Blood Parameter Response in Growing Alpine Goat Kids Fed Diets Containing Extruded Flaxseed or Pumpkin Seed Cake
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Management
2.2. Feed Analyses
2.3. Blood Sampling and Analyses
2.4. Statistical Analyses
3. Results
3.1. Average Daily Weight Gain
3.2. Hematological Parameters
3.3. Biochemical Parameters
4. Discussion
4.1. Average Daily Weight Gain
4.2. Hematological Parameters
4.3. Biochemical Parameters
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Habibu, B.; Kawu, M.U.; Makun, H.J.; Aluwong, T.; Yaqub, L.S. Seasonal variation in body mass index, cardinal physiological variables and serum thyroid hormones profiles in relation to susceptibility to thermal stress in goat kids. Small Rumin. Res. 2016, 145, 20–27. [Google Scholar] [CrossRef]
- Antunović, Z.; Mioč, B.; Klir Šalavardić, Ž.; Širić, I.; Držaić, V.; Đidara, M.; Novoselec, J. The effect of lactation stage on the hematological and serum-related biochemical parameters of the Travnik Pramenka ewes. Poljopr. Agric. 2021, 27, 56–62. [Google Scholar] [CrossRef]
- Abdelsattar, M.M.; Vargas-Bello-Pérez, E.; Zhuang, Y.; Fu, Y.; Zhang, N. Effects of age and dietary factors on the blood beta-hydroxybutyric acid, metabolites, immunoglobulins, and hormones of goats. Front. Vet. Sci. 2022, 8, 793427. [Google Scholar] [CrossRef]
- Redlberger, S.; Fischer, S.; Kohler, H.; Diller, R.; Reinhold, P. Age-dependent physiological dynamics in acid-base balance, electrolytes, and blood metabolites in growing goats. Vet. J. 2017, 229, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Fanta, Y.; Kechero, Y.; Yemane, N. Hematological parameters of sheep and goats fed diets containing various amounts of water hyacinth (Eichhornia crassipes). Front. Vet. Sci. 2024, 11, 1286563. [Google Scholar] [CrossRef]
- Antunović, Z.; Novoselec, J.; Klir, Ž. Body growth of goat kids in organic farming. Maced. J. Anim. Sci. 2015, 5, 59–62. [Google Scholar] [CrossRef]
- Ripoll, G.; Alcalde, M.J.; Horcada, A.; Panea, B. Suckling kid breed and slaughter weight discrimination using muscle colour and visible reflectance. Meat Sci. 2011, 87, 151–156. [Google Scholar] [CrossRef]
- Alcalde, M.J.; Ripoll, G.; Campo, M.M.; Horcada, A.; Panea, B. Relationship between consumers’ perceptions about goat kid meat and meat sensory appraisal. Animals 2023, 13, 2383. [Google Scholar] [CrossRef]
- Longobardi, F.; Sacco, D.; Casiello, G.; Ventrella, A.; Contessa, A.; Sacco, A. Garganica kid goat meat: Physico-chemical characterization and nutritional impacts. J. Food Compos. Anal. 2012, 28, 107–113. [Google Scholar] [CrossRef]
- Ivanović, S.; Pavlović, I.; Pisinov, B. The quality of goat meat and it’s impact on human health. Biotechnol. Anim. Husb. 2016, 32, 111–122. [Google Scholar] [CrossRef]
- Gawat, M.; Boland, M.; Singh, J.; Kaur, L. Goat meat: Production and quality attributes. Foods 2023, 12, 3130. [Google Scholar] [CrossRef]
- Pexas, G.; Doherty, B.; Kyriazakis, I. The future of protein sources in livestock feeds: Implications for sustainability and food safety. Front. Sustain. Food Syst. 2023, 7, 1188467. [Google Scholar] [CrossRef]
- Suriyapha, C.; Suntara, C.; Wanapat, M.; Cherdthong, A. Effects of substituting agro-industrial by-products for soybean meal on beef cattle feed utilization and rumen fermentation. Sci. Rep. 2022, 12, 21630. [Google Scholar] [CrossRef] [PubMed]
- Leguizamón, A. Modifying Argentina: GM soy and socio-environmental change. Geoforum 2014, 53, 149–160. [Google Scholar] [CrossRef]
- Nasir, N.A.N.M.; Kamaruddin, S.A.; Zakarya, I.A.; Islam, A.K.M.A. Sustainable alternative animal feeds: Recent advances and future perspective of using azolla as animal feed in livestock, poultry and fish nutrition. Sustain. Chem. Pharm. 2022, 25, 100581. [Google Scholar] [CrossRef]
- Klir, Z.; Castro-Montoya, J.M.; Novoselec, J.; Molkentin, J.; Domacinovic, M.; Mioc, B.; Dickhoefer, U.; Antunovic, Z. Influence of pumpkin seed cake and extruded linseed on milk production and milk fatty acid profile in Alpine goats. Animal 2017, 11, 1772–1778. [Google Scholar] [CrossRef]
- Boldea, I.M.; Dragomir, C.; Gras, M.A.; Ropotă, M. Inclusion of rapeseed and pumpkin seed cakes in diets for Murciano-Granadina goats alters the fatty acid profile of milk. S. Afrn. J. Anim. Sci. 2021, 51, 262–270. [Google Scholar] [CrossRef]
- Antunović, Z.; Klir, Ž.; Šperanda, M.; Sičaja, V.; Čolović, D.; Mioč, B.; Novoselec, J. Partial replacement of soybean meal with pumpkin seed cake in lamb diets: Effects on carcass traits, haemato-chemical parameters and fatty acids in meat. S. Afr. J. Anim. Sci. 2018, 48, 695–704. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, G.N.; Fang, X.P.; Zhao, C.; Wu, H.Y.; Lan, Y.X.; Che, L.; Sun, Y.K.; Lv, J.Y.; Zhang, Y.G.; et al. Effects of replacing soybean meal with pumpkin seed cake and dried distillers grains with solubles on milk performance and antioxidant functions in dairy cows. Animal 2021, 15, 100004. [Google Scholar] [CrossRef]
- Patel, S. Pumpkin (Cucurbita sp.) seeds as nutraceutic: A review on status quo and scopes. Mediterr. J. Nutr. Metab. 2013, 6, 183–189. [Google Scholar] [CrossRef]
- Valdez-Arjona, L.P.; Ramírez-Mella, M. Pumpkin waste as livestock feed: Impact on nutrition and animal health and on quality of meat, milk, and egg. Animals 2019, 9, 769. [Google Scholar] [CrossRef] [PubMed]
- Pospišil, M. Ratarstvo, II-Dio-Industrijsko Bilje [Plant Production, IInd Part-Industrial Plants]; Zrinski d.d.: Čakovec, Croatia, 2013; p. 84. [Google Scholar]
- Colonna, M.A.; Karatosidi, D.; Cosentino, C.; Freschi, P.; Carbonara, C.; Giannico, F.; Losacco, C.; Tufarelli, V.; Tarricone, S.; Selvaggi, M.; et al. Dietary supplementation with oregano and linseed in autochthonous “Facciuta Lucana” goats: Effects on meat quality traits in suckling kids. Animals 2023, 13, 3050. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.Y.; Yu, S.C.; Mu, C.T.; Wu, X.D.; Zhang, C.X.; Zhao, J.X.; Zhang, J.X. Replacing soybean meal with flax seed meal: Effects on nutrient digestibility, rumen microbial protein synthesis and growth performance in sheep. Animal 2020, 14, 1841–1848. [Google Scholar] [CrossRef] [PubMed]
- Ababakri, R.; Dayani, O.; Khezri, A.; Naserian, A.A. Effects of extruded flaxseed and dietary rumen undegradable protein on reproductive traits and the blood metabolites in Baluchi ewes. J. Anim. Feed. Sci. 2021, 30, 214–222. [Google Scholar] [CrossRef]
- Nudda, A.; Battacone, G.; Atzori, A.S.; Dimauro, C.; Rassu, S.P.G.; Nicolussi, P.; Bonelli, P.; Pulina, G. Effect of extruded linseed supplementation on blood metabolic profile and milk performance of Saanen goats. Animal 2013, 7, 1464–1471. [Google Scholar] [CrossRef]
- Alves Dutra, P.; Batista Pinto, L.F.; Cardoso-Neto, B.M.; Silva Mendes, C.; Moraes Pinheiro, A.; Pires Barbosa, L.; de Jesus Pereira, T.C.; Pinto de Carvalho, G.G. Flaxseed added to the diet of Alpine goats affects the nutrients intake and blood parameters. Trop. Anim. Health Prod. 2022, 54, 104. [Google Scholar] [CrossRef]
- Klir Šalavardić, Ž.; Novoselec, J.; Ronta, M.; Antunović, Z. Influence of pumpkin seed cake and extruded linseed on production traits of goat kids. Krmiva 2022, 1, 13–22. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids; The National Academies Press: Washington, DC, USA, 2007. [Google Scholar]
- Klir Šalavardić, Ž.; Novoselec, J.; Castro-Montoya, J.M.; Šperanda, M.; Đidara, M.; Molkentin, J.; Mioč, B.; Dickhoefer, U.; Antunović, Z. The effect of dietary pumpkin seed cake and extruded linseed on blood haemato-chemicals and milk quality in Alpine goats during early lactation. Mljekarstvo 2021, 71, 13–24. [Google Scholar] [CrossRef]
- Association of Official Agricultural Chemists, AOAC. Official Methods of Analysis of AOAC International; Association of Analytical Communities: Arlington, VA, USA, 2006. [Google Scholar]
- Menke, K.H.; Raab, L.; Salewski, A.; Steingass, H.; Fritz, D.; Schneider, W. The estimation of the digestibility and metabolizable energy content of ruminant feedingstuffs from the gas production when they are incubated with rumen liquor in vitro. J. Agr. Sci. 1979, 93, 217–222. [Google Scholar] [CrossRef]
- SAS; Version 9.4; SAS Institute Inc.: Cary, NC, USA.
- Mahouachi, M.; Mathlouthi, N.; Saïdi, C.; Atti, N. The effect of increasing extruded linseed level on nutrient digestibility, growth, carcass characteristics, and non-carcass components of lambs from two genotypes. Trop. Anim. Health Prod. 2023, 56, 1. [Google Scholar] [CrossRef]
- Novoselec, J.; Klir, Ž.; Steiner, Z.; Ronta, M.; Sičaja, V.; Antunović, Z. Production—Hematological parameters of lambs fed with diets containing pumpkin seed cake. Krmiva 2018, 59, 85–94. [Google Scholar] [CrossRef]
- Călin, I.; Răducută, I.; Dărăban, S.; Vlad, I.; Priseceanu, H.I.; Pascal, C.; Pădeanu, I. Research on quantitative skills in meat production direction at youth goats from Carpathian breed in relation with the rearing system. Agric. Agric. Sci. Procedia 2015, 6, 191–196. [Google Scholar] [CrossRef]
- Panayotov, D.; Sevov, S.; Georgiev, D. Live weight and intensity of growth of lambs from Lacaune breed raised in Bulgaria. Bulg. J. Agric. Sci. 2018, 24, 88–94. [Google Scholar]
- Vasta, V.; Nudda, A.; Cannas, A.; Lanza, M.; Priolo, A. Alternative feed resources and their effects on the quality of meat and milk from small ruminants. Anim. Feed. Sci. Technol. 2008, 147, 223–246. [Google Scholar] [CrossRef]
- Balogh, T.; Kőszegi, I.; Hoyk, E. The market of functional foods. Gradus 2020, 7, 161–166. [Google Scholar] [CrossRef]
- Abu El-Hamd, M.A.; El-Diahy, Y.M.; El-Maghraby, M.M.; Elshora, M.A. Effect of flaxseed oil on digestibility, blood parameters, immuno-response and productive performance of suckling friesian calves. J. Anim. Poult. Prod Mansoura Univ. 2015, 6, 755–765. [Google Scholar] [CrossRef]
- Al-Zuhairy, M.A.; Taher, M.G. Effects of feeding different levels of flaxseed on Performance traits and blood parameters in broiler. Diyala Agr. Sci. J. DASJ 2014, 6, 1–10. [Google Scholar]
- Calder, P.C.; Yaqoob, P.; Thies, F.; Wallace, F.A.; Mile, E.A. Fatty acids and lymphocyte functions. Brit. J. Nutr. 2002, 87, S31–S48. [Google Scholar] [CrossRef]
- Gandra, J.R.; Barletta, R.V.; Mingoti, R.D.; Verdurico, L.C.; Freitas, J.E.; Oliveira, L.J.; Takiya, C.S.; Kfoury, J.R.; Wiltbank, M.C.; Renno, F.P. Effects of whole flaxseed, raw soybeans, and calcium salts of fatty acids on measures of cellular immune function of transition dairy cows. J. Dairy Sci. 2016, 99, 4590–4606. [Google Scholar] [CrossRef]
- Calder, P.C. Mechanisms of action of (n-3) fatty acids. J. Nutr. 2012, 142, 592S–599S. [Google Scholar] [CrossRef]
- Lee, S.I.; Kang, K.S. Omega-3 fatty acids modulate cyclophosphamide induced markers of immunosuppression and oxidative stress in pigs. Sci. Rep. 2019, 9, 2684. [Google Scholar] [CrossRef] [PubMed]
- Momeni-Pooya, F.; Kazemi-Bonchenari, M.; Mirzaei, M.; Hossein Yazdi, M. Effects of linseed oil supplementation in Holstein dairy calves received starters based on either corn or barley grain on growth performance and immune response. J. Anim. Physiol. Anim. Nutr. 2023, 107, 329–339. [Google Scholar] [CrossRef] [PubMed]
- El-Saadany, A.S.; El-Barbary, A.M.; Shreif, E.Y.; Elkomy, A.; Khalifah, A.M.; El-Sabrout, K. Pumpkin and garden cress seed oils as feed additives to improve the physiological and productive traits of laying hens. Ital. J. Anim. Sci. 2022, 21, 1047–1057. [Google Scholar] [CrossRef]
- Jackson, P.G.G.; Cockcroft, P.D. Clinical Examination of Farm Animals; Blackwell Science Ltd.: Hoboken, NJ, USA, 2002; pp. 302–305. [Google Scholar]
- Lee, S.M.; Kyum Kim, H.; Lee, H.B.; Kwon, O.D.; Lee, E.B.; Cho, C.S.; Choi, Y.J.; Kang, S.K. Effects of flaxseed supplementation on omega-6 to omega-3 fatty acid ratio, lipid mediator profile, proinflammatory cytokines and stress indices in laying hens. J. Appl. Anim. Res. 2021, 49, 460–471. [Google Scholar] [CrossRef]
- Wijffels, G.; Sullivan, M.L.; Stockwell, S.; Briscoe, S.; Pearson, R.; Li, Y.; Macs, A.M.; Sejian, V.; McCulloch, R.; Olm, J.C.W.; et al. Comparing the responses of grain-fed feedlot cattle under moderate heat load and during subsequent recovery with those of feed-restricted thermoneutral counterparts: Blood cells and inflammatory markers. Int. J. Biometeorol. 2024, 68, 211–227. [Google Scholar] [CrossRef]
- Jain, N.C. Essentials of Veterinary Hematology; Lea & Febiger: Philadelphia, PA, USA, 1993; p. 417. [Google Scholar]
- Souza, D.F.; Paula, E.F.E.; Fernandes, S.R.; Regonato Franco, D.; Oliveira Koch, M.; Locatelli-Dittrich, R.; Barros Filho, I.R.; Gomes Monteiro, A.L. Dynamics of hematological parameters in female lambs during the first four months of life. Semin. Ciências Agrárias Londrina 2018, 39, 2465–2476. [Google Scholar] [CrossRef]
- Grundy, S.M. Cholesterol: Factors Determining Blood Levels. In Encyclopedia of Human Nutrition, 3rd ed.; Caballero, B., Ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 335–340. [Google Scholar]
- Hossein Abadi, M.; Ghoorchi, T.; Amirteymouri, E.; Poorghasemi, M. The effect of different processing methods of linseed on growth performance, nutrient digestibility, blood parameters and ruminate behaviour of lambs. Vet. Med. Sci. 2023, 9, 1771–1780. [Google Scholar] [CrossRef]
- Huerta, A.E. Role of Omega-3 Fatty Acids in Metabolic Syndrome. In Omega-3 Fatty Acids. Keys to Nutritional Health; Hegdje, M.W., Zanwar, A.A., Adekar, S.P., Eds.; Springer International Publishing: Cham, Switzerland, 2016; p. 198. [Google Scholar]
- Ockenden, E.M.; Russo, V.M.; Leury, B.J.; Giri, K.; Wales, W.J. Preweaning nutrition and its effects on the growth, immune competence and metabolic characteristics of the dairy calf. Animals 2023, 13, 829. [Google Scholar] [CrossRef]
- Mohapatra, A.; De, K.; Kumar Saxena, V.; Kumar Mallick, P.; Devi, I.; Singh, R. Behavioral and physiological adjustments by lambs in response to weaning stress. J. Vet. Behav. 2021, 41, 47–51. [Google Scholar] [CrossRef]
- Khan, M.A.; Lee, H.J.; Lee, W.S.; Kim, H.S.; Ki, K.S.; Hur, T.Y.; Suh, G.H.; Kang, S.J.; Choi, Y.J. Structural growth, rumen development, and metabolic and immune responses of Holstein male calves fed milk through step-down and conventional methods. J. Dairy Sci. 2007, 90, 3376–3387. [Google Scholar] [CrossRef]
- Qugley, J.D.; Caldwell, L.A.; Sinks, D.; Heitmann, R.N. Changes in blood glucose, nonesterified fatty acids, and ketones in response to weaning and feed intake in young calves. J. Dairy Sci. 1991, 74, 74250–74257. [Google Scholar] [CrossRef] [PubMed]
- Deelen, S.M.; Leslie, K.E.; Steele, M.A.; Eckert, E.; Brown, H.E.; DeVries, T.J. Validation of a calf-side β-hydroxybutyrate test and its utility for estimation of starter intake in dairy calves around weaning. J. Dairy Sci. 2016, 99, 7624–7633. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, A.O.; Ajayi, T.O.; Ajayi, O.S.; Yusuf, O.A. Nutritional manipulation in goats: Supplementation of high protein concentrate, effect on performance and resilience of internal parasites. Niger. J. Anim. Prod. 2019, 46, 193–201. [Google Scholar] [CrossRef]
- Osman, O.A.; Elkhair, N.M.; Abdoun, K.A. Effects of dietary supplementation with different concentration of molasses on growth performance, blood metabolites and rumen fermentation indices of Nubian goats. BMC Vet. Res. 2020, 16, 411. [Google Scholar] [CrossRef]
- Souza, D.F.; Reijers, T.S.S.S.; Gilaverte, S.; Cruz, T.A.; Hentz, F.; Castilhos, B.Q.; Dittrich, R.L.; Monteiro, A.L.G. Dynamics of biochemical parameters in lambs during the first four months of life. Rev. Bras. Zootec. 2020, 49, e20190167. [Google Scholar] [CrossRef]
- Santos, R.P.; Lima Macedo, G.J.; Silva, P.S.; Fernandes de Sousa, L.; Barbosa Andrade, M.E. Inclusion of propylene glycol in the diet of sheep and its effect on their lambs’ protein and mineral metabolites. Acta Sci. Anim. Sci. 2017, 39, 297–302. [Google Scholar] [CrossRef][Green Version]
- Makni, M.; Fetoui, H.; Gargouri, N.K.; Garoui, M.; Jaber, H.; Makni, J.; Boudawara, T.; Zeghal, N. Hypolipidemic and hepatoprotective effects of flax and pumpkin seed mixture rich in ω-3 and ω-6 fatty acids in hypercholesterolemic rats. Food Chem. Toxicol. 2008, 46, 3714–3720. [Google Scholar] [CrossRef] [PubMed]
- Nkosi, C.Z.; Opoku, A.R.; Terblanche, S.E. Effect of pumpkin seed (Cucurbita pepo) protein isolate on the activity levels of certain plasma enzymes in CCl4-induced liver injury in low-protein fed rats. Phytother. Res. 2005, 19, 341–345. [Google Scholar] [CrossRef]
- Nkosi, C.Z.; Opoku, A.R.; Terblanche, S.E. In Vitro antioxidative activity of pumpkin seed (Cucurbita pepo) protein isolate and its In Vivo effect on alanine transaminase and aspartate transaminase in acetaminophen-induced liver injury in low protein fed rats. Phytother. Res. 2006, 20, 780–783. [Google Scholar] [CrossRef]
- He, P.; Hua, H.; Tian, W.; Zhu, H.; Liu, Y.; Xu, X. Holly (Ilex latifolia Thunb.) polyphenols extracts alleviate hepatic damage by regulating ferroptosis following diquat challenge in a piglet model. Front. Nutr. 2020, 7, 604328. [Google Scholar] [CrossRef]
- Peng, K.; Shirley, D.C.; Xu, Z.; Huang, Q.; McAllister, T.A.; Chaves, A.V.; Acharya, S.; Liu, C.; Wang, S.; Wang, Y. Effect of purple prairie clover (Dalea purpurea Vent.) hay and its condensed tannins on growth performance, wool growth, nutrient digestibility, blood metabolites and ruminal fermentation in lambs fed total mixed rations. Anim. Feed. Sci. Technol. 2016, 222, 100–110. [Google Scholar] [CrossRef]
- Śpitalniak-Bajerska, K.; Szumny, A.; Pogoda-Sewerniak, K.; Kupczyński, R. Effects of n-3 fatty acids on growth, antioxidant status, and immunity of preweaned dairy calves. J. Dairy Sci. 2020, 103, 2864–2876. [Google Scholar] [CrossRef] [PubMed]
- Gangal, S. Modulation of immune response by omega-3 in health and disease. In Omega-3 Fatty Acids. Keys to Nutritional Health; Hegdje, M.W., Zanwar, A.A., Adekar, S.P., Eds.; Springer International Publishing: Cham, Switzerland, 2016; p. 308. [Google Scholar]
- Sembratowicz, I.; Zięba, G.; Cholewinska, E.; Czech, A. Effect of dietary flaxseed oil supplementation on the redox status, haematological and biochemical parameters of horses’ blood. Animals 2020, 10, 2244. [Google Scholar] [CrossRef] [PubMed]
- Al-Madhagy, S.; Ashmawy, N.S.; Mamdouh, A.; Eldahshan, O.A.; Farag, M.A. A comprehensive review of the health benefits of flaxseed oil in relation to its chemical composition and comparison with other omega-3-rich oils. Eur. J. Med. Res. 2023, 28, 240. [Google Scholar] [CrossRef] [PubMed]
- Barda, S.; Turki, M.; Khedir, S.B.; Mzid, M.; Rebai, T.; Ayadi, F.; Sahnoun, Z. The Effect of prickly pear, pumpkin, and linseed oils on biological mediators of acute inflammation and oxidative stress markers. BioMed Res. Int. 2020, 5643465, 11. [Google Scholar] [CrossRef]
- Domínguez-López, I.; Arancibia-Riveros, C.; Casas, R.; Tresserra-Rimbau, A.; Razquin, C.; Martínez-González, M.Á.; Hu, F.B.; Ros, E.; Fitó, M.; Estruch, R.; et al. Changes in plasma total saturated fatty acids and palmitic acid are related to pro-inflammatory molecule IL-6 concentrations after nutritional intervention for one year. Biomed. Pharmacother. 2022, 150, 113028. [Google Scholar] [CrossRef]
- Chen, S.J.; Yen, C.H.; Huang, Y.C.; Lee, B.J.; Hsia, S.; Lin, P.T. Relationships between inflammation, adiponectin, and oxidative stress in metabolic syndrome. PLoS ONE 2012, 7, e45693. [Google Scholar] [CrossRef]
- Li, P.; Li, L.; Zhang, C.; Cheng, X.; Zhang, Y.; Guo, Y.; Long, M.; Yang, S.; He, J. Palmitic acid and β-hydroxybutyrate induce inflammatory responses in bovine endometrial cells by activating oxidative stress-mediated NF-κB signaling. Molecules 2019, 24, 2421. [Google Scholar] [CrossRef]
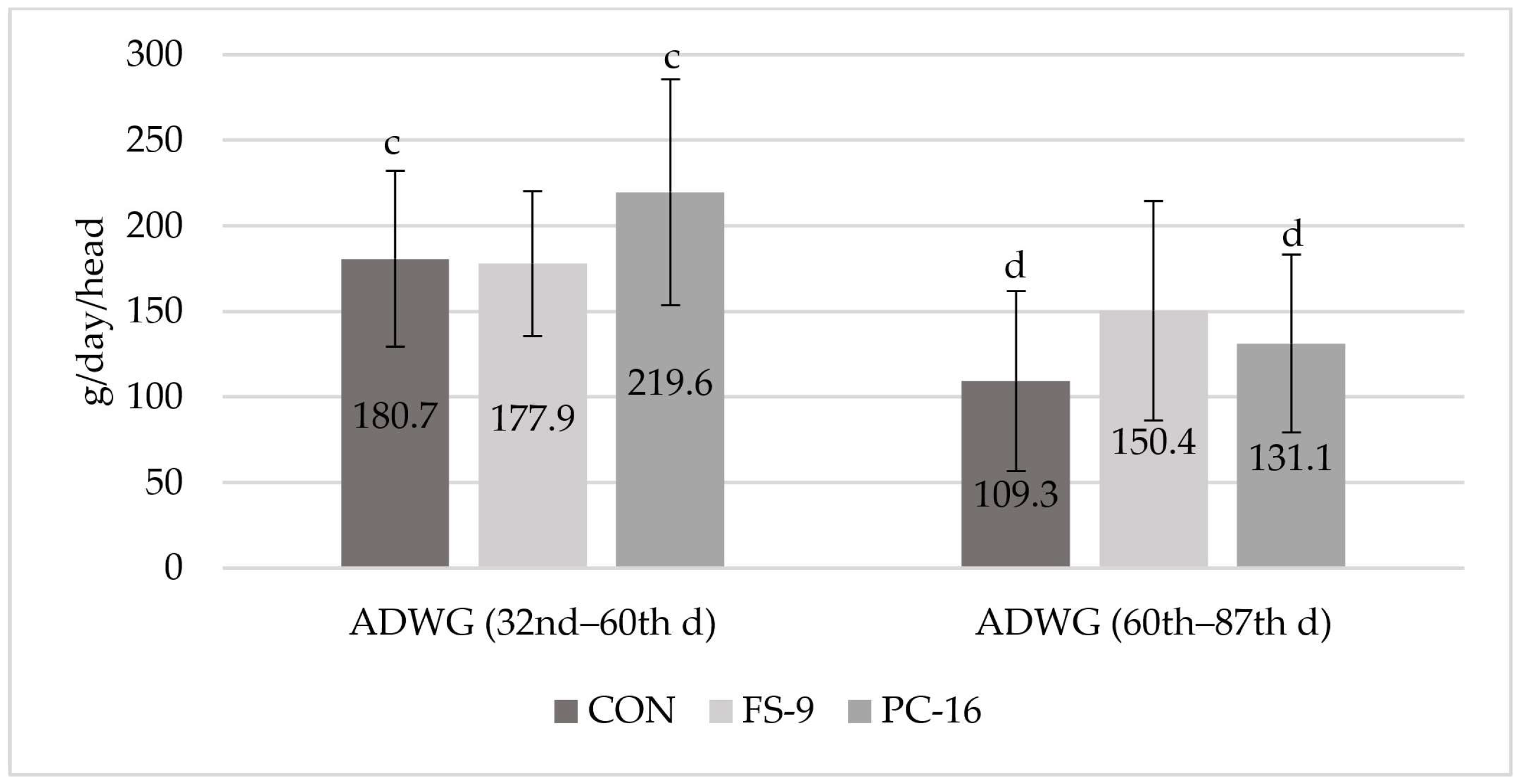
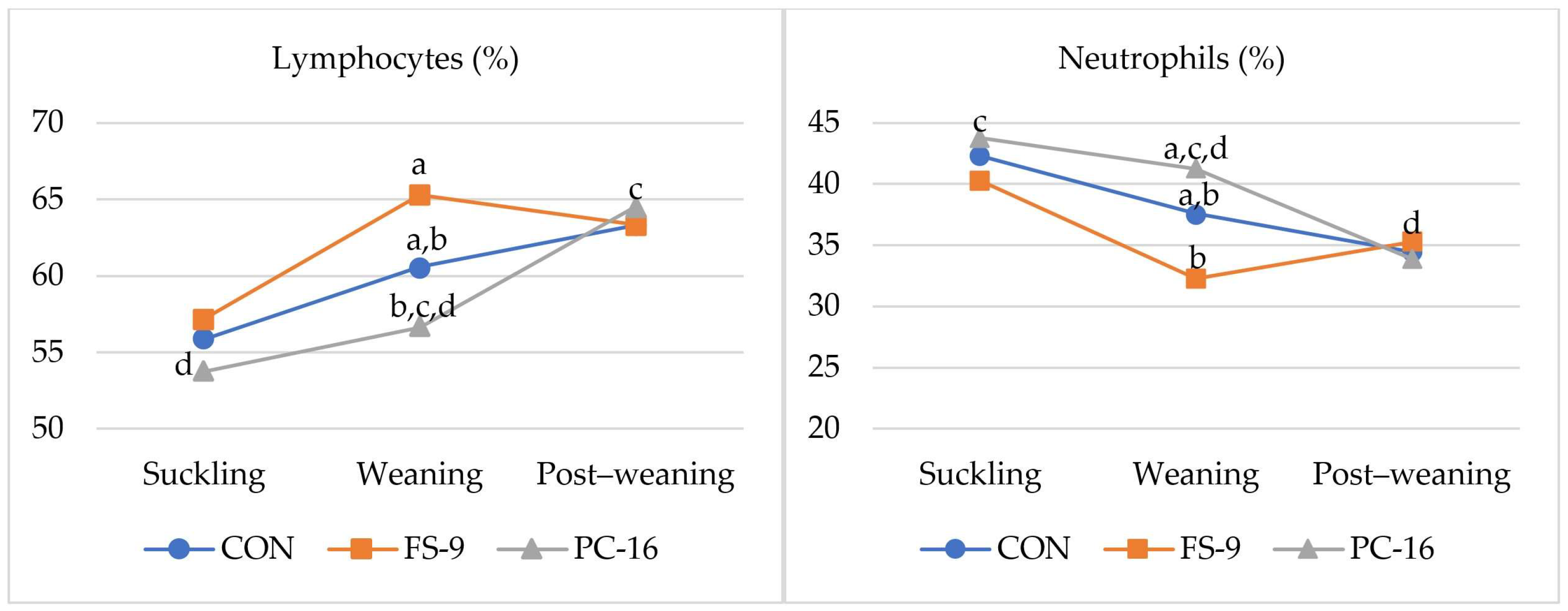
| Trait | Diets | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | FS-9 | PC-16 | |||||||
| Goat kids (n) | 9 | 11 | 11 | ||||||
| Age of goat kids (days) | 32 | 60 | 87 | 32 | 60 | 87 | 32 | 60 | 87 |
| Growth period | Suckling | Weaning | Post-weaning | Suckling | Weaning | Post-weaning | Suckling | Weaning | Post-weaning |
| Production traits 1 | |||||||||
| Live body weight (kg) | 7.48 | 12.54 | 15.49 | 8.07 | 13.05 | 17.11 | 8.23 | 14.38 | 17.92 |
| ADWG, 32nd–87th day (g) | 145.64 | 164.21 | 163.77 | ||||||
| Feeding | |||||||||
| Suckling milk | Ad libitum | Restricted 2 | - | Ad libitum | Restricted 2 | - | Ad libitum | Restricted 2 | - |
| Feed mixture | - | 0% extruded flaxseed or pumpkin seed cake | - | 9% extruded flaxseed | - | 16% pumpkin seed cake | |||
| Ad libitum | ~200 g | Ad libitum | ~200 g | Ad libitum | ~200 g | ||||
| Hay | - | Ad libitum | - | Ad libitum | - | Ad libitum | |||
| Ingredient, % | Concentrate Mixture | Hay | ||
|---|---|---|---|---|
| CON | FS-9 | PC-16 | ||
| Corn grain | 42.9 | 40.8 | 45.9 | |
| Barley grain | 8.0 | 8.0 | 9.0 | |
| Oat grain | 10.0 | 10.0 | 13.5 | |
| Wheat flour | 12.0 | 9.0 | 12.0 | |
| Extruded soybean | 15.0 | - | - | |
| Extruded linseed | - | 9.0 | - | |
| Pumpkin seed cake | - | - | 16.0 | |
| Alfalfa dehydrated | - | 4.0 | - | |
| Soybean meal (46% crude protein) | 8.5 | 15.7 | - | |
| Calcium carbonate | 1.6 | 1.5 | 1.6 | |
| Monocalcium phosphate | 0.5 | 0.5 | 0.5 | |
| Salt | 0.4 | 0.4 | 0.4 | |
| Pellet binder | 0.1 | 0.1 | 0.1 | |
| Mineral vitamin premix 1 | 1.0 | 1.0 | 1.0 | |
| Chemical composition, % | ||||
| DM (% fresh matter) | 87.6 | 87.4 | 87.3 | 92.5 |
| Crude protein | 16.2 | 16.2 | 16.3 | 11.0 |
| Crude fiber | 4.14 | 4.88 | 3.73 | 28.7 |
| Crude ash | 4.92 | 5.06 | 5.23 | 5.73 |
| Crude lipid | 5.64 | 5.83 | 5.63 | 1.33 |
| ME (MJ/kg DM) | 13.2 | 13.0 | 13.2 | 8.0 |
| Mineral composition (mg/kg DM) | ||||
| Ca | 8949 | 8108 | 8548 | 1572 |
| P | 7100 | 6147 | 6818 | 1410 |
| Mg | 2114 | 1944 | 2096 | 480 |
| Fe | 321 | 278 | 294 | 92.8 |
| Fatty acids (g/100 g FAME) | ||||
| C16:0 | 10.7 | 9.43 | 12.9 | 29.80 |
| C18:0 | 4.08 | 6.11 | 4.48 | 2.84 |
| C18:1 n-9 | 31.9 | 32.0 | 34.4 | 7.71 |
| C18:2 n-6 | 48.2 | 38.2 | 44.4 | 22.40 |
| C18:3 n-3 | 2.97 | 11.50 | 1.80 | 24.50 |
| Parameters | Growth Period | Diet | SEM | p Value 1 | ||
|---|---|---|---|---|---|---|
| CON | FS-9 | PC-16 | ||||
| WBCs (×109 L) | Suckling | 6.44 d | 8.49 d | 8.76 d | 0.530 | 0.185 |
| Weaning | 6.61 b,d | 9.84 a,c,d | 9.54 a,c,d | 0.455 | 0.005 | |
| Post-weaning | 10.76 c | 12.09 c | 12.58 c | 0.563 | 0.468 | |
| p value 2 | 0.011 | 0.003 | 0.027 | |||
| RBCs (×1012 L) | Suckling | 10.18 d | 11.35 | 11.85 | 0.503 | 0.442 |
| Weaning | 11.63 c,d | 12.00 | 13.03 | 0.505 | 0.556 | |
| Post-weaning | 13.54 c | 13.43 | 14.53 | 0.393 | 0.481 | |
| p value 2 | 0.017 | 0.232 | 0.064 | |||
| HGB (g/L) | Suckling | 85.86 | 83.20 d | 86.38 d | 3.568 | 0.927 |
| Weaning | 85.14 | 90.91 c,d | 96.38 c,d | 3.115 | 0.410 | |
| Post-weaning | 103.0 | 103.9 c | 110.3 c | 2.715 | 0.538 | |
| p value 2 | 0.076 | 0.038 | 0.013 | |||
| HCT (L/L) | Suckling | 0.485 | 0.540 | 0.408 | 0.032 | 0.231 |
| Weaning | 0.558 | 0.560 | 0.501 | 0.043 | 0.824 | |
| Post-weaning | 0.467 | 0.599 | 0.417 | 0.043 | 0.186 | |
| p value 2 | 0.720 | 0.851 | 0.323 | |||
| MCV (fL) | Suckling | 49.90 | 52.39 | 38.01 | 4.829 | 0.444 |
| Weaning | 56.77 | 60.33 | 40.74 | 5.422 | 0.302 | |
| Post-weaning | 48.20 | 61.55 | 36.31 | 5.200 | 0.117 | |
| p value 2 | 0.804 | 0.730 | 0.919 | |||
| MCH (pg) | Suckling | 8.02 | 7.38 | 7.33 | 0.178 | 0.050 |
| Weaning | 7.41 | 7.73 | 7.45 | 0.129 | 0.545 | |
| Post-weaning | 7.63 | 7.83 | 7.60 | 0.109 | 0.638 | |
| p value 2 | 0.111 | 0.203 | 0.562 | |||
| MCHC (g/L) | Suckling | 201.6 | 174.6 | 216.3 | 13.652 | 0.439 |
| Weaning | 159.9 | 165.1 | 214.8 | 14.990 | 0.290 | |
| Post-weaning | 194.0 | 158.5 | 241.9 | 15.001 | 0.079 | |
| p value 2 | 0.570 | 0.897 | 0.609 | |||
| Parameters, mmol/L | Growth Period | Diet | SEM | p Value 1 | ||
|---|---|---|---|---|---|---|
| CON | FS-9 | PC-16 | ||||
| Glucose | Suckling | 3.22 | 3.34 | 4.17 | 0.213 | 0.163 |
| Weaning | 3.80 | 3.94 | 4.29 | 0.195 | 0.621 | |
| Post-weaning | 3.94 | 3.94 | 4.03 | 0.108 | 0.926 | |
| p value 2 | 0.314 | 0.316 | 0.749 | |||
| Cholesterol | Suckling | 2.54 | 2.60 | 2.41 | 0.159 | 0.896 |
| Weaning | 2.27 | 2.21 | 2.40 | 0.130 | 0.830 | |
| Post-weaning | 2.66 | 3.05 | 2.86 | 0.191 | 0.740 | |
| p value 2 | 0.689 | 0.084 | 0.474 | |||
| TGCs | Suckling | 0.423 | 0.422 | 0.339 | 0.029 | 0.430 |
| Weaning | 0.291 | 0.323 | 0.384 | 0.033 | 0.569 | |
| Post-weaning | 0.397 | 0.395 | 0.454 | 0.037 | 0.780 | |
| p value 2 | 0.282 | 0.387 | 0.455 | |||
| HDL | Suckling | 1.49 | 1.58 | 1.36 | 0.066 | 0.384 |
| Weaning | 1.32 | 1.30 | 1.38 | 0.061 | 0.856 | |
| Post-weaning | 1.48 | 1.54 | 1.51 | 0.066 | 0.944 | |
| p value 2 | 0.633 | 0.088 | 0.654 | |||
| LDL | Suckling | 0.856 | 0.823 c,d | 0.900 | 0.094 | 0.949 |
| Weaning | 0.813 | 0.765 d | 0.846 | 0.071 | 0.893 | |
| Post-weaning | 0.998 | 1.33 c | 1.15 | 0.124 | 0.583 | |
| p value 2 | 0.788 | 0.035 | 0.433 | |||
| NEFAs | Suckling | 1.37 | 1.50 c | 1.69 c | 0.188 | 0.819 |
| Weaning | 0.590 | 0.242 d | 0.185 d | 0.077 | 0.111 | |
| Post-weaning | 0.443 | 0.324 d | 0.113 d | 0.103 | 0.485 | |
| p value 2 | 0.104 | <0.001 | <0.001 | |||
| BHB | Suckling | 0.463 | 0.396 c | 0.498 c | 0.033 | 0.436 |
| Weaning | 0.283 | 0.191 d | 0.210 d | 0.029 | 0.451 | |
| Post-weaning | 0.373 a | 0.229 b,d | 0.268 a,b,d | 0.023 | 0.036 | |
| p value 2 | 0.197 | <0.001 | 0.004 | |||
| Parameters (g/L) | Growth Period | Diet | SEM | p Value 1 | ||
|---|---|---|---|---|---|---|
| CON | FS-9 | PC-16 | ||||
| Urea (mmol/L) | Suckling | 4.15 | 4.90 | 4.06 | 0.236 | 0.254 |
| Weaning | 3.96 | 3.86 | 3.74 | 0.261 | 0.953 | |
| Post-weaning | 4.72 | 4.57 | 3.77 | 0.297 | 0.413 | |
| p value 2 | 0.614 | 0.271 | 0.793 | |||
| Proteins | Suckling | 53.21 d | 53.48 d | 57.11 d | 0.855 | 0.129 |
| Weaning | 53.53 d | 54.45 d | 53.64 d | 0.831 | 0.883 | |
| Post-weaning | 61.48 c | 64.42 c | 64.31 c | 0.786 | 0.303 | |
| p value 2 | 0.010 | <0.001 | <0.001 | |||
| Albumin | Suckling | 28.84 | 30.81 | 29.23 | 0.472 | 0.178 |
| Weaning | 29.07 | 28.75 | 29.09 | 0.378 | 0.766 | |
| Post-weaning | 30.45 | 30.44 | 31.00 | 0.362 | 0.787 | |
| p value 2 | 0.305 | 0.081 | 0.151 | |||
| Globulin | Suckling | 24.37 c,d | 22.67 d | 27.88 d | 0.940 | 0.057 |
| Weaning | 24.07 d | 25.70 d | 24.55 d | 0.679 | 0.605 | |
| Post-weaning | 31.03 c | 33.98 c | 33.31 c | 0.712 | 0.266 | |
| p value 2 | 0.032 | <0.001 | <0.001 | |||
| Ca (mmol/L) | Suckling | 2.36 | 2.45 | 2.54 | 0.029 | 0.053 |
| Weaning | 2.41 | 2.45 | 2.38 | 0.020 | 0.352 | |
| Post-weaning | 2.48 | 2.41 | 2.25 | 0.083 | 0.589 | |
| p value 2 | 0.191 | 0.680 | 0.415 | |||
| Mg (mmol/L) | Suckling | 0.931 | 0.967 | 0.880 d | 0.018 | 0.112 |
| Weaning | 0.869 | 0.945 | 0.960 d | 0.015 | 0.050 | |
| Post-weaning | 0.959 | 0.990 | 1.06 c | 0.020 | 0.144 | |
| p value 2 | 0.094 | 0.552 | <0.001 | |||
| Fe (μmol/L) | Suckling | 28.64 | 26.32 | 27.09 | 2.863 | 0.952 |
| Weaning | 30.67 | 26.68 | 33.58 | 2.567 | 0.333 | |
| Post-weaning | 25.48 | 23.26 | 22.69 | 1.259 | 0.710 | |
| p value 2 | 0.554 | 0.740 | 0.123 | |||
| P-inorganic (mmol/L) | Suckling | 3.06 | 3.15 | 3.10 | 0.078 | 0.906 |
| Weaning | 3.12 | 3.08 | 3.22 | 0.064 | 0.669 | |
| Post-weaning | 3.49 | 2.96 | 3.17 | 0.087 | 0.057 | |
| p value 2 | 0.187 | 0.491 | 0.809 | |||
| Enzyme (U/L) | Growth Period | Diet | SEM | p Value 1 | ||
|---|---|---|---|---|---|---|
| CON | FS-9 | PC-16 | ||||
| Aspartate aminotransferase (AST) | Suckling | 71.17 | 71.38 | 68.73 | 2.525 | 0.905 |
| Weaning | 97.15 | 80.72 | 74.57 | 5.984 | 0.377 | |
| Post-weaning | 101.06 | 82.81 | 77.19 | 3.974 | 0.072 | |
| p value 2 | 0.223 | 0.383 | 0.369 | |||
| Alanine aminotransferase (ALT) | Suckling | 11.76 | 18.78 | 20.45 | 2.885 | 0.498 |
| Weaning | 16.27 | 17.73 | 14.07 | 2.514 | 0.841 | |
| Post-weaning | 25.38 | 20.54 | 18.14 | 3.610 | 0.771 | |
| p value 2 | 0.251 | 0.927 | 0.694 | |||
| γ-glutamyl transferase (GGT) | Suckling | 34.23 d | 37.52 | 36.20 | 1.823 | 0.791 |
| Weaning | 43.97 c,d | 43.63 | 41.56 | 2.079 | 0.894 | |
| Post-weaning | 48.40 a,c | 39.52 a,b | 38.65 b | 1.606 | 0.040 | |
| p value 2 | 0.009 | 0.400 | 0.507 | |||
| Superoxide dismutase (SOD, U/mL) | Suckling | 0.340 | 0.402 | 0.464 | 0.066 | 0.803 |
| Weaning | 0.383 | 0.501 | 0.609 | 0.071 | 0.524 | |
| Post-weaning | 0.769 | 0.617 | 0.716 | 0.089 | 0.789 | |
| p value 2 | 0.209 | 0.323 | 0.610 | |||
| Glutathione peroxidase (GPx) | Suckling | 960.1 | 1061.4 d | 914.7 | 62.739 | 0.610 |
| Weaning | 1011.1 | 1104.2 c | 893.6 | 63.785 | 0.396 | |
| Post-weaning | 700.2 a,b | 809.7 a,d | 600.8 b | 35.716 | 0.039 | |
| p value 2 | 0.154 | 0.008 | 0.119 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klir Šalavardić, Ž.; Novoselec, J.; Đidara, M.; Antunović, Z. Blood Parameter Response in Growing Alpine Goat Kids Fed Diets Containing Extruded Flaxseed or Pumpkin Seed Cake. Agriculture 2024, 14, 1667. https://doi.org/10.3390/agriculture14101667
Klir Šalavardić Ž, Novoselec J, Đidara M, Antunović Z. Blood Parameter Response in Growing Alpine Goat Kids Fed Diets Containing Extruded Flaxseed or Pumpkin Seed Cake. Agriculture. 2024; 14(10):1667. https://doi.org/10.3390/agriculture14101667
Chicago/Turabian StyleKlir Šalavardić, Željka, Josip Novoselec, Mislav Đidara, and Zvonko Antunović. 2024. "Blood Parameter Response in Growing Alpine Goat Kids Fed Diets Containing Extruded Flaxseed or Pumpkin Seed Cake" Agriculture 14, no. 10: 1667. https://doi.org/10.3390/agriculture14101667
APA StyleKlir Šalavardić, Ž., Novoselec, J., Đidara, M., & Antunović, Z. (2024). Blood Parameter Response in Growing Alpine Goat Kids Fed Diets Containing Extruded Flaxseed or Pumpkin Seed Cake. Agriculture, 14(10), 1667. https://doi.org/10.3390/agriculture14101667







