The Dynamics of Soil Macropores and Hydraulic Conductivity as Influenced by the Fibrous and Tap Root Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Soil Samples
2.3. X-ray CT Scanning of Soil Samples, Image Processing, and Analysis
2.4. Measure of Saturated Hydraulic Conductivity
2.5. Statistical Analysis
3. Results
3.1. Soil Macropore Characteristics in Columns with the Two Plants
3.1.1. Macropore Indices in the Soil Columns with the Two Plants at Different Stages
3.1.2. Comparison of Macropore Indices of the Upper and Lower Layers
3.1.3. Variation in Number of Macropores with Depth
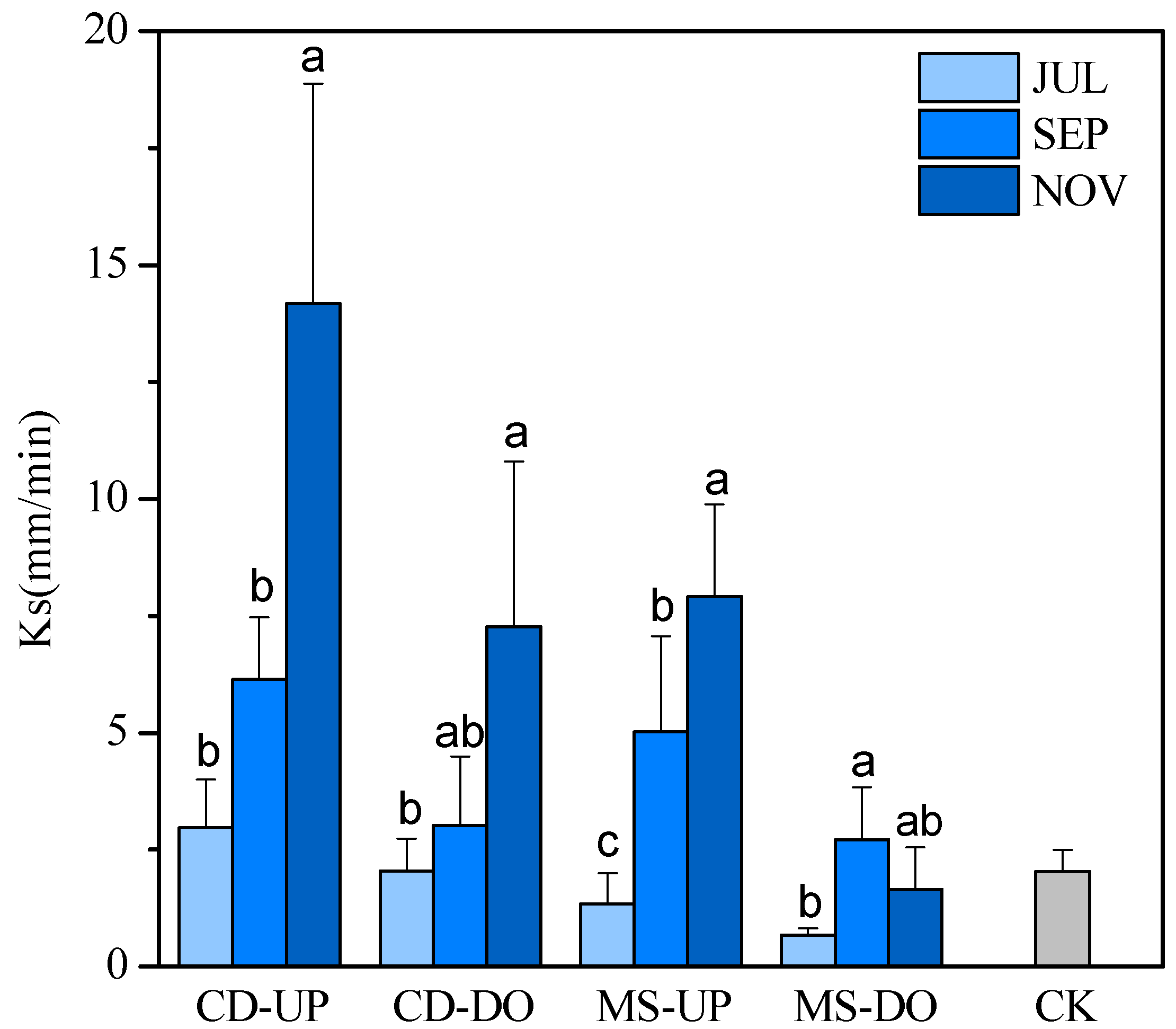
3.2. Trends in Saturated Hydraulic Conductivity (Ks)
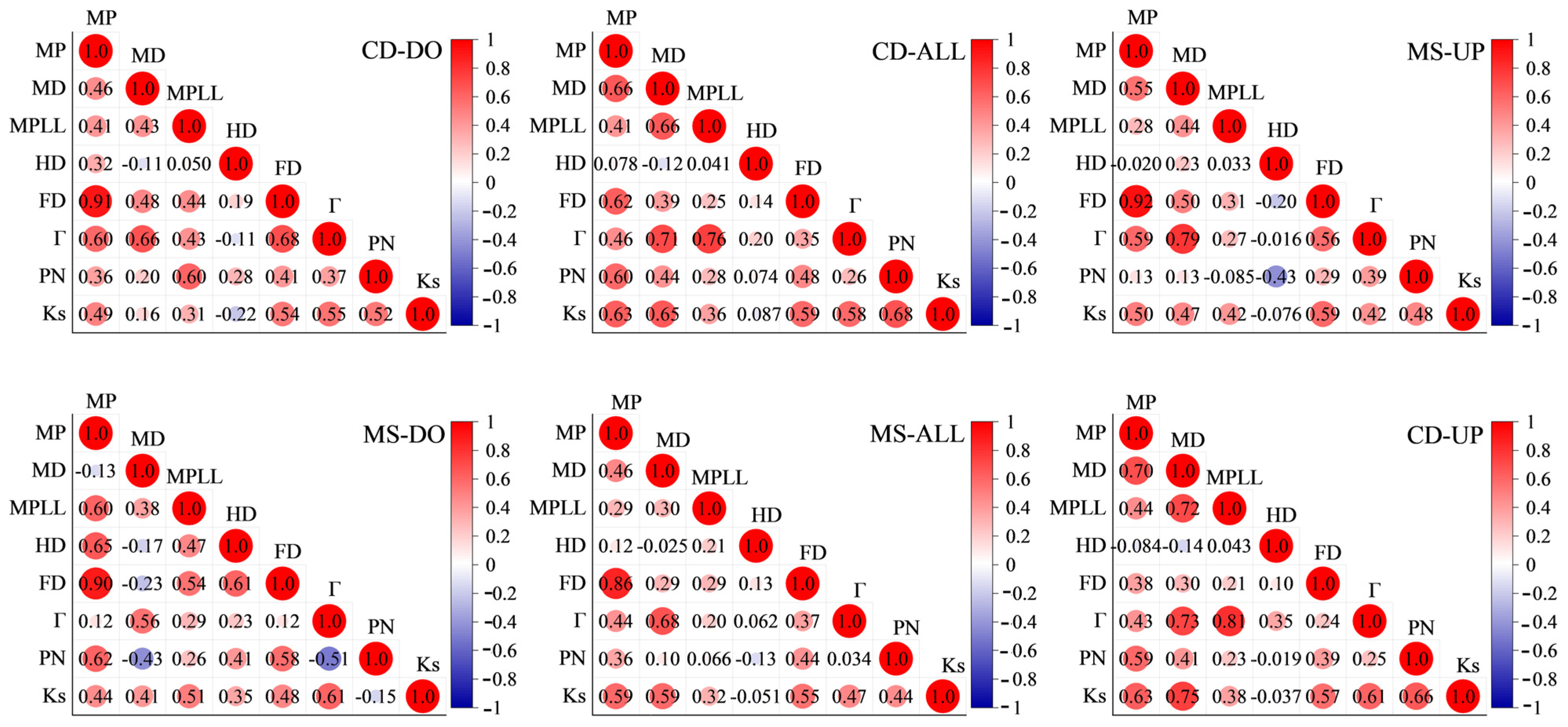
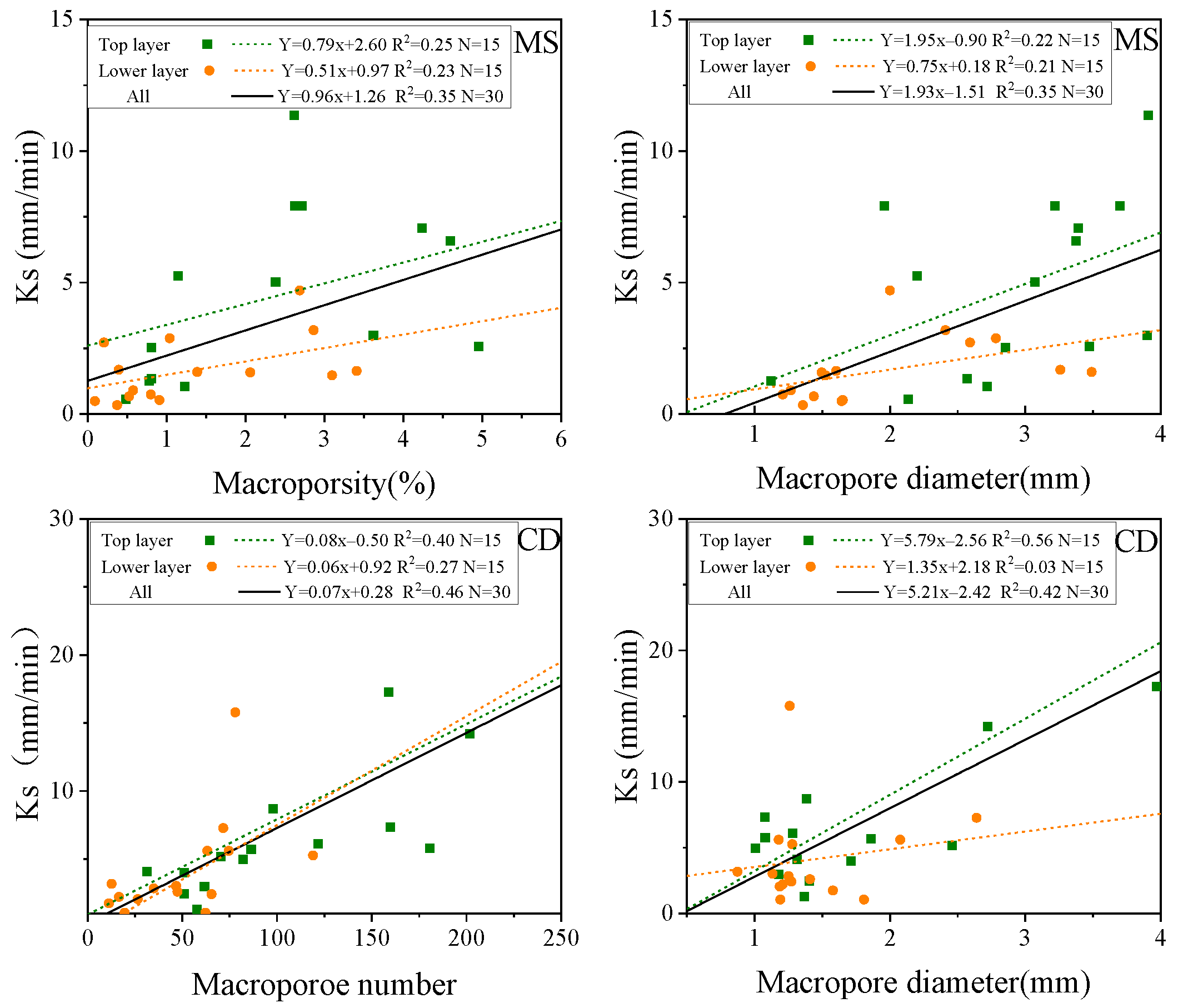
3.3. Relationships between Macropore Indexes and Saturated Hydraulic Conductivity
4. Discussion
4.1. The Response of Soil Macropores to the Growth of the Two Plant Species
4.2. The Mechanism That Determines Soil Hydraulic Conductivity with the Two Plants
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsvetnov, E.V.; Makarov, O.A.; Strokov, A.S.; Tsvetnova, O.B. The Role of Soils in Land Degradation Assessment: A Review. Eurasian Soil Sci. 2021, 54, 441–447. [Google Scholar] [CrossRef]
- Prats, S.A.; Malvar, M.C.; Coelho, C.O.A.; Wagenbrenner, J.W. Hydrologic and erosion responses to compaction and added surface cover in post-fire logged areas: Isolating splash, interrill and rill erosion. J. Hydrol. 2019, 575, 408–419. [Google Scholar] [CrossRef]
- Luo, J.; Zheng, Z.; Li, T.; He, S. Changes in micro-relief during different water erosive stages of purple soil under simulated rainfall. Sci. Rep. 2018, 8, 3483. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhong, Y.; Han, G.; Li, X.; Luo, L.; Cai, X.; Long, X.; Li, T.; Huang, L. Effect of different vegetation restoration on soil organic carbon dynamics and fractions in the Rainy Zone of Western China. J. Environ. Manag. 2023, 331, 117296. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Islam, M.; Elahi, T.E. Effectiveness of vetiver grass on stabilizing hill slopes: A numerical approach. In Geo-Congress 2020; American Society of Civil Engineers: Reston, VA, USA, 2020. [Google Scholar]
- Wang, B.; Zhang, G.; Shi, Y.; Li, Z.; Shan, Z. Effects of Near Soil Surface Characteristics on the Soil Detachment Process in a Chronological Series of Vegetation Restoration. Soil Sci. Soc. Am. J. 2015, 79, 1213–1222. [Google Scholar] [CrossRef]
- Hao, M.; Zhang, J.; Meng, M.; Chen, H.Y.H.; Guo, X.; Liu, S.; Ye, L. Impacts of changes in vegetation on saturated hydraulic conductivity of soil in subtropical forests. Sci. Rep. 2019, 9, 8372. [Google Scholar] [CrossRef]
- Stokes, A.; Douglas, G.B.; Fourcaud, T.; Giadrossich, F.; Gillies, C.; Hubble, T.; Kim, J.H.; Loades, K.W.; Mao, Z.; McIvor, I.R.; et al. Ecological mitigation of hillslope instability: Ten key issues facing researchers and practitioners. Plant Soil 2014, 377, 1–23. [Google Scholar] [CrossRef]
- Liu, M.; Cui, W.; Wu, D.; Liao, L.; Du, W. Soil Macropore Structures and their Effect on Preferential Flow. Appl. Mech. Mater. 2014, 522–524, 990–994. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Cao, L.; Qin, X.; Hou, D. Temporal variation in soil macropore properties and hydraulic conductivity in croplands in the dry-hot valley region of Southwest China. J. Soil Sediment. 2024, 24, 1576–1590. [Google Scholar] [CrossRef]
- Budhathoki, S.; Lamba, J.; Srivastava, P.; Williams, C.; Arriaga, F.; Karthikeyan, K.G. Impact of land use and tillage practice on soil macropore characteristics inferred from X-ray computed tomography. Catena 2022, 210, 105886. [Google Scholar] [CrossRef]
- Yu, X.; Fu, Y.; Lu, S. Characterization of the pore structure and cementing substances of soil aggregates by a combination of synchrotron radiation X-ray micro-computed tomography and scanning electron microscopy. Eur. J. Soil Sci. 2017, 68, 66–79. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, K.; Zhou, H.; Lin, H.; Li, D.; Peng, X. Linking saturated hydraulic conductivity and air permeability to the characteristics of biopores derived from X-ray computed tomography. J. Hydrol. 2019, 571, 1–10. [Google Scholar] [CrossRef]
- Budhathoki, S.; Lamba, J.; Srivastava, P.; Malhotra, K.; Way, T.R.; Katuwal, S. Temporal and spatial variability in 3D soil macropore characteristics determined using X-ray computed tomography. J. Soil Sediment. 2022, 22, 1263–1277. [Google Scholar] [CrossRef]
- Ju, X.; Jia, Y.; Li, T.; Gao, L.; Gan, M. Morphology and multifractal characteristics of soil pores and their functional implication. Catena 2021, 196, 104822. [Google Scholar] [CrossRef]
- Dal Ferro, N.; Sartori, L.; Simonetti, G.; Berti, A.; Morari, F. Soil macro- and microstructure as affected by different tillage systems and their effects on maize root growth. Soil Tillage Res. 2014, 140, 55–65. [Google Scholar] [CrossRef]
- Udawatta, R.P.; Anderson, S.H. CT-measured pore characteristics of surface and subsurface soils influenced by agroforestry and grass buffers. Geoderma 2008, 145, 381–389. [Google Scholar] [CrossRef]
- Naveed, M.; Moldrup, P.; Arthur, E.; Wildenschild, D.; Eden, M.; Lamandé, M.; Vogel, H.; de Jonge, L.W. Revealing Soil Structure and Functional Macroporosity along a Clay Gradient Using X-ray Computed Tomography. Soil Sci. Soc. Am. J. 2013, 77, 403–411. [Google Scholar] [CrossRef]
- Udawatta, R.P.; Anderson, S.H.; Gantzer, C.J.; Garrett, H.E. Agroforestry and Grass Buffer Influence on Macropore Characteristics. Soil Sci. Soc. Am. J. 2006, 70, 1763–1773. [Google Scholar] [CrossRef]
- Kim, H.; Anderson, S.H.; Motavalli, P.P.; Gantzer, C.J. Compaction effects on soil macropore geometry and related parameters for an arable field. Geoderma 2010, 160, 244–251. [Google Scholar] [CrossRef]
- Ben-Hur, M.; Yolcu, G.; Uysal, H.; Lado, M.; Paz-González, A. Soil structure changes: Aggregate size and soil texture effects on hydraulic conductivity under different saline and sodic conditions. Soil Res. 2009, 47, 688–696. [Google Scholar] [CrossRef]
- Gregory, P.J. RUSSELL REVIEW Are plant roots only “in” soil or are they “of” it? Roots, soil formation and function. Eur. J. Soil Sci. 2022, 73, e13219. [Google Scholar] [CrossRef]
- Shougrakpam, S.; Sarkar, R.; Dutta, S. An experimental investigation to characterise soil macroporosity under different land use and land covers of northeast India. J. Earth Syst. Sci. 2010, 119, 655–674. [Google Scholar] [CrossRef]
- Hu, X.; Li, X.; Wang, P.; Liu, Y.; Wu, X.; Li, Z.; Zhao, Y.; Cheng, Y.; Guo, L.; Lyu, Y.; et al. Influence of exclosure on CT-measured soil macropores and root architecture in a shrub-encroached grassland in northern China. Soil Tillage Res. 2019, 187, 21–30. [Google Scholar] [CrossRef]
- Lucas, M.; Nguyen, L.; Guber, A.; Kravchenko, A. Cover crop influence on pore size distribution and biopore dynamics: Enumerating root and soil faunal effects. Front. Plant Sci. 2022, 13, 928569. [Google Scholar] [CrossRef]
- Perkons, U.; Kautz, T.; Uteau, D.; Peth, S.; Geier, V.; Thomas, K.; Lütke Holz, K.; Athmann, M.; Pude, R.; Köpke, U. Root-length densities of various annual crops following crops with contrasting root systems. Soil Tillage Res. 2014, 137, 50–57. [Google Scholar] [CrossRef]
- Lu, E.; Yang, B.; Liu, W.; Zhu, X. Herbs perform better than woody plants at improving soil hydrological properties in rubber agroforestry systems. Agroforest Syst. 2023, 97, 1391–1404. [Google Scholar] [CrossRef]
- Liu, C.; Qiang, F.; Liu, G.; Ai, N.; Gao, R. Characteristics of understory herb communities across time during restoration in coal mine reclamation areas and their coupling with soil properties. Acta Pratacult. Sin. 2022, 31, 61–68. [Google Scholar] [CrossRef]
- Federica, G.; Chiara, V.; Rodolfo, G.; Anne, B.; Pierre, C.; Sandra, C.; Chiaradia, E.A. Root Characteristics of Herbaceous Species for Topsoil Stabilization in Restoration Projects. Land. Degrad. Dev. 2017, 28, 2074–2085. [Google Scholar] [CrossRef]
- Löbmann, M.T.; Geitner, C.; Wellstein, C.; Zerbe, S. The influence of herbaceous vegetation on slope stability—A review. Earth-Sci. Rev. 2020, 209, 103328. [Google Scholar] [CrossRef]
- Qin, M.; Cui, P.; Jiang, Y.; Guo, J.; Zhang, G.; Ramzan, M. Occurrence of shallow landslides triggered by increased hydraulic conductivity due to tree roots. Landslides 2022, 19, 2593–2604. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, J.; Zhang, M.; Wang, J. Effect of Cynodon dactylon community on the conservation and reinforcement of riparian shallow soil in the Three Gorges Reservoir area. Ecol. Process 2015, 4, 3. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, Y.; Cui, Z.; Fang, Y.; He, H.; Liu, B.; Wu, G. Soil water storage deficit of alfalfa (Medicago sativa) grasslands along ages in arid area (China). Field Crops Res. 2018, 221, 1–6. [Google Scholar] [CrossRef]
- Kang, Z.; Lou, G.; Guo, Y.; Xu, P. The anti-erosion potential of taproots and fibrous roots in alluvial loess of north China: A pot experiment. J. Soil Sediment. 2024, 24, 847–862. [Google Scholar] [CrossRef]
- Bacq-Labreuil, A.; Crawford, J.; Mooney, S.J.; Neal, A.L.; Ritz, K. Cover crop species have contrasting influence upon soil structural genesis and microbial community phenotype. Sci. Rep-Uk 2019, 9, 7473. [Google Scholar] [CrossRef]
- Zhu, P.; Zhang, G.; Zhang, B. Soil saturated hydraulic conductivity of typical revegetated plants on steep gully slopes of Chinese Loess Plateau. Geoderma 2022, 412, 115717. [Google Scholar] [CrossRef]
- Zhang, J.; Ran, Y.; Ma, D.; Chen, L.; Wu, Y.; Huang, P. Dynamic Characteristics of Cynodon Dactylon Root Growth and Its Influence on Soil Pore Evolution. Acta Pedol. Sin. 2024, in press. [Google Scholar]
- Li, J.; He, B.; Chen, Y.; Huang, R.; Tao, J.; Tian, T. Root distribution features of typical herb plants for slope protection and their effects on soil shear strength. Nongye Gongcheng Xuebao/Trans. Chin. Soc. Agric. Eng. 2013, 29, 144–152. [Google Scholar] [CrossRef]
- Iassonov, P.; Gebrenegus, T.; Tuller, M. Segmentation of X-ray computed tomography images of porous materials: A crucial step for characterization and quantitative analysis of pore structures. Water Resour. Res. 2009, 45. [Google Scholar] [CrossRef]
- Meira Cássaro, F.A.; Posadas Durand, A.N.; Gimenez, D.; Pedro Vaz, C.M. Pore-Size Distributions of Soils Derived using a Geometrical Approach and Multiple Resolution MicroCT Images. Soil Sci. Soc. Am. J. 2017, 81, 468–476. [Google Scholar] [CrossRef]
- Neerad, P.; Sumit, M.; Ashish, S.; Madhuri, J. Adaptive local thresholding for detection of nuclei in diversity stained cytology images. In Proceedings of the 2011 International Conference on Communications and Signal Processing, Kerala, India, 10–12 February 2011; pp. 218–220. [Google Scholar]
- Larsbo, M.; Koestel, J.; Jarvis, N. Relations between macropore network characteristics and the degree of preferential solute transport. Hydrol. Earth Syst. Sc. 2014, 18, 5255–5269. [Google Scholar] [CrossRef]
- Zhao, S.; Zhao, Y.; Wu, J. Quantitative analysis of soil pores under natural vegetation successions on the Loess Plateau. Sci. China Earth Sci. 2010, 53, 617–625. [Google Scholar] [CrossRef]
- Bagarello, V.; Sgroi, A. Using the simplified falling head technique to detect temporal changes in field-saturated hydraulic conductivity at the surface of a sandy loam soil. Soil Tillage Res. 2007, 94, 283–294. [Google Scholar] [CrossRef]
- Nijp, J.J.; Metselaar, K.; Limpens, J.; Gooren, H.P.A.; van der Zee, S.E.A.T. A modification of the constant-head permeameter to measure saturated hydraulic conductivity of highly permeable media. Methodsx 2017, 4, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, J. Prediction of the soil saturated hydraulic conductivity in a mining area based on CT scanning technology. J. Clean. Prod. 2023, 383, 135364. [Google Scholar] [CrossRef]
- Joshi, D.C.; Iden, S.C.; Peters, A.; Das, B.S.; Durner, W. Temperature Dependence of Soil Hydraulic Properties: Transient Measurements and Modeling. Soil Sci. Soc. Am. J. 2019, 83, 1628–1636. [Google Scholar] [CrossRef]
- Tang, Q.; Duan, X.; He, L.; Liao, D.; Cai, C.; Deng, Y. Variability and driving factors of the soil saturated hydraulic conductivity along the horizontal and vertical directions in the upper catchment of Benggang. Catena 2023, 222, 106810. [Google Scholar] [CrossRef]
- Athmann, M.; Kautz, T.; Pude, R.; Köpke, U. Root growth in biopores—Evaluation with in situ endoscopy. Plant Soil 2013, 371, 179–190. [Google Scholar] [CrossRef]
- Li, Z.; Hu, X.; Li, X. Characterization of Root Architectures and Soil Macropore Networks Under Different Ecosystems Using X-ray CT Scanning in the Qinghai Lake Watershed, NE Qinghai–Tibet Plateau. J. Soil Sci. Plant Nut. 2019, 19, 743–757. [Google Scholar] [CrossRef]
- Han, E.; Kautz, T.; Perkons, U.; Lüsebrink, M.; Pude, R.; Köpke, U. Quantification of soil biopore density after perennial fodder cropping. Plant Soil 2015, 394, 73–85. [Google Scholar] [CrossRef]
- Valentine, T.A.; Hallett, P.D.; Binnie, K.; Young, M.W.; Squire, G.R.; Hawes, C.; Bengough, A.G. Soil strength and macropore volume limit root elongation rates in many UK agricultural soils. Ann. Bot. Bot. 2012, 110, 259–270. [Google Scholar] [CrossRef]
- Bodner, G.; Leitner, D.; Kaul, H.P. Coarse and fine root plants affect pore size distributions differently. Plant Soil 2014, 380, 133–151. [Google Scholar] [CrossRef] [PubMed]
- Uteau, D.; Pagenkemper, S.K.; Peth, S.; Horn, R. Root and time dependent soil structure formation and its influence on gas transport in the subsoil. Soil Tillage Res. 2013, 132, 69–76. [Google Scholar] [CrossRef]
- Yu, Y.; Loiskandl, W.; Kaul, H.; Himmelbauer, M.; Wei, W.; Chen, L.; Bodner, G. Estimation of runoff mitigation by morphologically different cover crop root systems. J. Hydrol. 2016, 538, 667–676. [Google Scholar] [CrossRef]
- Chen, J.; Wu, Z.; Zhao, T.; Yang, H.; Long, Q.; He, Y. Rotation crop root performance and its effect on soil hydraulic properties in a clayey Utisol. Soil Tillage Res. 2021, 213, 105136. [Google Scholar] [CrossRef]
- Jiang, X.; Zhong, X.; Yu, G.; Zhang, X.; Liu, J. Different effects of taproot and fibrous root crops on pore structure and microbial network in reclaimed soil. Sci. Total Environ. 2023, 901, 165996. [Google Scholar] [CrossRef]
- Bai, W.; Zuo, Q.; Huang, Y.; Li, B. Effect of water supply on root growth and water uptake of alfalfa in wulanbuhe sandy region. Chin. J. Plant Ecol. 2001, 25, 29–35. [Google Scholar]
- Zhong, R.; Hu, J.; Bao, Y.; Wang, F.; He, X. Soil nutrients in relation to vertical roots distribution in the riparian zone of Three Gorges Reservoir, China. J. Mt. Sci. 2018, 15, 1498–1509. [Google Scholar] [CrossRef]
- Katuwal, S.; Norgaard, T.; Moldrup, P.; Lamandé, M.; Wildenschild, D.; de Jonge, L.W. Linking air and water transport in intact soils to macropore characteristics inferred from X-ray computed tomography. Geoderma 2015, 237–238, 9–20. [Google Scholar] [CrossRef]
- Baets, S.D.; Poesen, J.; Knapen, A.; Galindo, P. Impact of root architecture on the erosion-reducing potential of roots during concentrated flow. Earth Surf. Proc. Land. 2007, 32, 1323–1345. [Google Scholar] [CrossRef]
- Chen, G.; Weil, R.R. Penetration of cover crop roots through compacted soils. Plant Soil 2010, 331, 31–43. [Google Scholar] [CrossRef]
- Ghestem, M.; Sidle, R.C.; Stokes, A. The Influence of Plant Root Systems on Subsurface Flow: Implications for Slope Stability. BioScience 2011, 61, 869–879. [Google Scholar] [CrossRef]
- Li, J.; Yuan, X.; Ge, L.; Li, Q.; Li, Z.; Wang, L.; Liu, Y. Rhizosphere effects promote soil aggregate stability and associated organic carbon sequestration in rocky areas of desertification. Agric. Ecosyst. Environ. 2020, 304, 107126. [Google Scholar] [CrossRef]
- Xiao, T.; Li, P.; Fei, W.; Wang, J. Effects of vegetation roots on the structure and hydraulic properties of soils: A perspective review. Sci. Total Environ. 2024, 906, 167524. [Google Scholar] [CrossRef]
- Zhu, P.; Zhang, G.; Wang, H.; Xing, S. Soil infiltration properties affected by typical plant communities on steep gully slopes on the Loess Plateau of China. J. Hydrol. 2020, 590, 125535. [Google Scholar] [CrossRef]
- Rasse, D.P.; Smucker, A.J.M.; Santos, D. Alfalfa Root and Shoot Mulching Effects on Soil Hydraulic Properties and Aggregation. Soil Sci. Soc. Am. J. 2000, 64, 725–731. [Google Scholar] [CrossRef]


| Plant Species | Month | MP (%) | MPLL (%) | MD (mm) | HD (mm) | FD | Γ |
|---|---|---|---|---|---|---|---|
| CK | 0.55 ± 0.07 | 0.04 ± 0.005 | 1.10 ± 0.26 | 0.14 ± 0.001 | 1.46 ± 0.14 | 0.10 ± 0.04 | |
| CD | July | 0.60 ± 0.19 b | 0.06 ± 0.01 b | 1.36 ± 0.09 a | 0.23 ± 0.005 b | 1.57 ± 0.13 b | 0.12 ± 0.02 a |
| Sep | 1.77 ± 0.28 a | 0.41 ± 0.11 a | 1.51 ± 0.15 a | 0.23 ± 0.006 b | 2.07 ± 0.08 a | 0.21 ± 0.06 a | |
| Nov | 2.27 ± 0.55 a | 0.69 ± 0.38 a | 1.94 ± 0.33 a | 0.26 ± 0.008 a | 2.08 ± 0.13 a | 0.27 ± 0.07 a | |
| MS | July | 0.70 ± 0.10 b | 0.20 ± 0.06 b | 1.86 ± 0.66 b | 0.23 ± 0.003 | 1.63 ± 0.30 b | 0.29 ± 0.06 b |
| Sep | 2.39 ± 0.52 ab | 1.34 ± 0.29 a | 2.99 ± 0.65 a | 0.23 ± 0.003 | 2.02 ± 0.54 ab | 0.55 ± 0.09 a | |
| Nov | 3.05 ± 0.65 a | 1.31 ± 0.28 a | 2.48 ± 0.99 a | 0.25 ± 0.002 | 2.31 ± 0.61 a | 0.45 ± 0.09 a |
| Plant Species | ROI Depth (cm) | Value | MP (%) | MPLL (%) | MD (mm) | HD (mm) | FD | Γ |
|---|---|---|---|---|---|---|---|---|
| CD | 0–5 | Mean | 2.02 | 0.43 | 1.78 | 0.25 | 2.03 | 0.20 |
| CV% | 68.38 | 97.22 | 45.13 | 14.92 | 42.82 | 100.42 | ||
| 5–10 | Mean | 1.08 | 0.34 | 1.42 | 0.24 | 1.78 | 0.19 | |
| CV% | 92.37 | 94.00 | 38.81 | 20.51 | 35.44 | 52.46 | ||
| MS | 0–5 | Mean | 2.73 | 0.98 | 2.91 | 0.24 | 2.16 | 0.49 |
| CV% | 71.09 | 97.65 | 25.61 | 10.26 | 20.18 | 42.97 | ||
| 5–10 | Mean | 1.36 | 0.94 | 1.98 | 0.23 | 1.82 | 0.37 | |
| CV% | 81.53 | 78.67 | 36.20 | 5.19 | 32.16 | 76.89 |
| Plant Species | Equations | R2 | p | N |
|---|---|---|---|---|
| CD | Ks = −3.79 + 3.50 MD + 0.05 PN | 0.61 | 0.000 | 30 |
| Ks = −1.61 + 14 Γ+ 0.06 PN | 0.63 | 0.000 | 30 | |
| MS | Ks = −1.36 + 0.65 MP + 1.33 MD | 0.48 | 0.000 | 30 |
| Ks = −4.87 + 2.17 FD +1.54 MD | 0.52 | 0.000 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Pan, H.; Zhang, T.; Cao, L.; Wang, Y. The Dynamics of Soil Macropores and Hydraulic Conductivity as Influenced by the Fibrous and Tap Root Systems. Agriculture 2024, 14, 1676. https://doi.org/10.3390/agriculture14101676
Tang Y, Pan H, Zhang T, Cao L, Wang Y. The Dynamics of Soil Macropores and Hydraulic Conductivity as Influenced by the Fibrous and Tap Root Systems. Agriculture. 2024; 14(10):1676. https://doi.org/10.3390/agriculture14101676
Chicago/Turabian StyleTang, Yixuan, Heping Pan, Ting Zhang, Longxi Cao, and Yi Wang. 2024. "The Dynamics of Soil Macropores and Hydraulic Conductivity as Influenced by the Fibrous and Tap Root Systems" Agriculture 14, no. 10: 1676. https://doi.org/10.3390/agriculture14101676
APA StyleTang, Y., Pan, H., Zhang, T., Cao, L., & Wang, Y. (2024). The Dynamics of Soil Macropores and Hydraulic Conductivity as Influenced by the Fibrous and Tap Root Systems. Agriculture, 14(10), 1676. https://doi.org/10.3390/agriculture14101676





