Abstract
Seed treatment is a powerful technique for adding beneficial ingredients to plants during the seed preparation process. Biopolymers as drying agents and delivery systems in seed treatments were investigated for their biocompatibility with blastospores of the nematophagous fungus Pochonia chlamydosporia. To produce a novel seed treatment for the cover crop Phacelia tanacetifolia, xanthan gum TG and gellan gum were the most promising biopolymers in combination with potato starch and bentonite. The seed treatment process as well as the drying process were specially designed to be scalable, which make it suitable for applying the developed seed treatment in agriculture. Application of gellan gum in seed treatments led to 6.3% ± 1.6% of vital blastospores per seed compared to 3.8% ± 0.3% of vital blastospores when applying xanthan gum. Storage tests for seed treatments with 0.5% gellan gum indicated a higher stability at 4 °C compared to storage at 21 °C. After 42 days of storage at 4 °C, 54.1% ± 15.1% of the applied blastospores were viable compared to 0.3% ± 0.8% at 21 °C. This novel seed treatment application with P. chlamydosporia blastospores includes the seed treatment procedure, drying process, and storage tests and can easily be upscaled for application in agriculture.
1. Introduction
Seed treatments offer significant advantages for agricultural systems. Plant-growth promoting bacteria and fungi can help strengthen the plants while providing protection against pests, for example as active ingredients in seed treatments [1,2,3]. The direct application to seeds allows biological control agents and plant-strengthening microorganisms to establish themselves in the rhizosphere. In addition, the plant-growth-promoting additives are in close proximity to the plant root and can enter the plant roots directly or colonize the rhizosphere. Furthermore, seed treatment saves application time and costs compared to solid or liquid formulations, as no additional step, water, and specific machinery are required [3].
By treating cover crop seeds with biocontrol agents, the microorganisms can establish themselves and take effect before the main crop is even sown and germinated. This may lead to a reduced pest pressure for the main crop. To this end, the cover crop Phacelia tanacetifolia has various advantages. P. tanacetifolia is often cultivated and suitable in a wide range of crop rotations because it is phylogenetically distant to common crops like grains and vegetables [4]. Phacelia is sown for green manuring because it forms large amounts of biomass and a fine root system [5]. Moreover, Phacelia is less susceptible to plant diseases like plant-parasitic nematodes that affect many plants cultivated for food and feed [6]. Therefore, seed treatment with a biological control agent can improve the potential application of P. tanacetifolia and enables efficient green manuring while providing active pest control.
In order to develop a commercially exploitable seed treatment with biocontrol agents, it is important to apply biodegradable as well as low-cost ingredients that are registered for field application [3]. Biopolymers such as xanthan gum or gellan gum can be incorporated in seed treatments as food source and adhesives, and/or desiccants. Xanthan gum is a polysaccharide produced by Xanthomonas campestris in submerged fermentation and can be added as a fermentation nutrient [7]. Additionally, it has wide applications in the chemical industry, e.g., as a thickener for controlling drift of fungicide, herbicide, pesticide, and fertilizers during application in agriculture [8]. Gellan gum is a bacterial polysaccharide produced by Sphingomonas elodea and is known as cationic concentration-dependent gel former [9]. A further additive that can be included in seed treatments as filler, carrier material and absorbent to facilitate water acquisition during germination is bentonite, a highly versatile natural clay [10]. It is known for its strong water absorption and swelling capacity [11]. Finally, nutrient sources such as potato starch can be incorporated to the seed treatment to support the biocontrol agent and the plant in the early growth phase [12]. For applicability in agriculture, a low-cost and simple process of the seed treatment itself and of the biocontrol agent are indispensable [13].
The nematophagous fungus Pochonia chlamydosporia is a promising candidate as biocontrol agent [14,15,16] in seed treatments. Pochonia can rapidly be cultivated in a simple and low-cost liquid culture and is already effectively applied against various species of plant-parasitic nematodes in commercial formulations such as Rizotec® (Rizoflora Biotecnologia S.A., Viçosa, Brazil) or KlamiC® (Centro Nacional de Sanidad Agropecuaria, San José de las Lajas, La Habana, Cuba). They are available as powder or liquid including chlamydospores and none of these products are currently authorized on the European market. The drawback is that these products must be applied to the field separately, which increases costs for farmers, as they require more water, time, and machinery.
To reduce these costs, seed treatments are a promising alternative. Additionally, costs can be further reduced by applying blastospores derived from liquid culture. These yeast-like vegetative fungal cells are formed during in vitro-growth by specific culture conditions, as shown for Beauveria bassiana [17]. Blastospores are suitable for seed treatments, but due to their thin cell wall, blastospores are more sensitive to dehydration and loss of viability than chlamydospores [13]. On the other hand, blastospores are more infectious and, therefore, more effective against their target organism in comparison to chlamydospores [18]. Given this advantage, the required amount of blastospores could be reduced in case of seed treatments compared to powder or liquid formulations. Moreover, seed treatment allows for the biological control agent to be applied directly to where protection of young seedlings is required.
This work aimed for developing an effective treatment of seeds, where addition of biopolymers may improve the viability of P. chlamydosporia blastospores. For process optimization, P. chlamydosporia blastospores grown in liquid culture were dried in the presence of different biopolymers such as chitosan, pectin amide, carboxymethylcellulose, xanthan gum, and gellan gum to evaluate the drying properties of the biopolymers. Additionally, we applied blastospores to P. tanacetifolia seeds with bentonite, potato starch, and the biopolymers gellan gum or xanthan gum to test for increased blastospore survival after processing and drying. Lastly, we evaluated storage stability weekly over a period of 6 weeks in order to assess the complete process.
2. Materials and Methods
2.1. Pochonia Chlamydosporia Blastospore Cultivation
Pochonia chlamydosporia strain Pc001, deposited in the fungal culture collection at the Julius Kühn Institute, was originally isolated from surface-sterilized cysts of Heterodera schachtii collected from sugar beet fields located in North Rhine-Westphalia, Germany [19]. P. chlamydosporia was cultivated according to Uthoff et al. [20]. To this end, chlamydospores cultivated on potato dextrose (PDA, 39 g/L, Carl Roth GmbH & Co. KG, Karlsruhe, Germany) at 23 °C were used as pre-culture inoculum for blastospore cultivation. Blastospores were cultivated in sterile 250 mL flasks with baffled base, filled with 100 mL sterile potato dextrose broth (PDB, 26.5 g/L, Carl Roth GmbH & Co. KG, Karlsruhe, Germany) by incubation at 26 °C on a rotary shaker at 150 rpm (IKA KS 4000 ic, Staufen, Germany) for 7 days ([21], modified). To separate blastospores from mycelium, PDB liquid culture was filtered through a 5–10-µm Whatman filter (VWR, Darmstadt, Germany) under sterile conditions and centrifugated. Sterile 0.9% (w/v) NaCl solution was used to wash the blastospores two times. Blastospores were resuspended in NaCl solution and centrifugated (3600× g, 20 °C, 10 min) two times. In a Thoma cell counting chamber the blastospore number was counted under a light microscope (Axiostar plus; Carl Zeiss MicroImaging GmbH, Göttingen, Germany) at 200-fold magnification.
2.2. Preliminary Tests
For preliminary tests, the blastospore suspension was mixed with selected biopolymers to investigate the drying agent properties of the chosen biopolymers. For this purpose, 100 µL of the blastospore suspension (105 blastospores/mL) were added to 900 µL of the following biopolymer solution: 2% chitosan 80/60/A1 (Heppe Medical Chitosan GmbH, Halle/Saale, Germany), 2% pectin amide (Herbstreith & Fox KG, Neuenbuerg/Wuertt, Germany), 2% carboxymethylcellulose (Blanose 7M, Ashland Industries Deutschland GmbH, Düsseldorf, Germany), 1% xanthan gum TNAS and TG (Jungbunzlauer Suisse AG, Basel, Switzerland), or 1% gellan gum (Phytagel, Sigma-Aldrich Chemie GmbH, Taufkirchen,, Germany). A total of 100 µL of the mixture was dried in a desiccator for 4 days at 21 °C. For the positive control, blastospores were stored in 0.9% NaCl at 4 °C. For the negative control, blastospores were dried in 0.9% NaCl without any biopolymer. The dried blastospores were vortexed with 1 mL 0.1% Tween 80 for 10 min. A total of 100 µL sample per replicate (n = 3) was spread on selective PDA agar (39 g/L PDA) containing 0.1 g/L streptomycin, 0.05 g/L tetracycline, 0.1 g/L dodine, and 0.05 g/L cycloheximide [22]. After 5 days of growth in dark at 23 °C, colony-forming units (CFU) were counted in control and desiccated samples.
2.3. Seed Treatment
The seed treatment was performed in a self-constructed, stainless steel, lab-scale drum dryer (drum diameter 16.0 cm, drum speed 33 rpm) with biopolymers gellan gum or xanthan gum TG, each 0.25%, 0.5%, or 1%. To decrease contaminations on the seed surface, P. tanacetifolia seeds were first surface-sterilized for 3 min in 70% ethanol, 3 min in 3% (v/v) sodium hypochlorite, 3 min in 70% ethanol and washed 3 times in sterile ddH2O water. Sterilized P. tanacetifolia seeds (25 g) were mixed with potato starch superior (in total 30 g, Emsland-Stärke GmbH, Emlichheim, Germany) and bentonite (in total 12 g, Tonsil® 510ff, Clariant AG, Muttenz, Switzerland) in the drum under constant rotation. At the beginning of the process, the sterilized and washed seeds were added to the drum and rotated. A total of 10 g potato starch and 4 g bentonite were added alternately to the seeds to form the first layer of the coat. Afterwards, 6.5 mL of the biopolymer solution without P. chlamydosporia blastospores was added. This procedure was repeated three times. In the last step, 1 mL biopolymer solution with 107 P. chlamydosporia blastospores was added. All ingredients were rotated in the drum until a homogeneous seed treatment was formed. Afterwards, the treated seeds were dried by warm air stream (airflow temperature 50.0 to 55.0 °C) for 30–45 min with a maximum temperature of 30.0 °C on the seed surface, measured with an infrared sensor. At the end of the drying process, the water activity (aW) of the treated seeds was below 0.55 (LabMaster-aw, Novasina, Lachen, Switzerland). Every seed treatment was performed in three biological replicates by applying a freshly cultivated blastospore suspension each time. Then, 10 seeds with wet and dried coating from the same batch were washed in 0.9% sodium chloride (3 replicates from every batch). The washing suspension was plated on selective PDA plates [22] in 5 technical replicates. Plates were incubated at 23 °C in the dark and CFU were counted after five days to determine the seed treatment efficiency and survival of P. chlamydosporia blastospores during and after the process. To visualise dried blastospores in the seed treatment, dried coated seeds were sputtered with gold (EM SCD005, Leica 362 Microsystems, Wetzlar, Germany) prior to examination with a scanning electron microscope (SEM, S-450, Hitachi, Tokyo, Japan).
2.4. Storage Tests
For commercial seed treatments, a storability of at least four weeks at room temperature is required. Therefore, dried treated seeds were stored in clear, pre-sterilised 50-mL polypropylene tubes at 4 ± 1 °C and 21 ± 1 °C. After 7, 14, 21, 28, 35, and 42 days, 10 of the stored seeds were rinsed in 0.9% NaCl (3 technical replicates) and plated on selective potato-dextrose-agar plates, as described above.
2.5. Statistical Analysis
Figures were created and statistical analyses were calculated with RStudio, version 7.2 [23]. Data were presented as bar plots with mean and standard deviation. Figures were created using the ggplot2 package [24]. Analysis of normality and homogeneity of variance was calculated with Shapiro–Wilk and Levene’s test, respectively. Statistical analysis was calculated using Kruskal–Wallis test with Dunn’s Test for multiple comparisons with Benjamini and Hochberg [25] correction as post-hoc tests.
3. Results
3.1. Preliminary Tests—Drying P. chlamydosporia Blastospores in Biopolymers
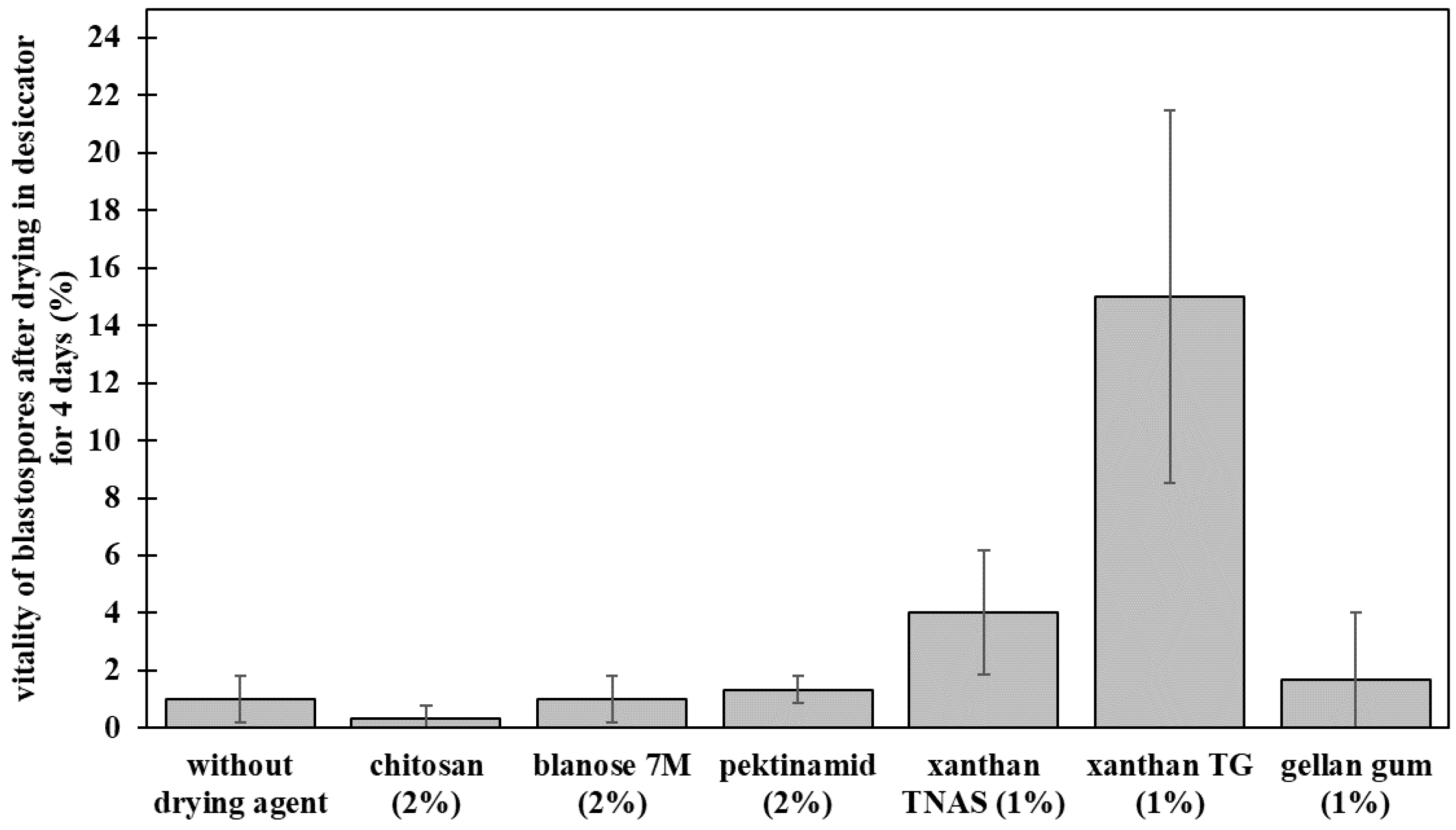
Preliminary tests aimed for investigating the general survival of P. chlamydosporia blastospores in selected polymers. After drying, highest viability was detected when blastospores were formulated with gellan gum or xanthan gum (Figure 1). The highest survival after drying was obtained with 1% xanthan gum TG (15.0% ± 6.5%) and 1% xanthan gum TNAS (4.0% ± 2.2%), followed by 1% gellan gum with 1.7% ± 2.4% viability of blastospores after drying.
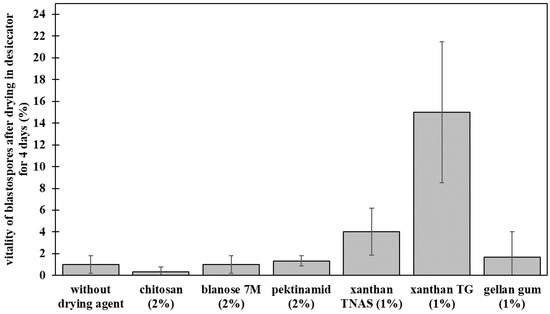
Figure 1.
Preliminary tests with biopolymers as the drying agent for Pochonia chlamydosporia blastospores. Bar plots show means ± standard deviation for the three biological replicates and the three technical replicates.
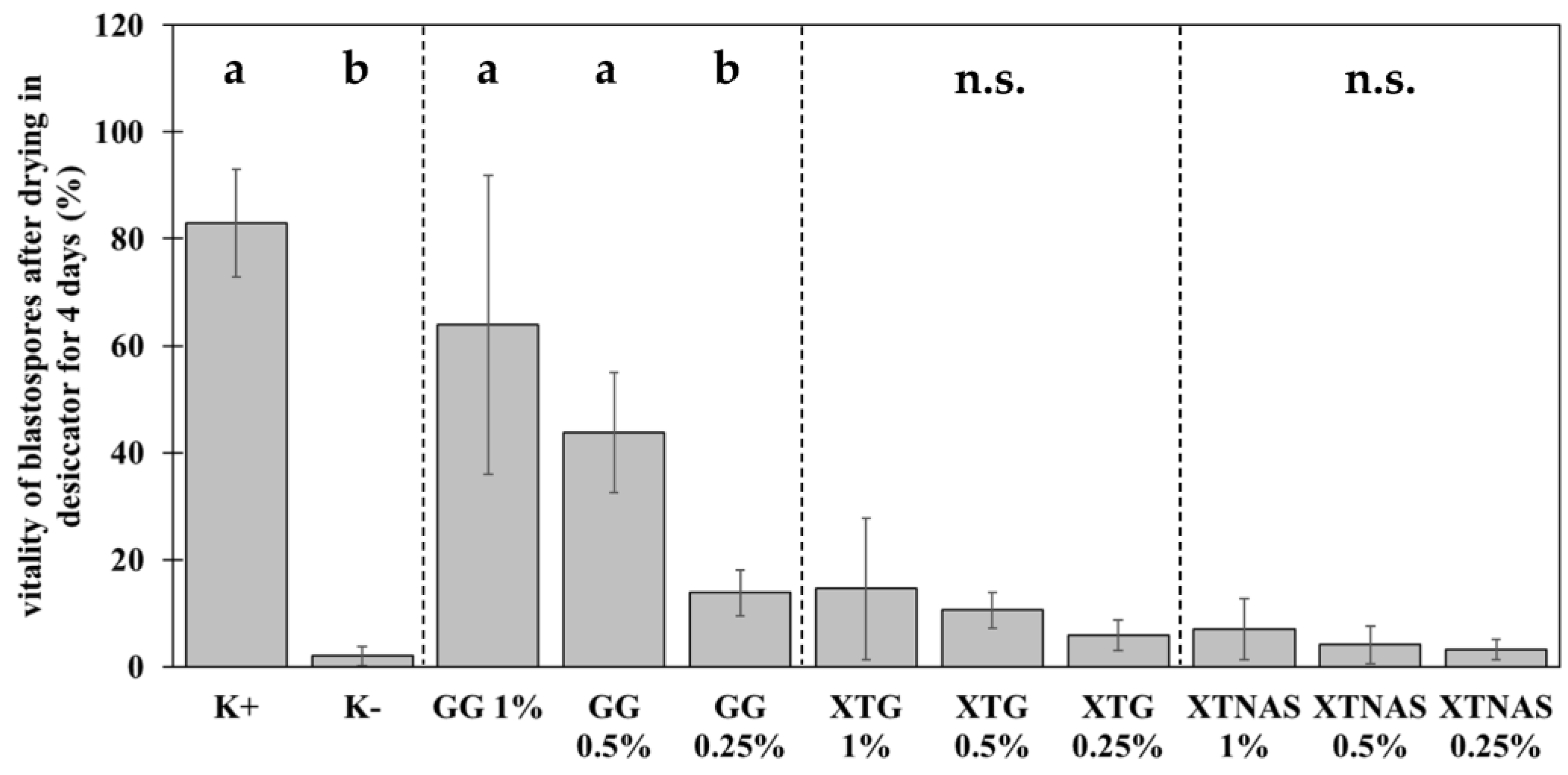
Based on the preliminary tests, further experiments with a concentration series (1%, 0.5%, 0.25%) of xanthan gum TG, xanthan gum TNAS, and gellan gum were carried out to detect the most efficient polymer concentration (Figure 2). The results marked three trends. First, the formulation with biopolymers led to a significant increase of blastospore viability after drying. Second, gellan gum performed best as the drying agent. Third, a dose-dependent effect was measured for gellan gum and xanthan gum. The positive control (K+) confirmed the blastospore viability without drying (83.0% ± 10.7%) and the negative control (K−) revealed a survival rate of 2.0% ± 1.9% of blastospores after drying without the addition of biopolymers. This experiment replicated the results from the preliminary tests with 1% xanthan gum TG (14.6% ± 14.0%). Reducing the xanthan gum TG concentration maintained the viability of blastospores (X2 = 5.46, df = 2, p = 0.07). The same could be demonstrated for xanthan gum TNAS (X2 = 1.48, df = 2, p = 0.48). The viability of blastospores after drying in 1% gellan gum was significantly higher compared to the previous experiments (64.0% ± 28.0%). However, the reduction of the gellan gum concentration led to a significant reduction of the survival (X2 = 18.9, df = 2, p < 0.001); 0.5% gellan gum 43.8% ± 11.2% vitality, and 0.25% gellan gum 13.8% ± 4.3%. In conclusion, gellan gum at high concentration protected P. chlamydosporia blastospores best during dehydration. For both xanthan gums, reducing the concentration maintained the viability of blastospores.
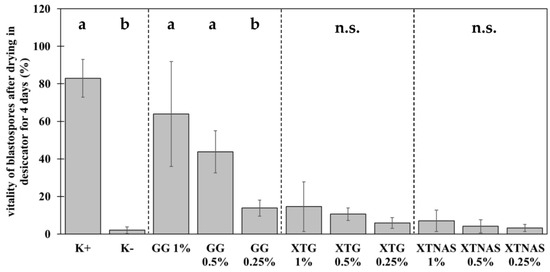
Figure 2.
Protective efficiency of xanthan gum TG (XTG), xanthan gum TNAS (XTNAS), and gellan gum (GG) on the viability of Pochonia chlamydosporia blastospores compared to positive control without drying (K+) and dried spores without biopolymer (negative control, K−). Bar plots show mean ± standard deviation for three biological replicates each with three technical replicates. Treatments with different concentrations of the same biopolymer were statistically compared using Kruskal–Wallis test with Dunn’s test for multiple comparisons as post-hoc test (p < 0.05). Different letters indicate significance of difference. n.s. indicates no significant differences between the treatments.
3.2. Protective Efficiency of Seed Treatments
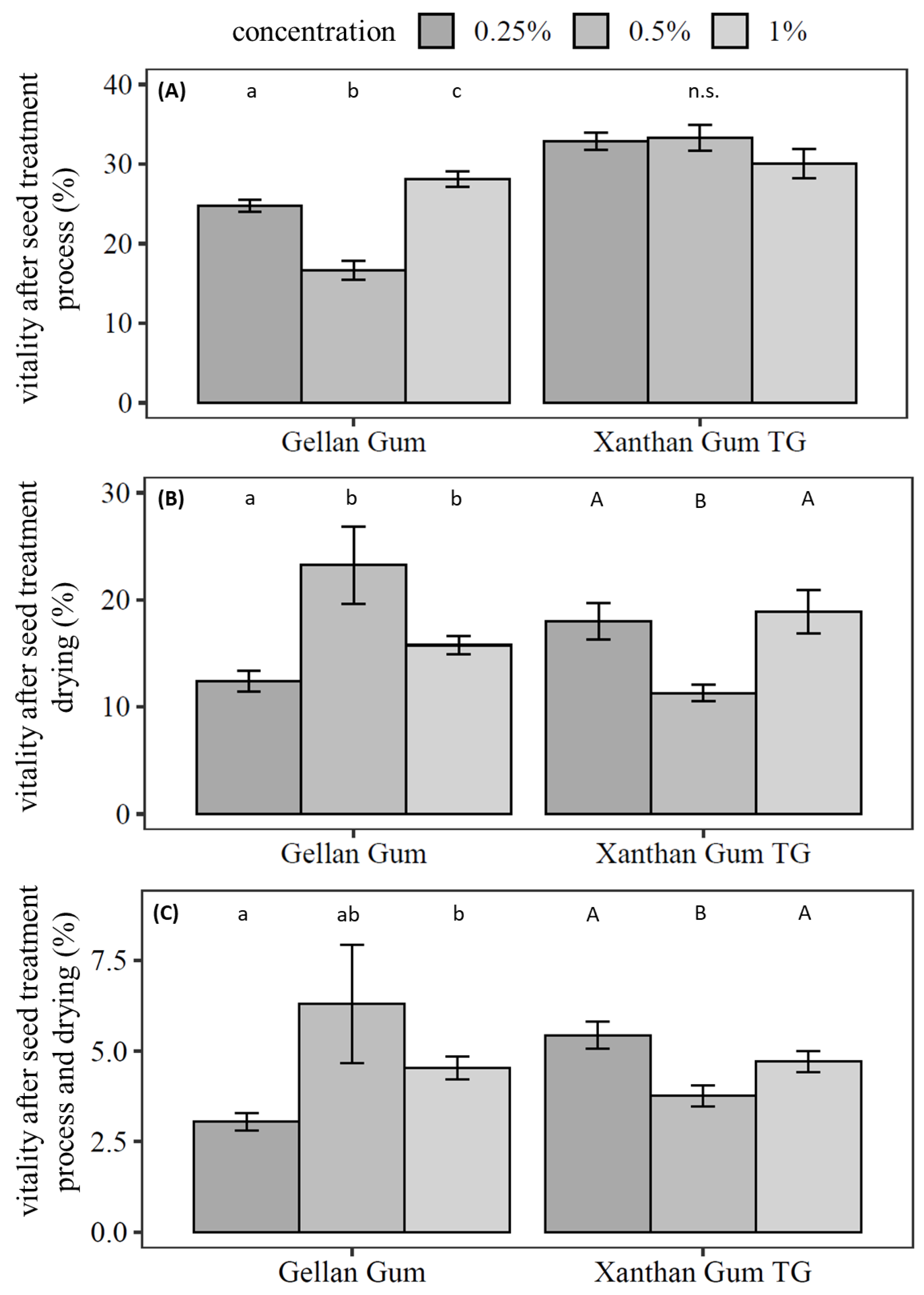
The seed treatment in the self-constructed drum dryer was carried out with xanthan gum TG and gellan gum at the previously tested concentrations of 1%, 0.5%, and 0.25% (Figure 3). Bentonite and potato starch were added as filler and substrate.
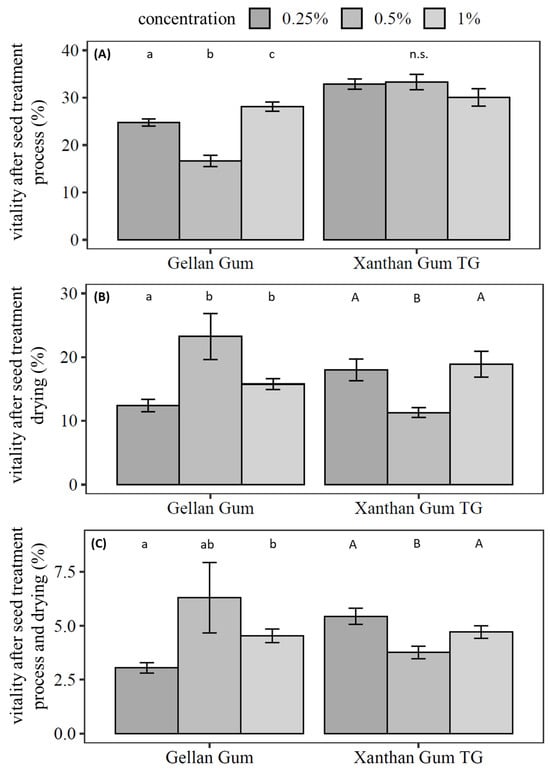
Figure 3.
Survival of Pochonia chlamydosporia blastospores during the seed treatment in the presence of gellan gum and xanthan gum TG. The seed formulation included potato starch and bentonite. Bar plots show mean ± SE for three independent biological replicates of seed treatments with five technical replicates of counting CFU. Different letters indicate significance of difference; n.s. indicates no significant differences between the treatments (Kruskal–Wallis test with Dunn’s test for multiple comparisons as the post-hoc test (p < 0.05)).
The germination of the seeds was not negatively affected by any of the seed treatments. The viability of blastospores decreased in a dose-dependent manner if gellan gum was applied. Survival of blastospores after the treatment process without drying was significantly higher with 1% gellan gum (28.1% ± 1.0%, χ2 = 52.0, df = 2, p < 0.001) compared to 0.5% gellan gum (Dunn’s Test: p < 0.001) and 0.25% gellan gum (Dunn’s test: p = 0.047). The viability of blastospores after the treatment process was significantly lower with 0.5% gellan gum than with 0.25% gellan gum (Dunn’s test: p < 0.001) (Figure 3A).
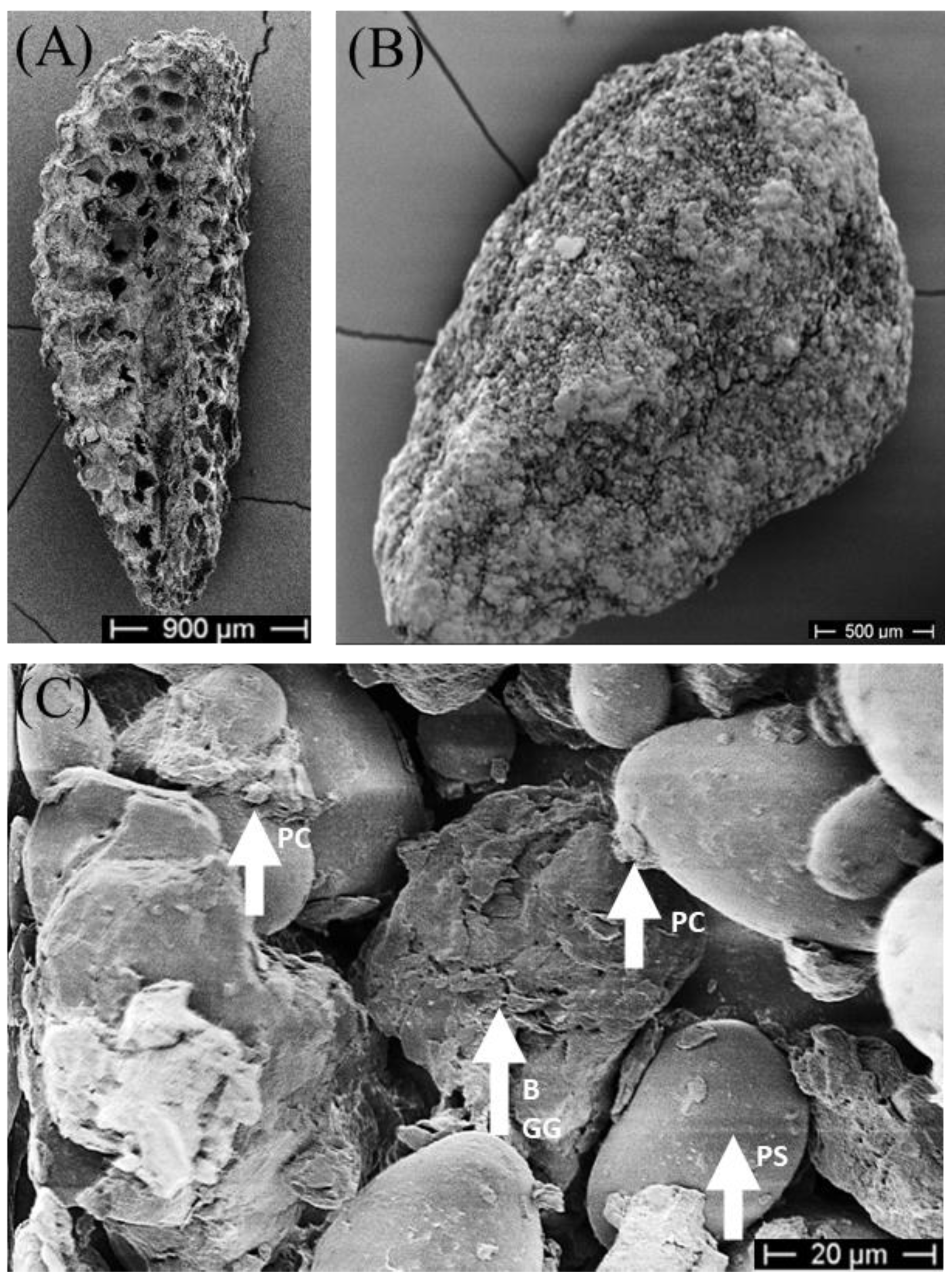
After drying, vital blastospores were significantly reduced in seed treatments with 0.25% gellan gum (χ2 = 11.6, df = 2, p = 0.003) compared to 1% gellan gum (Dunn’s test: p = 0.02) and compared to 0.5% gellan gum (Dunn’s test: p = 0.003) (Figure 3B). No statistical difference between treatments with 0.5% gellan gum and 1% gellan gum were calculated. In the overall observation of process and drying, 0.5% gellan gum had the highest number of viable blastospores (6.3% ± 1.6%). The gellan gum concentration had no effect below 0.5% on the survival of blastospores (Dunn’s test: p = 0.26, Figure 3C). To visualise the matrix and dried blastospores in seed treatment, dried treated seeds were analysed with SEM (Figure 4).
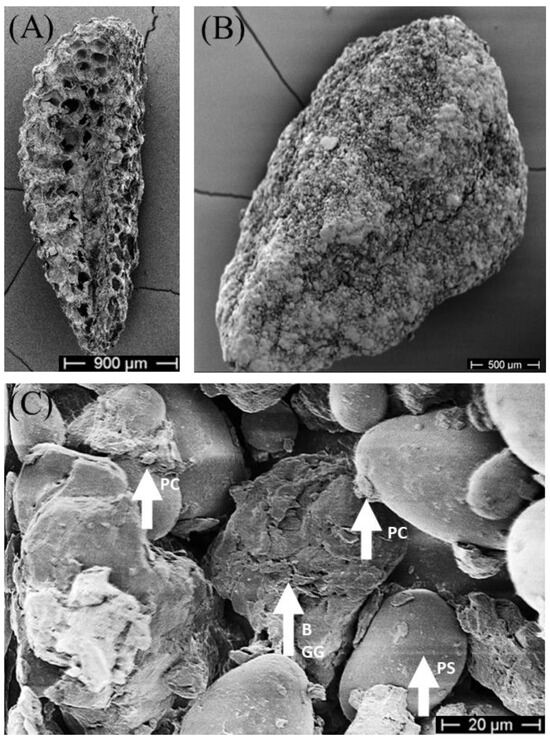
Figure 4.
SEM picture of Phacelia tanacetifolia. (A) seeds without coating (5 kV, 23× magnification), (B) treated seeds with bentonite, potato starch, and 0.5% gellan gum (5 kV, 40× magnification). (C) treated seeds with bentonite, potato starch, and 0.5% gellan gum (5 kV, 910× magnification). PC: presumably dried Pochonia chlamydosporia blastospores, B: bentonite, GG: 0.5% gellan gum, PS: potato starch.
The viability of blastospores after the treatment process remained unaffected by the xanthan gum TG concentration (χ2 = 3.26, df = 2, p = 0.19, Figure 3A); however, it was significantly reduced after drying in treatments with 0.5% xanthan gum TG compared to 1% xanthan gum TG (Dunn’s test: p = 0.021) and 0.25% (Dunn’s test: p = 0.019). In the overall process, a pattern could be observed. The number of vital blastospores when applying 0.5% xanthan gum TG (3.8% ± 0.3%, χ2 = 10.3, df = 2, p = 0.006) was significantly lower than with 0.25% xanthan gum TG (Dunn’s test: p = 0.005) and 1% xanthan gum TG (Dunn’s test: p = 0.04) in the overall observation of process and drying (Figure 3C).
In summary, 0.5% gellan gum resulted in the highest number of viable blastospores after seed treatment and drying, while xanthan gum TG performed best as an adhesive even at low concentrations.
3.3. Storage Tests
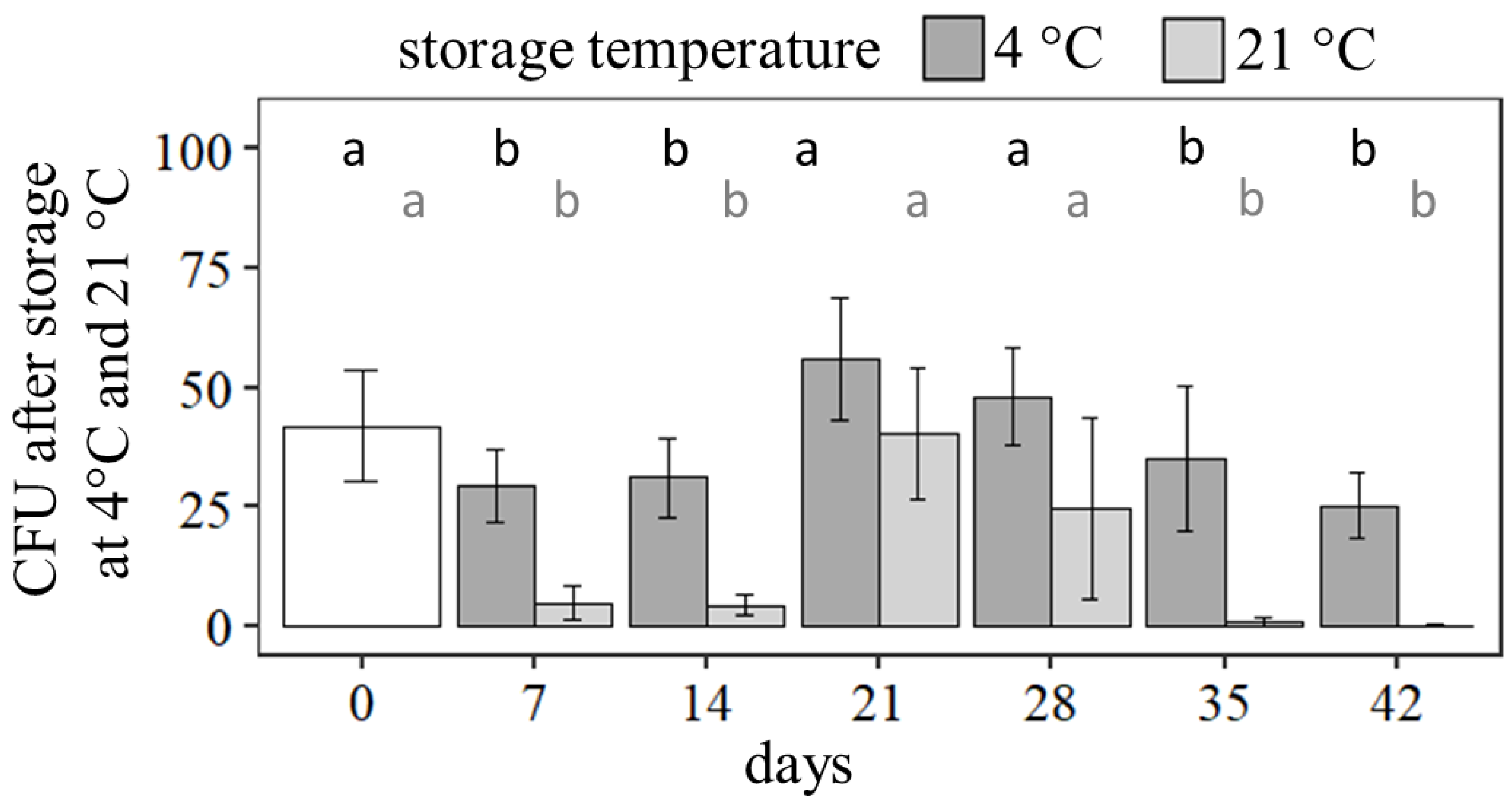
To develop commercial seed treatments, the viability of the active ingredient as well as the seeds during storage must be maintained for at least four weeks at room temperature. Dried seeds treated with bentonite, potato starch, and gellan gum were stored at 4 ± 1 °C and 21 ± 1 °C for up to 42 days.
Expectedly, storage at 4 °C led to higher numbers of CFU per seed compared to storage at 21 °C, and over storage time a reduction in viability was detected (Figure 5). At 4 °C the number of CFU was significantly reduced after storage for 7 days (62.6% ± 15.9% compared to day 0, X2 = 86.3, df = 6, p < 0.001) but after 21 and 28 days, an increasing tendency of CFU per seed was determined (day 21: 119.7% ± 27.3% compared to day 0 (p > 0.05); day 28: 102.3% ± 21.8% compared to day 0, p > 0.05). After 35 days (p > 0.05) and 42 days (p > 0.05) the number of CFU per seed did not decrease significantly compared to 7 days of storage at 4 °C.
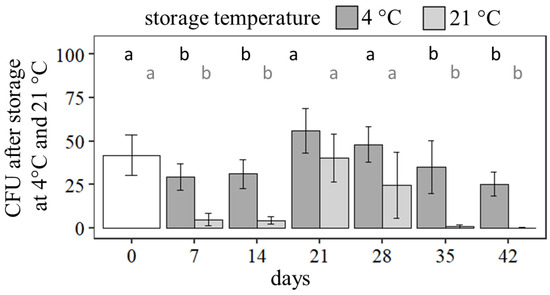
Figure 5.
CFU of germinating Pochonia chlamydosporia blastospores after storage tests for seeds treated with bentonite, potato starch, and 0.5% gellan gum at 4 °C and 21 °C. Bar plots show mean ± SD for five technical replicates. Different letters indicate significance of difference (Kruskal–Wallis test with Dunn’s test for multiple comparisons as post-hoc test (p < 0.05)).
The same tendency was investigated in the storage tests at 21 °C. After 7 days, the number of CFU was significantly reduced (X2 = 86.3, df = 6, p < 0.001) to 10.4% ± 7.8% compared to day 0 per seed. After 21 and 28 days, the CFU per seed reached higher values than after 7 days (day 21: 85.9% ± 29.1% compared to day 0, p > 0.05; day 28: 52.1% ± 40.5% compared to day 0, p > 0.05). For storage at 21 °C, the number of CFU per seed decreased not significantly after 35 (p > 0.05) and 42 days (p > 0.05) compared to storage for 7 days.
4. Discussion
Seed treatments are a common application method for pesticides and fertilisers [26]. This method is also suitable for applying biocontrol agents. However, process development is more complex compared to formulations without living entities. Seed formulations with biocontrol agents like P. chlamydosporia as the active ingredient are more attractive since they save costs and time [3]. Separate applications of other products rely on separate handling by the farmers, while pre-treated seeds are sown in the same way as untreated seeds. Thus, no additional machinery and further application steps are required. The demand for biological seed treatments is increasing [3] and further research to minimize the viability loss of incorporated biocontrol agents is highly needed.
Viability mostly decreases during distribution and storage prior to sowing. As shown in the present study, storage at 4 °C expectedly decreased the viability loss compared to storage at room temperature. These results are in line with the study of Duan et al. [27] for mycelium of Paecilomyces lilacinus and P. chlamydosporia. In contrast, storage tests for seed treatments with aerial conidia of Trichoderma koningiopsis showed up to 80% survival after 2 months [28]. Aerial conidia are thick-walled and serve as a natural survival form of fungi, which is why they are more resistant to drying than blastospores [13]. In practice, however, it is inefficient, costly, and uneconomical for farmers to store large amounts of seeds under cooled conditions. Therefore, it is important to investigate materials and drying processes that ensure the viability of biocontrol agents at room temperature during storage [3,29].
Furthermore, it is important that ingredients of seed treatments support and in the best case enhance sporulation of biocontrol agents. Therefore, starches, gums, oils, or cellulose derivatives [30,31] can be integrated in the seed treatment. The present study demonstrates the beneficial effect of gellan gum on the desiccation tolerance of P. chlamydosporia. This may be caused by the natural role of gellan gum in Sphingomonas spp. Gellan gum covers the cell surface as adhesive matrix to adhere to the targets [32]. Additionally, gellan gum layers around cells of Sphingomonas spp. may act as barrier protecting the cells from negative antimicrobial metabolites [33]. It may be hypothesized that this effect also occurs in P. chlamydosporia blastospores. The barrier of gellan gum or other biopolymers formed around cells may ameliorate negative abiotic stress impact, e.g., by slowing down the dehydration process or protecting sensitive structures like proteins on the cell surface from denaturation. The addition of gellan gum to starch also enhances overall starch performance in food by improving gas and moisture retention [28]. This effect likely supports sporulation of P. chlamydosporia blastospores after drying because of higher availability of oxygen and water in the seed treatment. Furthermore, compatible solutes could improve the viability of blastospores after drying. For example, trehalose is known to help prevent excessive protein aggregation and loss of membrane integrity in vitro but the exact functions and targets of trehalose during desiccation in vivo remain mostly unclear [34].
Importantly, the germination process of seeds is not negatively influenced by the seed treatment because gellan gum forms a firm but brittle gel. Water absorption, germination, and seedling growth may even be improved under these conditions. It is important that seeds are not overhydrated because water saturation limits oxygen diffusion for aerobic respiration, affects starch and energy metabolism, and inhibits seedling growth [35]. Additionally, gellan gum and potato starch can support growth of fungus and plant by serving as a carbon substrate [36,37].
In the present study, seed treatments were conducted with P. chlamydosporia blastospores as active ingredients. In general, published studies investigating complex seed treatments are limited because most of the developed seed formulation protocols are owned by biocontrol companies [38]. Additionally, only few studies are available where fungal blastospores were applied in seed treatments instead of aerial conidia or chlamydospores. Kuzhuppillymyal-Prabhakarankutty et al. [39] coated maize seeds with Beauveria bassiana blastospores in methylcellulose to control Spodoptera frugiperda. Unfortunately, no coating efficiency is mentioned in their study, but they were able to control the population of S. frugiperda in field experiments, which indicates a positive effect by B. bassiana after release from the seed coating. Various studies with aerial conidia as the active ingredient in seed treatments are available [28,40,41,42,43,44]. For a mixture of mycorrhizal fungi Rocha et al. [41] investigated a seed treatment for chickpea seeds to explore seed treatments as an effective inoculation method for field application. Seeds were dusted with Rhizophagus irregularis inoculum followed by biochar and gum arabic as a binding agent. Inoculation by seed treatment with several mycorrhizal fungi led to a significant increase in grain yield under field conditions. Their results emphasise the efficiency of a seed treatment for the application of microorganisms under field conditions. Cortés-Rojas et al. [28] coated rice seeds with T. koningiopsis with pregelatinized maize starch, Gelita® EC, or citric pectin in a rotating drum. In the first step the polymer and in a second step the aerial conidia suspended in the biopolymer were sprayed onto the seeds. Aluminium silicate derivative were added as a filler and polyethylene glycol was added. In their study the survival of conidia reached approximately 40–90% depending on biopolymer and initial conidia concentration. The high survival can be explained by the application of aerial conidia that are naturally more resistant to drying than blastospores [13].
In the present study, the coating efficiency with 0.5% gellan gum was 6.3% ± 1.6% and with 0.25% xanthan gum 5.4% ± 0.4% after processing and drying the P. chlamydosporia blastospores in seed treatment. The final concentration of viable blastospores on seeds in this study was approximately one order lower than the initial blastospore concentration. Rivas-Franco et al. [42] applied methylcellulose as a first layer and xanthan gum, canola oil and conidia suspension with various Metarhizium isolates as a second layer to maize seeds by mixing with a spatula. Then, bentonite and talc were added by hand for the final steps. The final concentration of CFU on the seeds was two exponential orders lower than the original conidial suspension in the biopolymer. This may indicate that not only the biopolymer plays an important role in the seed treatment, but also the layer in which the fungal spores are incorporated, as well as the (automatic) workflow of the process.
A previous study with the seed treatment described in this study has already shown its efficiency. Uthoff et al. [20] demonstrated the efficacy of P. chlamydosporia in the seed treatment against the root-knot nematode Meloidogyne hapla. P. chlamydosporia in seed treatment reduced the total number of M. hapla eggs by up to 95.6% in greenhouse experiments. Additionally, the reduced number of M. hapla eggs resulted in a lower gall index in a subsequently grown tomato plant.
In general, drying blastospores is still a challenge [13], especially for large-scale processes and thus for commercially suitable products. In some studies, blastospores were dried in solid materials such as diatomaceous earth [45,46]. The viability of B. bassiana blastospores after air-drying and spray-drying was greater than 80% [45]. Our investigation extends these treatments, since a complete technical process was designed that can easily be implemented on a large-scale production. The loss of viable blastospores due to the process still represents a challenge and further research will be needed on this topic. Additionally, drying can be optimised by adjusting the temperatures, the rotating speed of the drum, or the amount of water in the process. Another option is to conduct the coating process in the drum and optimise other drying methods such as fluidised bed drying. Furthermore, only P. tanacetifolia seeds, which have a rough surface, were investigated in this study and further research is needed to generalize the developed seed treatment to other seed species and varieties with a different surface texture.
5. Conclusions
The combination of biopolymers such as gellan gum and xanthan gum with potato starch and bentonite in seed treatments is a suitable method of applying P. chlamydosporia blastospores to P. tanacetifolia seeds. The shelf life under cold storage conditions was above 50% until 42 days of storage. To increase the number of viable blastospores per seed, the process needs further optimisation. On the one hand, the ingredients of the seed treatment could be optimised. For example, compatible solutes could protect the fungal blastospores from damage by desiccation. On the other hand, the technical process could be optimised by adjusting the drying temperature and drum speed or by selecting other drying methods such as fluidised bed drying. However, the present study already offers a promising strategy to applying P. chlamydosporia blastospores as the biocontrol agent to seeds.
Author Contributions
Conceptualization, J.U. and D.J.-S.; methodology, J.U.; validation, J.U.; formal analysis, J.U.; investigation, J.U.; resources, D.J.-S., K.-J.D. and A.P.; data curation, J.U.; writing—original draft preparation, J.U.; writing—review and editing, J.U., D.J.-S., K.-J.D. and A.P.; visualization, J.U.; supervision, K.-J.D. and A.P.; project administration, J.U., D.J.-S. and A.P.; funding acquisition, A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Federal Ministry of Education and Research (BMBF, Germany), grant number 13FH118PA8. The APC was funded by MDPI.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The data is not publicly available due to a cooperation agreement with the project partners.
Acknowledgments
The authors thank Rasha Haj Nuaima (Julius Kühn-Institut, Braunschweig) for providing Pochonia chlamydosporia P001 and Katharina Hermann as well as Kristin Schulte for their technical support in carrying out the trials.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Pedrini, S.; Merritt, D.J.; Stevens, J.; Dixon, K. Seed Coating: Science or Marketing Spin? Trends Plant Sci. 2017, 22, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y. Seed Coating with Beneficial Microorganisms for Precision Agriculture. Biotechnol. Adv. 2019, 37, 107423. [Google Scholar] [CrossRef]
- Rocha, I.; Ma, Y.; Souza-Alonso, P.; Vosátka, M.; Freitas, H.; Oliveira, R.S. Seed Coating: A Tool for Delivering Beneficial Microbes to Agricultural Crops. Front. Plant Sci. 2019, 10, 1357. [Google Scholar] [CrossRef]
- Petanidou, T. Introducing Plants for Bee-Keeping at Any Cost?—Assessment of Phacelia tanacetifolia as Nectar Source Plant under Xeric Mediterranean Conditions. Plant Syst. Evol. 2003, 238, 155–168. [Google Scholar] [CrossRef]
- Brant, V.; Neckář, K.; Pivec, J.; Duchoslav, M.; Holec, J.; Fuksa, P.; Venclová, V. Competition of Some Summer Catch Crops and Volunteer Cereals in the Areas with Limited Precipitation. Plant Soil. Environ. 2009, 55, 17–24. [Google Scholar] [CrossRef]
- Kubíková, Z.; Hutyrová, H.; Smejkalová, H.; Kintl, A.; Elbl, J. Application of Extended BBCH Scale for Studying the Development of Phacelia tanacetifolia Benth. Ann. Appl. Biol. 2022, 181, 332–346. [Google Scholar] [CrossRef]
- Babbar, S.B.; Jain, R. Xanthan Gum: An Economical Partial Substitute for Agar in Microbial Culture Media. Curr. Microbiol. 2006, 52, 287–292. [Google Scholar] [CrossRef]
- Lachke, A. Xanthan—A Versatile Gum. Reson 2004, 9, 25–33. [Google Scholar] [CrossRef]
- Tako, M.; Kitajima, S.; Yogi, T.; Uechi, K.; Onaga, M.; Tamaki, Y.; Uechi, S. Structure-Function Relationship of a Gellan Family of Polysaccharide, S-198 Gum, Produced by Alcaligenes ATCC31853. ABC 2016, 06, 55–69. [Google Scholar] [CrossRef]
- Grim, R.E. Applied Clay Mineralogy. Geol. Fören. I Stockh. Förh. 1962, 84, 533. [Google Scholar] [CrossRef]
- Mouzon, J.; Bhuiyan, I.U.; Hedlund, J. The Structure of Montmorillonite Gels Revealed by Sequential Cryo-XHR-SEM Imaging. J. Colloid Interface Sci. 2016, 465, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Velloso, C.C.V.; Lopes, M.M.; Badino, A.C.; Farinas, C.S. Exploring the Roles of Starch for Microbial Encapsulation through a Systematic Mapping Review. Carbohydr. Polym. 2023, 306, 120574. [Google Scholar] [CrossRef]
- Dietsch, R.; Jakobs-Schönwandt, D.; Grünberger, A.; Patel, A. Desiccation-Tolerant Fungal Blastospores: From Production to Application. Curr. Res. Biotechnol. 2021, 3, 323–339. [Google Scholar] [CrossRef]
- Kerry, B.R.; Kirkwood, I.A.; de Leij, F.A.A.M.; Barba, J.; Leijdens, M.B.; Brookes, P.C. Growth and Survival of Verticillium chlamydosporium Goddard, a Parasite of Nematodes, in Soil. Biocontrol Sci. Technol. 1993, 3, 355–365. [Google Scholar] [CrossRef]
- Tobin, J.D.; Haydock, P.P.J.; Hare, M.C.; Woods, S.R.; Crump, D.H. Effect of the Fungus Pochonia chlamydosporia and Fosthiazate on the Multiplication Rate of Potato Cyst Nematodes (Globodera pallida and G. rostochiensis) in Potato Crops Grown under UK Field Conditions. Biol. Control 2008, 46, 194–201. [Google Scholar] [CrossRef]
- Manzanilla-López, R.H.; Lopez-Llorca, L.V. (Eds.) Perspectives in Sustainable Nematode Management through Pochonia chlamydosporia Applications for Root and Rhizosphere Health; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-59222-0. [Google Scholar]
- Mascarin, G.M.; Jackson, M.A.; Kobori, N.N.; Behle, R.W.; Dunlap, C.A.; Delalibera Júnior, Í. Glucose Concentration Alters Dissolved Oxygen Levels in Liquid Cultures of Beauveria bassiana and Affects Formation and Bioefficacy of Blastospores. Appl. Microbiol. Biotechnol. 2015, 99, 6653–6665. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-B.; Yang, Z.-H.; Yu, J.-J.; Zhang, Y.-A.; Xue, J.-J.; Li, Z.; Li, J.-J.; Wang, C.-Y.; Wang, Z.; Hou, J.-G.; et al. Comparison between Conidia and Blastospores of Esteya vermicola, an Endoparasitic Fungus of the Pinewood Nematode, Bursaphelenchus xylophilus. World J. Microbiol. Biotechnol. 2013, 29, 2429–2436. [Google Scholar] [CrossRef] [PubMed]
- Nuaima, R.H.; Ashrafi, S.; Maier, W.; Heuer, H. Fungi Isolated from Cysts of the Beet Cyst Nematode Parasitized Its Eggs and Counterbalanced Root Damages. J. Pest Sci. 2021, 94, 563–572. [Google Scholar] [CrossRef]
- Uthoff, J.; Jakobs-Schönwandt, D.; Schmidt, J.H.; Hallmann, J.; Dietz, K.-J.; Patel, A. Biological Enhancement of the Cover Crop Phacelia tanacetifolia (Boraginaceae) with the Nematophagous Fungus Pochonia chlamydosporia to Control the Root-Knot Nematode Meloidogyne hapla in a Succeeding Tomato Plant. In BioControl; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Li, D.; Wang, B.; Zhang, K.; Niu, X. Yellow Pigment Aurovertins Mediate Interactions between the Pathogenic Fungus Pochonia chlamydosporia and Its Nematode Host. J. Agric. Food Chem. 2015, 63, 6577–6587. [Google Scholar] [CrossRef]
- Strasser, H.; Forer, A.; Schinner, F. Development of Media for the Selective Isolation and Maintenance of Virulence of Beauveria brongniartii. In Proceedings of the 3rd International Workshop on Microbial Control of Soil Dwelling Pests, Lincoln, New Zealand, 21–23 February 1996; pp. 125–130. [Google Scholar]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.r-project.org/ (accessed on 23 November 2023).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009; ISBN 978-3-319-24277-4. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Accinelli, C.; Abbas, H.K.; Shier, W.T. A Bioplastic-Based Seed Coating Improves Seedling Growth and Reduces Production of Coated Seed Dust. J. Crop Improv. 2018, 32, 318–330. [Google Scholar] [CrossRef]
- Duan, W.; Yang, E.; Xiang, M.; Liu, X. Effect of Storage Conditions on the Survival of Two Potential Biocontrol Agents of Nematodes, the Fungi Paecilomyces lilacinus and Pochonia chlamydosporia. Biocontrol Sci. Technol. 2008, 18, 605–612. [Google Scholar] [CrossRef]
- Cortés-Rojas, D.; Beltrán-Acosta, C.; Zapata-Narvaez, Y.; Chaparro, M.; Gómez, M.; Cruz-Barrera, M. Seed Coating as a Delivery System for the Endophyte Trichoderma koningiopsis Th003 in Rice (Oryza Sativa). Appl. Microbiol. Biotechnol. 2021, 105, 1889–1904. [Google Scholar] [CrossRef] [PubMed]
- Deaker, R. Legume Seed Inoculation Technology—A Review. Soil Biol. Biochem. 2004, 36, 1275–1288. [Google Scholar] [CrossRef]
- Hoben, H.J.; Aung, N.N.; Somasegaran, P.; Kang, U.-G. Oils as Adhesives for Seed Inoculation and Their Influence on the Survival of Rhizobium spp. and Bradyrhizobium spp. on Inoculated Seeds. World J. Microbiol. Biotechnol. 1991, 7, 324–330. [Google Scholar] [CrossRef]
- Burges, H.D. Formulation of Microbial Biopesticides—Beneficial Micro-Organisms, Nematodes and Seed Treatments. Phytochemistry 1998, 59, 361. [Google Scholar] [CrossRef]
- Giavasis, I.; Harvey, L.M.; McNeil, B. Gellan Gum. Crit. Rev. Biotechnol. 2000, 20, 177–211. [Google Scholar] [CrossRef]
- Evans, R.P.; Nelson, C.L.; Bowen, W.R.; Kleve, M.G.; Hickmon, S.G. Visualization of Bacterial Glycocalyx With a Scanning Electron Microscope. Clin. Orthop. Relat. Res. 1998, 347, 243–249. [Google Scholar] [CrossRef]
- Koshland, D.; Tapia, H. Desiccation Tolerance: An Unusual Window into Stress Biology. MBoC 2019, 30, 737–741. [Google Scholar] [CrossRef]
- Gorim, L.; Asch, F. Seed Coating Increases Seed Moisture Uptake and Restricts Embryonic Oxygen Availability in Germinating Cereal Seeds. Biology 2017, 6, 31. [Google Scholar] [CrossRef]
- Liu, X.Z.; Chen, S.Y. Nutritional Requirements of Pochonia chlamydosporia and ARF18, Fungal Parasites of Nematode Eggs. J. Invertebr. Pathol. 2003, 83, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Zeeman, S.C.; Kossmann, J.; Smith, A.M. Starch: Its Metabolism, Evolution, and Biotechnological Modification in Plants. Annu. Rev. Plant Biol. 2010, 61, 209–234. [Google Scholar] [CrossRef] [PubMed]
- Krell, V.; Jakobs-Schönwandt, D.; Patel, A.V. Application of Formulated Endophytic Entomopathogenic Fungi for Novel Plant Protection Strategies. In Endophytes for a Growing World; Cambridge University Press: Cambridge, UK, 2019; pp. 52–66. ISBN 978-1-108-47176-3. [Google Scholar]
- Kuzhuppillymyal-Prabhakarankutty, L.; Ferrara-Rivero, F.H.; Tamez-Guerra, P.; Gomez-Flores, R.; Rodríguez-Padilla, M.C.; Ek-Ramos, M.J. Effect of Beauveria bassiana-Seed Treatment on Zea mays L. Response against Spodoptera frugiperda. Appl. Sci. 2021, 11, 2887. [Google Scholar] [CrossRef]
- Poorna Chandrika, K.S.V.P.; Prasad, R.D.; Godbole, V. Development of Chitosan-PEG Blended Films Using Trichoderma: Enhancement of Antimicrobial Activity and Seed Quality. Int. J. Biol. Macromol. 2019, 126, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Rocha, I.; Duarte, I.; Ma, Y.; Souza-Alonso, P.; Látr, A.; Vosátka, M.; Freitas, H.; Oliveira, R.S. Seed Coating with Arbuscular Mycorrhizal Fungi for Improved Field Production of Chickpea. Agronomy 2019, 9, 471. [Google Scholar] [CrossRef]
- Rivas-Franco, F.; Hampton, J.G.; Morán-Diez, M.E.; Narciso, J.; Rostás, M.; Wessman, P.; Jackson, T.A.; Glare, T.R. Effect of Coating Maize Seed with Entomopathogenic Fungi on Plant Growth and Resistance against Fusarium graminearum and Costelytra giveni. Biocontrol Sci. Technol. 2019, 29, 877–900. [Google Scholar] [CrossRef]
- Turkan, S.; Mierek-Adamska, A.; Kulasek, M.; Konieczna, W.B.; Dąbrowska, G.B. New Seed Coating Containing Trichoderma viride with Anti-Pathogenic Properties. PeerJ 2023, 11, e15392. [Google Scholar] [CrossRef]
- Kuchlan, P.F.; Kuchlan, M.M.M.A.K.; Ansari, M.M. Efficient Application of Trichoderma viride on Soybean [Glycine max (L.) Merrill] Seed Using Thin Layer Polymer Coating. LR 2018, 42, 250–259. [Google Scholar] [CrossRef]
- Mascarin, G.M.; Jackson, M.A.; Behle, R.W.; Kobori, N.N.; Júnior, Í.D. Improved Shelf Life of Dried Beauveria bassiana Blastospores Using Convective Drying and Active Packaging Processes. Appl. Microbiol. Biotechnol. 2016, 100, 8359–8370. [Google Scholar] [CrossRef]
- Silva, D.M.; de Souza, V.H.M.; Moral, R.d.A.; Delalibera Júnior, I.; Mascarin, G.M. Production of Purpureocillium lilacinum and Pochonia chlamydosporia by Submerged Liquid Fermentation and Bioactivity against Tetranychus urticae and Heterodera glycines through Seed Inoculation. J. Fungi. 2022, 8, 511. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).