Balancing Yield and Antioxidant Capacity in Basil Microgreens: An Exploration of Nutrient Solution Concentrations in a Floating System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup and Design
2.2. Determination of Photosynthetic Pigments
2.3. Determination of Vitamin C Content
2.4. Measurement of Antioxidant Capacity, Polyphenols, Flavonoids, and Anthocyanin
2.5. Determination of the Antioxidant Potential Composite Index
2.6. Yield of Basil Microgreen
2.7. Statistical Analysis
3. Results
3.1. Content of Photosynthetic Pigments
3.2. Carotenoid Content
| Chl a | Chl b | Chl a+b | Car | Vit C | TFC | ACNs | TPC | AC | APCI | Y | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chl a | 1 | ||||||||||
| Chl b | 0.874 ** | 1 | |||||||||
| Chl a+b | 0.976 ** | 0.959 ** | 1 | ||||||||
| Car | 0.326 * | 0.193 | 0.278 * | 1 | |||||||
| Vit C | 0.377 ** | 0.232 | 0.325 * | 0.785 ** | 1 | ||||||
| TFC | 0.224 | 0.076 | 0.166 | 0.829 ** | 0.606 ** | 1 | |||||
| ACNs | 0.180 | 0.136 | 0.164 | 0.239 | 0.455 ** | 0.144 | 1 | ||||
| TPC | 0.394 ** | 0.154 | 0.299 * | 0.530 ** | 0.468 ** | 0.590 ** | 0.066 | 1 | |||
| AC | 0.372 ** | 0.201 | 0.309 * | 0.618 ** | 0.482 ** | 0.697 ** | −0.014 | 0.730 ** | 1 | ||
| APCI | 0.414 ** | 0.224 | 0.343 ** | 0.888 ** | 0.852 ** | 0.851 ** | 0.426 ** | 0.727 ** | 0.775 ** | 1 | |
| Y | −0.423 ** | −0.345 ** | −0.401 ** | 0.025 | 0.062 | −0.056 | −0.085 | −0.239 | −0.242 | −0.112 | 1 |
3.3. Vitamin C Content
3.4. Flavonoid Content
3.5. Anthocyanin Content
3.6. Total Polyphenols Content
3.7. Antioxidant Capacity
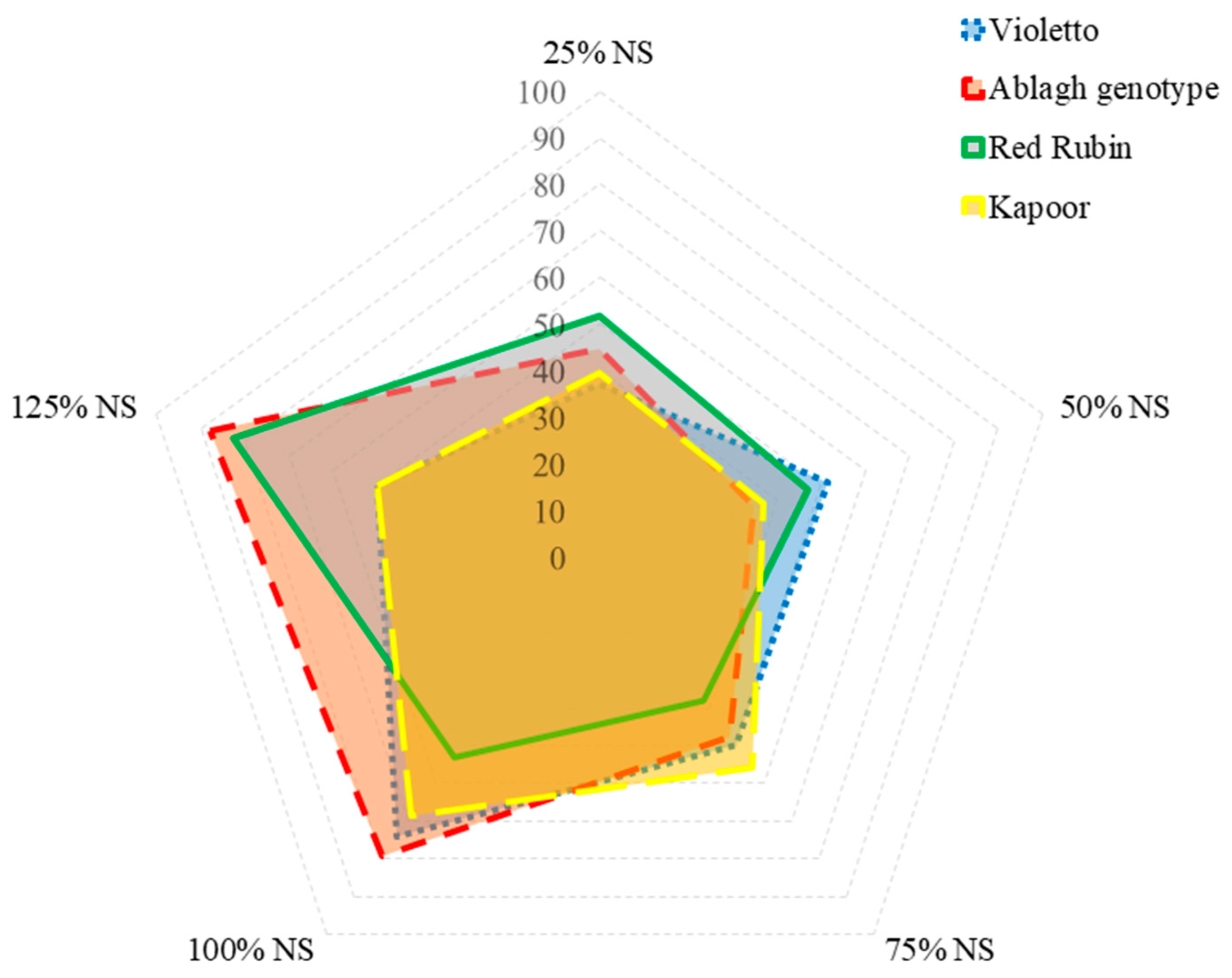
3.8. Antioxidant Potential Composite Index
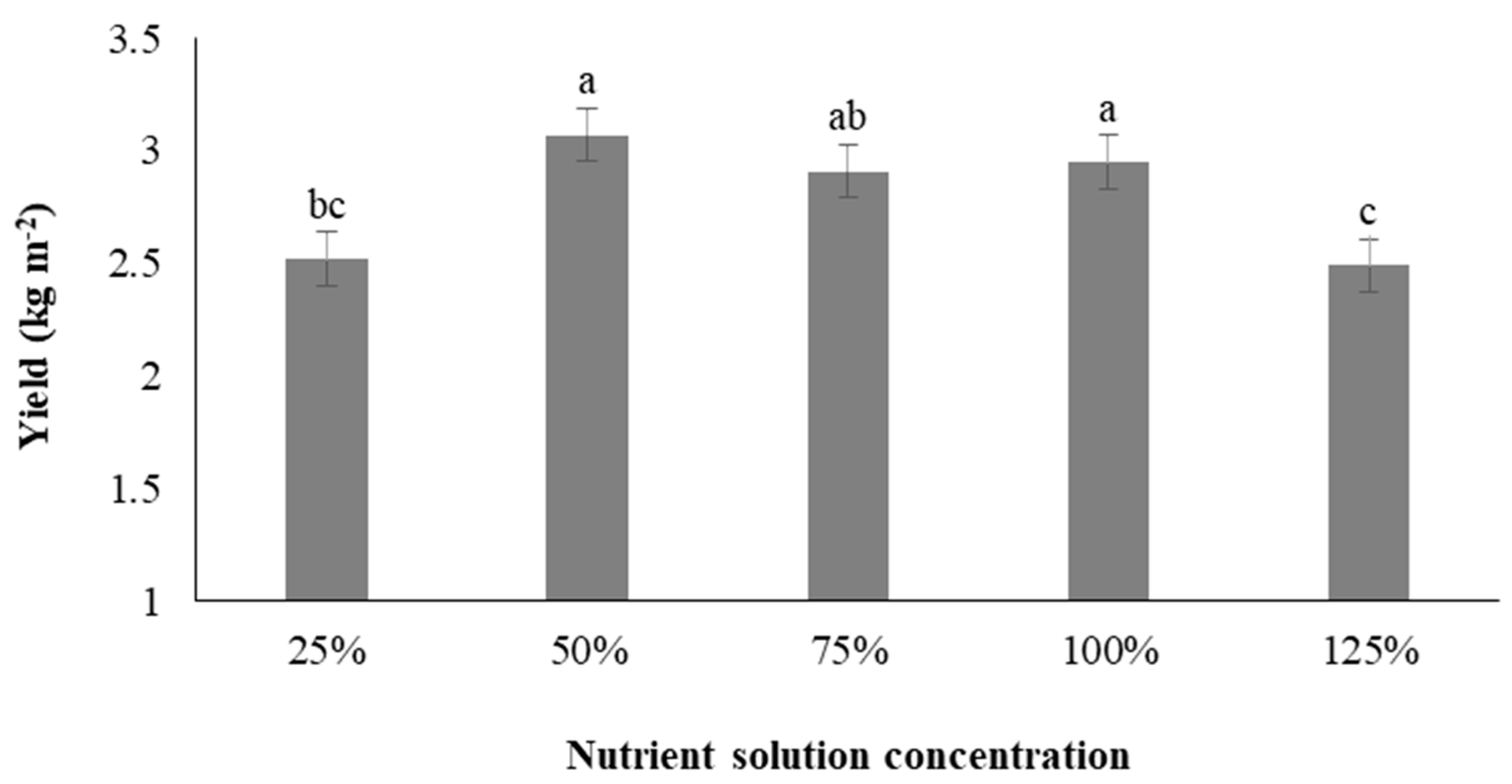
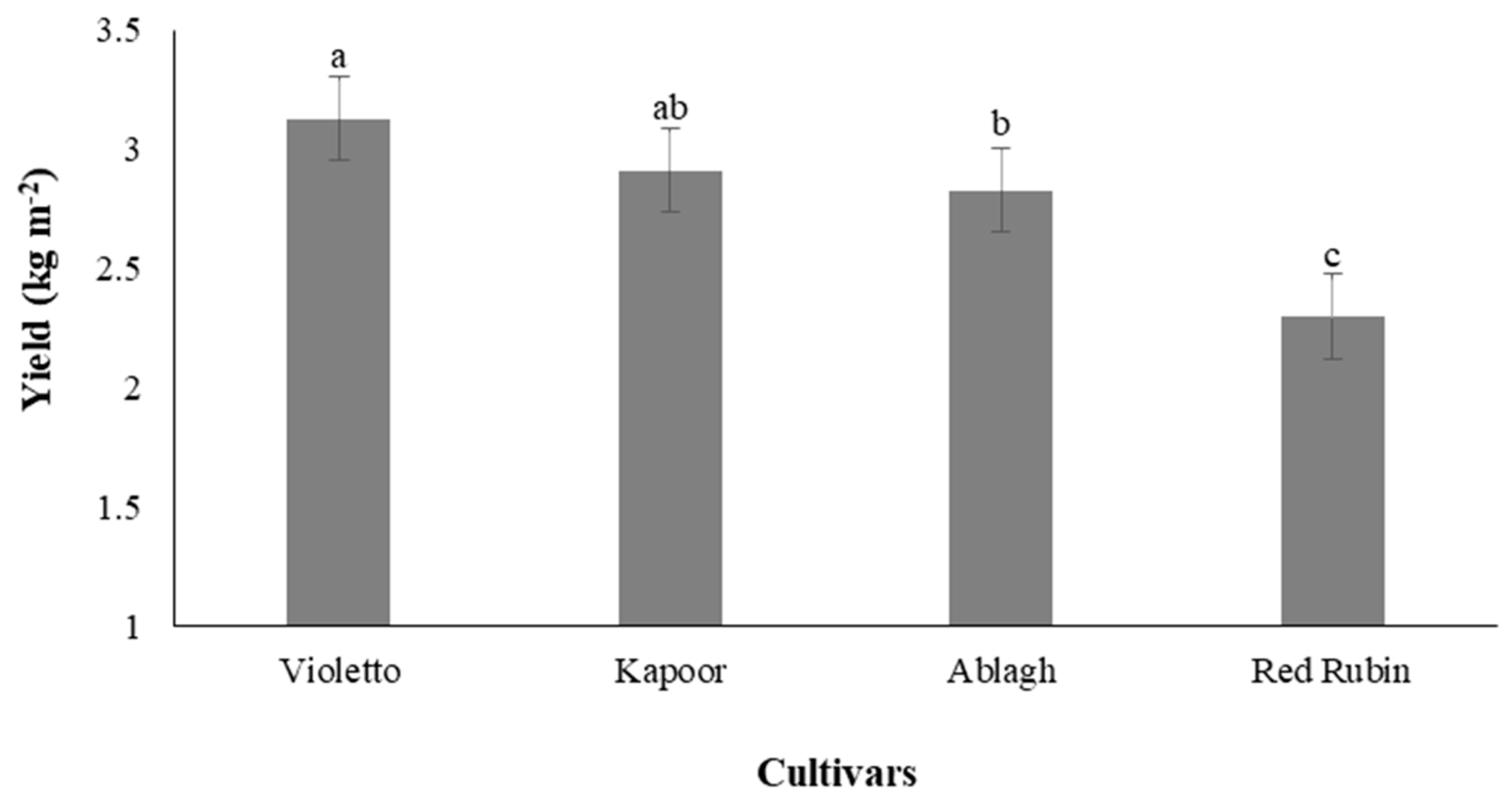
3.9. Yield of Microgreens
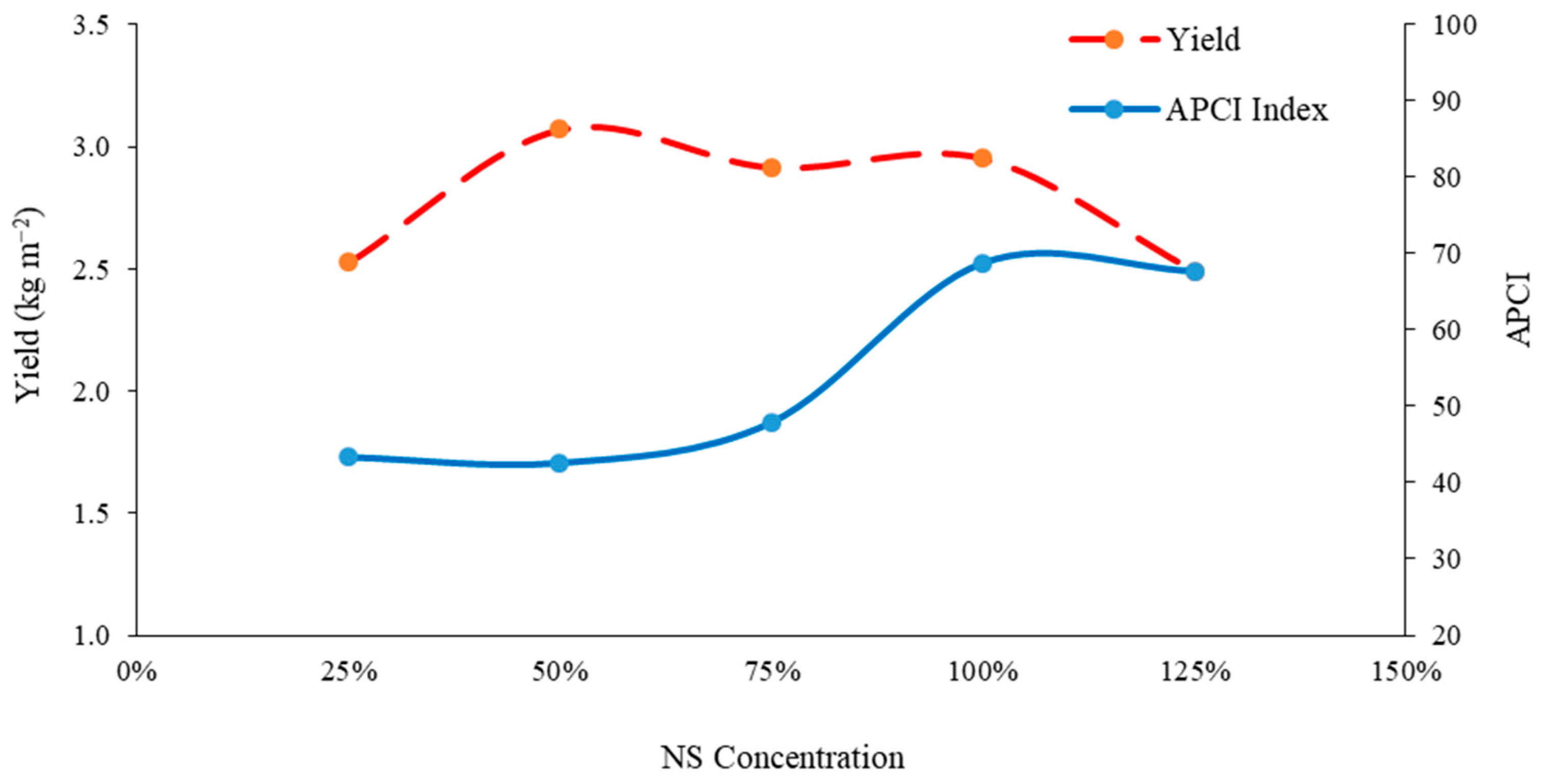
3.10. Balance of Yield and Antioxidant Accumulation under Different Concentrations of Nutrient Solution
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teklić, T.; Parađiković, N.; Špoljarević, M.; Zeljković, S.; Lončarić, Z.; Lisjak, M. Linking abiotic stress, plant metabolites, biostimulants and functional food. Ann. Appl. Biol. 2021, 178, 169–191. [Google Scholar] [CrossRef]
- Verma, N.; Shukla, S. Impact of various factors responsible for fluctuation in plant secondary metabolites. J. Appl. Res. Med. Aromat. Plants 2015, 2, 105–113. [Google Scholar] [CrossRef]
- Mir, S.A.; Shah, M.A.; Mir, M.M. Microgreens: Production, shelf life, and bioactive components. Crit. Rev. Food Sci. Nutr. 2017, 57, 2730–2736. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Li, J. Medicine is not health care, food is health care: Plant metabolic engineering, diet and human health. New Phytol. 2017, 216, 699–719. [Google Scholar] [CrossRef]
- Fayezizadeh, M.R.; Ansari, N.A.; Sourestani, M.M.; Hasanuzzaman, M. Biochemical Compounds, Antioxidant Capacity, Leaf Color Profile and Yield of Basil (Ocimum sp.) Microgreens in Floating System. Plants 2023, 12, 2652. [Google Scholar] [CrossRef]
- Larsen, D.H.; Li, H.; Shrestha, S.; Verdonk, J.C.; Nicole, C.; Marcelis, L.F.; Woltering, E.J. Lack of blue light regulation of antioxidants and chilling tolerance in Basil. Front. Plant Sci. 2022, 13, 852654. [Google Scholar] [CrossRef]
- Shomali, A.; Das, S.; Arif, N.; Sarraf, M.; Zahra, N.; Yadav, V.; Aliniaeifard, S.; Chauhan, D.K.; Hasanuzzaman, M. Diverse Physiological Roles of Flavonoids in Plant Environmental Stress Responses and Tolerance. Plants 2022, 11, 3158. [Google Scholar] [CrossRef]
- Lu, N.; Bernardo, E.L.; Tippayadarapanich, C.; Takagaki, M.; Kagawa, N.; Yamori, W. Growth and accumulation of secondary metabolites in perilla as affected by photosynthetic photon flux density and electrical conductivity of the nutrient solution. Front. Plant Sci. 2017, 8, 708. [Google Scholar] [CrossRef]
- Tan, L.; Nuffer, H.; Feng, J.; Kwan, S.H.; Chen, H.; Tong, X.; Kong, L. Antioxidant properties and sensory evaluation of microgreens from commercial and local farms. Food Sci. Human Wellness 2020, 9, 45–51. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; De Pascale, S.; Kyratzis, A.; Rouphael, Y. Microgreens as a Component of Space Life Support Systems: A Cornucopia of Functional Food. Front. Plant Sci. 2017, 8, 1587. [Google Scholar] [CrossRef]
- El-Nakhel, C.; Pannico, A.; Kyriacou, M.C.; Giordano, M.; De Pascale, S.; Rouphael, Y. Macronutrient deprivation eustress elicits differential secondary metabolites in red and green-pigmented butterhead lettuce grown in a closed soilless system. J. Sci. Food Agric. 2019, 99, 6962–6972. [Google Scholar] [CrossRef] [PubMed]
- Prinsi, B.; Negrini, N.; Morgutti, S.; Espen, L. Nitrogen Starvation and Nitrate or Ammonium Availability Differently Affect Phenolic Composition in Green and Purple Basil. Agronomy 2020, 10, 498. [Google Scholar] [CrossRef]
- Aktsoglou, D.-C.; Kasampalis, D.S.; Sarrou, E.; Tsouvaltzis, P.; Chatzopoulou, P.; Martens, S.; Siomos, A.S. Protein Hydrolysates Supplement in the Nutrient Solution of Soilless Grown Fresh Peppermint and Spearmint as a Tool for Improving Product Quality. Agronomy 2021, 11, 317. [Google Scholar] [CrossRef]
- Keutgen, N.; Hausknecht, M.; Tomaszewska-Sowa, M.; Keutgen, A.J. Nutritional and Sensory Quality of Two Types of Cress Microgreens Depending on the Mineral Nutrition. Agronomy 2021, 11, 1110. [Google Scholar] [CrossRef]
- Wieth, A.R.; Pinheiro, W.D.; Duarte, T.S. Purple cabbage microgreens grown in different substrates and nutritive solution concentrations. Rev. Caatinga 2019, 32, 976–985. [Google Scholar] [CrossRef]
- El-Nakhel, C.; Pannico, A.; Graziani, G.; Kyriacou, M.C.; Gaspari, A.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Nutrient Supplementation Configures the Bioactive Profile and Production Characteristics of Three Brassica L. Microgreens Species Grown in Peat-Based Media. Agronomy 2021, 11, 346. [Google Scholar] [CrossRef]
- Öztekin, G.B.; Uludağ, T.; Tüzel, Y. Growing spinach (Spinacia oleracea L.) in a floating system with different concentrations of nutrient solution. Appl. Ecol. Environ. Res. 2018, 16, 3333–3350. [Google Scholar] [CrossRef]
- Leibar-Porcel, E.; McAinsh, M.R.; Dodd, I.C. Elevated Root-Zone Dissolved Inorganic Carbon Alters Plant Nutrition of Lettuce and Pepper Grown Hydroponically and Aeroponically. Agronomy 2020, 10, 403. [Google Scholar] [CrossRef]
- Sakamoto, M.; Suzuki, T. Effect of Nutrient Solution Concentration on the Growth of Hydroponic Sweetpotato. Agronomy 2020, 10, 1708. [Google Scholar] [CrossRef]
- Yang, T.; Kim, H.-J. Characterizing Nutrient Composition and Concentration in Tomato-, Basil-, and Lettuce-Based Aquaponic and Hydroponic Systems. Water 2020, 12, 1259. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Chatzieustratiou, E.; Constantopoulou, E.; Kapotis, G. Yield and quality of lettuce and rocket grown in floating culture system. Not. Bot. Horti Agrobot. Cluj-Napoca 2016, 44, 603–612. [Google Scholar] [CrossRef]
- Spehia, R.S.; Devi, M.; Singh, J.; Sharma, S.; Negi, A.; Singh, S.; Sharma, J.C. Lettuce growth and yield in hoagland solution with an organic concoction. Int. J. Veg. Sci. 2018, 24, 557–566. [Google Scholar] [CrossRef]
- Arnon, A. Method of extraction of chlorophyll in the plants. Agron. J. 1967, 23, 112–121. [Google Scholar]
- Ochoa-Velasco, C.E.; Valadez-Blanco, R.; Salas-Coronado, R.; Sustaita-Rivera, F.; Hernández-Carlos, B.; García-Ortega, S.; Santos-Sánchez, N.F. Effect of nitrogen fertilization and Bacillus licheniformis biofertilizer addition on the antioxidants compounds and antioxidant activity of greenhouse cultivated tomato fruits (Solanum lycopersicum L. var. Sheva). Sci. Hortic. 2016, 201, 338–345. [Google Scholar] [CrossRef]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Dou, H.; Niu, G.; Gu, M.; Masabni, J.G. Responses of Sweet Basil to Different Daily Light Integrals in Photosynthesis, Morphology, Yield, and Nutritional Quality. Hortic. Sci. 2018, 53, 496–503. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E.; Eisele, T.; Giusti, M.M.; Hach, J.; Hofsommer, H.; Koswig, S.; Krueger, D.A.; Kupina, S.; et al. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Ghoora, M.D.; Babu, D.R.; Srividya, N. Nutrient composition, oxalate content and nutritional ranking of ten culinary microgreens. J. Food Compos. Anal. 2020, 91, 103495. [Google Scholar] [CrossRef]
- Islam, M.Z.; Lee, Y.-T.; Mele, M.A.; Choi, I.-L.; Kang, H.-M. The Effect of Phosphorus and Root Zone Temperature on Anthocyanin of Red Romaine Lettuce. Agronomy 2019, 9, 47. [Google Scholar] [CrossRef]
- Alberici, A.; Quattrini, E.; Penati, M.; Martinetti, L.; Marino Gallina, P.; Ferrante, A. Effect of the reduction of nutrient solution concentration on leafy vegetables quality grown in floating system. Acta Hortic. 2008, 801, 1167–1176. [Google Scholar] [CrossRef]
- Pompelli, M.F.; Arrieta, D.V.; Rodríguez, Y.Y.P.; Ramírez, A.M.J.; Bettin, A.M.V.; Avilez, M.A.Q.; Cárcamo, J.A.A.; Garcia-Castaño, S.G.; González, L.M.M.; Cordero, E.D.F.; et al. Can Chlorophyll a Fluorescence and Photobleaching Be a Stress Signal under Abiotic Stress in Vigna unguiculata L.? Sustainability 2022, 14, 15503. [Google Scholar] [CrossRef]
- Purwanto, S.; Salsabila, J. Growth response and yield of saline tolerant rice varieties to bio-fertilizer application at central Java north coastal saline paddy field. IOP Conf. Ser. Earth Environ. 2019, 406, 012001. [Google Scholar] [CrossRef]
- Tammam, A.; El-Aggan, W.; Helaly, A.; Badr, G.; El-Dakak, R. Proteomics and photosynthetic apparatus response to vermicompost attenuation of salinity stress Vicia faba leaves. Acta Physiol. Plant 2023, 45, 17. [Google Scholar] [CrossRef]
- Di Gioia, F.; Tzortzakis, N.; Rouphael, Y.; Kyriacou, M.C.; Sampaio, S.L.; Ferreira, I.C.F.R.; Ferreira, I.; Petropoulos, S.A. Grown to Be Blue—Antioxidant Properties and Health Effects of Colored Vegetables. Part II: Leafy, Fruit, and Other Vegetables. Antioxidants 2020, 9, 97. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Kopsell, D.E.; Curran-Celentano, J. Carotenoid pigments in kale are influenced by nitrogen concentration and form. J. Sci. Food Agric. 2007, 87, 900–907. [Google Scholar] [CrossRef]
- Dhami, N.; Cazzonelli, C.I. Environmental impacts on carotenoid metabolism in leaves. Plant Growth Regul. 2020, 92, 455–477. [Google Scholar] [CrossRef]
- Neugart, S.; Baldermann, S.; Hanschen, F.S.; Klopsch, R.; Wiesner-Reinhold, M.; Schreiner, M. The intrinsic quality of brassicaceous vegetables: How secondary plant metabolites are affected by genetic, environmental, and agronomic factors. Sci. Hortic. 2018, 233, 460–478. [Google Scholar] [CrossRef]
- Mampholo, B.M.; Maboko, M.M.; Soundy, P.; Sivakumar, D. Phytochemicals and overall quality of leafy lettuce (Lactuca sativa L.) varieties grown in closed hydroponic system. J. Food Qual. 2016, 39, 805–815. [Google Scholar] [CrossRef]
- Raiola, A.; Tenore, G.C.; Barone, A.; Frusciante, L.; Rigano, M.M. Vitamin E Content and Composition in Tomato Fruits: Beneficial Roles and Bio-Fortification. Int. J. Mol. Sci. 2015, 16, 29250–29264. [Google Scholar] [CrossRef]
- Paciolla, C.; Fortunato, S.; Dipierro, N.; Paradiso, A.; De Leonardis, S.; Mastropasqua, L.; de Pinto, M.C. Vitamin C in Plants: From Functions to Biofortification. Antioxidants 2019, 8, 519. [Google Scholar] [CrossRef] [PubMed]
- Gibson, A.R.; O’Leary, B.R.; Du, J.; Sarsour, E.H.; Kalen, A.L.; Wagner, B.A.; Stolwijk, J.M.; Falls-Hubert, K.C.; Alexander, M.S.; Carroll, R.S.; et al. Dual Oxidase-Induced Sustained Generation of Hydrogen Peroxide Contributes to Pharmacologic Ascorbate-Induced Cytotoxicity. Cancer Res. 2020, 80, 1401–1413. [Google Scholar] [CrossRef] [PubMed]
- 43. Kathi, S.; Laza, H.; Singh, S.; Thompson, L.; Li, W.; Simpson, C. Vitamin C biofortification of broccoli microgreens and resulting effects on nutrient composition. Front. Plant Sci. 2023, 14, 1145992. [Google Scholar] [CrossRef]
- De Souza, J.Z.; De Mello Prado, R.; Silva, S.L.; Farias, T.P.; Neto, J.G.; Souza Junior, J.P. Silicon Leaf Fertilization Promotes Biofortification and Increases Dry Matter, Ascorbate Content, and Decreases Post-Harvest Leaf Water Loss of Chard and Kale. Commun. Soil Sci. Plant Anal. 2019, 50, 164–172. [Google Scholar] [CrossRef]
- Garcia Neto, J.; Prado, R.M.; de Souza Júnior, J.P.; Silva, S.L.O.; Farias, T.P. Silicon leaf spraying increases biofortification production, ascorbate content and decreases water loss post-harvest from land cress and chicory leaves. J. Plant Nutr. 2022, 45, 1283–1290. [Google Scholar] [CrossRef]
- Dos Santos, M.M.M.; da Silva, G.P.; de Mello Prado, R.; Pinsetta Junior, J.S.; Mattiuz, B.H.; Braun, H. Biofortification of Tomato with Stabilized Alkaline Silicate and Silicic Acid, Nanosilica, and Potassium Silicate via Leaf Increased Ascorbic Acid Content and Fruit Firmness. J. Plant Nutr. 2021, 45, 896–903. [Google Scholar] [CrossRef]
- Kathi, S.; Laza, H.; Singh, S.; Thompson, L.; Li, W.; Simpson, C. Increasing vitamin C through agronomic biofortification of arugula microgreens. Sci. Rep. 2022, 12, 13093. [Google Scholar] [CrossRef]
- Sun, J.C.; Li, X.J.; Qu, Z.; Wang, H.R.; Cheng, Y.; Dong, S.J.; Zhao, H. Comparative proteomic analysis reveals novel insights into the continuous cropping induced response in Scrophularia ningpoensis. J. Sci. Food Agric. 2023, 103, 1832–1845. [Google Scholar] [CrossRef]
- Lillo, C.; Lea, U.S.; Ruoff, P. Nutrient depletion as a key factor for manipulating gene expression and product formation in different branches of the flavonoid pathway. Plant Cell Environ. 2008, 31, 587–601. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Photosynthesis: Physiological and ecological considerations. Plant Physiol. 2002, 9, 172–174. [Google Scholar]
- Oancea, S.; Oprean, L. Anthocyanins, from biosynthesis in plants to human health benefits. Acta Univ. Cinbinesis Ser. E Food Technol. 2011, 15, 3–16. [Google Scholar]
- Martínez-Ispizua, E.; Calatayud, Á.; Marsal, J.I.; Cannata, C.; Basile, F.; Abdelkhalik, A.; Soler, S.; Valcárcel, J.V.; Martínez-Cuenca, M.-R. The Nutritional Quality Potential of Microgreens, Baby Leaves, and Adult Lettuce: An Underexploited Nutraceutical Source. Foods 2022, 11, 423. [Google Scholar] [CrossRef] [PubMed]
- Toscano, S.; Trivellini, A.; Cocetta, G.; Bulgari, R.; Francini, A.; Romano, D.; Ferrante, A. Effect of preharvest abiotic stresses on the accumulation of bioactive compounds in horticultural produce. Front. Plant. Sci. 2019, 10, 1212. [Google Scholar] [CrossRef] [PubMed]
- El-Nakhel, C.; Pannico, A.; Graziani, G.; Kyriacou, M.C.; Giordano, M.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Variation in Macronutrient Content, Phytochemical Constitution and In Vitro Antioxidant Capacity of Green and Red Butterhead Lettuce Dictated by Different Developmental Stages of Harvest Maturity. Antioxidants 2020, 9, 300. [Google Scholar] [CrossRef]
- Žnidarčič, D.; Ban, D.; Šircelj, H. Carotenoid and chlorophyll composition of commonly consumed leafy vegetables in Mediterranean countries. Food Chem. 2011, 129, 1164–1168. [Google Scholar] [CrossRef] [PubMed]
- Scagel, C.F.; Lee, J.; Mitchell, J.N. Salinity from NaCl changes the nutrient and polyphenolic composition of basil leaves. Ind. Crops Prod. 2019, 127, 119–128. [Google Scholar] [CrossRef]
- Wada, K.C.; Mizuuchi, K.; Koshio, A.; Kaneko, K.; Mitsui, T.; Takeno, K. Stress Enhances the Gene Expression and Enzyme Activity of Phenylalanine Ammonia-Lyase and the Endogenous Content of Salicylic Acid to Induce Flowering in Pharbitis. J. Plant Physiol. 2014, 171, 895–902. [Google Scholar] [CrossRef]
- Mahajan, M.; Kuiry, R.; Pal, P.K. Understanding the consequence of environmental stress for accumulation of secondary metabolites in medicinal and aromatic plants. J. Appl. Res. Med. Aromat. Plants 2020, 18, 100255. [Google Scholar] [CrossRef]
- Corrado, G.; Vitaglione, P.; Chiaiese, P.; Rouphael, Y. Unraveling the Modulation of Controlled Salinity Stress on Morphometric Traits, Mineral Profile, and Bioactive Metabolome Equilibrium in Hydroponic Basil. Horticulturae 2021, 7, 273. [Google Scholar] [CrossRef]
- Maggio, A.; De Pascale, S.; Barbieri, G.; Raimondi, G.; Orsini, F. Yield and quality of hydroponically grown sweet basil cultivars. Acta Hort. 2006, 723, 357–360. [Google Scholar] [CrossRef]
- Ren, X.; Lu, N.; Xu, W.; Zhuang, Y.; Takagaki, M. Optimization of the Yield, Total Phenolic Content, and Antioxidant Capacity of Basil by Controlling the Electrical Conductivity of the Nutrient Solution. Horticulturae 2022, 8, 216. [Google Scholar] [CrossRef]
- Ciriello, M.; Pannico, A.; El-Nakhel, C.; Formisano, L.; Cristofano, F.; Duri, L.G.; Pizzolongo, F.; Romano, R.; De Pascale, S.; Colla, G.; et al. Sweet Basil Functional Quality as Shaped by Genotype and Macronutrient Concentration Reciprocal Action. Plants 2020, 9, 1786. [Google Scholar] [CrossRef] [PubMed]
- Al-Huqail, A.; El-Dakak, R.M.; Sanad, M.N.; Badr, R.H.; Ibrahim, M.M.; Soliman, D.; Khan, F. Effects of climate temperature and water stress on plant growth and accumulation of antioxidant compounds in sweet basil (Ocimum basilicum L.) leafy vegetable. Scientifica 2020, 2020, 3808909. [Google Scholar] [CrossRef]
- Jakovljević, D.; Topuzović, M.; Stanković, M. Nutrient Limitation as a Tool for the Induction of Secondary Metabolites with Antioxidant Activity in Basil Cultivars. Ind. Crops Prod. 2019, 138, 111462. [Google Scholar] [CrossRef]
- Munns, R.; James, R.A.; Läuchli, A. Approaches to increasing the salt tolerance of wheat and other cereals. J. Exp. Bot. 2006, 57, 1025–1043. [Google Scholar] [CrossRef]
- Brauer, A.M.; Shi, H.; Levin, P.A.; Huang, K.C. Physiological and regulatory convergence between osmotic and nutrient stress responses in microbes. Curr. Opin. Cell Biol. 2023, 81, 102170. [Google Scholar] [CrossRef]
- Hosseini, H.; Mozafari, V.; Roosta, H.R.; Shirani, H.; van de Vlasakker, P.C.H.; Farhangi, M. Nutrient Use in Vertical Farming: Optimal Electrical Conductivity of Nutrient Solution for Growth of Lettuce and Basil in Hydroponic Cultivation. Horticulturae 2021, 7, 283. [Google Scholar] [CrossRef]
- Zeng, H.; Wang, G.; Hu, X.; Wang, H.; Du, L.; Zhu, Y. Role of microRNAs in plant responses to nutrient stress. Plant Soil 2014, 374, 1005–1021. [Google Scholar] [CrossRef]
| Concentration of Nutrients (mg L−1) | 25% | 50% | 75% | 100% | 125% |
|---|---|---|---|---|---|
| N | 52.50 | 105 | 157.50 | 210 | 262.50 |
| K | 58.75 | 117.50 | 176.25 | 235 | 293.75 |
| Ca | 50 | 100 | 150 | 200 | 250 |
| P | 7.75 | 15.50 | 23.25 | 31 | 38.75 |
| S | 16 | 32 | 48 | 64 | 80 |
| Mg | 12 | 24 | 36 | 48 | 60 |
| Fe | 0.75 | 1.50 | 2.25 | 3 | 3.75 |
| B | 0.125 | 0.25 | 0.375 | 0.5 | 0.625 |
| Mn | 0.125 | 0.25 | 0.375 | 0.5 | 0.625 |
| Zn | 0.012 | 0.02 | 0.037 | 0.05 | 0.065 |
| Cu | 0.005 | 0.01 | 0.015 | 0.02 | 0.025 |
| Mo | 0.002 | 0.005 | 0.007 | 0.01 | 0.012 |
| EC (mS cm−1) y = 0.0237x + 0.0351, R2 = 0.99 | 0.60 | 1.24 | 1.85 | 2.42 | 2.97 |
| Factors | Chlorophyll a (mg g−1 FW) | Chlorophyll b (mg g−1 FW) | Total Chlorophyll (mg g−1 FW) | Carotenoids (mg g−1 FW) | |
|---|---|---|---|---|---|
| NS Concentration | Cultivar | ||||
| 25% | Violeto | 0.39 e | 0.20 ghi | 0.60 f | 0.10 h |
| Ablagh | 0.63 cd | 0.33 cdefgh | 0.97 cde | 0.25 f | |
| Red Rubin | 0.74 abc | 0.42 abcde | 1.17 abc | 0.31 e | |
| Kapoor | 0.68 c | 0.35 cdefg | 1.04 bcd | 0.24 f | |
| 50% | Violeto | 0.37 e | 0.15 i | 0.51 f | 0.39 d |
| Ablagh | 0.46 de | 0.25 fghi | 0.70 ef | 0.23 f | |
| Red Rubin | 0.48 de | 0.27 efghi | 0.76 def | 0.32 e | |
| Kapoor | 0.48 de | 0.24 ghi | 0.71 def | 0.30 e | |
| 75% | Violeto | 0.34 e | 0.15 i | 0.49 f | 0.38 d |
| Ablagh | 0.70 bc | 0.45 abc | 1.15 abc | 0.30 e | |
| Red Rubin | 0.75 abc | 0.53 ab | 1.28 abc | 0.18 g | |
| Kapoor | 0.45 e | 0.28 defghi | 0.73 def | 0.41 d | |
| 100% | Violeto | 0.39 e | 0.17 hi | 0.56 f | 0.41 d |
| Ablagh | 0.89 a | 0.53 ab | 1.41 a | 0.53 ab | |
| Red Rubin | 0.73 abc | 0.58 a | 1.31 ab | 0.32 e | |
| Kapoor | 0.48 de | 0.29 cdefghi | 0.77 def | 0.48 c | |
| 125% | Violeto | 0.46 de | 0.16 i | 0.62 f | 0.21 fg |
| Ablagh | 0.87 ab | 0.45 abc | 1.32 ab | 0.56 a | |
| Red Rubin | 0.89 a | 0.41 bcdef | 1.30 ab | 0.51 bc | |
| Kapoor | 0.46 de | 0.28 defghi | 0.75 def | 0.33 e | |
| Source of Variance | Vitamin C (mg g−1 FW) | Flavonoids (mg CAE g−1 FW) | Anthocyanin (mg 100 g−1 FW) | Polyphenols (mg GA 100 g−1 FW) | Antioxidant Capacity (%) | APCI Index | |
|---|---|---|---|---|---|---|---|
| NS Concentration | Cultivar | ||||||
| 25% | Violeto | 1.07 g | 0.99 h | 19.70 d | 788.44 ghi | 56.40 e | 37.25 jk |
| Ablagh | 1.09 g | 2.65 def | 11.90 gh | 1063.40 d | 66.17 cd | 44.87 i | |
| Red Rubin | 1.32 fg | 2.55 def | 21.00 cd | 934.22 e | 72.15 bc | 51.80 efg | |
| Kapoor | 2.22 d | 1.21 gh | 9.57 h | 866.97 efg | 46.53 f | 39.41 j | |
| 50% | Violeto | 1.83 e | 2.93 de | 23.53 bc | 836.88 fgh | 43.33 f | 51.62 efg |
| Ablagh | 1.09 g | 1.61 fgh | 15.23 ef | 838.42 fgh | 24.90 g | 34.74 k | |
| Red Rubin | 2.29 d | 2.12 efg | 24.17 bc | 746.71 ij | 30.23 g | 47.03 hi | |
| Kapoor | 1.53 f | 3.30 cd | 10.20 h | 700.51 j | 25.00 g | 36.92 jk | |
| 75% | Violeto | 2.56 c | 2.68 def | 25.17 b | 699.29 j | 26.13 g | 49.91 fgh |
| Ablagh | 2.68 c | 2.08 efgh | 18.00 de | 757.81 hij | 47.20 f | 47.71 ghi | |
| Red Rubin | 1.19 g | 1.69 fgh | 25.93 ab | 745.49 ij | 23.30 g | 37.98 jk | |
| Kapoor | 1.82 e | 5.46 b | 11.47 gh | 902.56 ef | 71.57 bc | 55.94 e | |
| 100% | Violeto | 4.34 a | 5.34 b | 28.77 a | 1024.56 d | 65.10 cd | 74.14 c |
| Ablagh | 4.23 a | 4.20 c | 23.87 bc | 1425.96 ab | 77.83 b | 79.41 b | |
| Red Rubin | 2.58 c | 2.15 efg | 26.43 ab | 866.97 efg | 43.63 f | 53.04 ef | |
| Kapoor | 2.64 c | 5.83 b | 14.50 fg | 1050.39 d | 93.10 a | 68.57 d | |
| 125% | Violeto | 1.25 g | 2.48 def | 20.21 d | 1395.30 ab | 56.20 e | 50.14 fgh |
| Ablagh | 3.63 b | 8.49 a | 24.97 b | 1444.92 a | 87.57 a | 87.82 a | |
| Red Rubin | 4.28 a | 5.37 b | 24.51 b | 1358.00 b | 90.27 a | 82.73 b | |
| Kapoor | 1.93 e | 3.37 cd | 6.52 i | 1268.69 c | 62.33 de | 50.14 fgh | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fayezizadeh, M.R.; Ansari, N.A.; Sourestani, M.M.; Hasanuzzaman, M. Balancing Yield and Antioxidant Capacity in Basil Microgreens: An Exploration of Nutrient Solution Concentrations in a Floating System. Agriculture 2023, 13, 1691. https://doi.org/10.3390/agriculture13091691
Fayezizadeh MR, Ansari NA, Sourestani MM, Hasanuzzaman M. Balancing Yield and Antioxidant Capacity in Basil Microgreens: An Exploration of Nutrient Solution Concentrations in a Floating System. Agriculture. 2023; 13(9):1691. https://doi.org/10.3390/agriculture13091691
Chicago/Turabian StyleFayezizadeh, Mohammad Reza, Naser Alemzadeh Ansari, Mohammad Mahmoodi Sourestani, and Mirza Hasanuzzaman. 2023. "Balancing Yield and Antioxidant Capacity in Basil Microgreens: An Exploration of Nutrient Solution Concentrations in a Floating System" Agriculture 13, no. 9: 1691. https://doi.org/10.3390/agriculture13091691
APA StyleFayezizadeh, M. R., Ansari, N. A., Sourestani, M. M., & Hasanuzzaman, M. (2023). Balancing Yield and Antioxidant Capacity in Basil Microgreens: An Exploration of Nutrient Solution Concentrations in a Floating System. Agriculture, 13(9), 1691. https://doi.org/10.3390/agriculture13091691











