Patterns of Nitrogen and Phosphorus along a Chronosequence of Tea Plantations in Subtropical China
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Sample Site
2.2. Collection of Soil and Leaf Samples
2.3. Chemical Analysis of Soil Sample
2.4. Chemical Analysis of Leaf Sample
2.5. Statistical Analysis
3. Results
3.1. Pattern of Soil Nitrogen Concentration
3.2. Pattern of Soil Phosphorus Concentration
3.3. Pattern of Tea Leaf Nitrogen and Phosphorus Concentrations
3.4. Correlations between Soil and Leaf Nitrogen Concentrations
3.5. Correlations between Soil and Leaf Phosphorus Concentrations
4. Discussion
4.1. Patterns of Nitrogen in Soil and Leaves
4.2. Patterns of Phosphorus in Soil and Leaves
4.3. Soil Controls Leaf Nitrogen and Phosphorus Concentrations
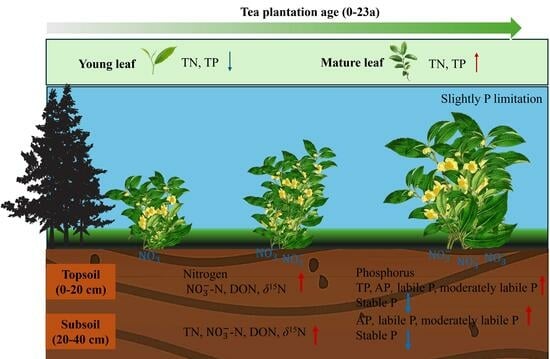
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oldroyd, G.E.D.; Leyser, O. A plant’s diet, surviving in a variable nutrient environment. Science 2020, 368, eaba0196. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Cheng, Y.H.; Chen, K.E.; Tsay, Y.F. Nitrate Transport, Signaling, and Use Efficiency. Annu. Rev. Plant Biol. 2018, 69, 85–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Y.F.; Wu, W.H. Potassium and phosphorus transport and signaling in plants. J. Integr. Plant Biol. 2021, 63, 34–52. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Wu, L.; Wang, D.; Fu, J.; Shen, C.; Li, X.; Zhang, L.; Zhang, L.; Fan, L.; Wenyan, H. Soil acidification in Chinese tea plantations. Sci. Total Environ. 2020, 715, 136963. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.K.; Wille, U.; Hu, H.-W.; Caruso, F.; Mumford, K.; Liang, X.; Pan, B.; Malcolm, B.; Roessner, U.; Suter, H.; et al. Next-generation enhanced-efficiency fertilizers for sustained food security. Nat. Food 2022, 3, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Jing, X.; Zhang, Q.; Zhan, X.; Zhang, A.; Hussain, H.A. Nitrogen and phosphorus trajectories (1998–2030) under regional development strategy of mainland China. Sci. Total Environ. 2021, 794, 148655. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Cabrera Serrenho, A. Greenhouse gas emissions from nitrogen fertilizers could be reduced by up to one-fifth of current levels by 2050 with combined interventions. Nat Food 2023, 4, 170–178. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, Z.; Pan, Z.; Wang, R.; Yan, G.; Liu, C.; Su, Y.; Zheng, X.; Butterbach-Bahl, K. Tea-planted soils as global hotspots for N2O emissions from croplands. Environ. Res. Lett. 2020, 15, 104018. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Fu, X.; Shen, J.; Chen, D.; Wang, Y.; Liu, X.; Xiao, R.; Wei, W.; Wu, J. Effect of controlled-release fertilizer on N(2)O emissions and tea yield from a tea field in subtropical central China. Environ. Sci. Pollut. Res. Int. 2018, 25, 25580–25590. [Google Scholar] [CrossRef]
- Ni, K.; Liao, W.Y.; Yi, X.Y.; Niu, S.G.; Ma, L.F.; Shi, Y.Z.; Zhang, Q.F.; Liu, M.Y.; Ruan, J.Y. Fertilization status and reduction potential in tea gardens of China. J. Plant Nutr. Fertil. 2019, 25, 421–432, [In Chinese with English Abstract]. [Google Scholar]
- Chien, S.C.; Krumins, J.A. Anthropogenic effects on global soil nitrogen pools. Sci. Total Environ. 2023, 902, 166238. [Google Scholar] [CrossRef] [PubMed]
- Bingham, A.H.; Cotrufo, M.F. Organic nitrogen storage in mineral soil: Implications for policy and management. Sci. Total Environ. 2016, 551–552, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.A.; Mahmood, R.; Ali, S. Soil urease inhibition by various plant extracts. PLoS ONE 2021, 16, e0258568. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, K.; Yanai, R. Nitrogen-phosphorous interactions in young northern hardwoods indicate P limitation: Foliar concentrations and resorption in a factorial N by P addition experiment. Oecologia 2019, 189, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yin, M.; Zhang, X.; Wang, Q.; Wang, R.; Yin, H. Differential aboveground-belowground adaptive strategies to alleviate N addition-induced P deficiency in two alpine coniferous forests. Sci. Total Environ. 2022, 849, 157906. [Google Scholar] [CrossRef] [PubMed]
- Krouk, G.; Kiba, T. Nitrogen and Phosphorus interactions in plants: From agronomic to physiological and molecular insights. Curr. Opin. Plant Biol. 2020, 57, 104–109. [Google Scholar] [CrossRef]
- Wang, L.; Yang, L.; Xiong, F.; Nie, X.; Li, C.; Xiao, Y.; Zhou, G. Nitrogen Fertilizer Levels Affect the Growth and Quality Parameters of Astragalus mongolica. Molecules 2020, 25, 381. [Google Scholar] [CrossRef]
- Giguere, A.T.; Taylor, A.E.; Myrold, D.D.; Mellbye, B.L.; Sayavedra-Soto, L.A.; Bottomley, P.J. Nitrite-oxidizing activity responds to nitrite accumulation in soil. FEMS Microbiol. Ecol. 2018, 94, fiy008. [Google Scholar] [CrossRef]
- Zhao, C.; He, X.; Dan, X.; He, M.; Zhao, J.; Meng, H.; Cai, Z.; Zhang, J. Soil dissolved organic matters mediate bacterial taxa to enhance nitrification rates under wheat cultivation. Sci. Total Environ. 2022, 828, 154418. [Google Scholar] [CrossRef]
- Bateman, A.S.; Kelly, S.D. Fertilizer nitrogen isotope signatures. Isot. Environ. Health Stud. 2007, 43, 237–247. [Google Scholar] [CrossRef]
- Yan, P.; Shen, C.; Fan, L.; Li, X.; Zhang, L.; Zhang, L.; Han, W. Tea planting affects soil acidification and nitrogen and phosphorus distribution in soil. Agric. Ecosyst. Environ. 2018, 254, 20–25. [Google Scholar] [CrossRef]
- Li, B.; Dinkler, K.; Zhao, N.; Ran, X.; Sobhi, M.; Dong, R.; Müller, J.; Xiong, W.; Huang, G.; Guo, J.; et al. Response of phosphorus speciation to organic loading rates and temperatures during anaerobic co-digestion of animal manures and wheat straw. Sci. Total Environ. 2022, 838, 155921. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.H.; Frizano, J.; Vann, D.R. Biogeochemical implications of labile phosphorus in forest soils determined by the Hedley fractionation procedure. Oecologia 2003, 135, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Fang, J.; Guo, D.; Zhang, Y. Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New Phytol. 2005, 168, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, B.; Gao, X.; Li, X.; Li, C. Nitrogen and phosphorus addition differentially affect plant ecological stoichiometry in desert grassland. Sci. Rep. 2019, 9, 18673. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Wu, S.; Guo, Q.; Wang, J.; Cao, C.; Wang, J. Leaf nitrogen assimilation and partitioning differ among subtropical forest plants in response to canopy addition of nitrogen treatments. Sci. Total Environ. 2018, 637–638, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Jia, X.; Yang, J.; Liu, J.; Shangguan, Z.; Yan, W. Inhibitors mitigate N2O emissions more effectively than biochar: A global perspective. Sci. Total Environ. 2023, 859, 160416. [Google Scholar] [CrossRef]
- Xie, H.; Chen, Z.; Feng, X.; Wang, M.; Luo, Y.; Wang, Y.; Xu, P. L-theanine exuded from Camellia sinensis roots regulates element cycling in soil by shaping the rhizosphere microbiome assembly. Sci. Total Environ. 2022, 837, 155801. [Google Scholar] [CrossRef]
- Gurmesa, G.A.; Hobbie, E.A.; Zhang, S.; Wang, A.; Zhu, F.; Zhu, W.; Koba, K.; Yoh, M.; Wang, C.; Zhang, Q.; et al. Natural 15N abundance of ammonium and nitrate in soil profiles: New insights into forest ecosystem nitrogen saturation. Ecosphere 2022, 13, e3998. [Google Scholar] [CrossRef]
- Keisuke, K.; Muneto, H.; Lina, K.; Ayato, K.; Hiroshi, T. Natural 15 N Abundance of Plants and Soil N in a Temperate Coniferous Forest. Ecosystems 2003, 6, 457–469. [Google Scholar]
- Retta, M.; Yin, X.; van der Putten, P.E.; Cantre, D.; Berghuijs, H.N.; Ho, Q.T.; Verboven, P.; Struik, P.C.; Nicolaï, B.M. Impact of anatomical traits of maize (Zea mays L.) leaf as affected by nitrogen supply and leaf age on bundle sheath conductance. Plant Sci. 2016, 252, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Bassi, D.; Menossi, M.; Mattiello, L. Nitrogen supply influences photosynthesis establishment along the sugarcane leaf. Sci. Rep. 2018, 8, 2327. [Google Scholar] [CrossRef] [PubMed]
- Du, E.; Terrer, C.; Pellegrini, A.F.A.; Ahlström, A.; van Lissa, C.J.; Zhao, X.; Xia, N.; Wu, X.; Jackson, R.B. Global patterns of terrestrial nitrogen and phosphorus limitation. Nat. Geosci. 2020, 13, 221–226. [Google Scholar] [CrossRef]
- Ding, D.; Arif, M.; Liu, M.; Li, J.; Hu, X.; Geng, Q.; Yin, F.; Li, C. Plant-soil interactions and C:N:P stoichiometric homeostasis of plant organs in riparian plantation. Front. Plant Sci. 2022, 13, 979023. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fang, X.; Tang, X.; Wang, W.; Zhou, G.; Xu, S.; Huang, W.; Wang, G.; Yan, J.; Ma, K.; et al. Patterns and controlling factors of plant nitrogen and phosphorus stoichiometry across China’s forests. Biogeochemistry 2019, 143, 191–205. [Google Scholar] [CrossRef]
- Nouri, A.; Lukas, S.; Singh, S.; Singh, S.; Machado, S. When do cover crops reduce nitrate leaching? A global meta-analysis. Glob. Chang. Biol. 2022, 28, 4736–4749. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.M.; Zhang, X.L.; Zong, Y.Y.; Zhang, Y.; Wan, S.Z.; Bu, W.S.; Chen, F.S. Soil phosphorus functional fractions and tree tissue nutrient concentrations influenced by stand density in subtropical Chinese fir plantation forests. PLoS ONE 2017, 12, e0186905. [Google Scholar] [CrossRef]
- Zhang, Z.; Pan, M.; Zhang, X.; Liu, Y. Responses of invasive and native plants to different forms and availability of phosphorus. Am. J. Bot. 2022, 109, 1560–1567. [Google Scholar] [CrossRef]
- McLaren, T.I.; Smernik, R.J.; McLaughlin, M.J.; McBeath, T.M.; Kirby, J.K.; Simpson, R.J.; Guppy, C.N.; Doolette, A.L.; Richardson, A.E. Complex Forms of Soil Organic Phosphorus-A Major Component of Soil Phosphorus. Environ. Sci. Technol. 2015, 49, 13238–13245. [Google Scholar] [CrossRef]
- Lambers, H. Phosphorus Acquisition and Utilization in Plants. Annu. Rev. Plant Biol. 2022, 73, 17–42. [Google Scholar] [CrossRef]





| Soil Layer | TN of Young Leaf | TN of Mature Leaf | |||||
|---|---|---|---|---|---|---|---|
| n | r | p | n | r | p | ||
| Topsoil (0–20 cm) | TN | 12 | 0.33 | =0.30 | 15 | −0.36 | =0.19 |
| -N | 12 | −0.23 | =0.47 | 15 | 0.75 | <0.01 | |
| -N | 12 | −0.38 | =0.23 | 15 | −0.39 | =0.15 | |
| -N | 12 | −0.76 | <0.01 | 15 | 0.56 | =0.03 | |
| DON | 12 | 0.55 | =0.06 | 15 | 0.40 | =0.14 | |
| δ15N | 12 | −0.45 | =0.14 | 15 | 0.77 | <0.01 | |
| Subsoil (20–40 cm) | TN | 12 | 0.07 | =0.84 | 15 | 0.58 | =0.02 |
| -N | 12 | −0.01 | =0.98 | 15 | 0.89 | <0.001 | |
| -N | 12 | −0.38 | =0.22 | 15 | 0.09 | =0.75 | |
| -N | 12 | 0.03 | =0.93 | 15 | 0.20 | =0.47 | |
| DON | 12 | 0.34 | =0.28 | 15 | 0.44 | =0.10 | |
| δ15N | 12 | 0.01 | =0.99 | 15 | 0.72 | <0.01 | |
| Soil Layer | TP of Young Leaf | TP of Mature Leaf | |||||
|---|---|---|---|---|---|---|---|
| n | r | p | n | r | p | ||
| Topsoil (0–20 cm) | TP | 12 | −0.49 | =0.11 | 15 | 0.89 | <0.001 |
| AP | 12 | −0.67 | =0.02 | 15 | 0.88 | <0.001 | |
| H2O-Pi | 12 | −0.38 | =0.22 | 15 | 0.74 | <0.01 | |
| NaHCO3-Pi | 12 | −0.83 | <0.01 | 15 | 0.81 | <0.001 | |
| NaHCO3-Po | 12 | 0.46 | =0.13 | 15 | 0.19 | =0.51 | |
| NaOH-Pi | 12 | −0.31 | =0.33 | 15 | 0.82 | <0.001 | |
| NaOH-Po | 12 | −0.45 | =0.14 | 15 | −0.20 | =0.48 | |
| D.HCl-Pi | 12 | −0.71 | =0.01 | 15 | 0.70 | <0.01 | |
| C.HCl-Pi | 12 | 0.13 | =0.68 | 15 | −0.22 | =0.42 | |
| C.HCl-Po | 12 | 0.30 | =0.34 | 15 | −0.45 | =0.09 | |
| Residual-Pt | 12 | 0.40 | =0.20 | 15 | −0.45 | =0.09 | |
| Subsoil (20–40 cm) | TP | 12 | −0.47 | =0.12 | 15 | 0.51 | =0.05 |
| AP | 12 | −0.74 | <0.01 | 15 | 0.89 | <0.001 | |
| H2O-Pi | 12 | 0.49 | =0.11 | 15 | 0.44 | =0.10 | |
| NaHCO3-Pi | 12 | −0.66 | =0.02 | 15 | 0.68 | <0.01 | |
| NaHCO3-Po | 12 | 0.36 | =0.26 | 15 | 0.48 | =0.07 | |
| NaOH-Pi | 12 | −0.74 | <0.01 | 15 | 0.81 | <0.001 | |
| NaOH-Po | 12 | 0.36 | =0.24 | 15 | −0.11 | =0.68 | |
| D.HCl-Pi | 12 | −0.61 | =0.04 | 15 | 0.25 | =0.38 | |
| C.HCl-Pi | 12 | 0.22 | =0.48 | 15 | −0.35 | =0.20 | |
| C.HCl-Po | 12 | 0.48 | =0.12 | 15 | −0.24 | =0.39 | |
| Residual-Pt | 12 | 0.59 | =0.04 | 15 | −0.74 | <0.01 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, S.; Huang, C.; Chen, Y.; Bai, X.; Li, W.; He, B. Patterns of Nitrogen and Phosphorus along a Chronosequence of Tea Plantations in Subtropical China. Agriculture 2024, 14, 110. https://doi.org/10.3390/agriculture14010110
Zou S, Huang C, Chen Y, Bai X, Li W, He B. Patterns of Nitrogen and Phosphorus along a Chronosequence of Tea Plantations in Subtropical China. Agriculture. 2024; 14(1):110. https://doi.org/10.3390/agriculture14010110
Chicago/Turabian StyleZou, Shun, Chumin Huang, Yang Chen, Xiaolong Bai, Wangjun Li, and Bin He. 2024. "Patterns of Nitrogen and Phosphorus along a Chronosequence of Tea Plantations in Subtropical China" Agriculture 14, no. 1: 110. https://doi.org/10.3390/agriculture14010110
APA StyleZou, S., Huang, C., Chen, Y., Bai, X., Li, W., & He, B. (2024). Patterns of Nitrogen and Phosphorus along a Chronosequence of Tea Plantations in Subtropical China. Agriculture, 14(1), 110. https://doi.org/10.3390/agriculture14010110