Abstract
Some of the key pests of tomatoes are virus vectors, such as whiteflies, green peach aphids, and thrips, mainly because there is a lack of full resistance to the transmitted viruses. Alternatives to reduce this problem include the use of a push-and-pull strategy. Hence, this work assessed the association between Solanum habrochaites PI 1344117, used as a companion plant, and commercial tomato varieties over tomato virus vectors and the yield quality and quantity. Field and greenhouse trials were run during the 2015, 2016, 2018, and 2019 seasons. The treatments were BRS Tospodoro and BRS Tyão cultivated as monocrops and associated with PI-134417. The number of these insects was assessed by directly counting ten plants per plot and using captures on yellow sticky traps settled in the center of the plots. The yield and the number of irregularly ripening fruits (a symptom caused by whiteflies) were also measured. Both commercial cultivars gained from the protective effect of PI 134417, expressed by the significant reduction in the virus vectors on the plants. It was also noticed that there was a reduction in the number of irregularly ripening fruits, improving the fruit quality. These results encourage the use of wild and commercial tomato genotypes in association with implementing strategies to control tomato virus vectors, reducing the need to rely only on a chemical control.
1. Introduction
Tomato (Solanum lycopersicum L.) is one of the most significant solanaceous crops, and it is grown both in open fields and protected environments [1]. Improvements in production systems have led to an enhanced significance of tomatoes, a great source of vitamins and mineral salts [2].
Tomato cultivation is limited, though, by key pests, such as the virus vectors whitefly (Bemisia tabaci Gennadius (Hemiptera: Aleyrodidae)) [3], green peach aphid (Myzus persicae Sulzer (Hemiptera: Aphididae)) [4], and onion thrips (Thrips tabaci Lindeman (Thysanoptera: Thripidae)) [5]. Direct damage from these pests includes sap feeding and honeydew excretion, a substrate for black sooty mold that reduces photosynthesis and decreases yield [6]. However, the most significant damage comes from the transmission of viruses to tomatoes [6].
As Ong [7] stated, B. tabaci is a vector of several virus families: Geminiviridae, Betaflexiviridae, Closteroviridae, and Potyviridae. The green aphid can transmit Potato virus Y (PVY), Potato leafroll virus (PLRV), and Pepper yellow mosaic virus (PepYMV) [8], and onion thrips are a vector of orthotospoviruses, such as Tomato spotted wilt virus (TSWV) and Tomato yellow ring virus (TYRV) [5]. Most viruses are controlled by host resistance or a vector control; in tomatoes, this involves spraying insecticides against the vectors based on their presence on the plants [9]. The harmful effects of the indiscriminate use of insecticides are the contamination of the environment and human beings [10], the depletion of natural enemies [11], and the selection of populations resistant to insecticides [12]. Additionally, protective vector control measures, such as insecticides, are not an option for many nonpersistent plant viruses, since infection is too quick [13].
Sources of pest resistance previously detected in the Solanum germplasm highlight the significance of the S. habrochaites accession PI 134417 as a source of resistance to Leptinotarsa decemlineata Say (Coleoptera: Chrysomelidae), Spodoptera exigua Hübner (Lepidoptera: Noctuidae), B. tabaci, M. persicae, and Keiferia lycopersicella (Walsingham) (Lepidoptera: Gelechiidae) [14]. Despite these findings, these authors also highlight that no tomato commercial genotype possesses insect resistance. In this situation, a viable way to gain some protection against these pests includes the associated cultivation between susceptible commercial genotypes and resistant wild genotypes. This scheme is an associative resistance [15], although it can also be considered as a modality of the push-and-pull strategy [16], where the wild genotype works as the push component and the commercial cultivars work as the pull component. In any case, the term associational resistance will be adopted throughout the text as a way to encompass a broader concept.
The association of two genotypes that vary in their susceptibility to pests makes it possible for the susceptible genotypes to gain from the protection conferred by the resistant genotype through the liberation of stimuli that change the pest behavior, modifying its abundance and distribution [17]. This is possible because the companion plants generally synthesize deterrent and repellent compounds [17]. Concerning the S. habrochaites accession PI 134417, it is considered a valuable source of insect resistance due to the repellent and toxic effects [18], and the different foliar trichomes involved in the chemical and morphological basis of resistance [19]. Therefore, it is hypothesized that this genotype could be efficiently used in association with commercial cultivars to control B. tabaci, M. persicae, and T. tabaci in tomatoes.
In addition, according to the philosophy of integrated pest management, integrating multiple tactics should reduce the need to interfere with the pest population through chemical control, and the protection gained by the association of the plants with varying degrees of resistance can contribute to achieving that. For instance, the association of susceptible and resistant plants or genotypes [20] can either (1) attract pests to a more susceptible plant (pull effect) or (2) repel pests from the susceptible genotypes (push effect) because of the presence of repellent or toxic chemicals. This strategy must be considered with pests that present control failures and are challenging, such as B. tabaci, M. persicae, and T. tabaci.
Hence, this work aimed to assess the association between Solanum habrochaites PI 1344117, used as a companion plant, and commercial tomato varieties over tomato virus vectors and their influence on the yield quality and quantity.
2. Materials and Methods
2.1. General Conditions
The trials were run during four different seasons: 2015, 2016, 2018, and 2019. All cultivations were located at “Fazenda Água Limpa”, Vargem Bonita, Brasília, Distrito Federal, Brazil. In the 2015 and 2016 seasons, trials were run under field conditions, while in the 2018 and 2019 seasons, they took place in a greenhouse, with plants cultivated in 5 L polyethylene pots containing one plant per pot.
Seedlings were produced at Embrapa Hortaliças facilities, located at Rodovia BR 060 Km 9, Distrito Federal, Brazil, using 128-cell foam trays filled with the substrate Plantmax® (Plantmax, Eucatex Agro®, Paulínea, São Paulo, Brazil). These seedlings were transplanted 30 days after planting when they had three to four true leaves. The treatments were Solanum lycopersicum cv. BRS Tospodoro (Tospodoro) and S. lycopersicum cv. BRS Tyão (Tyão) monocropped and cultivated with the S. habrochaites accession PI 134417. In both trials (field and greenhouse trials), there were isolated (monocrop) plots cultivated with the S. habrochaites accession PI 134417. However, these plots were not included in the comparisons because, as a wild-resistant genotype, PI 134417 is not infested with insects; when infested, the insects die fast. Also, the plants do not yield fruits that have any commercial value.
2.2. Field Trials
Each field had 850.5 m2 (27 × 31.5 w × l) and was fertilized with cattle manure at 800 Kg ha−1, 4-30-16 (N-P-K) at 1200 kg ha−1, and ammonium sulfate at 350 kg ha−1. Additionally, two top-dressing fertilizations with 4-30-16 (N-P-K) at 1200 Kg ha−1 were made 29 days after transplanting (DAT) and during the flowering period. The drip irrigation system supplied the water demand, and weeds were manually grazed in the area.
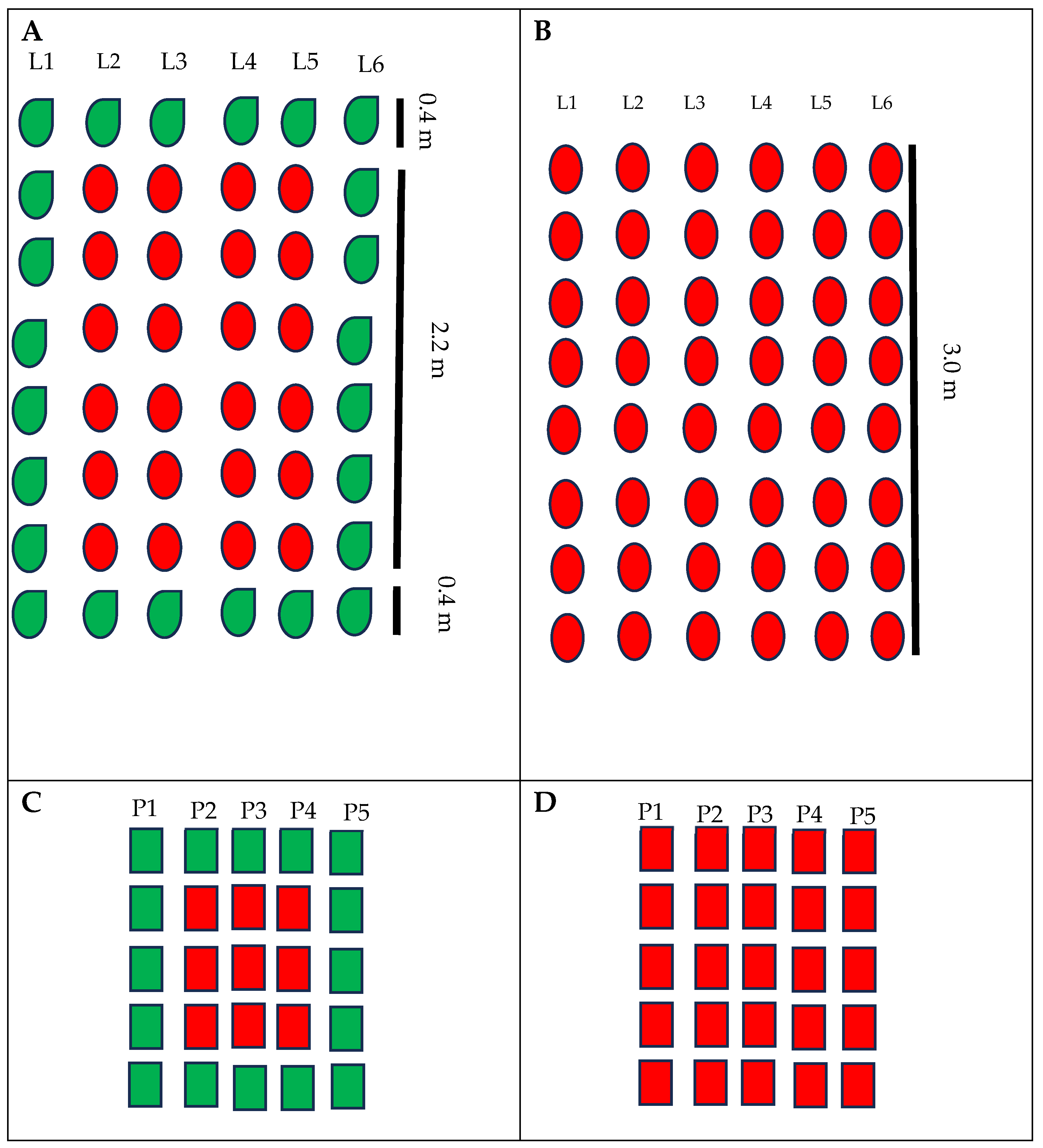
The experimental design was composed of completely randomized blocks with four replicates, and each experimental plot had six rows of three meters in length. Treatments were the commercial varieties of industrial tomatoes, BRS Tospodoro and BRS Tyão, cultivated as monocrops and associated with PI 134417. Each plot adopted a buffer zone (without any plants) of 2 m (w × l) to avoid treatment interference. Monocrop plots had the whole area cultivated with only one genotype (BRS Tospodoro or BRS Tyão) (Figure 1B,D), while in the plots developed in association, the BRS Tospodoro and BRS Tyão plants were surrounded by PI 134417 (Figure 1A,C). The surroundings were arranged by the cultivation of PI 134417 in the first and last rows, and the first 0.40 m of the initial and final four remaining rows (Figure 1A,C), which resulted in the plots surrounded in the whole perimeter by one line of PI 134417.
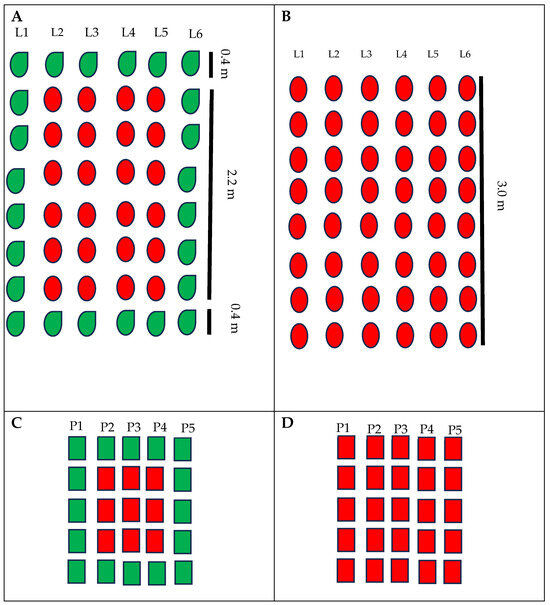
Figure 1.
Experimental plot schemes for field (A,B) and greenhouse trials (C,D). The red color represents commercial genotypes of tomatoes and the green color represents Solanum habrochaites PI 134417 plants. L1–6—rows of plants in field trials; P1–5—pots of plants in greenhouse trials.
2.2.1. Surveys
Field evaluations were performed at 36, 43, 52, 64, 71, 78, 85, 92, 99, and 106 days after planting (DAP) in 2015, and 28, 35, 41, 49, 56, 63, and 71 DAP in 2016 by directly counting the number of nymphs and adults of B. tabaci biotype B, M. persicae, and T. tabaci found on ten BRS Tospodoro and BRS Tyão plants per plot. Sampled plants were located on the central rows (four midrows positioned at the center of the plots, excluding the first and final 1 m of the rows).
2.2.2. Traps
The Bemisia tabaci biotype B, M. persicae, and T. tabaci densities were also assessed in two periods with regard to the 2015 season (at 44 and 71 DAT), and three periods concerning the 2016 season (49, 63, and 78 DAT), using yellow sticky panels (30 × 10 cm) having two sticky sides and fixed on wood sticks of a height of 1.50 m. These traps were placed in the center of the plot, removed after 14 days, wrapped in PVC transparent film, and taken to the Plant Protection Laboratory at the University of Brasília (UnB) for insect counting under a stereomicroscope (40× magnification) (Coleman®, Santo André, São Paulo, Brazil).
2.2.3. Fruit Evaluation
The fructification of the plants was also visually monitored until ripening; at this time, ten plants per plot were randomly chosen. Fruits from these plants were harvested, sorted (typical or showing irregular ripening disorder), and weighed for yield estimation. Based on these data, the percentage of distinctively ripening fruits was calculated.
2.3. Greenhouse Trials
In these trials, the experimental plot was formed by 25 black plastic pots (5 rows with 5 pots in each row), and the space between the plots was 0.50 m and 0.60 m between the blocks. In the associated plots, commercial cultivars were surrounded by the wild species PI 134417. Hence, these plots had nine pots of commercial cultivars (three rows with three pots in each row), and in the first and last of these rows, there were six pots of PI 134417. Moreover, two other lines of PI 134417 (five pots each) were located at the initial and final plots (Figure 1C). Monocrop plots had the whole plot cultivated with one of the commercial cultivars, BRS Tospodoro or BRS Tyão (Figure 1D). Treatments were designed as completely randomized blocks with three replicates. Fertilization practices were similar to the field trials.
Adults of B. tabaci biotype B used in the 2018 and 2019 trials came from Embrapa Vegetables mass-rearing facilities. Each year, 5000 viruliferous adults of B. tabaci biotype B were released. PCR tests were run in the Virology Laboratory of Embrapa Vegetables to test the viruliferous condition of these insects.
2.3.1. Surveys
Plant inspections were done at 29, 35, 43, 50, 56, 64, 71, 78, and 85 DAT in 2018, and 28, 35, 42, 49, 57, 63, and 71 DAT in 2019, by counting the number of nymphs and adults of B. tabaci biotype B, M. persicae, and T. tabaci found on five BRS Tospodoro and BRS Tyão plants per plot. The surveys were done on four midrow plants, excluding the first and last 1 m of the row.
2.3.2. Insects Caught on Traps
Bemisia tabaci biotype B, M. persicae, and T. tabaci were also assessed in the 2018 season (at 43, 50, 57, 64, 71, 78, 85, 92, 99, 105, and 113 DAT) and the 2019 season (at 28, 35, 42, 49, 57, 63, and 71 DAT) using yellow sticky panels (30 × 10 cm) fixed on wood sticks of a height of 1.50 m and having two sticky sides. These traps were placed in the center of the plot, removed after seven days, wrapped in PVC transparent film, and taken to the Plant Protection Laboratory at the University of Brasília (UnB) for insect counting under a stereomicroscope (40× magnification) (Coleman®, Santo André, São Paulo, Brazil).
2.3.3. Fruit Evaluation
The fruit of the plants was visually monitored from their emergence until ripening by sampling ten plants per plot randomly assigned and located at the same position used to monitor the insects on the plants. At the mature fruiting stage, the fruits were harvested and classified according to the number and weight of regular and irregular ripening disorders (caused by whiteflies), and weighed on a precision scale (LG 0.01 g, Bel®, Piracicaba, São Paulo, Brazil). The percentage of distinctively ripening fruits was calculated.
2.4. Data Analysis
Direct counting from field and greenhouse trials was reduced to the means per plant. In contrast, data coming from yellow sticky trap capture were reduced to the means per trap per day, and both were expressed as the percentages of increase or decrease in the insect densities in the monocrop plants compared to the plants growing in association with PI 134417. The absolute densities and standard error mean were also presented. Data coming from field evaluations were pairwise-compared—monocrops versus commercial genotypes growing with PI 134417—by repeated measures ANOVA [21] at p < 0.05 to seek the treatment effects on different evaluation days. This procedure was adopted because the measures on the same plants in different weeks of the same season were related and nonindependent. Data from the yellow sticky traps, and the yield quality and quantity, were also pairwise-compared for the treatment effects on each evaluation date or at harvest by one-way ANOVA [21] at p < 0.05. In all situations, only significant data were represented.
3. Results
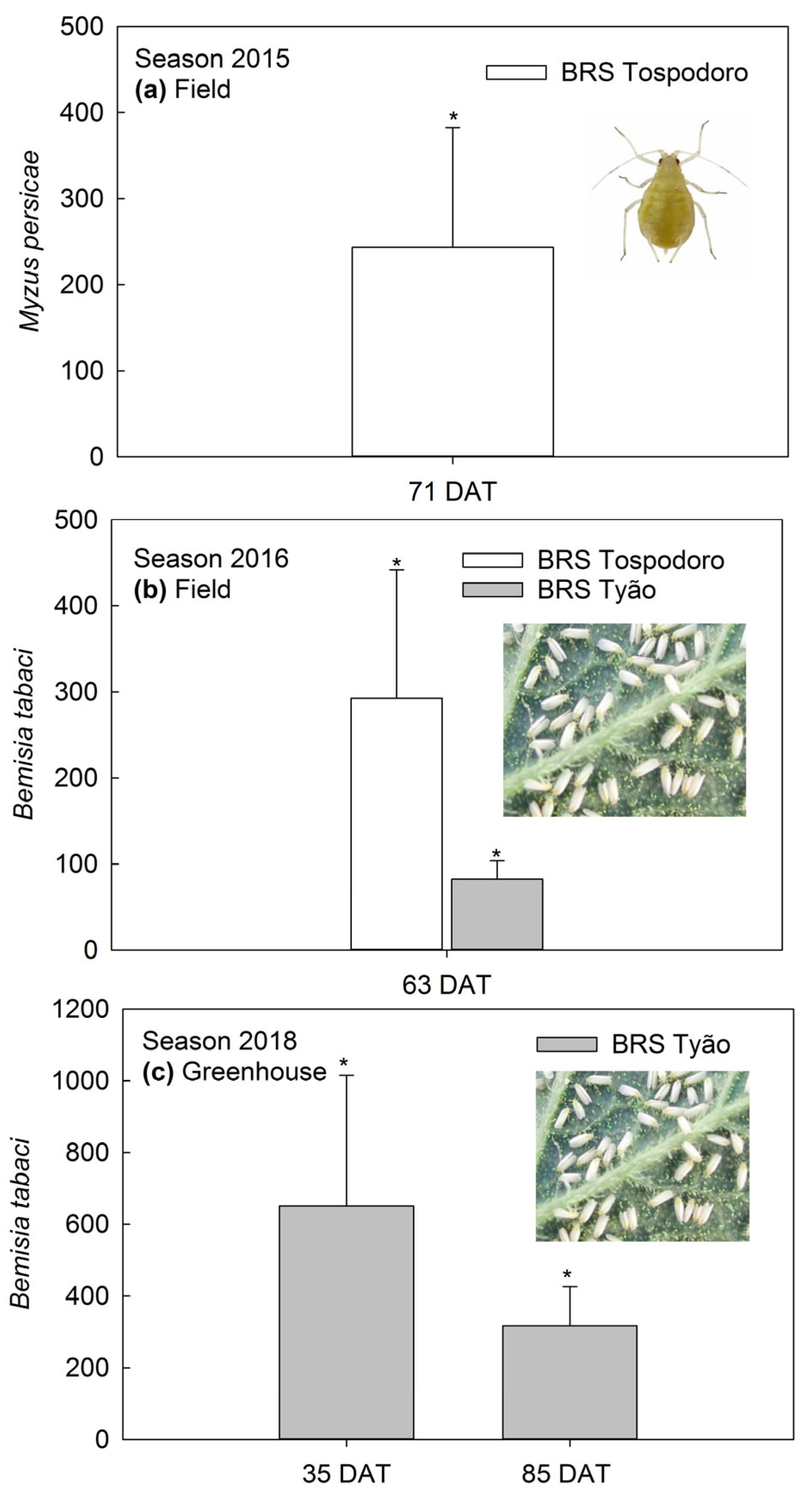
Densities of M. persicae were greater in the BRS Tospodoro monocrop than in BRS Tospodoro surrounded by PI 134417 at 71 DAT in the 2015 season (Figure 2a). This trend was also observed for the B. tabaci biotype B adults at 63 DAT in the 2016 season, with the BRS Tospodoro and BRS Tyão monocrops having 335% and 84% more infestation than the plants cultivated in the association (Figure 2b). Additionally, BRS Tyão were ≈650% and 320% more infested at 35 and 85 DAT in the 2018 season, respectively, when compared to BRS Tyão in association with PI 134417 (Figure 2c). The absolute densities, followed by the SEM and the comparisons according to the ANOVA by repeated measures, are also represented in the legend (Figure 2).
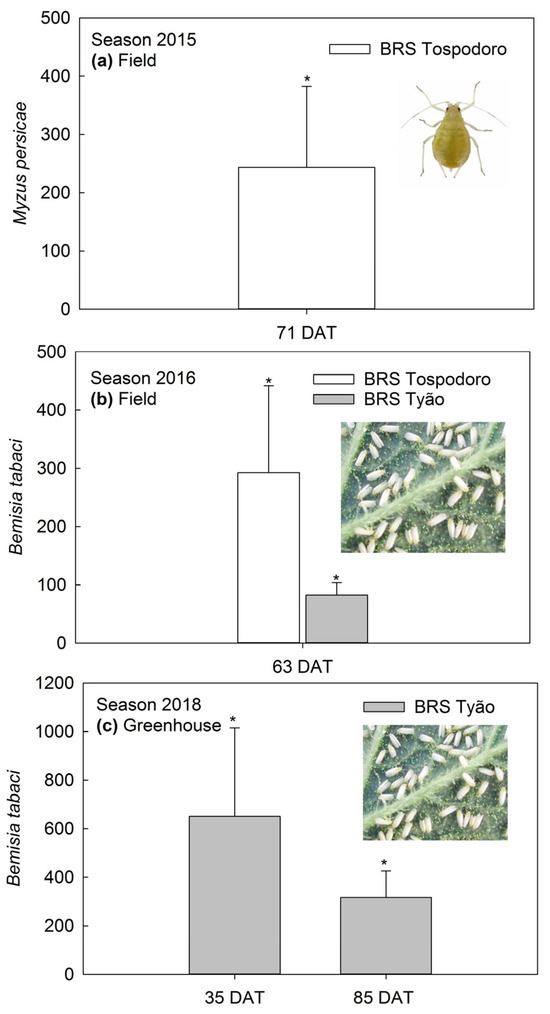
Figure 2.
(a) Percentage of increase (mean +SEM) in Myzus persicae densities in unaccompanied “BRS Tospodoro” tomato plants at 71 days after transplanting (DAT) (2015 field experiment); mean densities: (F1,3 = 24.02; p = 0.0163), BRS Tospodoro + PI 134417 = 0.73 ± 0.03 B, BRS Tospodoro = 0.97 ± 0.08 A; (b) Percentage of the increase in Bemisia tabaci biotype B densities in unaccompanied “BRS Tospodoro” and “BRS Tyão” at 63 DAT (2016 field experiment) (F1,3 = 18.31; p = 0.0234), BRS Tospodoro + PI 134417 = 0.77 ± 0.04 B, BRS Tospodoro = 1.30 ± 0.14 A; (F1,3 = 23.12; p = 0.0171), BRS Tyão + PI 134417 = 0.77 ± 0.06 B, BRS Tyão = 1.14 ± 0.05 A; (c) Percentage of the increase in B. tabaci biotype B densities in unaccompanied “BRS Tyão” at 35 and 85 DAT (2018 greenhouse experiment); 35 DAT: (F1,2 = 117.69; p = 0.0084), BRS Tyão + PI 134417 = 1.53 ± 0.42 B, BRS Tyão = 2.62 ± 0.47 A; 85 DAT: (F1,2 = 24.33; p = 0.0387), BRS Tyão + PI 134417 = 1.10 ± 0.20 B, BRS Tyão = 1.65 ± 0.30 A. Insects were evaluated through direct counting on the plants. The percentages were estimated by comparing unaccompanied “BRS Tospodoro and BRS Tyão” and “BRS Tospodoro and BRS Tyão” surrounded by Solanum habrochaites f. glabratum PI134417. * indicate differences according to the F-test at α = 0.05.
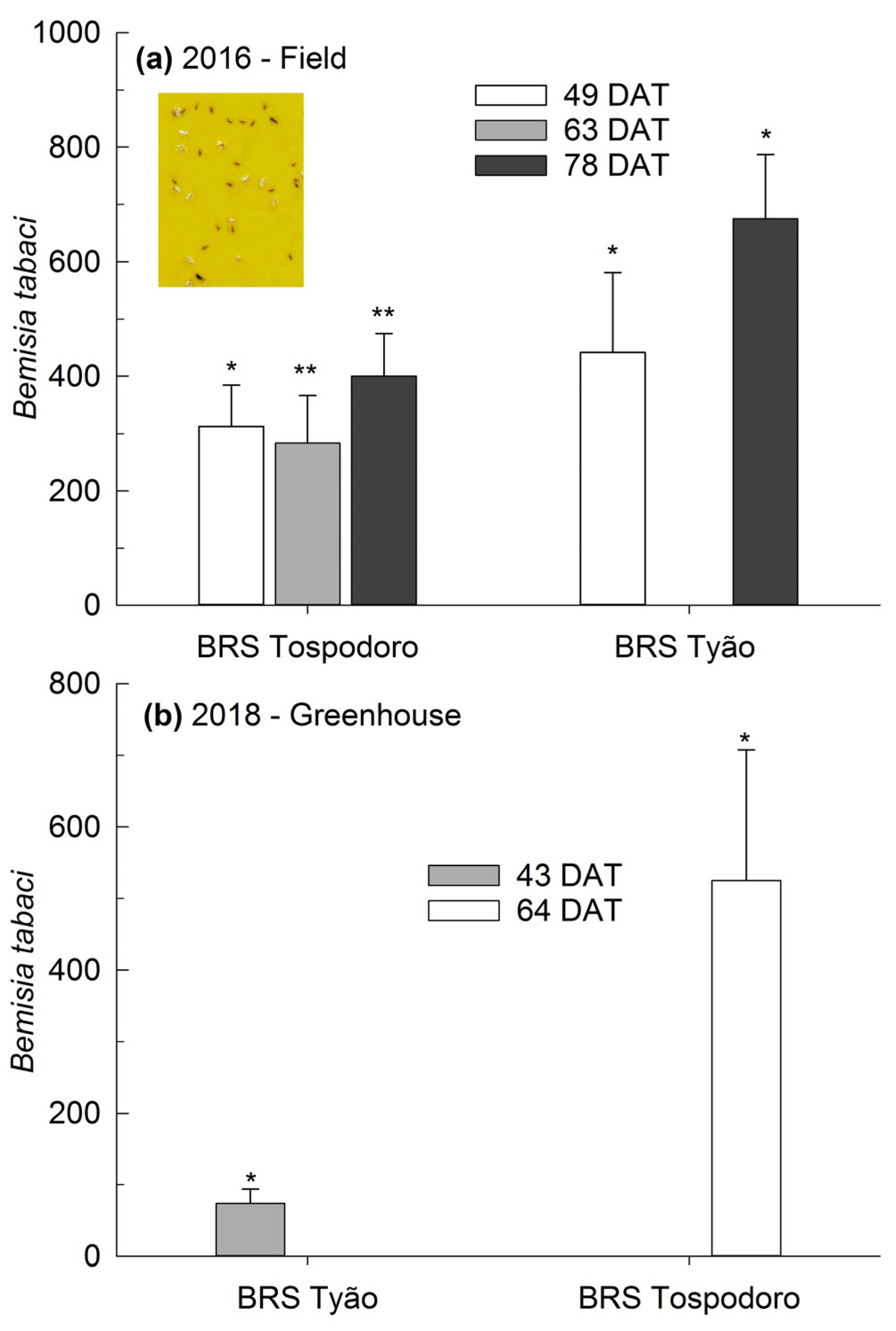
There was a significant increase from ≈300 to 400% in B. tabaci biotype B adults collected on yellow sticky traps settled in the monocrop plots of BRS Tospodoro cultivated in the field and sampled at 49, 63, and 78 DAT compared to the plots surrounded by PI 134417 (Figure 3a). A similar trend was observed for BRS Tyão sampled at 49 and 78 DAT, with ≈400 to 700% increases under the same environmental conditions (Figure 3a). When tomatoes were cultivated in the greenhouse, the number of B. tabaci biotype B adults trapped in the yellow sticky panels was ≈75% and ≈500% greater on the monocrops of BRS Tyão sampled at 43 DAT and BRS Tospodoro sampled at 64 DAT, respectively, compared to the plants cultivated in association with PI 134417 (Figure 3b). The absolute densities, followed by the SEM and the comparisons according to the one-way ANOVA, are also represented in the legend (Figure 3).
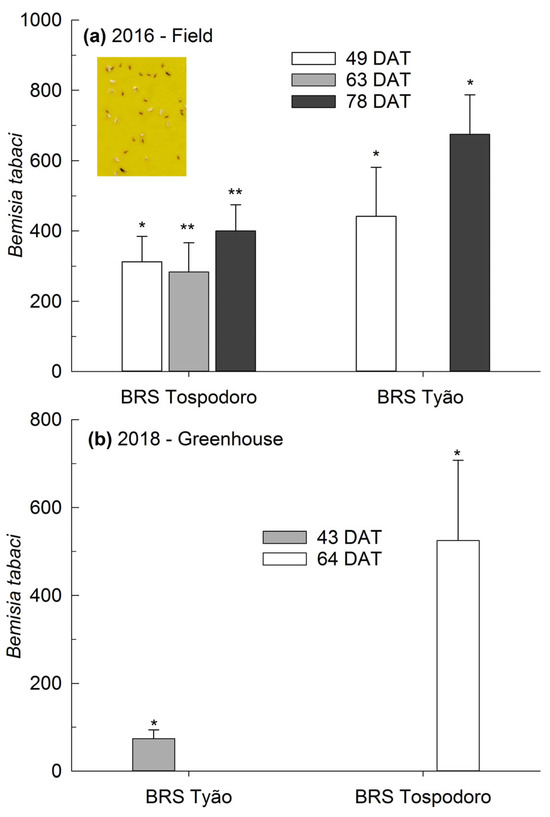
Figure 3.
Percentage of increase (mean + SEM) of Bemisia tabaci biotype B adults captured in yellow sticky traps in unaccompanied “BRS Tospodoro” and “BRS Tyão” tomato plants at 49, 63, and 78 days after transplanting (DAT) in the 2016 season; 49 DAT: (F1,3: 121.0; p = 0.0016), BRS Tospodoro + PI 134417 = 2.25 ± 0.63 B, BRS Tospodoro = 7.75 ± 0.85 A; (F1,3: 25.00; p = 0.0154), BRS Tyão + PI 134417 = 2.25 ± 0.48 B, BRS Tyão = 9.75 ± 1.38 A; 63 DAT: (F1,3: 10.8; p = 0.0462), BRS Tospodoro + PI 134417 = 1.00 ± 0.71 B, BRS Tospodoro = 4.00 ± 0.82 A; 78 DAT: (F1,3: 37.8; p = 0.0087), BRS Tospodoro + PI 134417 = 1.25 ± 0.48 B, BRS Tospodoro = 6.50 ± 0.87 A; (F1,3: 24.03; p = 0.0162), BRS Tyão + PI 134417 = 0.50 ± 0.29 B, BRS Tyão = 7.25 ± 1.31 A (a), and at 43 and 64 DAT in the 2018 season; 43 DAT: (F1,2: 27.00; p = 0.0351), BRS Tyão + PI 134417 = 5.33 ± 1.86 B, BRS Tyão = 8.33 ± 1.45 A; 64 DAT: (F1,2: 18.75; p = 0.0494), BRS Tospodoro + PI 134417 = 7.00 ± 4.51 B, BRS Tospodoro = 27.00 ± 8.74 A (b). The percentages were estimated by comparing the unaccompanied “BRS Tospodoro and BRS Tyão” and “BRS Tospodoro and BRS Tyão” surrounded by Solanum habrochaites PI134417. * and ** indicate the differences according to the F-test at α = 0.05 and α = 0.01, respectively.
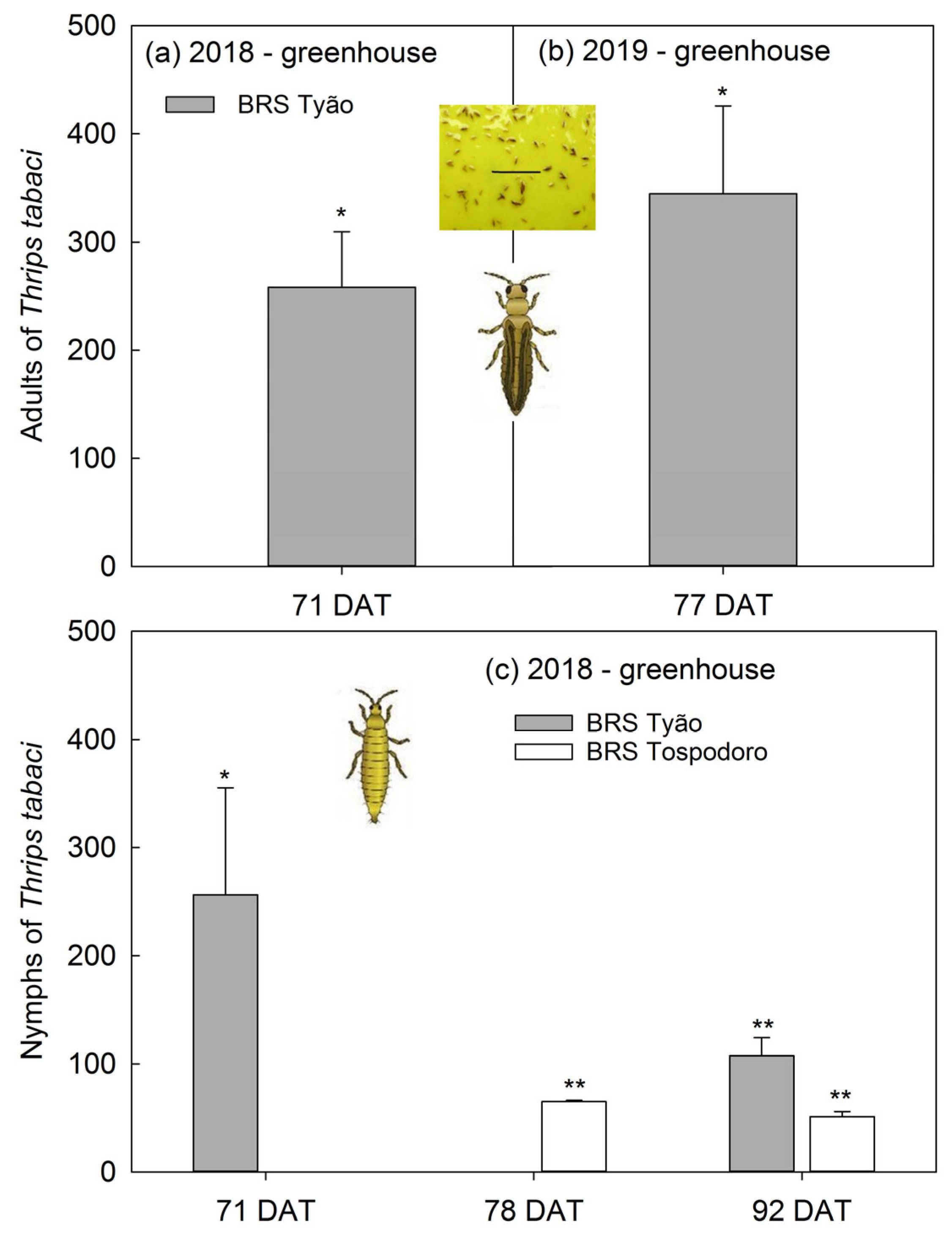
Adults of Thrips tabaci captured on the yellow sticky traps at 71 DAT in the 2018 season were 250% greater in the BRS Tyão monocrop than in the BRS Tyão associated with PI 134417 (Figure 4a). A similar trend was observed at 77 DAT in the 2019 season, with an increase of 350% in thrips trapped (Figure 4b). The number of T. tabaci nymphs trapped at 78 and 92 DAT in the 2018 season in the monocrops of BRS Tospodoro was ≈50% greater than BRS Tospodoro + PI 134417 plots (Figure 4c). A similar trend was observed for BRS Tyão at 71 and 92 DAT in the 2018 season, with increased rates of ≈100 to 250% in monocrop plots (Figure 4b). Absolute densities followed by SEM and the comparisons according to one-way ANOVA are also represented in the legend (Figure 4).
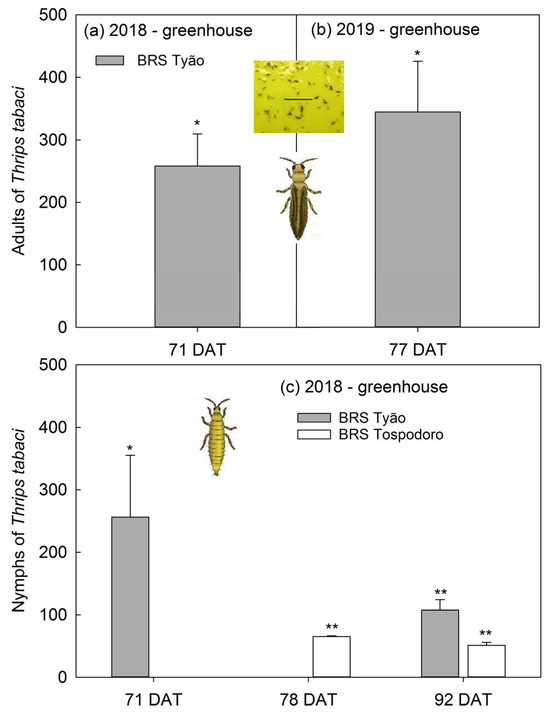
Figure 4.
Percentage of increase (mean + SEM) of Thrips tabaci adults and nymphs captured in yellow sticky traps in unaccompanied “BRS Tyão” tomato plants at 71 and 77 days after transplanting (DAT) in 2018 (a); 71 DAT: (F1,2: 18.75; p = 0.0494), BRS Tyão + PI 134417 = 2.00 ± 1.15 B, BRS Tyão = 7.00 ± 2.00 A; in 2019 (b); 77 DAT: (F1,2: 22.23; p = 0.0422), BRS Tyão + PI 134417 = 2.00 ± 2.00 B, BRS Tyão = 7.67 ± 3.18 A; and in the unaccompanied “BRS Tospodoro” and “BRS Tyão” at 71, 78, and 92 DAT in the 2018 season (c); 71 DAT: (F1,2: 26.18; p = 0.0361), BRS Tyão + PI 134417 = 30.00 ± 12.00 B, BRS Tyão = 69.67 ± 16.50 A; 78 DAT: (F1,2: 281.19; p = 0.0035), BRS Tospodoro + PI 134417 = 156.00 ± 6.08 B, BRS Tospodoro = 258.00 ± 12.12 A; 92 DAT: (F1,2: 612.89; p = 0.0016), BRS Tospodoro + PI 134417 = 89.33 ± 15.86 B, BRS Tospodoro = 133.00 ± 17.62 A; (F1,2: 379.11; p = 0.0026), BRS Tyão + PI 134417 = 100.33 ± 20.04 B, BRS Tyão = 198.33 ± 15.90 A. All plants were cultivated in greenhouse. The percentages were estimated by comparing unaccompanied “BRS Tospodoro and BRS Tyão” and “BRS Tospodoro and BRS Tyão” surrounded by Solanum habrochaites PI134417. * and ** indicate differences according to F-test at α = 0.05 and α = 0.01, respectively.
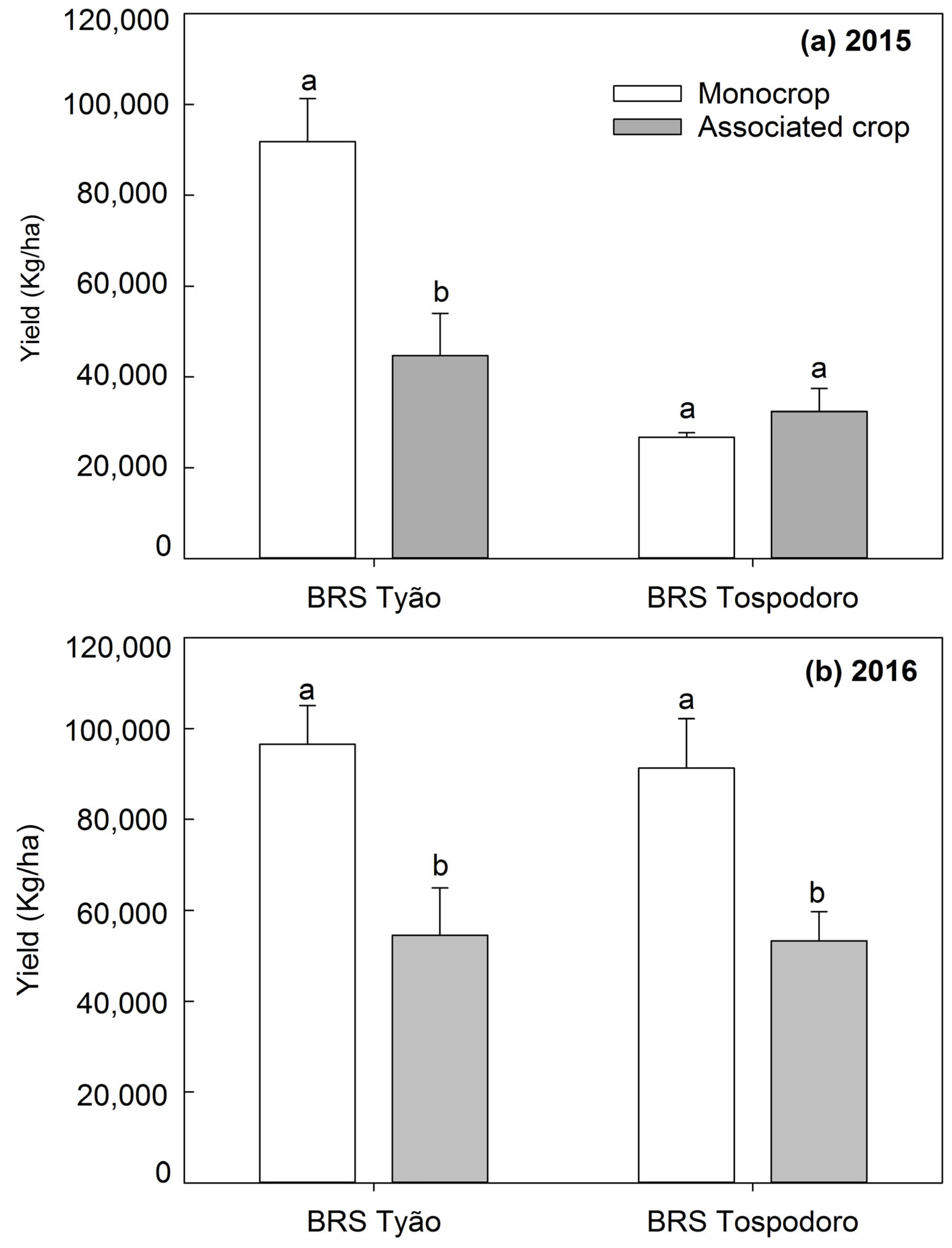
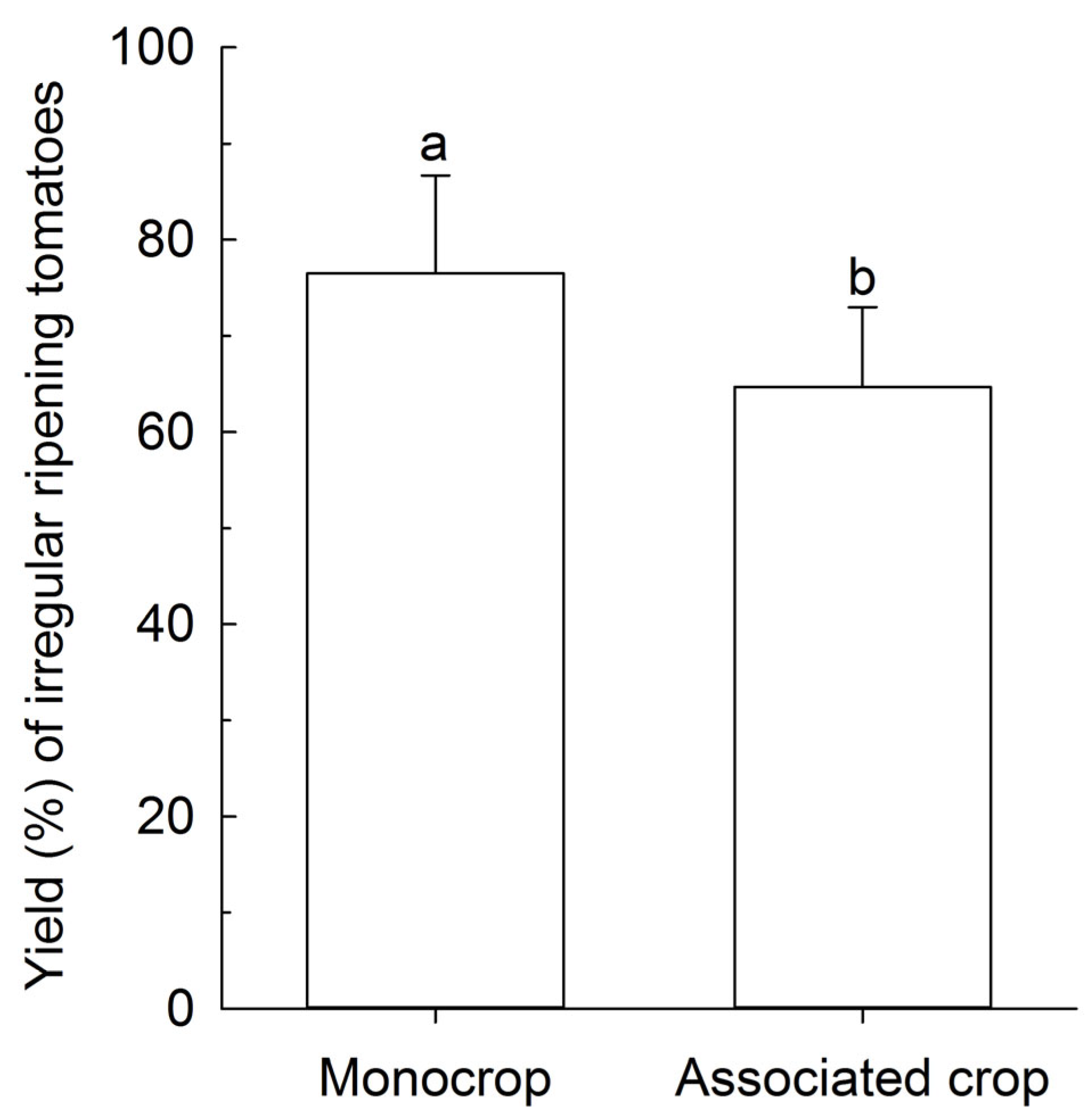
The yield of BRS Tyão associated with PI 134417 in a field trial in the 2015 season was the lowest compared to the remaining treatments that did not differ among them (Figure 5a), while in the 2016 field trial, both BRS Tospodoro and BRS Tyão cultivated in association with PI 134417 showed the lowest yield compared to the monocrop plots (Figure 5b). Nonetheless, the most significant proportion of the irregular ripening of fruits found in the greenhouse trial in the 2018 season was observed in the BRS Tospodoro monocrop when compared with BRS Tospodoro associated with PI 134417 (Figure 6).
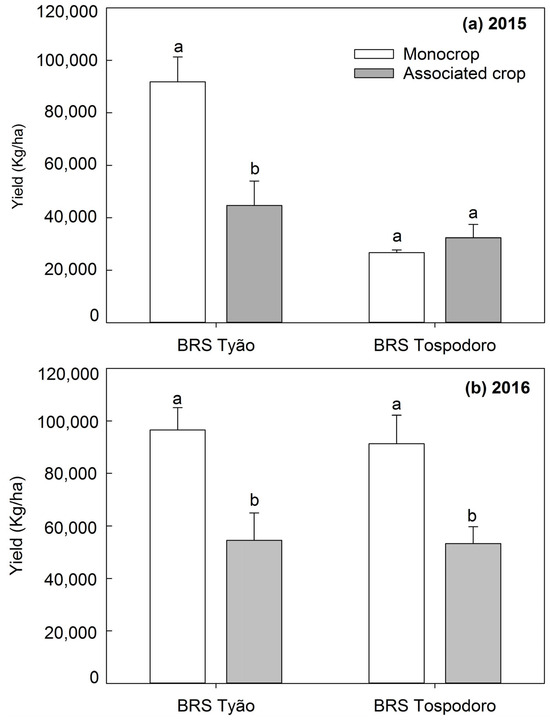
Figure 5.
Yield (Kg/ha) (mean + SEM) of unaccompanied “BRS Tospodoro” and “BRS Tyão” plants (monocrop), and “BRS Tospodoro” and “BRS Tyão” surrounded by Solanum habrochaites PI134417 (associated crop), in the 2015 (a) and 2016 (b) field seasons. Means followed by the same letters do not differ by the F-test (p < 0.05).
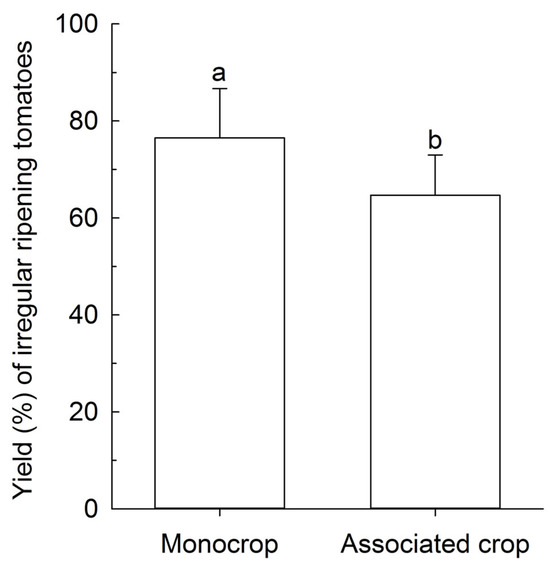
Figure 6.
Percentage yield (Kg/ha) of the irregular ripening fruits in unaccompanied “BRS Tospodoro” (monocrop) and “BRS Tospodoro” surrounded by Solanum habrochaites PI 134417 (associated crop) in the greenhouse trial in the 2018 season. Means followed by the same letter do not differ by the F-test (p < 0.05).
4. Discussion
The primary sources of resistance to virus vectors are wild species of tomato. The chemical causes of this resistance are acylsugars from Solanum penelli [22,23], sesquiterpenoids, such as methyl ketones and zingiberenes, from S. habrochaites [24], which are allelochemicals conferring resistance of these wild species to B. tabaci biotype B [25], M. persicae [26], and T. tabaci [24].
The insect resistance of the S. habrochaites accession PI 134417 has been reported in the literature for more than 50 years, and one of the first records was provided by Gentile and Stoner [27]. Priyadarshan [28] pointed out that finding a resistance source is the first step to developing a resistant cultivar. At the same time, the next step involves transferring these characteristics using appropriate approaches and breeding methods [29]. Despite the great accumulated knowledge on the sources and mechanisms of the resistance of tomato wild species to B. tabaci biotype B, M. persicae, and T. tabaci, resistant cultivars still need to be made available [30]. Most of this is caused by the incompatibility between resistant traits and agronomical characteristics. Also, vector management through insecticide sprays is limited by the existence of trade-offs between the low densities used as thresholds—the presence of the insects—and the expected efficacy [31], reported insecticide resistance [32], and the low number of modes of action available to control this group [33]. Hence, the control of tomato virus vectors by insecticide sprays and the adoption of resistant varieties are still not practicable [3]. In this context, Midega et al. [34] endorse that an alternative way to manage these pests is the push-and-pull strategy, exploiting companion plants’ stimuli to change the pests’ abundance and survival in the agroecosystems.
In an overview of this research, it is possible to notice that B. tabaci biotype B, M. persicae, and T. tabaci have their densities reduced by the association of PI 134417 with BRS Tospodoro and BRS Tyão. This could be explained by the repellent effects of this wild species [18], caused by the chemical and morphological factors present in the foliar trichomes of PI 134417 [19]. Two methyl ketones, 2-tridecanone and 2-undecanone, present in trichomes found in this species are responsible for antibiosis and antixenosis effects on several pests, reducing their feeding, oviposition, and survival [35]. Previous studies found that PI 134417 not only proportionated low survival, reduced the growth index, and increased the development time from egg to adult of psyllids, but also reduced the adult feeding time by 98% [36], increasing the chances of the adults to abandon the plants [37] or emigrate.
These effects explain the protection conferred by this wild species when used in association with tomato-susceptible commercial cultivars, independently of seasons (2015, 2016, 2018, and 2019) and environments (field or greenhouse). It is worth noticing that the protection gained from the association with a wild species was diminished under a greenhouse environment, probably due to the high initial whitefly population (5000 adults) and the protection promoted by the greenhouse environment from biotic and abiotic mortality factors acting with the high inside temperatures, both of which contribute to population increase. Similar findings were observed by Conboy et al. [20], who noticed that the addition of Calendula officinalis plants after an increase in the T. vaporariorum population was ineffective in reducing pest infestation on tomato plants.
Although the yield of commercial cultivars was diminished in the 2015 season for BRS Tyão, and in the 2016 season for both BRS Tyão and Tospodoro, it is essential to point out that, even if subjected to these hazardous conditions, the association with PI 134417 was advantageous to commercial plants because BRS Tospodoro + PI 134417 had fewer irregularly ripening fruits compared to the BRS Tospodoro monocrop. The reduction in the yield was probably a result of the vigorous growing of PI 134417 compared to the commercial cultivars. The wild PI 134417 was not bred for possessing commercial attributes. Because of that, it kept growing in height and diameter, presenting a significant vegetative growth, confirmed by a greater leaf area and the accumulation of dry matter compared to other commercial and wild genotypes [38]. Therefore, the commercial plants associated with PI 134417 probably suffered more with the established competition, which was reflected in a reduced yield. Further effort to design a proper row spacing should be done. Keeping a higher distance between the wild and commercial genotypes when they grow in association, or managing PI 134417 through pruning, are some possibilities.
The variation in the yield of BRS Tospodoro between the 2015 and 2016 seasons was probably due to the enrichment of the soil fertility from one year to the next. In 2015, the tomatoes were growing for the first time in the area. Then, in the subsequent year, the plants benefited from the enrichment proportionated to the soil, where they were growing for the second time and which was fertilized with mineral and organic fertilizers, as seen for other crops [39]. However, despite the difference on the yield between seasons, the relevant data here are that the increase in the yields allowed the differentiation between monocrops and plants growing in association with PI 134417, something not noticed when the yield was lower in 2015.
National yield averages of industrial tomatoes range from 34.6 to 70.4 t/ha, varying with the region where the tomatoes are growing inside of Brazil [40], and worldwide averages, without a distinction between fresh and processing tomatoes, are 37.1 t/ha [41]. Based on these averages, the yields obtained in the associated plots are not considered so low, mostly when no additional intervention was taken to control the virus vectors.
Another relevant fact is that the wild species PI 134417 has resistance to the Tomato yellow vein streak virus (TYVSV) [42]. Also, epidemics caused by the Alfalfa mosaic virus (AMV) in canning tomatoes, which is transmitted by aphids, pushed the development of alternatives; while doing that, it was noticed that PI 134417 possesses the genes that confer complete resistance to several isolates of the AMV [43]. Therefore, PI 134417 could be helpful in tomato virus management programs, mainly because managing the virus is partially dependent on managing the vectors. The management of the viruses through the management of the vectors, for instance, is highly reliant on a chemical control, which needs to achieve an efficacy of nearly 100% to avoid the infection of the plants [44]. Therefore, an alternative is having plants resistant to pathogens and insects in the system, reducing the chances of population increase and inoculum dissemination.
Because insects infesting PI 134417 show reduced feeding time [37] and increased chances of emigrating [36], plants that are part of a system together with PI 134417 are less likely to be infested and infected. For example, Tomato yellow leaf curl virus can be PCR-detected in the cibarium and midgut of the whitefly after 5 to 40 min of feeding [45]. Therefore, a reduction in feeding can avoid infection with the virus by the insect, and consequently reduce transmission to the plants. In addition, the repellent volatiles discourage infestation [18]. For those insects that remain and feed on PI 134417, the presence of toxins that poison the insects [35] will make them more susceptible to other control measures, such as insecticides, because of the additive effects between plant resistance and insecticides [46], increasing the chances of achieving the proper control of the population that persists in the system. All these facts encourage the use of PI 134417 as a part of such systems to manage the complex involving both categories of pests, viruses, and virus vectors.
The advantages of using PI 134417 as a companion plant to manage tomato virus vectors are not only justified by the presence of resistant traits; instead, this genotype is also a perennial species possessing an indeterminate growth habit and wide adaptability, growing fast even under low temperatures [30]. Like the Desmodium plant used in Africa’s push-and-pull strategy, the S. habrochaites accession PI 134417 covers the soil and allows better moisture retention [47]. Additionally, the accession PI 134417 of S. habrochaites is an autogamous, highly prolific species [48], producing many viable seeds over decades. Despite that, the risk of this species becoming a weed is primarily low because cultural practices efficiently manage these plants.
The proposed method described here matches the fourth level of the technology readiness level (TRL) [49], meaning that the proof-of-concept was demonstrated over four years in greenhouse and field trials. In these trials, a reduction in tomato virus vectors and a decrease in irregular ripening disorder fruits in plots containing the association between the commercial cultivars and the wild genotype PI 134417 were observed. These facts encourage the incorporation of this strategy into the management of tomato virus vectors, reducing the need to depend exclusively on a chemical control.
The following steps of this research will include the validation of this model on a farm (TRL = 5), increasing the number of growers and areas testing the model (TRL = 6), and, finally, validating this system in different regions and seasons (TRL = 7) [50]. These steps are essential to determining the most suitable schemes to overcome the slight decrease in yield when commercial cultivars were associated with PI 134417. Additionally, during this technology dissemination, other plant species commonly cultivated by horticultural growers that grow tomatoes can be added to the system. For instance, brassica species, such as cabbage, kale, and Chinese cabbage, which are not susceptible to the common viruses vectored by whiteflies and are very attractive to them. Therefore, they can also act as pull species, attracting adult whiteflies away from commercial tomatoes, which work as pull species in the current proposed system.
5. Conclusions
Despite the differences in yield seen in this study, where the commercial tomato cultivars in the associated plots produced less than when cultivated as monocrops, the most important, especially concerning the integrated pest management approach, is how often intervention is needed, because it increases the production cost and reduces the liquid profitability [51]. For insects, such as the virus vectors that are managed based on the presence on the plants, considerable reductions in the occurrence, as seen in the present work, may alter the frequency of decision-making and increase the activity’s profitability. Therefore, the commercial cultivars of tomato, BRS Tyão and Tospodoro, gained from the protective effect conferred by PI 134417, attested by the significant reduction in the virus vectors on the plants and the yellow sticky traps. Also, the association proportionated a reduction in the number of irregularly ripening fruits, improving the fruit quality.
Author Contributions
Conceptualization, C.S.B., F.A.S. and J.B.T.; methodology, C.S.B., F.A.S. and J.B.T.; formal analysis, C.S.B., F.A.S. and J.B.T.; investigation, D.M., K.L.C., J.S.d.S., C.O.S. and C.S.B.; resources, F.A.S. and C.S.B.; data curation, D.M., K.L.C., J.S.d.S., C.O.S. and C.S.B.; writing—original draft preparation, D.M., K.L.C., J.S.d.S., C.O.S. and C.S.B.; writing—review and editing, C.S.B., F.A.S. and J.B.T.; supervision, C.S.B., F.A.S., and J.B.T.; project administration, F.A.S. and C.S.B.; funding acquisition, F.A.S. and C.S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundação De Apoio à Pesquisa do Distrito Federal (FAP-DF), grant number 0193.001481/2016.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The datasets and analysis protocols used during the current study are available from the corresponding author on request.
Acknowledgments
Thanks to the “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)” for the scholarship and to the “Conselho Nacional de Pesquisa e Desenvolvimento Tecnológico (CNPq)” for the scholarships and fellowships.
Conflicts of Interest
Fábio Akiyoshi Suinaga was employed by Embrapa Hortaliças (CNPH). The remaining authors declare that the research was conducted without commercial or financial relationships that could be construed as a potential conflicts of interest.
References
- Abewoy Fentik, D. Review on Genetics and Breeding of Tomato (Lycopersicon esculentum Mill). Adv. Crop Sci. Technol. 2017, 5, 306. [Google Scholar] [CrossRef]
- Costa, J.M.; Heuvelink, E. The Global Tomato Industry. In Tomatoes; Heuvelink, E., Ed.; CABI: Wallingford, UK, 2018; pp. 1–26. ISBN 978-1-78064-193-5. [Google Scholar]
- Li, Y.; Mbata, G.N.; Punnuri, S.; Simmons, A.M.; Shapiro-Ilan, D.I. Bemisia tabaci on Vegetables in the Southern United States: Incidence, Impact, and Management. Insects 2021, 12, 198. [Google Scholar] [CrossRef]
- Ali, J. The Peach Potato Aphid (Myzus Persicae): Ecology and Management; CRC Press: Boca Raton, FL, USA, 2023; ISBN 1-00-099677-8. [Google Scholar]
- Loredo Varela, R.C.; Fail, J. Host Plant Association and Distribution of the Onion Thrips, Thrips tabaci Cryptic Species Complex. Insects 2022, 13, 298. [Google Scholar] [CrossRef] [PubMed]
- Castañé, C.; Van Der Blom, J.; Nicot, P.C. Tomatoes. In Integrated Pest and Disease Management in Greenhouse Crops; Gullino, M.L., Albajes, R., Nicot, P.C., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 487–511. ISBN 978-3-030-22303-8. [Google Scholar]
- Ong, S.N.; Taheri, S.; Othman, R.Y.; Teo, C.H. Viral Disease of Tomato Crops (Solanum lycopersicum L.): An Overview. J. Plant Dis. Prot. 2020, 127, 725–739. [Google Scholar] [CrossRef]
- Võ, T.T.; Dehne, H.-W.; Hamacher, J. Transmission of Tomato Chlorotic Dwarf Viroid by Myzus persicae Assisted by Potato Leafroll Virus. J. Plant Dis. Prot. 2018, 125, 259–266. [Google Scholar] [CrossRef]
- Roditakis, E.; Stavrakaki, M.; Grispou, M.; Achimastou, A.; Van Waetermeulen, X.; Nauen, R.; Tsagkarakou, A. Flupyradifurone Effectively Manages Whitefly Bemisia tabaci MED (Hemiptera: Aleyrodidae) and Tomato Yellow Leaf Curl Virus in Tomato. Pest Manag. Sci. 2017, 73, 1574–1584. [Google Scholar] [CrossRef]
- Dudley, N.; Attwood, S.J.; Goulson, D.; Jarvis, D.; Bharucha, Z.P.; Pretty, J. How Should Conservationists Respond to Pesticides as a Driver of Biodiversity Loss in Agroecosystems? Biol. Conserv. 2017, 209, 449–453. [Google Scholar] [CrossRef]
- Barratt, B.I.P.; Moran, V.C.; Bigler, F.; Van Lenteren, J.C. The Status of Biological Control and Recommendations for Improving Uptake for the Future. BioControl 2018, 63, 155–167. [Google Scholar] [CrossRef]
- Balabanidou, V.; Grigoraki, L.; Vontas, J. Insect Cuticle: A Critical Determinant of Insecticide Resistance. Curr. Opin. Insect Sci. 2018, 27, 68–74. [Google Scholar] [CrossRef]
- Sarwar, M. Insects as Transport Devices of Plant Viruses. In Applied Plant Virology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 381–402. ISBN 978-0-12-818654-1. [Google Scholar]
- Stout, M.J.; Kurabchew, H.; Leite, G.L.D. Host-Plant Resistance in Tomato. In Sustainable Management of Arthropod Pests of Tomato; Elsevier: Amsterdam, The Netherlands, 2018; pp. 217–236. ISBN 978-0-12-802441-6. [Google Scholar]
- Bastos, C.S.; Ribeiro, A.V.; Suinaga, F.A.; Brito, S.M.; Oliveira, A.A.; Barbosa, T.M.; dos Santos, P.D.; Oliveira, D.V.; Teichmann, Y.S. Resistência de Plantas a Insetos: Contextualização e Inserção No MIP. In Avanços Tecnológicos Aplicados à Pesquisa na Produção Vegetal; Universidade Federal de Viçosa: Viçosa, Brazil, 2015; pp. 31–72. [Google Scholar]
- Pickett, J.A.; Woodcock, C.M.; Midega, C.A.; Khan, Z.R. Push–Pull Farming Systems. Curr. Opin. Biotechnol. 2014, 26, 125–132. [Google Scholar] [CrossRef]
- Cook, S.M.; Khan, Z.R.; Pickett, J.A. The Use of Push-Pull Strategies in Integrated Pest Management. Annu. Rev. Entomol. 2007, 52, 375–400. [Google Scholar] [CrossRef] [PubMed]
- Antonious, G.; Snyder, J. Natural Products: Repellency and Toxicity of Wild Tomato Leaf Extracts to the Two-Spotted Spider Mite, Tetranychus urticae Koch. J. Environ. Sci. Health Part B 2006, 41, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Lucatti, A.F.; Meijer-Dekens, F.R.; Mumm, R.; Visser, R.G.; Vosman, B.; Van Heusden, S. Normal Adult Survival but Reduced Bemisia Tabaci Oviposition Rate on Tomato Lines Carrying an Introgression from S. habrochaites. BMC Genet. 2014, 15, 142. [Google Scholar] [CrossRef] [PubMed]
- Conboy, N.J.A.; McDaniel, T.; Ormerod, A.; George, D.; Gatehouse, A.M.R.; Wharton, E.; Donohoe, P.; Curtis, R.; Tosh, C.R. Companion Planting with French Marigolds Protects Tomato Plants from Glasshouse Whiteflies through the Emission of Airborne Limonene. PLoS ONE 2019, 14, e0213071. [Google Scholar] [CrossRef] [PubMed]
- SAS. SAS System, Version 9.1. 3; SAS Institute Inc.: Cary, NC, USA, 2008. [Google Scholar]
- Mutschler, M.A.; Doerge, R.W.; Liu, S.C.; Kuai, J.P.; Liedl, B.E.; Shapiro, J.A. QTL Analysis of Pest Resistance in the Wild Tomato Lycopersicon pennellii: QTLs Controlling Acylsugar Level and Composition. Theor. Appl. Genet. 1996, 92, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Mutschler, M.A.; Kennedy, G.G.; Ullman, D.E. Acylsugar-Mediated Resistance as Part of a Multilayered Defense against Thrips, Orthotospoviruses, and Beyond. Curr. Opin. Insect Sci. 2023, 56, 101021. [Google Scholar] [CrossRef]
- Kortbeek, R.W.J.; Galland, M.D.; Muras, A.; Therezan, R.; Maia, S.; Haring, M.A.; Schuurink, R.C.; Bleeker, P.M. Genetic and Physiological Requirements for High-Level Sesquiterpene-Production in Tomato Glandular Trichomes. Front. Plant Sci. 2023, 14, 1139274. [Google Scholar] [CrossRef]
- Andrade, M.C.; Da Silva, A.A.; Neiva, I.P.; Oliveira, I.R.C.; De Castro, E.M.; Francis, D.M.; Maluf, W.R. Inheritance of Type IV Glandular Trichome Density and Its Association with Whitefly Resistance from Solanum galapagense Accession LA1401. Euphytica 2017, 213, 52. [Google Scholar] [CrossRef]
- Akhtar, K.P.; Saleem, M.Y.; Asghar, M.; Ahmad, M.; Sarwar, N. Resistance of Solanum Species to Cucumber Mosaic Virus Subgroup IA and Its Vector Myzus persicae. Eur. J. Plant Pathol. 2010, 128, 435–450. [Google Scholar] [CrossRef]
- Gentile, A.G.; Stoner, A.K. Resistance in Lycopersicon Spp. to the Tobacco Flea Beetle13. J. Econ. Entomol. 1968, 61, 1347–1349. [Google Scholar] [CrossRef]
- Priyadarshan, P.M. Host Plant Resistance Breeding. In Plant Breeding: Classical to Modern; Springer: Singapore, 2019; pp. 379–412. [Google Scholar]
- Sabesan, T.; Saravanan, K. Breeding Procedures for Developing Insect-Resistant Crops. In Experimental Techniques in Host-Plant Resistance; Kumar Chakravarthy, A., Selvanarayanan, V., Eds.; Springer: Singapore, 2019; pp. 285–293. ISBN 9789811326516. [Google Scholar]
- Zeist, A.R.; de Resende, J.T.; Faria, M.V.; Gabriel, A.; da Silva, I.F.L.; Lima Filho, R.B.D. Base Temperature for Node Emission and Plastochron Determination in Tomato Species and Their Hybrids. Pesqui. Agropecuária Bras. 2018, 53, 307–315. [Google Scholar] [CrossRef]
- Michereff Filho, M.; Lins Junior, J.; Quezado-Duval, A.; Inoue-Nagata, A.; Lima, M. Manejo Integrado de Pragas Do Tomate Para Mesa; Embrapa Hortaliças: Brasília, Brazil, 2022; 59p. [Google Scholar]
- Horowitz, A.R.; Ghanim, M.; Roditakis, E.; Nauen, R.; Ishaaya, I. Insecticide Resistance and Its Management in Bemisia tabaci Species. J. Pest Sci. 2020, 93, 893–910. [Google Scholar] [CrossRef]
- Brasil Ministério da Agricultura. Agrofit. Available online: https://agrofit.agricultura.gov.br/agrofit_cons/principal_agrofit_cons (accessed on 5 November 2023).
- Midega, C.A.O.; Pittchar, J.O.; Pickett, J.A.; Hailu, G.W.; Khan, Z.R. A Climate-Adapted Push-Pull System Effectively Controls Fall Armyworm, Spodoptera frugiperda (J E Smith), in Maize in East Africa. Crop Prot. 2018, 105, 10–15. [Google Scholar] [CrossRef]
- Rakha, M.; Hanson, P.; Ramasamy, S. Identification of Resistance to Bemisia tabaci Genn. in Closely Related Wild Relatives of Cultivated Tomato Based on Trichome Type Analysis and Choice and No-Choice Assays. Genet. Resour. Crop Evol. 2017, 64, 247–260. [Google Scholar] [CrossRef]
- Liu, D.; Trumble, J.T. Interactions of Plant Resistance and Insecticides on the Development and Survival of Bactericerca cockerelli [Sulc] (Homoptera: Psyllidae). Crop Prot. 2005, 24, 111–117. [Google Scholar] [CrossRef]
- Liu, D.; Trumble, J.T. Tomato Psyllid Behavioral Responses to Tomato Plant Lines and Interactions of Plant Lines with Insecticides. J. Econ. Entomol. 2004, 97, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Zeist, A.R.; Faria, M.V.; Resende, J.T.V.; Gabriel, A.; Nonato, J.J.; Santos, M.H.D. Biomass Association in Specimens and Interspecific Hybrids of Tomatoes. Acta Sci. Agron. 2019, 42, e42806. [Google Scholar] [CrossRef]
- Fan, T.; Stewart, B.A.; Yong, W.; Junjie, L.; Guangye, Z. Long-Term Fertilization Effects on Grain Yield, Water-Use Efficiency and Soil Fertility in the Dryland of Loess Plateau in China. Agric. Ecosyst. Environ. 2005, 106, 313–329. [Google Scholar] [CrossRef]
- EMBRAPA Hortaliças, 2006. Cultivo de Tomate Para Industrialização. Available online: https://sistemasdeproducao.cnptia.embrapa.br/FontesHTML/Tomate/TomateIndustrial_2ed/index.htm (accessed on 11 December 2023).
- Food and Agriculture Organizations of the United Nations—FAO Database. Available online: http://www.fao.org/faostat/en/#data (accessed on 11 December 2023).
- Matos, E.S.; Siqueira, W.J.; Lourenção, A.L.; Melo, A.M.T.; Sawazaki, H.E.; Souza-Dias, J.A.C.; Colariccio, A. Resistência de Genótipos de Tomateiro a Um Isolado de Geminivírus Do Cinturão Verde de Campinas, São Paulo. Fitopatol. Bras. 2003, 28, 159–165. [Google Scholar] [CrossRef]
- Parrella, G.; Hochu, I.; Gebre-Selassié, K.; Gognalons, P.; Moretti, A.; Marchoux, G.; Caranta, C. Molecular Tagging of the Am Gene from Lycopersicon hirsutum f. glabratum PI 134417 Using AFLP Markers. Acta Physiol. Plant. 2000, 22, 291–293. [Google Scholar] [CrossRef]
- Esashika, D.A.; Michereff-Filho, M.; Bastos, C.S.; Inoue-Nagata, A.K.; Dias, A.M.; Ribeiro, M.G. Suscetibilidade de Adultos de Bemisia tabaci Biótipo B a Inseticidas. Hortic. Bras. 2016, 34, 189–195. [Google Scholar] [CrossRef]
- Ghosh, S.; Ghanim, M. Factors Determining Transmission of Persistent Viruses by Bemisia tabaci and Emergence of New Virus–Vector Relationships. Viruses 2021, 13, 1808. [Google Scholar] [CrossRef]
- Hanson, A.A.; Koch, R.L. Interactions of Host-Plant Resistance and Foliar Insecticides for Soybean Aphid Management. Crop Prot. 2018, 112, 232–238. [Google Scholar] [CrossRef]
- Khan, Z.R.; Pickett, J.A.; Hassanali, A.; Hooper, A.M.; Midega, C.A.O. Desmodium Species and Associated Biochemical Traits for Controlling Striga Species: Present and Future Prospects. Weed Res. 2008, 48, 302–306. [Google Scholar] [CrossRef]
- Sifres, A.; Blanca, J.; Nuez, F. Pattern of Genetic Variability of Solanum habrochaites in Its Natural Area of Distribution. Genet. Resour. Crop Evol. 2011, 58, 347–360. [Google Scholar] [CrossRef]
- Mankins, J.C. Technology Readiness Levels. White Pap. April 1995, 6, 1995. [Google Scholar]
- Cartalos, O.; Rozakis, S.; Tsiouki, D. A Method to Assess and Support Exploitation Projects of University Researchers. J. Technol. Transf. 2018, 43, 986–1006. [Google Scholar] [CrossRef]
- Araujo Robusti, E.; Godoy Androcioli, H.; Ventura, M.U.; Hata, F.T.; Soares Júnior, D.; Menezes Júnior, A.D.O. Integrated Pest Management versus Conventional System in the Common Bean Crop in Brazil: Insecticide Reduction and Financial Maximization. Int. J. Pest Manag. 2023, 1–11. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).