The Importance of Lentils: An Overview
Abstract
:1. Introduction
2. Biological Nitrogen Fixation (BNF)
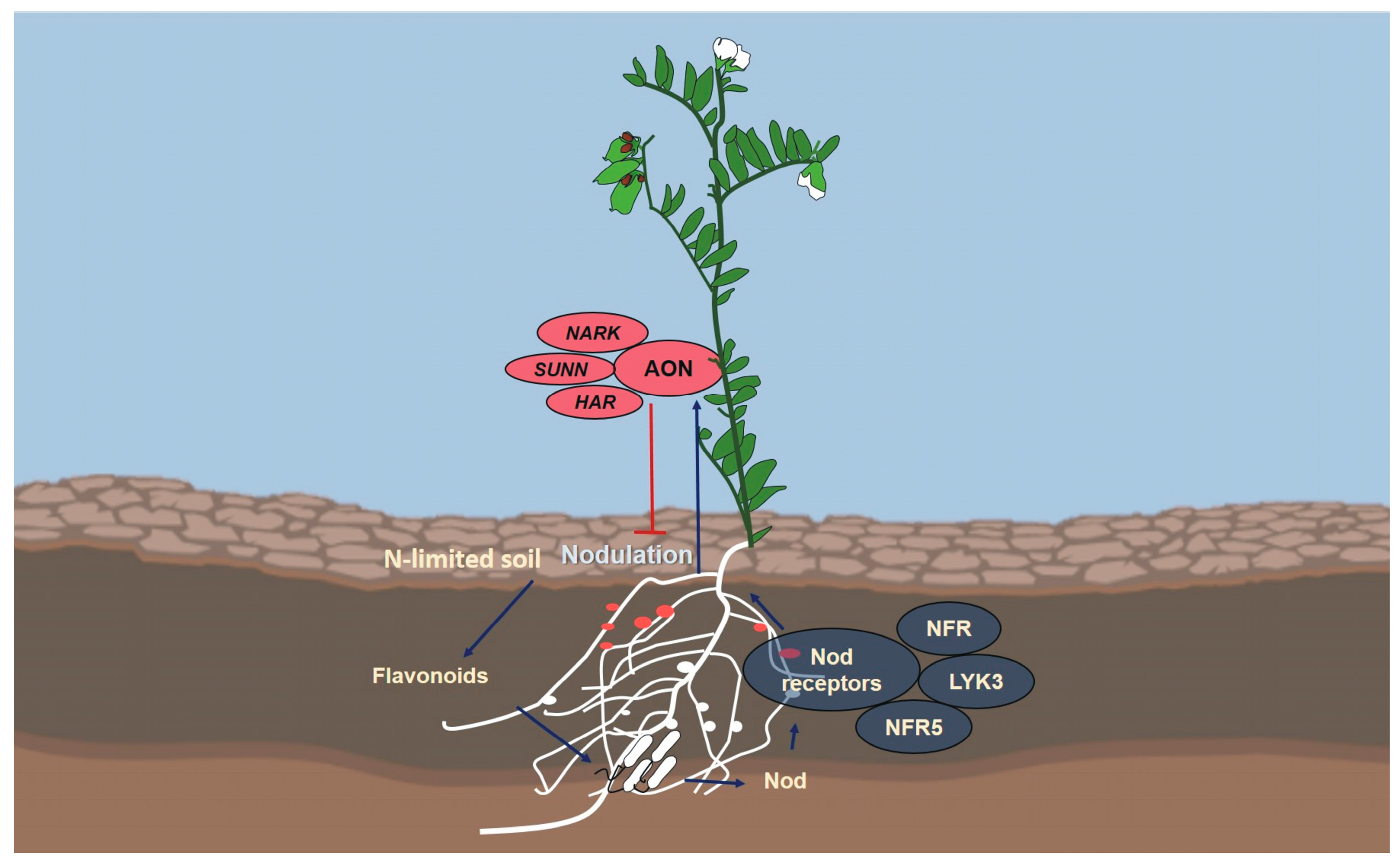
3. Lentil Nodulation
4. Nutritional Importance of Lentils
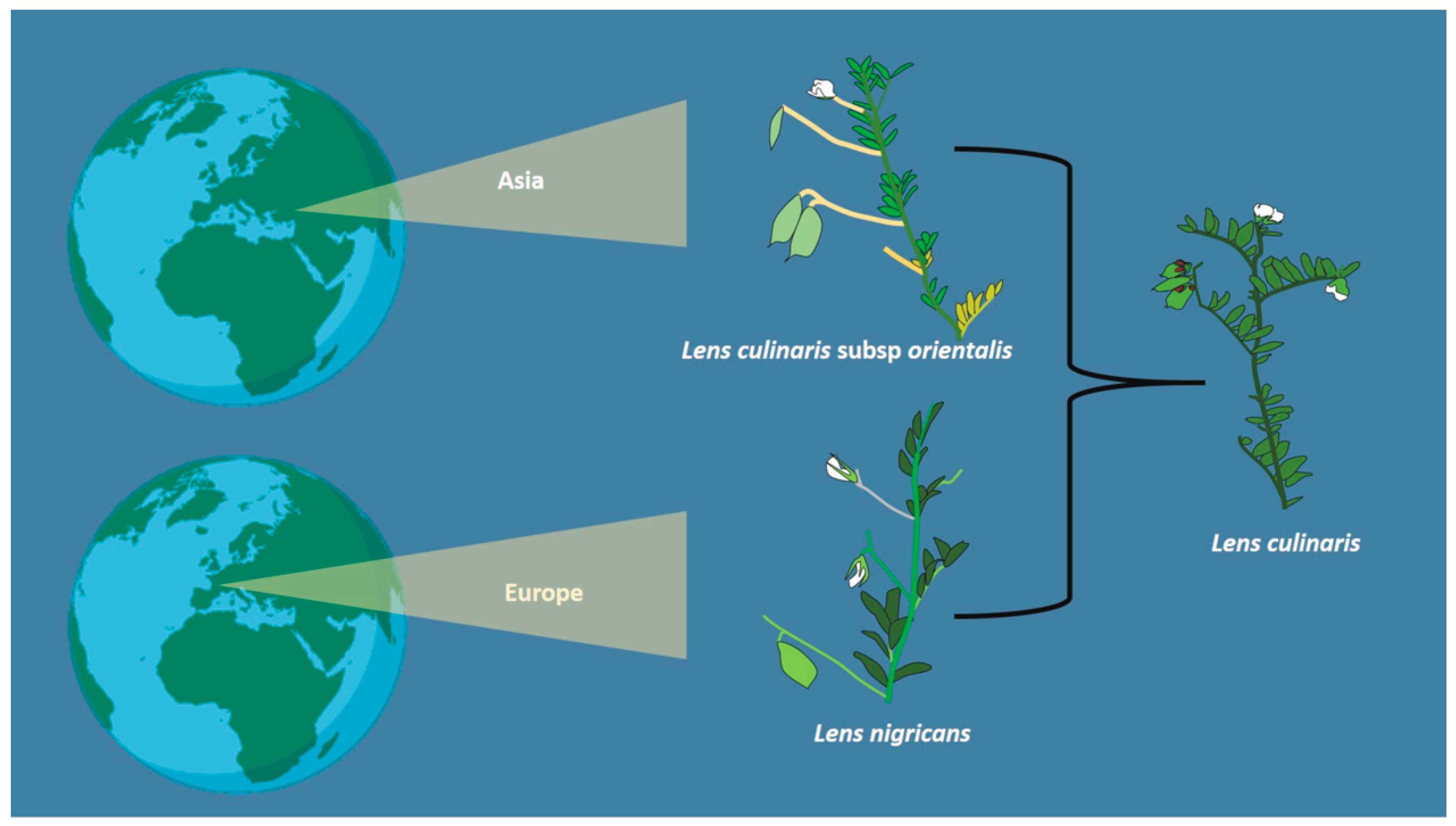
5. Role of Wild Lentils in Lens culinaris (Medik.) Domestication
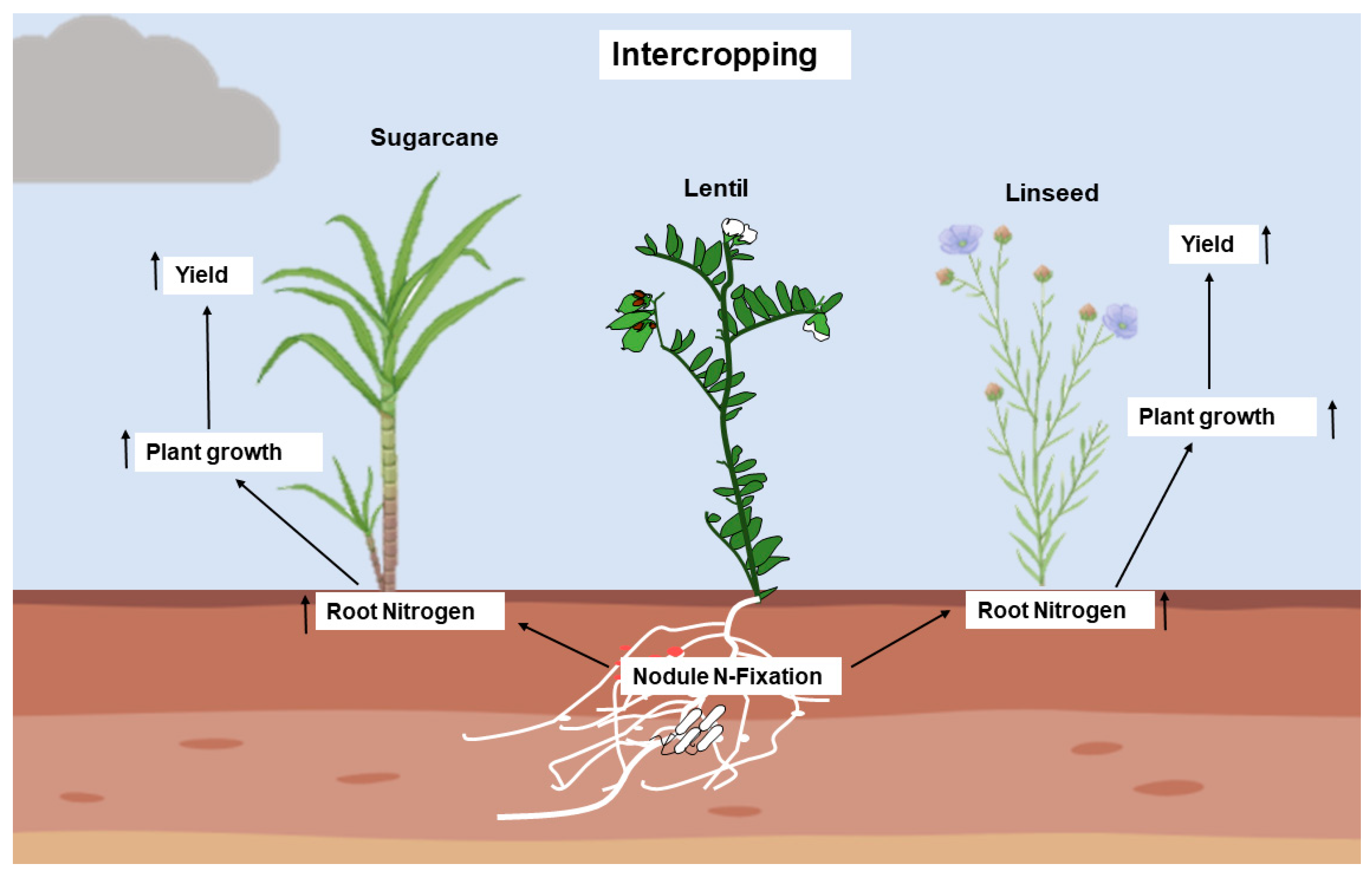
6. Lentils in Sustainable Agricultural Systems
7. Lentil Crop Constraints in a Changing Environment
8. Lentil Medical Implications
9. Use of Lentils in Soil Bioremediation
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- LPWG. Legume phylogeny and classification in the 21st century: Progress, prospects and lessons for other species-rich clades. Taxon 2013, 62, 217–248. [Google Scholar] [CrossRef]
- LPWG. A new subfamily classification of the Leguminosae based on a taxonomically comprehensive phylogeny. Taxon 2017, 66, 44–77. [Google Scholar] [CrossRef]
- Schaefer, H.; Hechenleitner, P.; Santos-Guerra, A.; Menezes de Sequeira, M.; Pennington, R.T.; Kenicer, G.; Carine, M.A. Systematics, biogeography, and character evolution of the legume tribe Fabeae with special focus on the middle-Atlantic island lineages. BMC Evol. Biol. 2012, 12, 250. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, T.R.M.; Ortiz, P.R.H.; Rodríguez, M.O.; de la Fe, M.C.F.; Lamz, P.A. Comportamiento agronómico de la lenteja (Lens culinaris Medik.) en la localidad de Tapaste, Cuba. Cultiv. Trop. 2014, 35, 92–99. [Google Scholar]
- Zafar, M.; Maqsood, M.; Anser, M.R.; Ali, Z. Growth and yield of lentil as affected by phosphorus. Int. J. Agric. Biol. 2003, 5, 98–100. [Google Scholar]
- Nutritionvalue.org. Nutrition Value: Find Nutritional Value of a Product. Available online: https://www.nutritionvalue.org/ (accessed on 19 April 2023).
- FAOSTAT. Statistics division, Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/faostat/en/#data/QI (accessed on 15 December 2023).
- Zhang, B.; Peng, H.; Deng, Z.; Tsao, R. Phytochemicals of lentil (Lens culinaris) and their antioxidant and anti-inflammatory effects. J. Food Bioact. 2018, 1, 93–103. [Google Scholar] [CrossRef]
- Andrews, M.; Raven, J.A.; Lea, P.J. Do plants need nitrate? The mechanisms which nitrogen form affects plants. Ann. Appl. Biol. 2013, 163, 174–199. [Google Scholar] [CrossRef]
- Ladizinsky, G. The origin of lentil and its wild genepool. Euphytica 1979, 28, 179–187. [Google Scholar] [CrossRef]
- Sonnante, G.; Hammer, K.; Pignone, D. From the cradle of agriculture a handful of lentils: History of domestication. Rend. Lincei. Sci. Fis. Nat. 2009, 20, 21–37. [Google Scholar] [CrossRef]
- Renfrew, J.M. The archeological evidence for the domesticastion of plants: Methods and problems. In The Domestication and Exploitation of Plants and Animals; Ucko, P.J., Dimbleby, G.W., Eds.; Aldine: Chicago, IL, USA, 1969. [Google Scholar]
- Havey, M.J.; Muehlbauer, F.J. Variability for restriction fragment lengths and phylogenies in lentil. TAG Theor. Appl. Genetics. Theor. Und Angew. Genet. 1989, 77, 839–843. [Google Scholar] [CrossRef]
- Ladizinsky, G.; Braun, D.; Goshen, D.; Muehlbauer, F.J. Evidence for domestication of Lens nigricans (M. Bieb.) Godron in S. Europe. Bot. J. Linn. Soc. 1983, 87, 169–176. [Google Scholar] [CrossRef]
- Zohary, D.; Hopf, M.; Weiss, E. Domestication of Plants in the Old World: The Origin and Spread of Domesticated Plants in Southwest Asia, Europe, and the Mediterranean Basin; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Maxted, N.; Kell, S.; Toledo, A.; Dulloo, M.; Heywood, V.; Hodgkin, T.; Hunter, D.; Guarino, L.; Jarvis, A.; Ford-Lloyd, B. A global approach to crop wild relative conservation: Securing the gene pool for food and agriculture. Kew Bull. 2010, 65, 561–576. [Google Scholar] [CrossRef]
- Ferguson, M.; Acikgoz, N.; Ismail, A.; Cinsoy, A. An ecogeographic survey of wild Lens species in Aegean and south west Turkey. Anadolu 1996, 6, 159–166. [Google Scholar]
- van Oss, H.; Aron, Y.; Ladizinsky, G. Chloroplast DNA variation and evolution in the genus Lens Mill. Theor. Appl. Genet. 1997, 94, 452–457. [Google Scholar] [CrossRef]
- Zohary, D. The wild progenitor and the place of origin of the cultivated lentil: Lens culinaris. Econ. Bot. 1972, 26, 326–332. [Google Scholar] [CrossRef]
- Canfield, D.E.; Glazer, A.N.; Falkowski, P.G. The evolution and future of Earth’s nitrogen cycle. Science 2010, 330, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Keramidas, K.; Tchung-Ming, S.; Diaz-Vazquez, A.; Weitzel, M.; Vandyck, T.; Després, J.; Schmitz, A.; Rey, L.; Los Santos, K.; Wojtowicz, B.; et al. Soria-Ramirez, Global Energy and Climate Outlook 2018: Sectoral Mitigation Options Towards a Low-Emissions Economy—Global Context to the EU Strategy for Long-Term Greenhouse Gas Emissions Reduction; EUR 29462 EN; Publications Office of the European Union: Luxembourg, 2018; ISBN 978-92-79-97462-5. [Google Scholar]
- Saikia, S.P.; Jain, V. Biological nitrogen fixation with non-legumes: An achievable target or a dogma. Curr. Sci. 2007, 92, 317–322. [Google Scholar]
- Sammauria, R.; Kumawat, S.; Kumawat, P.; Singh, J.K.; Jatwa, T. Microbial inoculants: Potential tool for sustainability of agricultural production systems. Arch. Microbiol. 2020, 202, 677–693. [Google Scholar] [CrossRef]
- Muehlbauer, F.; Cho, S.; Sarker, A.; Mcphee, K.; Clarice, C.; Rajesh, P.; Ford, R. Application of biotechnology in breeding lentil for resistance to biotic and abiotic stress. Euphytica 2006, 147, 149–165. [Google Scholar] [CrossRef]
- Sarker, A.; Kumar, S. Lentils in production and food systems in West Asia and Africa. International Center for Agricultural Research in the Dry Areas (ICARDA), Aleppo, Syria. Grain Legumes 2011, 57, 46–48. [Google Scholar]
- Teng, Y.; Wang, X.; Li, L.; Li, Z.; Luo, Y. Rhizobia and their bio-partners as novel drivers for functional remediation in contaminated soils. Front. Plant Sci. 2015, 6, 32–42. [Google Scholar] [CrossRef]
- Caetano-Anollés, G.; Gresshoff, P.M. Plant genetic control of nodulation. Annu. Rev. Microbiol. 1991, 45, 345–382. [Google Scholar] [CrossRef] [PubMed]
- Caetano-Anollés, G.; Gresshoff, P.M. Alfalfa controls nodulation during the onset of Rhizobum-induced cortical cell division. Plant Physiol. 1991, 95, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Delves, A.C.; Mathews, A.; Day, D.A.; Carter, A.S.; Carroll, B.J.; Gresshoff, P.M. Regulation of the soybean-Rhizobium nodule symbiosis by shoot and root factors. Plant Physiol. 1986, 82, 588–590. [Google Scholar] [CrossRef] [PubMed]
- Reid, D.E.; Ferguson, B.J.; Hayashi, S.; Lin, Y.H.; Gresshoff, P.M. Molecular mechanisms controlling legume autoregulation of nodulation. Ann. Bot. 2011, 108, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Liu, W.; Nandety, R.S.; Crook, A.; Mysore, K.S.; Pislariu, C.I.; Frugoli, J.; Dickstein, R.; Udvardi, M.K. Celebrating 20 Years of Genetic Discoveries in Legume Nodulation and Symbiotic Nitrogen Fixation. Plant Cell 2020, 32, 15–41. [Google Scholar] [CrossRef]
- Oldroyd, G.E. Speak, friend, and enter: Signalling systems that promote beneficial symbiotic associations in plants. Nat. Rev. Microbiol. 2013, 11, 252–263. [Google Scholar] [CrossRef]
- Streeter, J.; Wong, P.P. Inhibition of legume nodule formation and N2 fixation by nitrate. Crit. Rev. Plant Sci. 1998, 7, 1–23. [Google Scholar] [CrossRef]
- Xuan, X.; Chunmei, M.; Shoukun, D.; Yao, X.; Zhenping, G. Effects of nitrogen concentrations on nodulation and nitrogenase activity in dual root systems of soybean plants. Soil Sci. Plant Nutr. 2017, 63, 470–482. [Google Scholar]
- Lyu, X.; Li, M.; Li, X.; Li, S.; Yan, C.; Ma, C.; Gong, Z. Assessing the Systematic Effects of the Concentration of Nitrogen Supplied to Dual-Root Systems of Soybean Plants on Nodulation and Nitrogen Fixation. Agronomy 2020, 10, 763. [Google Scholar] [CrossRef]
- Du, M.; Gao, Z.; Li, X.; Liao, H. Excess nitrate induces nodule greening and reduces transcript and protein expression levels of soybean leghaemoglobins. Ann Bot. 2020, 126, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Sachs, J.L.; Skophammer, R.G.; Regus, J.U. Evolutionary transitions in bacterial symbiosis. Proc. Natl. Acad. Sci. USA 2011, 108, 10800–10807. [Google Scholar] [CrossRef] [PubMed]
- Ampomah, O.Y.; Huss-Danell, K. Genetic diversity of rhizobia nodulating native Vicia spp. in Sweden. Syst. Appl. Microbiol. 2016, 39, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.H.; Schäfer, H.; Gonzalez, J.; Wink, M. Genetic diversity of rhizobia nodulating lentil (Lens culinaris) in Bangladesh. Syst. Appl. Microbiol. 2012, 35, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Taha, K.; Berraho, E.B.; El Attar, I.; Dekkiche, S.; Aurag, J.; Béna, G. Rhizobium laguerreae is the main nitrogen-fixing symbiont of cultivated lentil (Lens culinaris) in Morocco. Syst. Appl. Microbiol. 2018, 41, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.; Charles, T.; Glick, B. Isolation and characterization of new plant growth-promoting bacterial endophytes. Appl. Soil Ecol. 2012, 61, 217–224. [Google Scholar] [CrossRef]
- Duca, D.; Lorv, J.; Patten, C.L.; Rose, D.; Glick, B.R. Indole-3-acetic acid in plant-microbe interactions. Antonie Van Leeuwenhoek 2014, 106, 85–125. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Charles, T.C.; Glick, B.R. Delay of flower senescence by bacterial endophytes expressing 1-aminocyclopropane deaminase. J. Appl. Microbiol. 2012, 113, 1139–1144. [Google Scholar] [CrossRef]
- Sepúlveda-Caamaño, M.; Gerding, M.; Vargas, M.; Moya-Elizondo, M.; Oyarzúa, P.; Campos, J. Lentil (Lens culinaris L.) growth promoting rhizobacteria and their effect on nodulation in co-inoculation with rhizobia. Arch. Agron. Soil Sci. 2018, 64, 244–256. [Google Scholar] [CrossRef]
- Iqbal Khalid, M.; Shahzad, S.; Ahmad, M.; Soleman, N.; Akhtar, N. Integrated use of Rhizobium leguminosarum, Plant Growth Promoting Rhizobacteria and Enriched Compost for Improving Growth, Nodulation and Yield of Lentil (Lens culinaris Medik.). Chil. J. Agric. Res. 2012, 72, 104–110. [Google Scholar] [CrossRef]
- Sutton, M.A.; Oenema, O.; Erisman, J.W.; Leip, A.; van Grinsven, H.; Winiwarter, W. Too much of a good thing. Nature 2011, 472, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Itav, S.; Rothschild, D.; Meijer, M.T.; Levy, M.; Moresi, C.; Dohnalová, L.; Braverman, S.; Rozin, S.; Malitsky, S.; et al. Persistent microbiome alterations modulate the rate of post-dieting weight regain. Nature 2016, 540, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.M.; Surette, M.; Bercik, P. The interplay between the intestinal microbiota and the brain. Nat. Rev. Microbiol. 2012, 10, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Rodriguez, E.; Egea-Zorrilla, A.; Plaza-Díaz, J.; Aragón-Vela, J.; Muñoz-Quezada, S.; Tercedor-Sánchez, L.; Abadia-Molina, F. The Gut Microbiota and Its Implication in the Development of Atherosclerosis and Related Cardiovascular Diseases. Nutrients 2020, 12, 605. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, J.; Wang, L. Role and Mechanism of Gut Microbiota in Human Disease. Front. Cell Infect. Microbiol. 2021, 11, 625913. [Google Scholar] [CrossRef] [PubMed]
- Siva, N.; Johnson, C.R.; Richard, V.; Jesch, E.D.; Whiteside, W.; Abood, A.A.; Thavarajah, P.; Duckett, S.; Thavarajah, D. Lentil (Lens culinaris Medikus) Diet Affects the Gut Microbiome and Obesity Markers in Rat. J. Agric. Food Chem. 2018, 66, 8805–8813. [Google Scholar] [CrossRef] [PubMed]
- Verni, M.; Demarinis, C.; Rizzello, C.G.; Baruzzi, F. Design and characterization of a novel fermented beverage from lentil grains. Foods Foods 2020, 9, 893. [Google Scholar] [CrossRef]
- Adsule, R.N.; Kadam, S.S.; Leung, H.K. Lentil. In Handbook of World Food Legumes: Nutritional Chemistry, Processing Technology, and Utilization; Salunkhe, D.K., Kadam, S.S., Eds.; CRC Press: Boca Raton, FL, USA, 1989; Volume II, pp. 133–152. [Google Scholar]
- Lombardi-Boccia, G.; Ruggeri, S.; Aguzzi, A.; Cappelloni, M. Globulins enhance in vitro iron but not zinc dialysability: A study on six legume species. J. Trace Elem. Med. Biol. 2013, 17, 1–5. [Google Scholar] [CrossRef]
- Asif, M.; Rooney, L.W.; Ali, R.; Riaz, M.N. Application and opportunities of pulses in food system: A review. Crit. Rev. Food. Sci. Nutr. 2013, 53, 1168–1179. [Google Scholar] [CrossRef]
- Bhatty, R.S. Protein subunits and amino acid composition of wild lentil. Phytochem 1986, 25, 641–644. [Google Scholar] [CrossRef]
- Tripathi, K.; Gore, P.G.; Pandey, A.; Bhardwaj, R.; Singh, N.; Chawla, G.; Kumar, A. Seed morphology, quality traits and imbibition behavior study of atypical lentil (Lens culinaris Medik.) from Rajasthan, India. Genet. Resour. Crop. Evol. 2019, 66, 697–706. [Google Scholar] [CrossRef]
- Ali, J.A.F.; Shaikh, N.A. Genetic exploitation of lentil through induced mutations. Pak. J. Bot. 2007, 39, 2379–2388. [Google Scholar]
- Hammer, K. Resolving the challenge posed by agrobiodiversity and plant genetic resources—An attempt. JARTS 2004, 76, 184. [Google Scholar]
- Horneburg, B. Developing locally adapted varieties. A study with landraces of lentils. In Schriften zu Genetischen Ressourcen; ZADI: Bonn, Germany, 2003; p. 21. [Google Scholar]
- Ladizinsky, G. The genetics of several morphological traits in lentil. J. Hered. 1979, 70, 135–137. [Google Scholar] [CrossRef]
- Ladizinsky, G. The genetics of hard seed coat in the genus Lens. Euphytica 1985, 34, 539–543. [Google Scholar] [CrossRef]
- Wong, M.M.; Gujaria-Verma, N.; Ramsay, L.; Yuan, H.Y.; Caron, C.; Diapari, M.; Vandenberg, A.; Bett, K.E. Classification and characterization of species within the genus lens using genotyping-by-sequencing (GBS). PLoS ONE 2015, 10, e0122025. [Google Scholar] [CrossRef] [PubMed]
- Mudgal, V.; Mehta, M.K.; Rane, A.S. Lentil straw (Lens culinaris): An alternative and nutritious feed resource for kids. Anim. Nutr. 2018, 4, 417–421. [Google Scholar] [CrossRef]
- Schmidtke, K.; Neumann, A.; Hof, C.; Rauber, R. Soil and atmospheric nitrogen uptake by lentil (Lens culinaris Medik.) and barley (Hordeum vulgare ssp. nudum L.) as monocrops and intercrops. Field Crops Res. 2004, 87, 245–256. [Google Scholar] [CrossRef]
- Hossain, Z.; Wang, X.; Hamel, C.; Knight, J.D.; Morrison, M.J.; Gan, Y. Biological nitrogen fixation by pulse crops on semiarid Canadian prairies.Can. J. Plant Sci. 2016, 97, 119–131. [Google Scholar]
- Zhao, J.; Chen, J.; Beillouin, D.; Lambers, H.; Yang, Y.; Smith, P.; Zhaohai Zeng, Z.; Olesen, J.E.; Zang, H. Global systematic review with meta-analysis reveals yield advantage of legume-based rotations and its drivers. Nat. Commun. 2022, 13, 4926. [Google Scholar] [CrossRef] [PubMed]
- Aloo, B.N.; Mbega, E.R.; Makumba, B.A.; Tumuhairwe, J.B. Effects of agrochemicals on the beneficial plant rhizobacteria in agricultural systems. Environ. Sci. Pollut. Res. 2021, 28, 60406–60424. [Google Scholar] [CrossRef] [PubMed]
- Weese, D.J.; Heath, K.D.; Dentinger, B.T.; Lau, J.A. Long-term nitrogen addition causes the evolution of less-cooperative mutualists. Evol. Int. J. Org. Evol. 2015, 69, 631–642. [Google Scholar] [CrossRef]
- Oyejola, B.A.; Mead, R. Statistical assessment of different ways of calculating land equivalent ratios (LER). Exp. Agric. 1982, 18, 125–138. [Google Scholar] [CrossRef]
- Sekhon, H.S.; Singh, G.; Ram, H. Lentil–based cropping systems. In Lentil: An Ancient Crop for Modern Times; Yadav, S.S., McNeil, D., Stevenson, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 107–126. [Google Scholar]
- Miah, A.A.; Rahman, M.M. Agronomy of lentil in Bangladesh. In Lentil in South Asia; Erskine, W., Saxena, M.C., Eds.; International Center for Agricultural Research in the Dry Areas, ICARDA: Aleppo, Syria, 1993; pp. 128–138. [Google Scholar]
- Singh, M.K.; Thakur, R.; Pal, S.K.; Verma, U.N.; Upasani, R.R. Plant density and row arrangement of lentil (Lens culinaris) and mustard (Brassica juncea) intercropping for higher productivity under Bihar plateau. Indian J. Agron. 2000, 45, 284–287. [Google Scholar]
- Devi, K.N.; Shamurailatpam, D.; Singh, B.; Athokpam, H.; Singh, N.; Singh, G.; Singh, L.; Singh, A.; Chanu, O.; Singh, S.; et al. Performance of lentil (Lens culinaris M.) and mustard (Brassica juncea L.) intercropping under rainfed conditions. Aust. J. Crop. Sci. 2014, 8, 284–289. [Google Scholar]
- Sarkar, R.K.; Malik, G.C.; Pal, P.K. Effect of intercropping lentil (Lens culinaris) and linseed (Linum usitatissimum) under varying plant density and row arrangement on productivity and advantages in system under rainfed upland. Indian J. Agron. 2004, 49, 241–243. [Google Scholar] [CrossRef]
- Rana, N.S.; Kumar, S.; Saini, S.K.; Panwar, G.S. Production potential and profitability of autumn sugarcane-based intercropping systems as influenced by intercrops and row spacing. Indian J. Agron. 2006, 51, 31–33. [Google Scholar] [CrossRef]
- McKenzie, B.A.; Hill, G.D. Growth, yield and water use of lentils (Lens culinaris) in Canterbury, New Zealand. J. Agric. Sci. 1990, 114, 309–320. [Google Scholar] [CrossRef]
- Xie, J.; Schoenau, J.; Warkentin, T.D. Yield and uptake of nitrogen and phosphorus in soybean, pea, and lentil and effects on soil nutrient supply and crop yield in the succeeding year in Saskatchewan, Canada. Can. J. Plant Sci. 2017, 98, 5–16. [Google Scholar] [CrossRef]
- Erskine, W.; Sarker, A.; Kumar, S. Crops that feed the world 3. Investing in lentil improvement toward a food secure world. Food Secur. 2011, 3, 127–139. [Google Scholar] [CrossRef]
- Kumar, S.; Barpete, S.; Kumar, J.; Gupta, P.; Sarker, A. Global lentil production: Constraints and strategies. SATSA Mukhapatra Annu. Tech. Issue 2013, 17, 1–13. [Google Scholar]
- Oweis, T.; Hachum, A.; Pala, M. Lentil production under supplemental irrigation in a Mediterranean environment. Agric. Water Manag. 2004, 68, 251–265. [Google Scholar] [CrossRef]
- Delahunty, A.; Nuttall, J.; Nicolas, M.; Brand, J. Response of lentil to high temperature under variable water supply and carbon dioxide enrichment. Crop Pasture Sci. 2018, 69, 1103–1112. [Google Scholar] [CrossRef]
- Wright, D.M.; Neupane, S.; Heidecker, T.; Haile, T.A.; Chan, C.; Coyne, C.J.; McGee, R.J.; Udupa, S.; Henkrar, F.; Barilli, E.; et al. Understanding photothermal interactions will help expand production range and increase genetic diversity of lentil (Lens culinaris Medik.). Plants People Planet 2021, 3, 171–181. [Google Scholar] [CrossRef]
- Singh, M.; Bisht, I.S.; Dutta, M.; Kumar, K.; Kumar, S.; Bansal, K.C. Genetic studies on morpho-phenological traits in lentil (Lens culinaris Medikus) wide crosses. J. Genet. 2014, 93, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Dadu, R.H.R.; Sambasivam, P.; Bar, I.; Singh, M.; Beera, N.; Biju, S. Toward climate-resilient lentils: Challenges and opportunities. In Genomic Designing of Climate-Smart Pulse Crops; Kole, C., Ed.; Springer: Cham, Switzerland, 2019; pp. 165–234. [Google Scholar]
- Coyne, C.J.; Kumar, S.; von Wettberg, E.J.; Marques, E.; Berger, J.D.; Redden, R.J.; Ellis, T.H.N.; Brus, J.; Zablatzká, L.; Smýkal, P. Potential and limits of exploitation of crop wild relatives for pea, lentil, and chickpea improvement. Legume Sci. 2020, 2, e36. [Google Scholar] [CrossRef]
- Rajpal, V.R.; Singh, A.; Kathpalia, R.; Thakur, R.K.; Khan, M.; Pandey, A.; Hamurcu, M.; Raina, S.N. The prospects of gene introgression from crop wild relatives into cultivated lentil for climate change mitigation. Front. Plant Sci. 2023, 14, 1127239. [Google Scholar] [CrossRef]
- Civantos-Gómez, I.; Rubio Teso, M.L.; Galeano, J.; Rubiales, D.; Iriondo, J.M.; García-Algarra, J. Climate change conditions the selection of rust-resistant candidate wild lentil populations for in situ conservation. Front. Plant Sci. 2022, 13, 1010799. [Google Scholar] [CrossRef]
- Martinelli, F.; Vollheyde, A.L.; Cebrián-Piqueras, M.A.; von Haaren, C.; Lorenzetti, E.; Barberi, P.; Loreto, F.; Piergiovanni, A.R.; Totev, V.V.; Bedini, A.; et al. LEGU-MED: Developing biodiversity-based agriculture with legume cropping systems in the mediterranean basin. Agronomy 2022, 12, 132. [Google Scholar] [CrossRef]
- Wang, W.; Li, Q.; Wu, J.; Hu, Y.; Wu, G.; Yu, C.; Xu, K.; Liu, X.; Wang, Q.; Huang, W.; et al. Lentil lectin derived from Lens culinaris exhibit broad antiviral activities against SARS-CoV-2 variants. Emerg. Microbes Infect. 2021, 10, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Peng, L.; Xiong, H.; Wang, J.; Tsao, R.; Peng, X.; Jiang, L.; Sun, Y. Free and Bound Phenolics of Laird Lentil (Lens culinaris) Hulls and the Anti-inflammatory Activity of their Digestive Products via Crosstalk between NF-κB and Keap1-Nrf2 Signaling Pathways in HT-29 Cells. J. Agric. Food Chem. 2022, 70, 13251–13263. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.S.; Lee, S.H.; Chun, S.Y.; Kim, D.H.; Jang, B.I.; Han, M.H.; Lee, S.O. In Vitro and In Vivo Protective Effects of Lentil (Lens culinaris) Extract against Oxidative Stress-Induced Hepatotoxicity. Molecules 2022, 27, 59. [Google Scholar] [CrossRef]
- Rainbird, B.; Bentham, R.H.; Soole, K.L. Rhizoremediation of residual sulfonylurea herbicides in agricultural soils using Lens culinaris and a commercial supplement. Int. J. Phytoremediation 2018, 20, 104–113. [Google Scholar] [CrossRef]
- Mercado, S.A.S.; Caleño, J.D.Q. Use of Lens culinaris Med test as environmental bioindicator to identify the cytogenotoxic effect of paraquat pesticide. Environ. Sci. Pollut. Res. 2021, 28, 51321–51328. [Google Scholar] [CrossRef]

| Country | 2011 | 2021 | ||||
|---|---|---|---|---|---|---|
| Production (t × 1000) | Area Harvested (ha × 1000) | Yield (t ha−1) | Production (t × 1000) | Area Harvested (ha × 1000) | Yield (t ha−1) | |
| Canada | 1574 | 1005 | 1.57 | 1606 | 1716 | 0.94 |
| India | 944 | 1597 | 0.59 | 1490 | 1734 | 0.86 |
| Australia | 288 | 173 | 1.67 | 854 | 501 | 1.70 |
| Türkiye | 406 | 215 | 1.89 | 263 | 297 | 0.89 |
| Nepal | 207 | 208 | 1.00 | 246 | 202 | 1.22 |
| Bangladesh | 80 | 83 | 0.97 | 186 | 146 | 1.27 |
| Russia | 33 | 30 | 1.11 | 176 | 161 | 1.09 |
| China | 150 | 60 | 2.50 | 165 | 65 | 2.54 |
| USA | 215 | 166 | 1.29 | 151 | 222 | 0.68 |
| Ethiopia | 128 | 110 | 1.16 | 123 | 87 | 1.41 |
| Syria | 112 | 140 | 0.80 | 94 | 111 | 0.84 |
| Iran | 50 | 103 | 0.49 | 80 | 133 | 0.60 |
| Kazakhstan | 7 | 7 | 1.07 | 56 | 72 | 0.77 |
| Morocco | 45 | 58 | 0.78 | 42 | 42 | 1.00 |
| Argentina | 24 | 17 | 1.40 | 20 | 28 | 0.72 |
| Mexico | 8 | 7 | 1.21 | 10 | 9 | 1.12 |
| World | 4382 | 4119 | 1.06 | 5610 | 5586 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montejano-Ramírez, V.; Valencia-Cantero, E. The Importance of Lentils: An Overview. Agriculture 2024, 14, 103. https://doi.org/10.3390/agriculture14010103
Montejano-Ramírez V, Valencia-Cantero E. The Importance of Lentils: An Overview. Agriculture. 2024; 14(1):103. https://doi.org/10.3390/agriculture14010103
Chicago/Turabian StyleMontejano-Ramírez, Vicente, and Eduardo Valencia-Cantero. 2024. "The Importance of Lentils: An Overview" Agriculture 14, no. 1: 103. https://doi.org/10.3390/agriculture14010103
APA StyleMontejano-Ramírez, V., & Valencia-Cantero, E. (2024). The Importance of Lentils: An Overview. Agriculture, 14(1), 103. https://doi.org/10.3390/agriculture14010103







