1. Introduction
Today, there is an increasing interest in a healthy and balanced diet, but the explosive population growth makes supplying the human diet with all necessary nutrients a challenge for the food industry [
1]. Thus, it is important to develop several strategies aimed at improving foodstuffs’ composition with several nutritive compounds that can help the proper functioning of the human body and prevent some diseases, in order to achieve the nutritive requirements for a healthy lifestyle [
2]. The most important strategy used to influence animals’ food products, leading to nutritionally enriched foodstuffs, is the animal feeding system, but the commonly used feed resources (e.g., soya) are limited and some of them are unavailable or expensive. Thus, the valorisation of several industry byproducts as raw materials for animals’ nutrition represents a sustainable alternative [
3,
4,
5]. Yeast is an important feed ingredient in ruminants’ diets. Several studies have examined the impact of including live yeast in ruminants’ diets (e.g.,
Saccharomyces cerevisiae), obtaining contradictory results depending on the level of yeast inclusion. The addition of 10 g of live yeast in cows’ diet enhanced their dry matter intake and milk output. However, no significant differences in these parameters were identified at a lower inclusion level (4.0 g/day) [
6,
7].
Also, brewery byproducts can be used as feedstuffs for ruminants. It has been reported that brewer’s yeast, liquid brewer’s yeast, or brewer’s spent grain can be used as alternative feedstuffs to commonly used protein sources (such as soybeans), without affecting ingesta behaviour, gas kinetics, or ruminal fermentation [
8,
9,
10,
11]. Brewer’s spent yeast (BSY) represents one of the major byproducts obtained from the brewery industry. BSY is generated from the fermentation of yeast that is no longer useful in the fermentation process and must be discarded. A small portion of it can be used to initiate a new fermentation cycle, but the majority of it represents the spent yeast (BSY) [
12]. In the brewery process, BSY can be considered to be waste and disposed of by the brewers [
13]. However, from the economical point of view, and considering its composition, BSY may have potential application in ruminants’ nutrition, as an alternative feedstuff to commonly used protein sources [
12]. In addition to being a protein source, the composition of BSY suggests that its inclusion in ruminants’ diets may have positive effects, e.g., by influencing the ruminal environment. Because BSY is rich in α- and β-acids, it has potential inhibitory activity against hyper-ammonia bacteria and Gram-positive bacteria; therefore, the inclusion of BSY in ruminants’ diets can reduce the ruminal concentration of ammonia and methane production, which is important from the environmental point of view [
14]. Also, BSY may have effects on milk quality, considering its rich composition of bioactive compounds, such as vitamins, minerals, β-glucans, and phenolic compounds, known for their anti-inflammatory, antitumor, and antioxidant activities [
12,
15]. Despite its potential, little attention has been paid to the effects of BSY on ruminal fermentation parameters and milk quality. Moreover, very few publications are available in the literature that discuss the effects of the other inactivated yeasts in ruminants. Several studies have suggested that BSY or other live yeasts may have the potential to improve milk production and milk fat; however, the effects on milk minor constituents have not been fully elucidated [
12,
16,
17,
18]. The ability to change the minor constituents of the milk may be of interest, especially in the periods when the milk yield is decreasing, such as during late lactation.
The aim of this study was to investigate the effect of BSY’s inclusion in late-lactation dairy sheep’s diets on the parameters describing ruminal fermentation and several milk quality parameters, given that BSY can positively influence ruminal fermentation and increase milk’s antioxidant capacity.
4. Discussion
The diversity of the processing technologies, the variability of the raw materials, etc., induce a great variability in the byproducts in terms of characteristics that are relevant from a nutritional point of view. Brewery is no exception; therefore, it is important to consider the nutritional particularities of the studied byproduct.
The crude protein content of the BSY used in the experiment was below the range of the values reported in the literature (45–60%) [
12], suggesting particularities of the processing technology. Indeed, the chemical composition of BSY may vary based on diversity of yeast strains used for pitching, the characteristics of the growth medium, the wort gravity and density, etc. [
29]. The data regarding the protein content of BSY used in our experiment were closer to the data obtained for the SBM (44.23%). However, it was higher than the protein content of the concentrates commonly used in ruminants’ nutrition, such as sunflower or rapeseed cakes (27.9–33.7% crude protein), [
30] or other minor oilseeds cakes such as linseed or safflower cakes (32–33% crude protein) [
31]. This places BSY between this group and soybean meal, which opens the possibility of replacing the latter in some animal categories that have intermediate protein requirements. The resemblance to the soybean meal is also given by its low fat content (0.36%). For the BSY used in our experiment, the determined level was within the range of 0.02–6.5%, reported in the literature [
32,
33,
34].
Also, the trace mineral concentrations of the BSY were within the ranges of the data presented in the literature [
29,
30,
32,
34], with the exception of zinc, whose concentration was lower than the values reported in other studies [
32]. The data regarding the mineral composition of BSY presented in the literature are contradictory and dependent on the technological flux of beer production. The determination of antioxidant potential (antioxidant capacity, total polyphenols, and vitamin E content) confirmed that the studied BSY is an important source of bioactive compounds, showing remarkable antioxidant potential, with a total polyphenols content of 2.77 mg/g gallium acid equivalents, a vitamin E content of 125.69 mg/kg, and an antioxidant capacity of 13.26 mM Trolox equivalents.
Ruminal pH value was not affected by the treatments, being close to the “safe zone” from the point of view of the rumen’s normal activity (fibre degradation, microbial protein synthesis, etc.). Our results suggest that BSY can influence the rumen-level digestion by acting on both nitrogen and energy metabolism. Thus, the sheep in the BSY group tended to had a higher level of ruminal ammonia (+23%), which stands in contrast to the fact that active substances from BSY may actually inhibit the rumen’s degradation of proteins, e.g., by decreasing the population of hyper-ammonia-producing bacteria [
35,
36].
Compared to the control group, the inclusion of BSY considerably influenced the profile of the primary VFAs, leading to a lower propionate concentration, a tendency towards a higher butyrate concentration, and a higher acetate: propionate ratio. Brewery byproducts, including BSY, presumably contain active substances from hop cones [
14,
35,
36]. Various studies have also highlighted the capacity of yeasts to absorb the polyphenols [
37] and α- and β-acids from hops during fermentation [
14]. However, our results are in contrast to those of [
38], who reported that ruminal bacteria produced less acetate in the presence of hop beta-acids. The inclusion of BSY is known to decrease the production of methane during in vitro incubations with ruminal microorganisms [
8]; this is related to changes (e.g., in the ruminal populations) that also influence the VFA profiles.
Our results suggest that BSY has the potential to influence processes involved in ruminal methanogenesis (via carbohydrate metabolism, as evidenced by changes in the volatile fatty acid profile, e.g., an increase in the acetate: propionate ratio), but additional research, such as assessing microbial populations, will be required to confirm this finding [
35].
In our study, BSY did not significantly influence the primary chemical composition of milk. In both groups, the protein content was at the upper limit of the range reported in the literature for various sheep breeds [
39]. Very few data about the effects of BSY on milk composition are presented in the literature; therefore, we also compared our results with those for other yeast-based ingredients, inactivated or not. Studies on the use of BSY slurry or
Saccharomyces cerevisiae in the diets of ruminants (e.g., cows and sheep) have reported significant increases in the milk fat content by improving the composition of several fatty acids, such as C18:3 [
18,
40]. Data obtained by other authors [
41] revealed an increase in the milk protein content after
Saccharomyces cerevisiae supplementation in dairy cows, but these results are less comparable, considering that BSY contains inactivated yeast. Other milk parameters, such as total casein content, lactose content, and pH, were not affected by the inclusion of BSY according to data presented in the literature [
42,
43,
44].
The fact that BSY, in our study, did not significantly influence the primary chemical composition of milk is consistent with the fact that the level of BSY dietary inclusion was limited, and that the diets were designed to be isonitrogenous and isoenergetic.
The fatty acid profiles were also analysed to better observe the effects of BSY on fat quality. The inclusion of BSY induced a significant increase in saturated fatty acid (SFA) contents and a significant decrease in monounsaturated fatty acid (MUFA) and polyunsaturated fatty acid (PUFA) contents. In the literature, there are no data on the effects of BSY on milk’s FA profile. Several studies have reported that
Saccharomyces cerevisiae supplementation did not significantly change the fatty acid profile of cow milk, but unlike BSY they used live yeasts and, consequently, a lower inclusion rate [
45,
46]. Also, the mechanisms involved in their influence on digestion are different.
On the other hand, the inclusion of BSY induced significant decreases in milk’s contents of Ω6 fatty acids, which contributed to the significant decrease in the Ω6/Ω3 ratio. These are important for consumers’ health, as it is known that a low Ω6/Ω3 ratio in the consumed lipids may help prevent some autoimmune, inflammatory, and cardiovascular diseases [
47].
The fact that the inclusion of BSY induced a significant increase in SCFA (caproic acid) and MCFA (capric and lauric acids) contents may have potential beneficial implications for human health. Various studies have reported that SCFAs and MCFAs are better sources of energy for humans, compared with long-chain fatty acids, due to their smaller molecules and to the pathways of their transport through the organism [
48]. Other studies have suggested that SCFAs and MCFAs may play a potential protective role against the growth of pathogens and can exert anti-inflammatory activity [
49]. Moreover, recent studies have highlighted that SCFAs can have beneficial effects in the context of cardiovascular diseases such as coronary artery calcification [
50].
Considering the particularities of BSY, the milk samples were also compared from the point of view of fine constituents that can influence the quality of milk and can improve human health. Among the noticeable effects of the BSY was the modification of the levels of some fine constituents of milk. The inclusion of BSY induced a significant increase in calcium content, which is important from the point of view of consumer health. Some authors even consider Ca to be a “super-nutrient” due to its capacity to reduce the risk of developing diseases such as osteoporosis and hypertension, while recent studies have revealed its potential to reduce the risk of developing colon cancer [
51].
In terms of milk’s antioxidant potential, the inclusion of BSY was linked to an increase in total polyphenol content; this is important from the perspective of milk quality and consumer health, given the polyphenols’ ability to block or delay lipid oxidation [
52]. The increased in milk’s total polyphenol content can be explained by the capacity of BSY to absorb the bioactive compounds (such as polyphenols) from wort during the brewery fermentation process. This opens the possibility to transfer these bioactive compounds, through animal feeding, into the final products, such as milk [
37].
On the other hand, the antioxidant capacity of the milk was not significantly influenced by the inclusion of BSY. There are no reports in the literature on the effects of the inclusion of BSY on milk’s antioxidant capacity; however, in a study performed on sows’ colostrum and milk, a significant increase in milk’s antioxidant status was observed when
Saccharomyces cerevisiae was included in their diet [
53].
Vitamin E is another antioxidant compound that can be found in milk, and it is important due to its powerful capacity to inhibit peroxidation [
54]; however, in our study, its concentration was not significantly changed by the inclusion of BSY. It is known that BSY is not a rich source of vitamin E, but the antioxidant compounds could influence the absorption and deposition of vitamin E [
55].
The antioxidant capacity of milk is considered to be a priority subject, considering that fatty acids’ oxidation is one of the most important processes that can lead to changes in milk’s flavour and nutritional quality [
56]. Studies on the effects of BSY on TBARS parameters are still lacking; however, the same trend was observed in sows’ colostrum and milk with the inclusion of a yeast extract [
57]. After performing the TBARS analysis adapted for milk samples [
25], it was observed that BSY did not influence the TBARS parameters, with the exception of the absorbance read at 495 nm (specifically for saturated aldehydes), which was significantly increased.
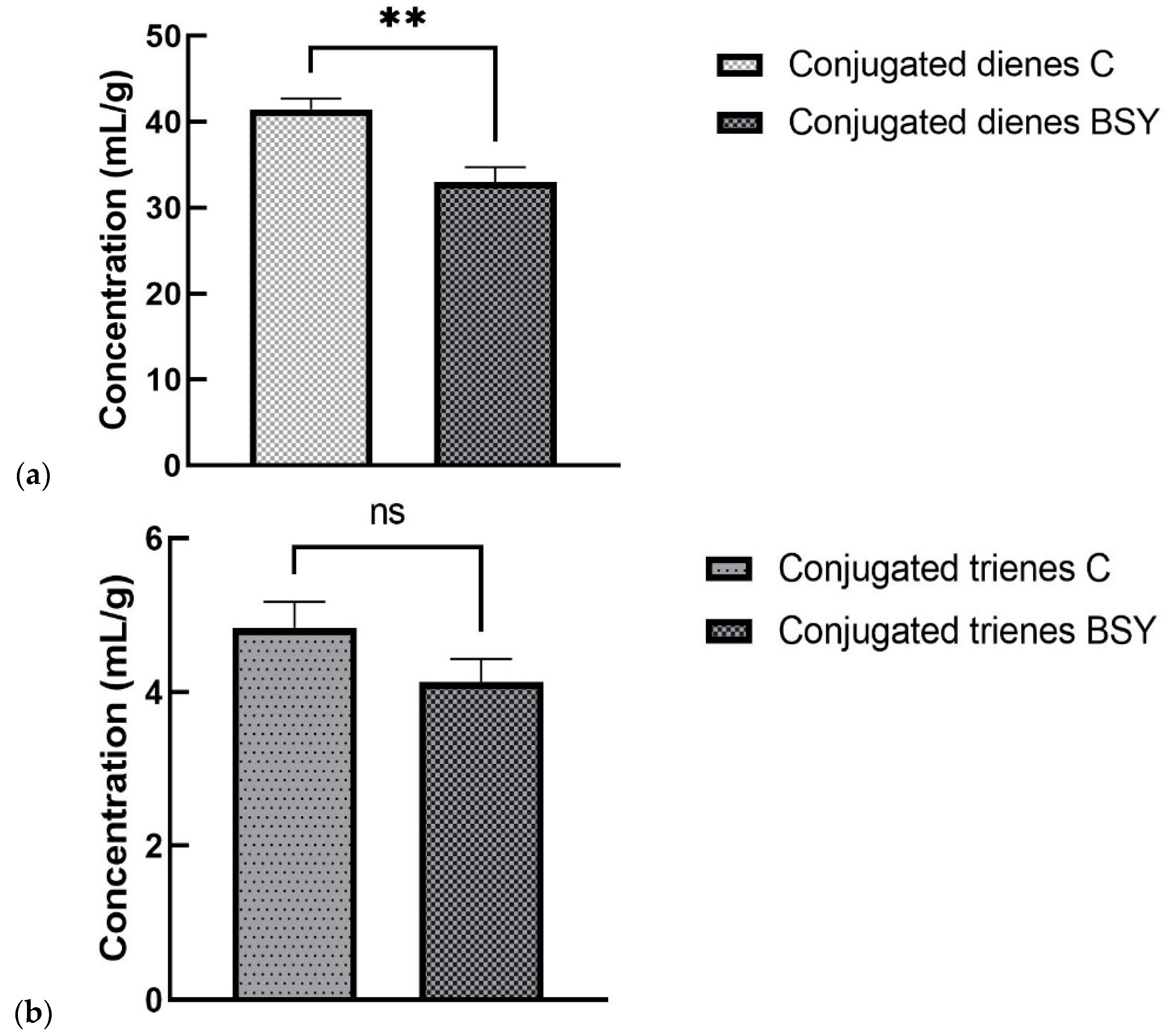
The process of fatty acids’ oxidation occurs in different stages; therefore, it is important to quantify the primary (conjugated dienes and conjugated trienes) and secondary (TBARS) degradation products. Our results suggested that BSY significantly decreased the contents of conjugated dienes, but the contents of conjugated trienes were not influenced by the inclusion of BSY. Very few publications can be found regarding the degradation products of milk obtained from ruminants fed diets including BSY; however, in one study performed on dairy cows to investigate the effects of the inclusion of malt bagasse and selenium-enriched
Saccharomyces cerevisiae on animals’ performance, it was observed that the contents of conjugated dienes were significantly increased in the experimental group, in contrast with data presented in our study [
16].
The heatmap results (
Figure 2) confirmed that the presence of antioxidant compounds in milk can slow down the lipids’ oxidation processes. The same pattern of negative correlations was presented between both parameters of lipid oxidation and the antioxidant potential of milk (secondary degradation parameters with antioxidant capacity and vitamin E content, and primary degradation products with total polyphenols content), as also reported by other studies [
58]. The contents of Ω6 fatty acids were positively correlated with all milk lipid degradation parameters, reflecting that a decrease in Ω6 fatty acids (influenced by the inclusion of BSY) can slow down the milk lipids’ oxidation process by decreasing the contents of primary oxidation products of milk lipids (i.e., conjugated dienes).
The primary chemical composition of milk was not correlated with any of the milk lipid oxidation products, but the contents of milk protein and caseins were positively correlated with the milk’s total antioxidant capacity. Indeed, the scientific literature confirms the correlation between antioxidant capacity and milk protein content, as it is also known that proteins can protect the molecules of bioactive compounds, such as polyphenols, and can enhance the antioxidant activity [
59]. Moreover, it is known that several peptides present in milk, such as casein fractions, are directly related to the antioxidant capacity of milk, due to their antioxidant potential [
60].