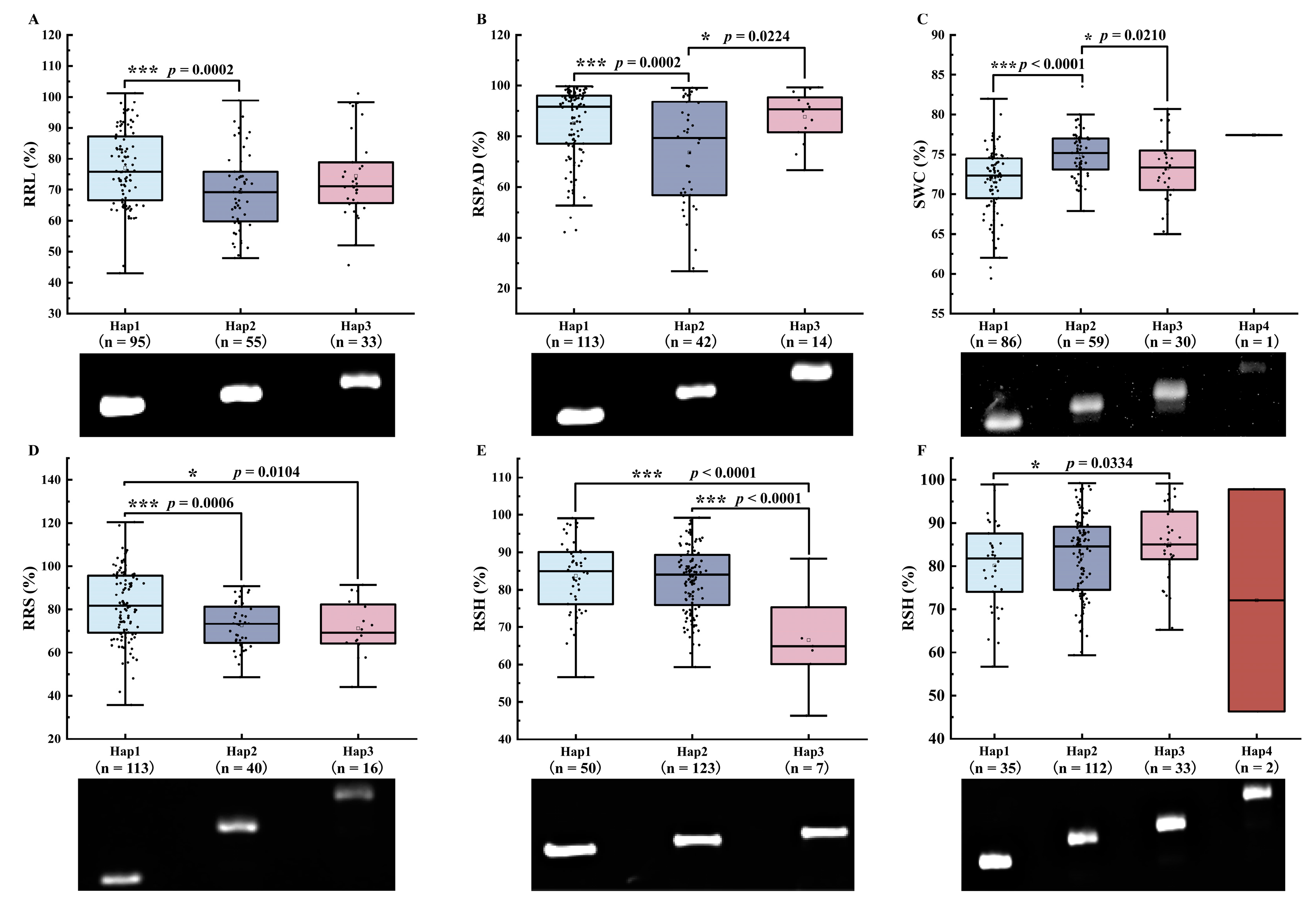
3.8.3. Analysis of Differences in Salt Tolerance among Different Haplotypes at Significantly Associated Loci
4. Discussion
4.1. Genome-Wide Distribution Characteristics of InDel Loci in Different Rice Types in Relation to Rice Domestication
4.2. Development and Application of InDel Markers in Rice Breeding
4.3. Comparison with the Salt-Tolerance-Related QTLs Reported Previously
4.4. Analysis of Cloned Genes within or near the InDel-Linked Locus Regions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, W.; Mauleon, R.; Hu, Z.; Chebotarov, D.; Tai, S.; Wu, Z.; Li, M.; Zheng, T.; Fuentes, R.R.; Zhang, F.; et al. Genomic variation in 3010 diverse accessions of Asian aultivated rice. Nature 2018, 557, 43–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Xia, X.; Zhang, Z.; Nong, B.; Zeng, Y.; Xiong, F.; Wu, Y.; Gao, J.; Deng, G.; Li, D. QTL mapping by whole genome re-sequencing and analysis of candidate genes for nitrogen use efficiency in rice. Front. Plant Sci. 2017, 8, 1634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Feng, Q.; Qian, Q.; Zhao, Q.; Wang, L.; Wang, A.; Guan, J.; Fan, D.; Weng, Q.; Huang, T.; et al. High-throughput genotyping by whole-genome resequencing. Genome Res. 2009, 19, 1068–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, W.; Feng, Q.; Yu, H.; Huang, X.; Zhao, Q.; Xing, Y.; Yu, S.; Han, B.; Zhang, Q. Parent-independent genotyping for constructing an ultrahigh-density linkage map based on population sequencing. Proc. Natl. Acad. Sci. USA 2010, 107, 10578–10583. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Y.; Shen, H.; Yang, W. In silico identification and experimental validation of insertion-deletion polymorphisms in tomato genome. DNA Res. 2014, 21, 429–438. [Google Scholar] [CrossRef] [Green Version]
- Lü, Y.; Cui, X.; Li, R.; Huang, P.; Zong, J.; Yao, D.; Li, G.; Zhang, D.; Yuan, Z. Development of genome-wide insertion/deletion markers in rice based on graphic pipeline platform. J. Integr. Plant Biol. 2015, 57, 980–991. [Google Scholar] [CrossRef] [Green Version]
- Pan, G.; Li, Z.; Huang, S.; Tao, J.; Shi, Y.; Chen, A.; Li, J.; Tang, H.; Chang, L.; Deng, Y.; et al. Genome-wide development of insertion-deletion (InDel) markers for Cannabis and its uses in genetic structure analysis of Chinese germplasm and sex-linked marker identification. BMC Genom. 2021, 22, 595. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, Y.; Xiao, C.; Yuan, J.; Liu, Y.; Zhang, Q. Development of a set of InDel markers for characterizing three japonica rice (Oryza sativa L.) varieties. Plant Genet. Resour. 2023, 20, 304–307. [Google Scholar] [CrossRef]
- Shen, Y.; Jiang, H.; Jin, J.; Zhang, Z.; Xi, B.; He, Y.; Wang, G.; Wang, C.; Qian, L.; Li, X.; et al. Development of genome-wide DNA polymorphism database for map-based cloning of rice genes. Plant Physiol. 2004, 135, 1198–1205. [Google Scholar] [CrossRef] [Green Version]
- Yuan, H.; Yang, W.; Zou, J.; Cheng, M.; Fan, F.; Liang, T.; Yu, Y.; Qiu, R.; Li, S.; Hu, J. InDel markers based on 3K whole-genome re-sequencing data characterise the subspecies of rice (Oryza sativa L.). Agriculture 2021, 11, 655. [Google Scholar] [CrossRef]
- Ji, H.; Shin, Y.; Lee, C.; Oh, H.; Yoon, I.S.; Baek, J.; Cha, Y.-S.; Lee, G.-S.; Kim, S.L.; Kim, K.-H. Genomic variation in Korean japonica rice varieties. Genes 2021, 12, 1749. [Google Scholar] [CrossRef]
- Yano, K.; Yamamoto, E.; Aya, K.; Takeuchi, H.; Lo, P.; Hu, L.; Yamasaki, M.; Yoshida, S.; Kitano, H.; Hirano, K.; et al. Genome-wide association study using whole-genome sequencing rapidly identifies new genes influencing agronomic traits in rice. Nat. Genet. 2016, 48, 927–934. [Google Scholar] [CrossRef]
- Hossain, H.; Rahman, M.A.; Alam, M.S.; Singh, R.K. Mapping of quantitative trait loci associated with reproductive-stage salt tolerance in rice. J. Agron. Crop Sci. 2015, 201, 17–31. [Google Scholar] [CrossRef]
- Lei, L.; Zheng, H.; Bi, Y.; Yang, L.; Liu, H.; Wang, J.; Sun, J.; Zhao, H.; Li, X.; Li, J.; et al. Identification of a major QTL and candidate gene analysis of salt tolerance at the bud burst stage in rice (Oryza sativa L.) using QTL-seq and RNA-seq. Rice 2020, 13, 55. [Google Scholar] [CrossRef]
- Qin, H.; Li, Y.; Huang, R. Advances and challenges in the breeding of salt-tolerant rice. Int. J. Mol. Sci. 2020, 21, 8385. [Google Scholar] [CrossRef]
- Shi, Y.; Gao, L.; Wu, Z.; Zhang, X.; Wang, M.; Zhang, C.; Zhang, F.; Zhou, Y.; Li, Z. Genome-wide association study of salt tolerance at the seed germination stage in rice. BMC Plant Biol. 2017, 17, 92. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Norton, G.J.; Price, A.H. Genome-wide association mapping for salt tolerance of rice seedlings grown in hydroponic and soil systems using the Bengal and assam Aus Panel. Front. Plant Sci. 2020, 11, 576479. [Google Scholar] [CrossRef]
- Ren, Z.; Gao, J.; Li, L.; Cai, X.; Huang, W.; Chao, D.; Zhu, M.; Wang, Z.; Luan, S.; Lin, H. A rice quantitative trait locus for salt tolerance encodes a sodium transporter. Nat. Genet. 2005, 37, 1141–1146. [Google Scholar] [CrossRef]
- Yu, J.; Zao, W.; He, Q.; Kim, T.-S.; Park, Y.-J. Genome-wide association study and gene set analysis for understanding candidate genes involved in salt tolerance at the rice seedling stage. Mol. Genet. Genom. 2017, 292, 1391–1403. [Google Scholar] [CrossRef]
- Song, J.; Yang, H.; Qiao, C.; Zhu, C.; Bai, T.; Du, H.; Ma, S.; Wang, N.; Luo, C.; Zhang, Y.; et al. Natural variations of chlorophyll fluorescence and ion transporter genes influenced the differential response of japonica rice germplasm with different salt tolerances. Front. Plant Sci. 2023, 14, 1095929. [Google Scholar] [CrossRef]
- Ma, S.; Tian, R.; Hu, H.; Lv, J.; Tian, L.; Luo, C.; Zhang, Y.; Li, P. Comprehensive evaluation and selection of rice (Oryza sativa japonica) germplasm for saline tolerance at seedling stage. J. Plant Genet. Resour. 2020, 21, 1089–1101. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The genome analysis toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cingolani, P.; Platts, A.; Wang, L.L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, X.; Ruden, D.M. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 2012, 6, 80–92. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wang, L.; Shou, L. Modified CTAB method for extracting genomic DNA from wheat leaf. J. Agric. Sci. Technol. 2013, 14, 946–949. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Van Meir, E.G. A simple genotyping method to detect small CRISPR-Cas9 induced indels by agarose gel electrophoresis. Sci. Rep. 2019, 9, 4437. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Muse, S.V. PowerMarker: An integrated analysis environment for genetic marker analysis. Bioinformatics 2005, 21, 2128–2129. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Guo, X.; Zhao, P.; Ruan, M.; Yu, X.; Zou, L.; Yang, Y.; Li, X.; Deng, D.; Xiao, J.; et al. Molecular diversity analysis, drought related marker-traits association mapping and discovery of excellent alleles for 100-day old plants by EST-SSRs in cassava germplasms (Manihot esculenta Cranz). PLoS ONE 2017, 12, e0177456. [Google Scholar] [CrossRef] [Green Version]
- Falush, D.; Stephens, M.; Pritchard, J.K. Inference of population structure using multilocus genotype data: Linked loci and correlated allele frequencies. Genetics 2003, 164, 1567–1587. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [Green Version]
- Remington, D.L.; Thornsberry, J.M.; Matsuoka, Y.; Wilson, L.M.; Whitt, S.R.; Doebley, J.; Kresovich, S.; Goodman, M.M.; Buckler, E.S. Structure of linkage disequilibrium and phenotypic associations in the maize genome. Proc. Natl. Acad. Sci. USA 2001, 98, 11479–11484. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—An update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [Green Version]
- Bradbury, P.J.; Zhang, Z.; Kroon, D.E.; Casstevens, T.M.; Ramdoss, Y.; Buckler, E.S. TASSEL: Software for association mapping of complex traits in diverse samples. Bioinformatics 2007, 23, 2633–2635. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Wei, X.; Sang, T.; Zhao, Q.; Feng, Q.; Zhao, Y.; Li, C.; Zhu, C.; Lu, T.; Zhang, Z.; et al. Genome-wide association studies of 14 agronomic traits in rice landraces. Nat. Genet. 2010, 42, 961–967. [Google Scholar] [CrossRef]
- Nayyeripasand, L.; Garoosi, G.A.; Ahmadikhah, A. Genome-wide association study (GWAS) to identify salt-tolerance QTLs carrying novel candidate genes in rice during early vegetative stage. Rice 2021, 14, 9. [Google Scholar] [CrossRef]
- Bizimana, J.B.; Luzi-Kihupi, A.; Murori, R.W.; Singh, R.K. Identification of quantitative trait loci for salinity tolerance in rice (Oryza sativa L.) using IR29/Hasawi mapping population. J. Genet. 2017, 96, 571–582. [Google Scholar] [CrossRef]
- Naveed, S.A.; Zhang, F.; Zhang, J.; Zheng, T.-Q.; Meng, L.-J.; Pang, Y.-L.; Xu, J.-L.; Li, Z.-K. Identification of QTN and candidate genes for salinity tolerance at the germination and seedling stages in rice by genome-wide association analyses. Sci. Rep. 2018, 8, 6505. [Google Scholar] [CrossRef] [Green Version]
- Batayeva, D.; Labaco, B.; Ye, C.; Li, X.; Usenbekov, B.; Rysbekova, A.; Dyuskalieva, G.; Vergara, G.; Reinke, R.; Leung, H. Genome-wide association study of seedling stage salinity tolerance in temperate japonica rce germplasm. BMC Genet. 2018, 19, 2. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.Y.; Zaidem, M.; Gutaker, R.; Dorph, K.; Singh, R.K.; Purugganan, M.D. The complex geography of domestication of the African rice Oryza glaberrima. PLoS Genet. 2019, 15, e1007414. [Google Scholar] [CrossRef] [Green Version]
- Cubry, P.; Tranchant-Dubreuil, C.; Thuillet, A.-C.; Monat, C.; Ndjiondjop, M.-N.; Labadie, K.; Cruaud, C.; Engelen, S.; Scarcelli, N.; Rhoné, B.; et al. The rise and fall of African rice cultivation revealed by analysis of 246 new genomes. Curr. Biol. 2018, 28, 2274–2282.e6. [Google Scholar] [CrossRef] [Green Version]
- Veltman, M.A.; Flowers, J.M.; van Andel, T.R.; Schranz, M.E. Origins and geographic diversification of African rice (Oryza glaberrima). PLoS ONE 2019, 14, e0203508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Yu, Y.; Haberer, G.; Marri, P.R.; Fan, C.; Goicoechea, J.L.; Zuccolo, A.; Song, X.; Kudrna, D.; Ammiraju, J.S.S.; et al. The genome sequence of African rice (Oryza glaberrima) and evidence for independent domestication. Nat. Genet. 2014, 46, 982–988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Zhang, D.; Fan, Y.; Li, C.; Xu, P.; Li, W.; Sun, Q.; Huang, X.; Zhang, C.; Wu, L.; et al. The identification of grain size genes by RapMap reveals directional selection during rice domestication. Nat. Commun. 2021, 12, 5673. [Google Scholar] [CrossRef] [PubMed]
- Chai, C.; Shankar, R.; Jain, M.; Subudhi, P.K. Genome-wide discovery of DNA polymorphisms by whole genome sequencing differentiates weedy and cultivated rice. Sci. Rep. 2018, 8, 14218. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhao, X.; Zhu, J.; Wu, W. Genome-wide investigation of intron length polymorphisms and their potential as molecular markers in rice (Oryza sativa L.). DNA Res. 2006, 12, 417–427. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; He, H.; Chen, L.; Li, L.; Liang, M.; Wang, X.; Liu, X.; He, G.; Chen, R.; Ma, L.; et al. A genome-wide transcription analysis reveals a close correlation of promoter INDEL polymorphism and heterotic gene expression in rice hybrids. Mol. Plant 2008, 1, 720–731. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Zhou, T.; Wang, P.; Wang, B.; Song, J.; Han, Z.; Chen, L.; Liu, K.; Xing, Y. Development of whole-genome agarose-resolvable LInDel markers in rice. Rice 2020, 13, 1. [Google Scholar] [CrossRef]
- Ma, X.; Tang, J.; Zhang, J.; Cui, D.; Li, H.; Li, M.; Han, L. Development of molecular markers polymorphic between Dongxiang wild rice and Geng rice cultivar ‘Nipponbare’. Zuo Wu Xue Bao 2019, 45, 316–321. [Google Scholar] [CrossRef]
- Yuan, R.; Zhao, N.; Usman, B.; Luo, L.; Liao, S.; Qin, Y.; Nawaz, G.; Li, R. Development of chromosome segment substitution lines (CSSLs) derived from guangxi wild rice (Oryza rufipogon Griff.) under rice (Oryza sativa L.) background and the identification of QTLs for plant architecture, agronomic traits and cold tolerance. Genes 2020, 11, 980. [Google Scholar] [CrossRef]
- Li, Z.; Xue, Y.; Zhou, H.; Li, Y.; Usman, B.; Jiao, X.; Wang, X.; Liu, F.; Qin, B.; Li, R.; et al. High-resolution mapping and breeding application of a novel brown planthopper resistance gene derived from wild rice (Oryza. rufipogon Griff). Rice 2019, 12, 41. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Yao, W.; Ouyang, Y.; Yang, W.; Wang, G.; Lian, X.; Xing, Y.; Chen, L.; Xie, W. RiceVarMap: A comprehensive database of rice genomic variations. Nucleic Acids Res. 2015, 43, D1018–D1022. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, J.; Xing, D. Phosphatidylinositol 3-kinase plays a vital role in regulation of rice seed vigor via altering NADPH oxidase activity. PLoS ONE 2012, 7, e33817. [Google Scholar] [CrossRef]
- Delteil, A.; Gobbato, E.; Cayrol, B.; Estevan, J.; Michel-Romiti, C.; Dievart, A.; Kroj, T.; Morel, J.-B. Several wall-associated kinases participate positively and negatively in basal defense against rice blast fungus. BMC Plant Biol. 2016, 16, 17. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Liu, C.; Gao, Z.; Zhang, Y.; Zhang, A.; Zhu, L.; Hu, J.; Ren, D.; Yu, L.; Xu, G.; et al. Variation in the abundance of OsHAK1 transcript underlies the differential salinity tolerance of an indica and a japonica rice cultivar. Front. Plant Sci. 2018, 8, 2216. [Google Scholar] [CrossRef] [Green Version]
- Deng, M.; Hu, B.; Xu, L.; Liu, Y.; Wang, F.; Zhao, H.; Wei, X.; Wang, J.; Yi, K. OsCYCP1;1, a PHO80 homologous protein, negatively regulates phosphate starvation signaling in the roots of rice (Oryza sativa L.). Plant Mol. Biol. 2014, 86, 655–669. [Google Scholar] [CrossRef]
- You, X.; Zhu, S.; Zhang, W.; Zhang, J.; Wang, C.; Jing, R.; Chen, W.; Wu, H.; Cai, Y.; Feng, Z.; et al. OsPEX5 regulates rice spikelet development through modulating jasmonic acid biosynthesis. New Phytol. 2019, 224, 712–724. [Google Scholar] [CrossRef]
- Kim, S.-K.; You, Y.N.; Park, J.C.; Joung, Y.; Kim, B.-G.; Ahn, J.C.; Cho, H.S. The rice thylakoid lumenal cyclophilin OsCYP20-2 confers enhanced environmental stress tolerance in tobacco and Arabidopsis. Plant Cell Rep. 2012, 31, 417–426. [Google Scholar] [CrossRef]
- Wu, T.; Peng, C.; Li, B.; Wu, W.; Kong, L.; Li, F.; Chu, Z.; Liu, F.; Ding, X. OsPGIP1-mediated resistance to bacterial leaf streak in rice is beyond responsive to the polygalacturonase of Xanthomonas oryzae pv. oryzicola. Rice 2019, 12, 90. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Deng, Z.; Gong, C.; Wang, T. The rice eukaryotic translation initiation factor 3 subunit f (OseIF3f) is involved in microgametogenesis. Front. Plant Sci. 2016, 7, 532. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Ying, J.; Peng, L.; Sun, S.; Huang, C.; Li, C.; Wang, Z.; He, Y. A genome-wide association study reveals that the cytochrome B5 involved in seed reserve mobilization during seed germination in rice. Theor. Appl. Genet. 2021, 134, 4067–4076. [Google Scholar] [CrossRef]
- Choi, J.; Lee, J.; Kim, K.; Cho, M.; Ryu, H.; An, G.; Hwang, I. Functional identification of OsHk6 as a homotypic cytokinin receptor in rice with preferential affinity for iP. Plant Cell Physiol. 2012, 53, 1334–1343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.-K.; Eom, J.-S.; Hwang, S.-K.; Shin, D.; An, G.; Okita, T.W.; Jeon, J.-S. Plastidic phosphoglucomutase and ADP-glucose pyrophosphorylase mutants impair starch synthesis in rice pollen grains and cause male sterility. J. Exp. Bot. 2016, 67, 5557–5569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, W.; Li, M.; Yang, S.; Gao, C.; Su, Y.; Zeng, X.; Jiao, Z.; Xu, W.; Zhang, M.; Xia, K. MiR2105 and the kinase OsSAPK10 co-regulate OsbZIP86 to mediate drought-induced ABA biosynthesis in rice. Plant Physiol. 2022, 189, 889–905. [Google Scholar] [CrossRef] [PubMed]
- Chinpongpanich, A.; Phean-O-Pas, S.; Thongchuang, M.; Qu, L.-J.; Buaboocha, T. C-terminal extension of calmodulin-like 3 protein from Oryza sativa L.: Interaction with a high mobility group target protein. Acta Biochim. Biophys. Sin. 2015, 47, 880–889. [Google Scholar] [CrossRef]
- Han, M.-J.; Jung, K.-H.; Yi, G.; Lee, D.-Y.; An, G. Rice immature pollen 1 (RIP1) is a regulator of late pollen development. Plant Cell Physiol. 2006, 47, 1457–1472. [Google Scholar] [CrossRef] [Green Version]
- Kubo, T.; Takashi, T.; Ashikari, M.; Yoshimura, A.; Kurata, N. Two tightly linked genes at the hsa1 locus cause both F1 and F2 hybrid sterility in rice. Mol. Plant 2016, 9, 221–232. [Google Scholar] [CrossRef] [Green Version]
- Nan, N.; Wang, J.; Shi, Y.; Qian, Y.; Jiang, L.; Huang, S.; Liu, Y.; Wu, Y.; Liu, B.; Xu, Z. Rice plastidial NAD -dependent malate dehydrogenase 1 negatively regulates salt stress response by reducing the vitamin B6 content. Plant Biotechnol. J. 2019, 18, 172–184. [Google Scholar] [CrossRef]
- Lou, D.; Wang, H.; Yu, D. The sucrose non-fermenting-1-related protein kinases SAPK1 and SAPK2 function collaboratively as positive regulators of salt stress tolerance in rice. BMC Plant Biol. 2018, 18, 203. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Atienza, J.; Jiang, X.; Garciadeblas, B.; Mendoza, I.; Zhu, J.-K.; Pardo, J.M.; Quintero, F.J. Conservation of the salt overly sensitive pathway in rice. Plant Physiol. 2007, 143, 1001–1012. [Google Scholar] [CrossRef] [Green Version]

| Chromosome | Physical Length (Mb) | Japonica Landraces | Japonica Cultivars | ||
|---|---|---|---|---|---|
| Count/Genome | Frequency (InDels/Mb) | Count/Genome | Frequency (InDels/Mb) | ||
| 1 | 45.06 | 12,229 | 217.4 | 7013 | 155.6 |
| 2 | 36.82 | 9141 | 248.3 | 4567 | 124.0 |
| 3 | 37.25 | 9670 | 259.6 | 4049 | 108.7 |
| 4 | 35.86 | 13,626 | 380.0 | 7893 | 220.1 |
| 5 | 30.04 | 9802 | 326.3 | 3029 | 100.8 |
| 6 | 32.12 | 13,448 | 418.7 | 9168 | 258.4 |
| 7 | 30.35 | 9500 | 313.0 | 3909 | 128.8 |
| 8 | 28.53 | 12,543 | 439.6 | 6368 | 223.2 |
| 9 | 23.77 | 6547 | 275.4 | 3173 | 133.5 |
| 10 | 23.66 | 13,095 | 553.5 | 4866 | 205.7 |
| 11 | 30.83 | 12,985 | 421.1 | 11,938 | 387.2 |
| 12 | 27.75 | 10,952 | 394.7 | 8842 | 318.6 |
| Average | 31.84 | 11,128 | 354.0 | 6235 | 197.0 |
| Chromosome | Chromosome Length (Mb) | Number of Markers | Mean Interval (Mb) |
|---|---|---|---|
| 1 | 45.06 | 40 | 1.12 |
| 2 | 36.82 | 32 | 1.15 |
| 3 | 37.25 | 34 | 1.10 |
| 4 | 35.86 | 37 | 0.97 |
| 5 | 30.04 | 30 | 1.00 |
| 6 | 32.12 | 31 | 1.04 |
| 7 | 30.35 | 36 | 0.84 |
| 8 | 28.53 | 35 | 0.82 |
| 9 | 23.77 | 29 | 0.82 |
| 10 | 23.66 | 32 | 0.74 |
| 11 | 30.83 | 33 | 0.93 |
| 12 | 27.75 | 33 | 0.84 |
| Trait | Min | Max | Range | Median | Mean | SD | CV (%) |
|---|---|---|---|---|---|---|---|
| STS | 1.4 | 7.5 | 6.1 | 4.8 | 4.6 | 1.5 | 31.6 |
| LDP | 10.0 | 100.0 | 90.0 | 70.7 | 66.1 | 22.3 | 33.7 |
| RSH | 46.3 | 99.2 | 52.9 | 83.9 | 82.3 | 9.8 | 11.8 |
| RRL | 43.1 | 101.2 | 58.1 | 73.2 | 74.4 | 12.9 | 17.4 |
| RSPAD | 26.8 | 99.7 | 72.9 | 89.4 | 82.7 | 16.7 | 20.2 |
| RSFW | 24.2 | 99.8 | 75.6 | 56.0 | 57.3 | 17.7 | 31.3 |
| RRFW | 22.5 | 98.6 | 76.1 | 53.4 | 55.3 | 16.4 | 29.9 |
| RSDW | 30.4 | 96.2 | 65.8 | 66.6 | 67.0 | 16.3 | 23.8 |
| RRDW | 19.8 | 98.7 | 78.9 | 51.3 | 54.7 | 16.4 | 30.2 |
| RPDW | 24.6 | 95.1 | 70.5 | 61.3 | 63.2 | 15.6 | 25.2 |
| RRS | 35.8 | 120.4 | 84.6 | 79.6 | 79.5 | 15.3 | 19.4 |
| SWC | 59.4 | 83.5 | 24.1 | 73.6 | 73.2 | 4.0 | 5.0 |
| Trait | STS | LDP | RSH | RRL | RSPAD | RSFW | RRFW | RSDW | RRDW | RPDW | RRS | SWC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| STS | 1.000 | |||||||||||
| LDP | −0.766 ** | 1.000 | ||||||||||
| RSH | 0.447 ** | −0.318 ** | 1.000 | |||||||||
| RRL | −0.056 | 0.043 | 0.257 ** | 1.000 | ||||||||
| RSPAD | 0.720 ** | −0.587 ** | 0.412 ** | −0.046 | 1.000 | |||||||
| RSFW | 0.597 ** | −0.475 ** | 0.364 ** | 0.115 | 0.488 ** | 1.000 | ||||||
| RRFW | 0.438 ** | −0.288 ** | 0.293 ** | 0.135 | 0.280 ** | 0.710 ** | 1.000 | |||||
| RSDW | 0.579 ** | −0.418 ** | 0.449 ** | 0.081 | 0.454 ** | 0.753 ** | 0.681 ** | 1.000 | ||||
| RRDW | 0.407 ** | −0.317 ** | 0.267 ** | 0.143 | 0.271 ** | 0.715 ** | 0.772 ** | 0.685 ** | 1.000 | |||
| RPDW | 0.541 ** | −0.417 ** | 0.368 ** | 0.055 | 0.379 ** | 0.725 ** | 0.684 ** | 0.927 ** | 0.724 ** | 1.000 | ||
| RRS | −0.281 ** | 0.187 * | −0.223 ** | 0.096 | −0.315 ** | −0.097 | 0.073 | −0.266 ** | 0.345 ** | −0.101 | 1.000 | |
| SWC | 0.298 ** | −0.305 ** | 0.058 | −0.042 | 0.412 ** | 0.422 ** | 0.302 ** | 0.211 ** | 0.194 ** | 0.232 ** | −0.145 | 1.000 |
| Traits | Chromosome | Locus-Allele | −log10(p) | R2 (%) | Known QTLs |
|---|---|---|---|---|---|
| RSH | 3 | 3IM14.70Mb-1 | 4.44 | 9.28 | |
| 3 | 3IM14.70Mb-2 | 3.64 | 7.68 | ||
| 10 | 10IM11.19Mb-3 | 3.78 | 7.97 | ||
| 12 | 12IM7.43Mb-1 | 4.42 | 9.25 | ||
| 12 | 12IM7.43Mb-2 | 4.01 | 8.42 | ||
| 12 | 12IM7.43Mb-3 | 5.09 | 10.56 | ||
| 12 | 12IM14.92Mb-1 | 3.72 | 7.85 | ||
| 12 | 12IM14.92Mb-3 | 3.70 | 7.81 | ||
| 12 | 12IM14.92Mb-4 | 3.80 | 8.01 | ||
| 12 | 12IM24.74Mb-2 | 3.93 | 8.28 | ||
| 12 | 12IM24.74Mb-3 | 3.78 | 7.94 | ||
| 12 | 12IM24.74Mb-4 | 3.71 | 7.83 | ||
| 12 | 12IM24.74Mb-5 | 3.86 | 8.13 | ||
| RRL | 1 | 1IM25.45Mb-2 | 4.02 | 7.71 | |
| RSPAD | 2 | 2IM25.33Mb-2 | 3.62 | 7.74 | qSFWn2.2 [35] |
| 9 | 9IM10.36Mb-1 | 3.68 | 7.81 | qRFW9.1 [36] | |
| RSFW | 10 | 10IM6.24Mb-1 | 3.63 | 8.62 | |
| 10 | 10IM6.24Mb-2 | 4.26 | 10.03 | qRFWs10.1 [35] qRLs10.1 [35] | |
| RRFW | 10 | 10IM6.24Mb-2 | 4.63 | 11.22 | qRFWs10.1 [35] |
| qRLs10.1 [35] | |||||
| RRDW | 10 | 10IM6.24Mb-2 | 4.15 | 9.44 | qRFWs10.1 [35] qRLs10.1 [35] |
| RPDW | 10 | 10IM6.24Mb-2 | 4.00 | 9.41 | qRFWs10.1 [35] qRLs10.1 [35] |
| RRS | 5 | 5IM19.41Mb-1 | 3.70 | 9.07 | |
| SWC | 1 | 1IM14.63Mb-1 | 4.19 | 7.57 | qSNC1 [37] |
| 1 | 1IM14.63Mb-2 | 3.82 | 6.83 | ||
| 4 | 4IM19.96Mb-3 | 3.65 | 7.96 | qSNa12_4.1 [38] | |
| 5 | 5IM0.17Mb-2 | 4.17 | 9.04 | ||
| 10 | 10IM1.48Mb-1 | 3.69 | 8.04 | ||
| 10 | 10IM1.48Mb-2 | 3.69 | 8.04 |
| Gene | Chr | Related Traits | Position of Associated InDel (bp) | Gene MSU ID | Gene Function | References |
|---|---|---|---|---|---|---|
| Osrboh1 | 1 | SWC | 1IM14.63Mb | LOC_Os01g25820 | respiratory burst oxidase | Liu et al., 2012 [52] |
| OsWAK14 | 2 | RSPAD | 2IM25.33Mb | LOC_Os02g42150 | OsWAK14—OsWAK receptor-like protein kinase | Delteil et al., 2016 [53] |
| OsHAK1 | 4 | SWC | 4IM19.96Mb | LOC_Os04g32920 | potassium transporter | Chen et al., 2018 [54] |
| OsCYCP1;1 | 5 | RRS | 5IM19.41Mb | LOC_Os05g33040 | cyclin, putative, expressed | Deng et al., 2014 [55] |
| OsPEX14 | 5 | SWC | 5IM0.17Mb | LOC_Os05g01090 | pex14, putative, expressed | You et al., 2019 [56] |
| OsCYP20-2 | 5 | SWC | 5IM0.17Mb | LOC_Os05g01270 | peptidyl-prolyl cis-trans isomerase, putative, expressed | Kim et al., 2011 [57] |
| OsPGIP1 | 5 | SWC | 5IM0.17Mb | LOC_Os05g01380 | polygalacturonase inhibitor precursor, putative, expressed | Wu et al., 2019 [58] |
| OseIF3f | 5 | SWC | 5IM0.17Mb | LOC_Os05g01450 | eukaryotic translation initiation factor 3 subunit F, putative, expressed | Li et al., 2016 [59] |
| OsCyb5 | 9 | RSPAD | 9IM10.36Mb | LOC_Os09g16920 | desaturase/cytochrome b5 protein, putative, expressed | Huang et al., 2021 [60] |
| OsHk5 | 10 | RSH | 10IM11.19Mb | LOC_Os10g21810 | histidine kinase, putative, expressed | Choi et al., 2012 [61] |
| OspPGM | 10 | RSFW RRFW RRDW RPDW | 10IM6.24Mb | LOC_Os10g11140 | phosphoglucomutase, putative, expressed | Lee et al., 2016 [62] |
| OsbZIP86 | 12 | RSH | 12IM7.43Mb | LOC_Os12g13170 | transcription factor, putative, expressed | Gao et al., 2022 [63] |
| OsCML3 | 12 | RSH | 12IM14.92Mb | LOC_Os12g03816 | OsCML3—Calmodulin-related calcium sensor protein, expressed | Chinpongpanich et al., 2015 [64] |
| RIP1 | 12 | RSH | 12IM14.92Mb | LOC_Os12g03822 | WD domain, G-beta repeat domain-containing protein, expressed | Han et al., 2006 [65] |
| HSA1b | 12 | RSH | 12IM24.74Mb | LOC_Os12g39920 | expressed protein | Kubo et al., 2016 [66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Song, J.; Qiao, C.; Duan, K.; Feng, P.; Kong, W.; Bai, T.; Zhu, C.; Ma, S.; Zhang, Y.; et al. Genome-Wide Association Studies of Salt-Tolerance-Related Traits in Rice at the Seedling Stage Using InDel Markers Developed by the Genome Re-Sequencing of Japonica Rice Accessions. Agriculture 2023, 13, 1573. https://doi.org/10.3390/agriculture13081573
Yang H, Song J, Qiao C, Duan K, Feng P, Kong W, Bai T, Zhu C, Ma S, Zhang Y, et al. Genome-Wide Association Studies of Salt-Tolerance-Related Traits in Rice at the Seedling Stage Using InDel Markers Developed by the Genome Re-Sequencing of Japonica Rice Accessions. Agriculture. 2023; 13(8):1573. https://doi.org/10.3390/agriculture13081573
Chicago/Turabian StyleYang, Hui, Jiawei Song, Chengbin Qiao, Kairong Duan, Peiyuan Feng, Weiru Kong, Tianliang Bai, Chunyan Zhu, Shuaiguo Ma, Yinxia Zhang, and et al. 2023. "Genome-Wide Association Studies of Salt-Tolerance-Related Traits in Rice at the Seedling Stage Using InDel Markers Developed by the Genome Re-Sequencing of Japonica Rice Accessions" Agriculture 13, no. 8: 1573. https://doi.org/10.3390/agriculture13081573





