Suppressive Activity of Glechoma hederacea Extracts against the Phytopathogenic Oomycete Plasmopara viticola, and First Screening of the Active Metabolites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Procedures
2.2. Plant Material and Microorganisms
2.3. Production of G. hederacea Aqueous Extracts and Activity Evaluation against P. viticola
2.4. Obtaining G. hederacea Extracts, and Isolation and Identification of Secondary Metabolites
2.5. Evaluation of G. hederacea Extracts and Secondary Metabolites against P. viticola
2.6. Statistical Analysis
3. Results and Discussion
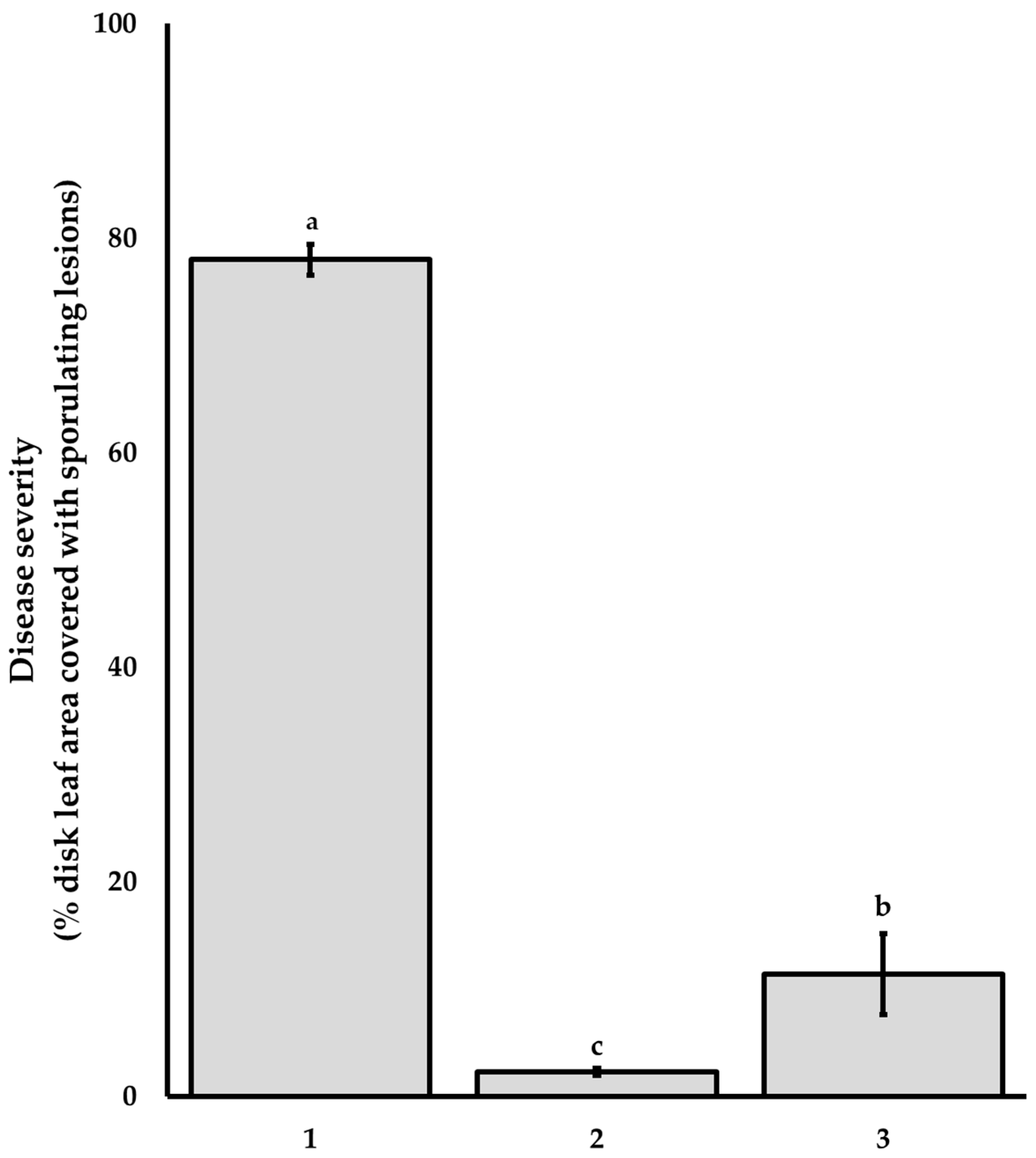
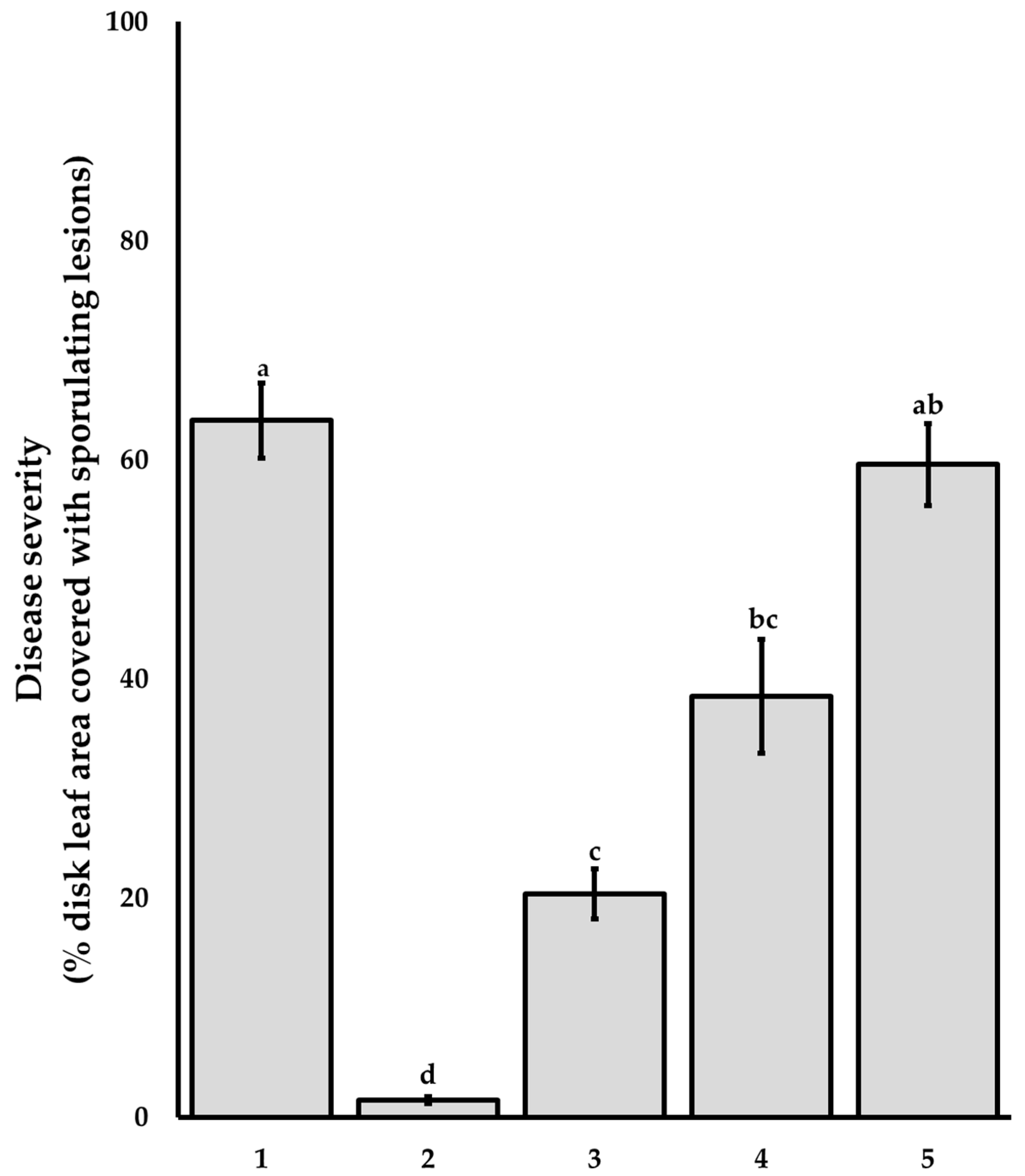
3.1. Activity of G. hederacea Aqueous Extracts against P. viticola on Grapevine Leaf Discs
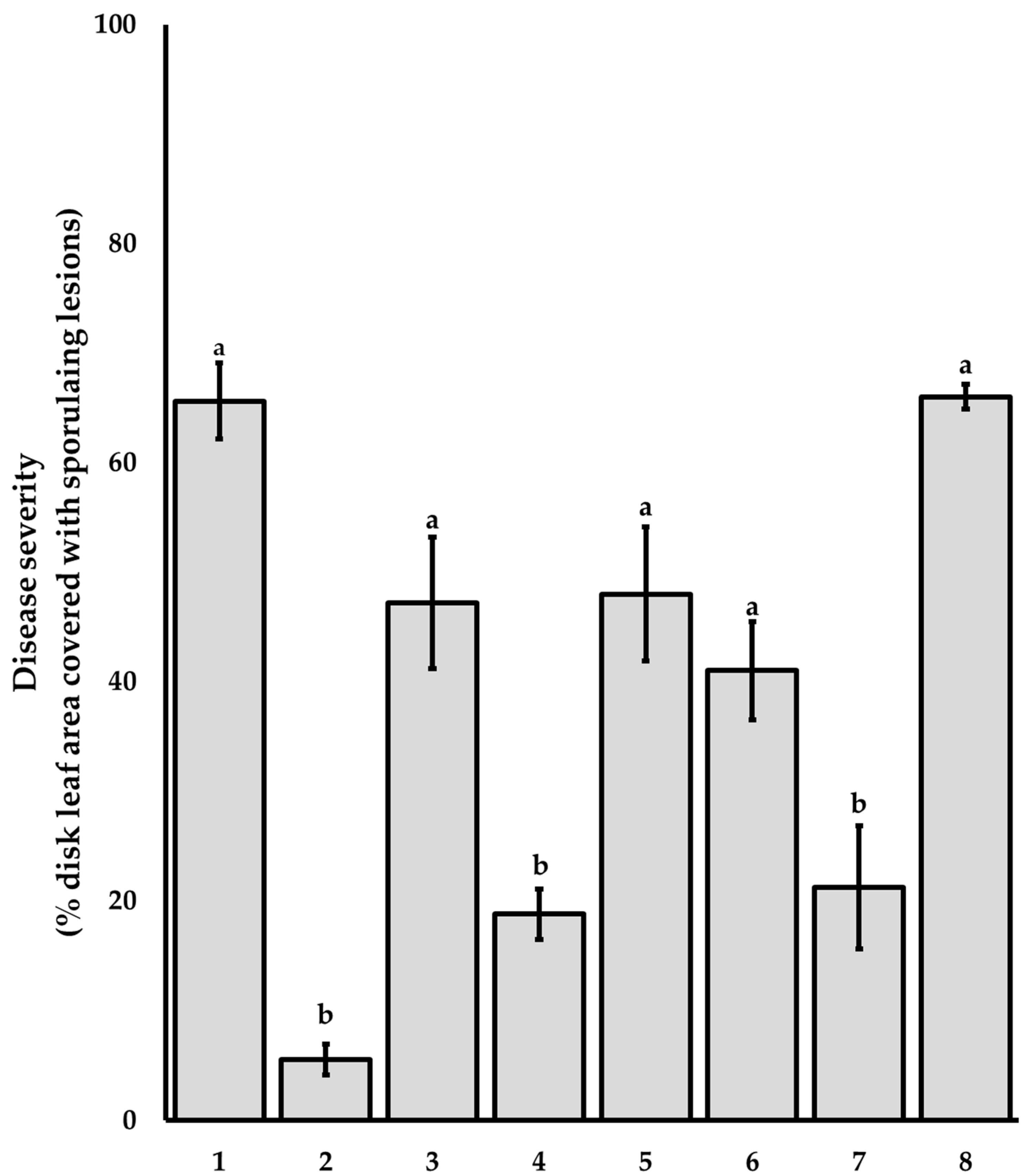
3.2. Bioguided Isolation and Supressive Activity of Pure Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jermini, M.; Blaise, P.; Gessler, C. Quantitative effect of leaf damage caused by downy mildew (Plasmopara viticola) on growth and yield quality of grapevine ‘Merlot’ (Vitis vinifera). Vitis 2010, 49, 77–85. [Google Scholar]
- Gava, A.; Emer, C.D.; Ficagna, E.; de Andrade, S.F.; Fuentefria, A.M. Occurrence and impact of fungicides residues on fermentation during wine production—A review. Food Addit. Contam. 2021, 38, 943–961. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, M.C.; Labbé, F.; Dussert, Y.; Delière, L.; Richart-Cervera, S.; Giraud, T.; Delmotte, F. Europe as a bridgehead in the worldwide invasion history of grapevine downy mildew, Plasmopara viticola. Curr. Biol. 2021, 31, 2155–2166.e4. [Google Scholar] [CrossRef] [PubMed]
- Burruano, S.; Conigliaro, G.; Piccolo, S.L.; Alfonzo, A.; Torta, L. Plasmopara viticola: Three decades of observation in Sicily. In Proceedings of the 5th International Workshop on Grapevine Downy and Powdery Mildew, Trentino, Italy, 18–23 June 2016, 1st ed.; Pertot, I., Gessler, C., Gubler, W., Kassemeyer, H.-H., Magarey, P., Eds.; (San Michele all’Adige: SafeCrop Centre). S. Michele all’Adige: Trentino, Italy, 2006. [Google Scholar]
- Massi, F.; Torriani, S.F.F.; Borghi, L.; Toffolatti, S.L. Fungicide Resistance Evolution and Detection in Plant Pathogens: Plasmopara viticola as a Case Study. Microorganisms 2021, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Jermini, M.; Blaise, P.; Gessler, C. Response of ‘Merlot’ (Vitis vinifera) grapevine to defoliation caused by downy mildew (Plasmopara viticola) during the following growing season. Vitis 2010, 49, 161–166. [Google Scholar]
- Gessler, C.; Pertot, I.; Perazzolli, M. Plasmopara viticola: A review of knowledge on downy mildew of grapevine and effective disease management. Phytopathol. Mediterr. 2011, 50, 3–44. [Google Scholar]
- Fröbel, S.; Zyprian, E. Colonization of Different Grapevine Tissues by Plasmopara viticola—A Histological Study. Front. Plant Sci. 2019, 10, 951. [Google Scholar] [CrossRef]
- Cabús, A.; Pellini, M.; Zanzotti, R.; Devigili, L.; Maines, R.; Giovannini, O.; Mattedi, L.; Mescalchin, E. Efficacy of reduced copper dosages against Plasmopara viticola in organic agriculture. Crop Prot. 2017, 96, 103–108. [Google Scholar] [CrossRef]
- Ballabio, C.; Panagos, P.; Lugato, E.; Huang, J.-H.; Orgiazzi, A.; Jones, A.; Fernández-Ugalde, O.; Borrelli, P.; Montanarella, L. Copper distribution in European topsoils: An assessment based on LUCAS soil survey. Sci. Total Environ. 2018, 636, 282–298. [Google Scholar] [CrossRef]
- Thuerig, B.; James, E.E.; Schärer, H.J.; Langat, M.; Mulholland, D.; Treutwein, J.; Kleeberg, I.; Ludwig, M.; Jayarajah, P.; Giovannini, O.; et al. Reducing copper use in the environment: The use of larixol and larixyl acetate to treat downy mildew caused by Plasmopara viticola in Viticulture. Pest Manag. Sci. 2018, 74, 477–488. [Google Scholar] [CrossRef]
- Schnee, S.; Queiroz, E.F.; Voinesco, F.; Marcourt, L.; Dubuis, P.H.; Wolfender, J.L.; Gindro, K. Vitis vinifera canes, a new source of antifungal compounds against Plasmopara viticola, Erysiphe necator, and Botrytis cinerea. J. Agric. Food Chem. 2013, 61, 5459–5467. [Google Scholar] [CrossRef]
- Gabaston, J.; Richard, T.; Cluzet, S.; Pinto, A.P.; Dufour, M.-C.; Corio-Costet, M.-F.; Mérillon, J.-M. Pinus pinaster Knot: A Source of Polyphenols against Plasmopara viticola. J. Agric. Food Chem. 2017, 65, 8884–8891. [Google Scholar] [CrossRef] [PubMed]
- Godard, S.; Slacanin, I.; Viret, O.; Gindro, K. Induction of defence mechanisms in grapevine leaves by emodin- and anthra-quinone-rich plant extracts and their conferred resistance to downy mildew. Plant Physiol. Biochem. 2009, 47, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Dagostin, S.; Schärer, H.-J.; Pertot, I.; Tamm, L. Are there alternatives to copper for controlling grapevine downy mildew in organic viticulture? Crop Prot. 2011, 30, 776–788. [Google Scholar] [CrossRef]
- Thuerig, B.; Ramseyer, J.; Hamburger, M.; Oberhänsli, T.; Potterat, O.; Schärer, H.J.; Tamm, L. Efficacy of a Juncus effusus extract on grapevine and apple plants against Plasmopara viticola and Venturia inaequalis, and identification of the major active con-stituent. Pest Manag. Sci. 2016, 72, 1718–1726. [Google Scholar] [CrossRef] [PubMed]
- Gabaston, J.; Richard, T.; Biais, B.; Waffo-Teguo, P.; Pedrot, E.; Jourdes, M.; Corio-Costet, M.-F.; Mérillon, J.-M. Stilbenes from common spruce (Picea abies) bark as natural antifungal agent against downy mildew (Plasmopara viticola). Ind. Crop Prod. 2017, 103, 267–273. [Google Scholar] [CrossRef]
- Andreu, V.; Levert, A.; Amiot, A.; Cousin, A.; Aveline, N.; Bertrand, C. Chemical composition and antifungal activity of plant extracts traditionally used in organic and biodynamic farming. Environ. Sci. Pollut. Res. 2018, 25, 29971–29982. [Google Scholar] [CrossRef]
- La Torre, A.; Righi, L.; Iovino, V.; Battaglia, V. Evaluation of copper alternative products to control grape downy mildew in organic farming. Plant Pathol. J. 2019, 101, 1005–1012. [Google Scholar] [CrossRef]
- Taillis, D.; Pébarthé-Courrouilh, A.; Lepeltier, E.; Petit, E.; Palos-Pinto, A.; Gabaston, J.; Mérillon, J.M.; Richard, T.; Cluzet, S. A grapevine by-product extract enriched in oligomerised stilbenes to control downy mildews: Focus on its modes of action to-wards Plasmopara viticola. Oeno One 2022, 56, 55–68. [Google Scholar] [CrossRef]
- Harm, A.; Kassemeyer, H.-H.; Seibicke, T.; Regner, F. Evaluation of chemical and natural resistance inducers against downy mildew (Plasmopara viticola) in grapevine. Am. J. Enol. Vitic. 2011, 62, 184–192. [Google Scholar] [CrossRef]
- Mulholland, D.A.; Thuerig, B.; Langat, M.K.; Tamm, L.; Nawrot, D.A.; James, E.E.; Qayyum, M.; Shen, D.; Ennis, K.; Jones, A.; et al. Efficacy of extracts from eight economically important forestry species against grapevine downy mildew (Plasmopara viticola) and identification of active constituents. J. Crop Prot. 2017, 102, 104–109. [Google Scholar] [CrossRef]
- Ramseyer, J.; Thuerig, B.; De Mieri, M.; Schärer, H.J.; Oberhänsli, T.; Gupta, M.; Tamm, L.; Hamburger, M.; Potterat, O. Eu-desmane sesquiterpenes from Verbesina lanata with inhibitory activity against grapevine downy mildew. J. Nat. Prod. 2017, 80, 3296–3304. [Google Scholar] [CrossRef] [PubMed]
- Rienth, M.; Crovadore, J.; Ghaffari, S.; Lefort, F. Oregano essential oil vapour prevents Plasmopara viticola infection in grapevine (Vitis Vinifera) and primes plant immunity mechanisms. PLoS ONE 2019, 14, e0222854. [Google Scholar] [CrossRef] [PubMed]
- Heng, M.Y.; Thuerig, B.; Danton, O.; Ramseyer, J.; Gupta, M.P.; Tamm, L.; Hamburger, M.; Potterat, O. Ingadosides A-C, acacic acid-type saponins from Inga sapindoides with potent inhibitory activity against downy mildew. Phytochemistry 2022, 199, 113183. [Google Scholar] [CrossRef] [PubMed]
- Thuerig, B.; Ramseyer, J.; Hamburger, M.; Ludwig, M.; Oberhänsli, T.; Potterat, O.; Schärer, H.-J.; Tamm, L. Efficacy of a Magnolia officinalis bark extract against grapevine downy mildew and apple scab under controlled and field conditions. Crop Prot. 2018, 114, 97–105. [Google Scholar] [CrossRef]
- Dagostin, S.; Formolo, T.; Giovannini, O.; Pertot, I.; Schmitt, A. Salvia officinalis extract can protect grapevine against Plasmopara viticola. Plant Dis. 2010, 94, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.G.; Fraga-Corral, M.; García-Oliveira, P.; Jimenez-Lopez, C.; Lourenço-Lopes, C.; Carpena, M.; Otero, P.; Gullón, P.; Prieto, M.A.; Simal-Gandara, J. Culinary and nutritional value of edible wild plants from northern Spain rich in phenolic compounds with potential health benefits. Food Funct. 2020, 11, 8493–8515. [Google Scholar] [CrossRef]
- Gwiazdowska, D.; Uwineza, P.A.; Frąk, S.; Juś, K.; Marchwińska, K.; Gwiazdowski, R.; Waśkiewicz, A. Antioxidant, anti-microbial and antibiofilm properties of Glechoma hederacea extracts obtained by supercritical fluid extraction, using different extraction conditions. Appl. Sci. 2022, 12, 3572. [Google Scholar] [CrossRef]
- Endo, E.H.; Cortez, D.A.G.; Ueda-Nakamura, T.; Nakamura, C.V.; Filho, B.P.D. Potent antifungal activity of extracts and pure compound isolated from pomegranate peels and synergism with fluconazole against Candida albicans. Res. Microbiol. 2010, 161, 534–540. [Google Scholar] [CrossRef]
- Jmii, G.; Molinillo, J.M.; Zorrilla, J.G.; Haouala, R. Allelopathic activity of Thapsia garganica L. leaves on lettuce and weeds, and identification of the active principles. S. Afr. J. Bot. 2020, 131, 188–194. [Google Scholar] [CrossRef]
- Mejías, F.J.; Durán, A.G.; Zorrilla, J.G.; Varela, R.M.; Molinillo, J.M.G.; Valdivia, M.M.; Macías, F.A. Acyl derivatives of eudes- manolides to boost their bioactivity: An explanation of behavior in the cell membrane using a molecular dynamics approach. ChemMedChem 2021, 16, 1297–1307. [Google Scholar] [CrossRef]
- Puopolo, G.; Giovannini, O.; Pertot, I. Lysobacter capsici AZ78 can be combined with copper to effectively control Plasmopara viticola on grapevine. Microbiol. Res. 2014, 169, 633–642. [Google Scholar] [CrossRef]
- European and Mediterranean Plant Protection Organization. Guidelines for the efficacy evaluation of fungicides: Plasmopara viticola. EPPO Bull. 2001, 31, 313–317. [Google Scholar] [CrossRef]
- Trindade, G.G.; Thrivikraman, G.; Menezes, P.P.; França, C.M.; Lima, B.S.; Carvalho, Y.M.; Souza, E.P.; Duarte, M.C.; Shanmugam, S.; Quintans-Júnior, L.J.; et al. Carvacrol/β-cyclodextrin inclusion complex inhibits cell proliferation and migration of prostate cancer cells. Food Chem. Toxicol. 2019, 125, 198–209. [Google Scholar] [CrossRef]
- Suleimenov, E.M.; Raldugin, V.A.; Adekenov, S.M. Cirsimaritin from Stizolophus balsamita. Chem. Nat. Compd. 2008, 44, 398. [Google Scholar] [CrossRef]
- Osigwe, C.C.; Akah, P.A.; Nworu, C.S.; Okoye, F.B.C. Apigenin: A methanol fraction component of Newbouldia laevis leaf, as a potential antidiabetic agent. J. Phytopharm. 2017, 6, 38–44. [Google Scholar] [CrossRef]
- Xiang, M.; Su, H.; Hu, J.; Yan, Y. Isolation, identification and determination of methyl caffeate, ethyl caffeate and other phenolic compounds from Polygonum amplexicaule var. sinense. J. Med. Plants Res. 2011, 5, 1685–1691. [Google Scholar]
- Świsłocka, R. Spectroscopic (FT-IR, FT-Raman, UV absorption, 1H and 13C NMR) and theoretical (in B3LYP/6-311++ G** level) studies on alkali metal salts of caffeic acid. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 100, 21–30. [Google Scholar] [CrossRef]
- Lu, Y.; Foo, L.Y. Rosmarinic acid derivatives from Salvia officinalis. Phytochemistry 1999, 51, 91–94. [Google Scholar] [CrossRef]
- Le Claire, E.; Schwaiger, S.; Banaigs, B.; Stuppner, H.; Gafner, F. Distribution of a new rosmarinic acid derivative in Eryngium alpinum L. and other Apiaceae. J. Agric. Food Chem. 2005, 53, 4367–4372. [Google Scholar] [CrossRef]
- Radulović, N.; Đorđević, N.; Marković, M.; Palić, R. Volatile constituents of Glechoma hirsute Waldst. & Kit. and G. hederacea L. (Lamiaceae). Bull. Chem. Soc. Ethiop. 2010, 24, 67–76. [Google Scholar]
- Marinelli, L.; Di Stefano, A.; Cacciatore, I. Carvacrol and its derivatives as antibacterial agents. Phytochem. Rev. 2018, 17, 903–921. [Google Scholar] [CrossRef]
- Cala, A.; Salcedo, J.R.; Torres, A.; Varela, R.M.; Molinillo, J.M.G.; Macías, F.A. A Study on the phytotoxic potential of the seasoning herb marjoram (Origanum majorana L.) leaves. Molecules 2021, 26, 3356. [Google Scholar] [CrossRef]
- Nikolova, M.; Asenov, A. Surface flavonoid aglycones in newly studied plant species. Nat. Prod. Res. 2006, 20, 103–106. [Google Scholar] [CrossRef]
- Silva, M.J.D.; Simonet, A.M.; Silva, N.C.; Dias, A.L.T.; Vilegas, W.; Macías, F.A. Bioassay-guided isolation of fungistatic compounds from Mimosa caesalpiniifolia Leaves. J. Nat. Prod. 2019, 82, 1496–1502. [Google Scholar] [CrossRef]
- El-Aasr, M.; Nohara, T.; Ikeda, T.; Abu-Risha, S.E.; Elekhnawy, E.; Tawfik, H.O.; Shoeib, N.; Attia, G. LC-MS/MS metabolomics profiling of Glechoma hederacea L. methanolic extract; in vitro antimicrobial and in vivo with in silico wound healing studies on Staphylococcus aureus infected rat skin wound. Nat. Prod. Res. 2023, 37, 1730–1734. [Google Scholar] [CrossRef]
- Topal, M.; Gülçin, I. Rosmarinic acid: A potent carbonic anhydrase isoenzymes inhibitor. Turk. J. Chem. 2014, 38, 894–902. [Google Scholar] [CrossRef]
- Chao, W.W.; Chan, W.C.; Ma, H.T.; Chou, S.T. Phenolic acids and flavonoids-rich Glechoma hederacea L. (Lamiaceae) water extract against H2O2-induced apoptosis in PC12 cells. J. Food Biochem. 2022, 46, e14032. [Google Scholar] [CrossRef]
- Aziz, N.H.; Farag, S.E.; Mousa, L.A.; Abo-Zaid, M.A. Comparative antibacterial and antifungal effects of some phenolic compounds. Microbios 1998, 93, 43–54. [Google Scholar]
- Alcerito, T.; Barbo, F.E.; Negri, G.; Santos, D.Y.; Meda, C.I.; Young, M.C.M.; Chávez, D.; Blatt, C.T. Foliar epicuticular wax of Arrabidaea brachypoda: Flavonoids and antifungal activity. Biochem. Syst. Ecol. 2002, 30, 677–683. [Google Scholar] [CrossRef]
- Boubaker, H.; Karim, H.; El Hamdaoui, A.; Msanda, F.; Leach, D.; Bombarda, I.; Vanloot, P.; Abbad, A.; Boudyach, E.; Ben Aoumar, A.A. Chemical characterization and antifungal activities of four Thymus species essential oils against postharvest fungal pathogens of citrus. Ind. Crop Prod. 2016, 86, 95–101. [Google Scholar] [CrossRef]
- Lee, H.; Woo, E.-R.; Lee, D.G. Apigenin induces cell shrinkage in Candida albicans by membrane perturbation. FEMS Yeast Res. 2018, 18, foy003. [Google Scholar] [CrossRef] [PubMed]
- Lima, T.C.; Ferreira, A.R.; Silva, D.F.; Lima, E.O.; de Sousa, D.P. Antifungal activity of cinnamic acid and benzoic acid esters against Candida albicans strains. Nat. Prod. Res. 2018, 32, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, S.; Du, S.; Chen, S.; Sun, H. Antifungal activity of thymol and carvacrol against postharvest pathogens Botrytis cinerea. J. Food Sci. Technol. 2019, 56, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, M.; Kostić, M.; Stojković, D.; Soković, M. Rosmarinic acid–modes of antimicrobial and antibiofilm activities of a common plant polyphenol. S. Afr. J. Bot. 2022, 146, 521–527. [Google Scholar] [CrossRef]
- Saghrouchni, H.; El Barnossi, A.; Mssillou, I.; Lavkor, I.; Ay, T.; Kara, M.; Alarfaj, A.A.; Hirad, A.H.; Nafidi, H.-A.; Bourhia, M.; et al. Potential of carvacrol as plant growth-promotor and green fungicide against fusarium wilt disease of perennial ryegrass. Front. Plant Sci. 2023, 14, 973207. [Google Scholar] [CrossRef] [PubMed]
- Mattivi, F.; Vrhovsek, U.; Malacarne, G.; Masuero, D.; Zulini, L.; Stefanini, M.; Moser, C.; Velasco, R.; Guella, G. Profiling of resveratrol oligomers, important stress metabolites, accumulating in the leaves of hybrid Vitis vinifera (Merzling × Teroldego) genotypes infected with Plasmopara viticola. J. Agric. Food Chem. 2011, 59, 5364–5375. [Google Scholar] [CrossRef]
- Figueiredo, A.; Sebastiana, M.; Martins, J.; Monteiro, F.; Coelho, A.; Pais, M. Early events of grapevine resistance towards downy mildew by a systems biology approach. Rev. Fac. Cienc. Agrar. 2015, 38, 124–130. [Google Scholar]
- Maia, M.; Ferreira, A.E.N.; Nascimento, R.; Monteiro, F.; Traquete, F.; Marques, A.P.; Cunha, J.; Eiras-Dias, J.E.; Cordeiro, C.; Figueiredo, A.; et al. Integrating metabolomics and targeted gene expression to uncover potential biomarkers of fungal/oomycetes-associated disease susceptibility in grapevine. Sci. Rep. 2020, 10, 15688. [Google Scholar] [CrossRef]
- Balachandran, C.; Duraipandiyan, V.; Al-Dhabi, N.A.; Balakrishna, K.; Kalia, N.P.; Rajput, V.S.; Khan, I.A.; Ignacimuthu, S. Antimicrobial and antimycobacterial activities of methyl caffeate isolated from Solanum torvum Swartz fruit. Indian J. Microbiol. 2012, 52, 676–681. [Google Scholar] [CrossRef]
- Baser, K.H.C. Biological and pharmacological activities of carvacrol and carvacrol bearing essential oils. Curr. Pharm. Des. 2008, 14, 3106–3119. [Google Scholar] [CrossRef]
- Abd-ElGawad, A.M.; El Gendy, A.E.-N.G.; Assaeed, A.M.; Al-Rowaily, S.L.; Alharthi, A.S.; Mohamed, T.A.; Nassar, M.I.; Dewir, Y.H.; Elshamy, A.I. Phytotoxic effects of plant essential oils: A systematic review and structure-activity relationship based on chemometric analyses. Plants 2020, 10, 36. [Google Scholar] [CrossRef]
- Chami, N.; Chami, F.; Bennis, S.; Trouillas, J.; Remmal, A. Antifungal treatment with carvacrol and eugenol of oral candidiasis in immunosuppressed rats. Braz. J. Infect. Dis. 2004, 8, 217–226. [Google Scholar] [CrossRef]
- Pina-Vaz, C.; Rodrigues, A.G.; Pinto, E.; Costa-De-Oliveira, S.; Tavares, C.; Salgueiro, L.; Cavaleiro, C.; Gonçalves, M.; Martinez-De-Oliveira, J. Antifungal activity of Thymus oils and their major compounds. J. Eur. Acad. Dermatol. Venereol. 2003, 18, 73–78. [Google Scholar] [CrossRef]
- Chami, N.; Bennis, S.; Chami, F.; Aboussekhra, A.; Remmal, A. Study of anticandidal activity of carvacrol and eugenol in vitro and in vivo. Oral Microbiol. Immunol. 2005, 20, 106–111. [Google Scholar] [CrossRef]
- Kordali, S.; Cakir, A.; Ozer, H.; Cakmakci, R.; Kesdek, M.; Mete, E. Antifungal, phytotoxic and insecticidal properties of es-sential oil isolated from Turkish Origanum acutidens and its three components, carvacrol, thymol and p-cymene. Bioresour. Technol. 2008, 99, 8788–8795. [Google Scholar] [CrossRef]
- Lima, I.O.; Pereira, F.D.O.; Oliveira, W.A.D.; Lima, E.D.O.; Menezes, E.A.; Cunha, F.A.; Diniz, M.D.F.F.M. Antifungal activity and mode of action of carvacrol against Candida albicans strains. J. Essent. Oil Res. 2013, 25, 138–142. [Google Scholar] [CrossRef]
- Jesus, F.P.K.; Ferreiro, L.; Bizzi, K.S.; Loreto, S.; Pilotto, M.B.; Ludwig, A.; Alves, S.H.; Zanette, R.A.; Santurio, J.M. In vitro activity of carvacrol and thymol combined with antifungals or antibacterials against Pythium insidiosum. J. Med. Mycol. 2015, 25, e89–e93. [Google Scholar] [CrossRef]
- Dai, G.H.; Andary, C.; Mondolot-Cosson, L.; Boubals, D. Involvement of phenolic compounds in the resistance of grapevine callus to downy mildew (Plasmopara viticola). Eur. J. Plant Pathol. 1995, 101, 541–547. [Google Scholar] [CrossRef]
- Sánchez-Maldonado, A.F.; Schieber, A.; Gänzle, M.G. Antifungal activity of secondary plant metabolites from potatoes (So-lanum tuberosum L.): Glycoalkaloids and phenolic acids show synergistic effects. J. Appl. Microbiol. 2016, 120, 955–965. [Google Scholar] [CrossRef] [PubMed]
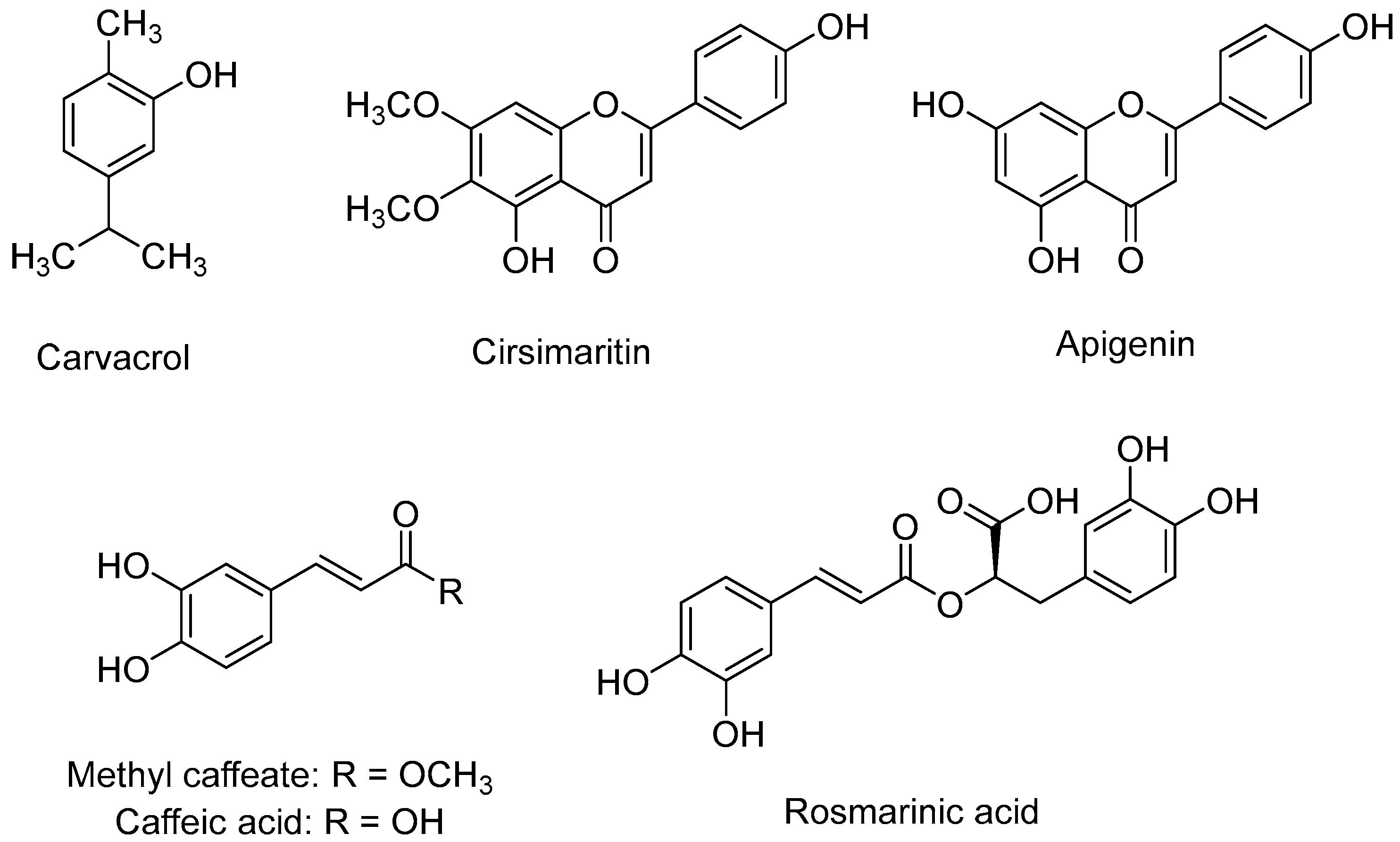
| Compound | Carvacrol | Cirsimaritin | Apigenin | Methyl Caffeate | Caffeic Acid | Rosmarinic Acid |
|---|---|---|---|---|---|---|
| Clog P | 3.35 | 2.86 | 2.91 | 1.20 | 0.98 | 1.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zorrilla, J.G.; Giovannini, O.; Nadalini, S.; Zanini, A.; Russo, M.T.; Masi, M.; Puopolo, G.; Cimmino, A. Suppressive Activity of Glechoma hederacea Extracts against the Phytopathogenic Oomycete Plasmopara viticola, and First Screening of the Active Metabolites. Agriculture 2024, 14, 58. https://doi.org/10.3390/agriculture14010058
Zorrilla JG, Giovannini O, Nadalini S, Zanini A, Russo MT, Masi M, Puopolo G, Cimmino A. Suppressive Activity of Glechoma hederacea Extracts against the Phytopathogenic Oomycete Plasmopara viticola, and First Screening of the Active Metabolites. Agriculture. 2024; 14(1):58. https://doi.org/10.3390/agriculture14010058
Chicago/Turabian StyleZorrilla, Jesús G., Oscar Giovannini, Stefano Nadalini, Alberto Zanini, Maria Teresa Russo, Marco Masi, Gerardo Puopolo, and Alessio Cimmino. 2024. "Suppressive Activity of Glechoma hederacea Extracts against the Phytopathogenic Oomycete Plasmopara viticola, and First Screening of the Active Metabolites" Agriculture 14, no. 1: 58. https://doi.org/10.3390/agriculture14010058
APA StyleZorrilla, J. G., Giovannini, O., Nadalini, S., Zanini, A., Russo, M. T., Masi, M., Puopolo, G., & Cimmino, A. (2024). Suppressive Activity of Glechoma hederacea Extracts against the Phytopathogenic Oomycete Plasmopara viticola, and First Screening of the Active Metabolites. Agriculture, 14(1), 58. https://doi.org/10.3390/agriculture14010058










