Transcriptome Analysis Reveals Key Genes and Pathways Associated with Cadmium Stress Tolerance in Solanum aculeatissimum C. B. Clarke
Abstract
:1. Introduction
2. Materials and Methods
2.1. S. aculeatissimum Seed Pretreatment
2.2. Experimental Design
2.3. Soil Heavy Metal Treatment
2.4. Plant Establishment and Management
2.5. Measurement of Seedling Growth Parameters
2.5.1. Determination of Morphological Indicators
2.5.2. Observation of Morphological Indicators
2.6. Content of Membrane Lipid Peroxidation and Antioxidant Enzyme Activity
2.7. Cd Concentration Determination and Transfer Coefficient
2.8. cDNA Library Construction, Deep Sequencing, and De Novo Assembly
2.9. RNA Isolation
2.10. Library Construction and Transcriptome Sequencing
2.11. Gene Expression Level and Differential Analysis
2.12. Functional Analysis of Differentially Expressed Genes GO and KEGG
2.13. Screening and qRT-PCR Validation of Cd Tolerance and Uptake Enrichment Genes in S. aculeatissimum
3. Results
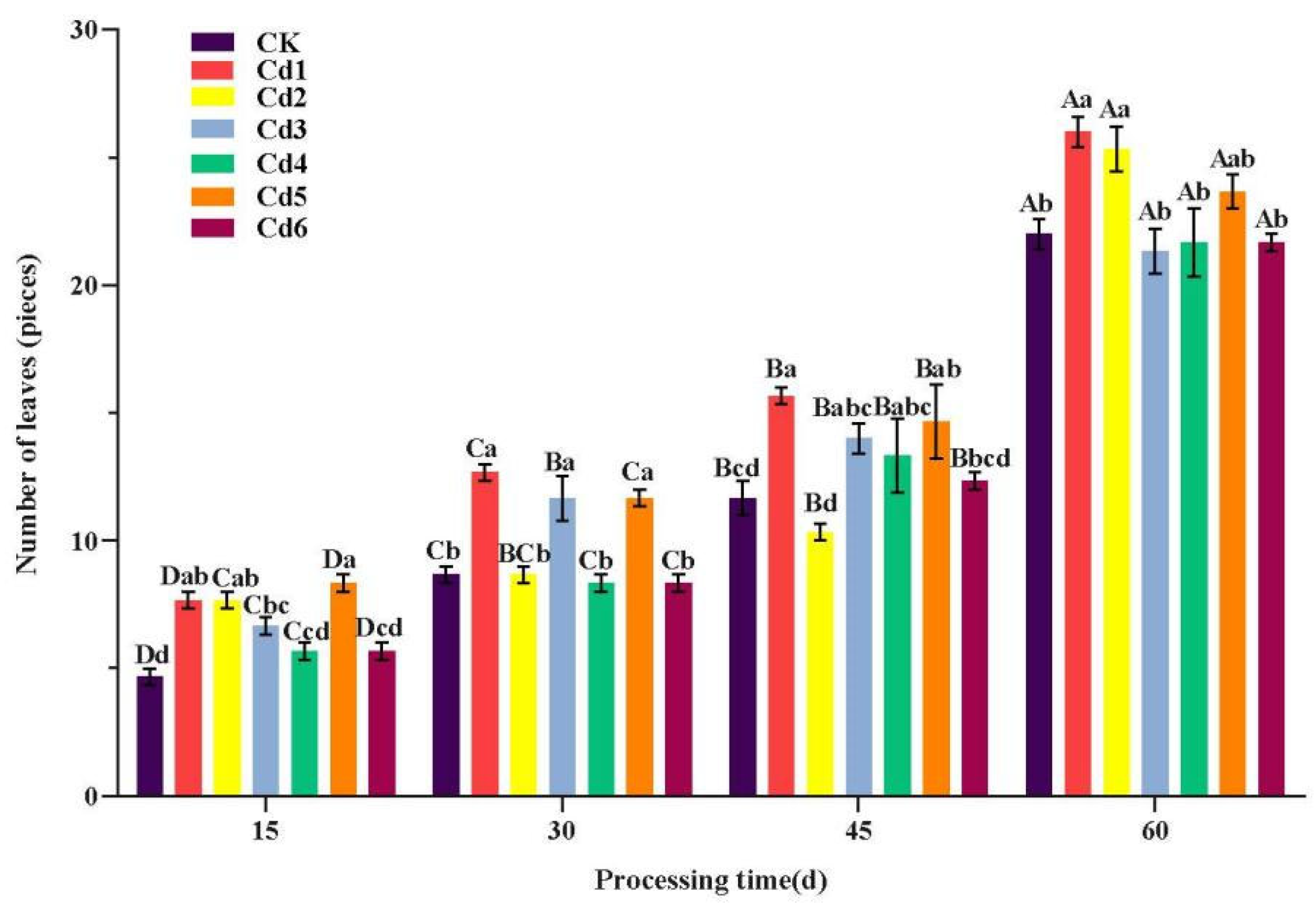
3.1. Response of Plant Growth to Cd Stress
Changes in Phenotypes due to Cd Stress on S. aculeatissimum
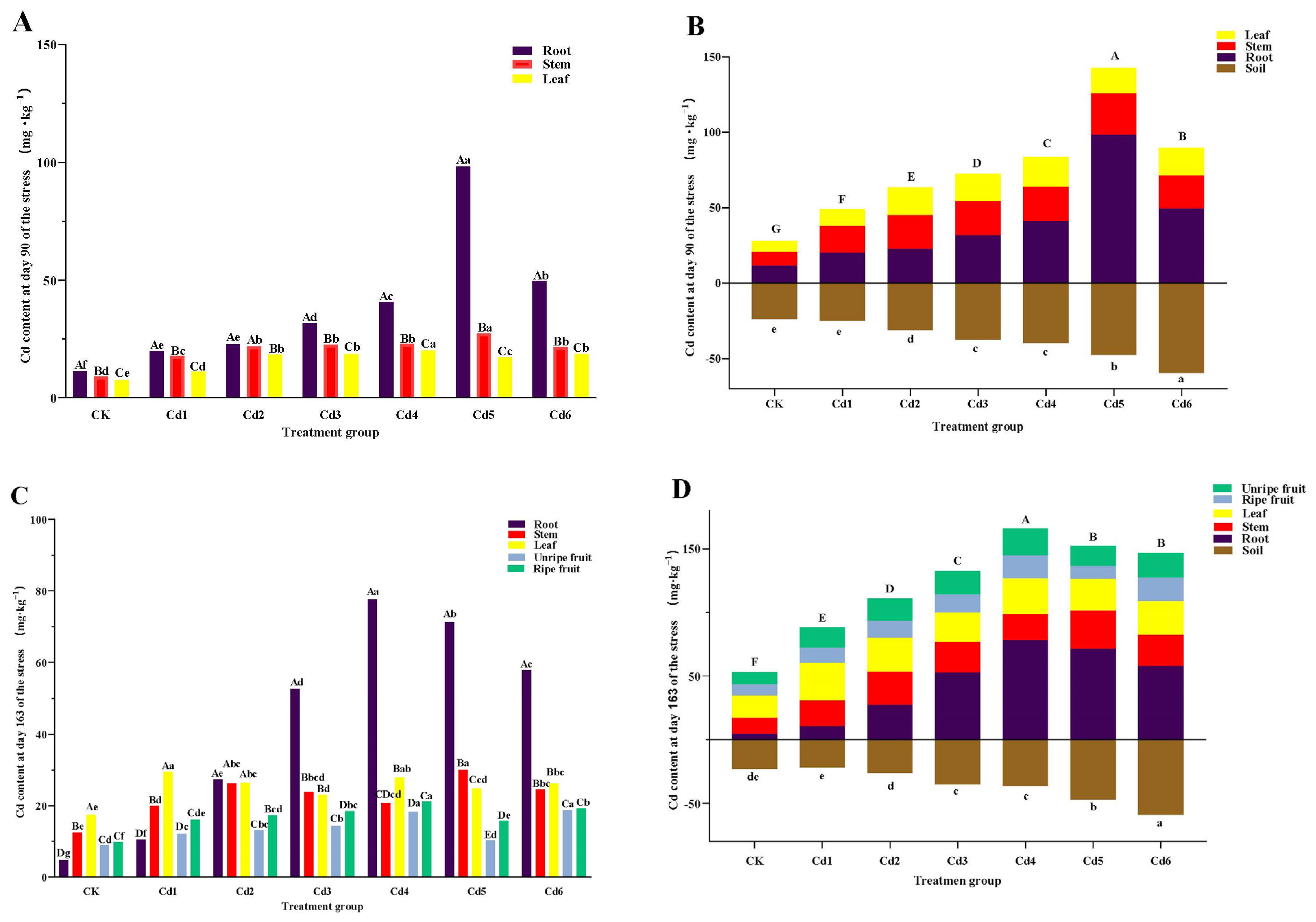
3.2. Cd Absorption and Transport by S. aculeatissimum
3.2.1. Cd Content of Plant Organs
3.2.2. Enrichment Transport Characteristics
3.3. Effect of Cd Treatment on Membrane Lipid Peroxidation and Antioxidant Enzymes
3.4. Transcriptome Sequencing, De Novo Assembly, and Functional Annotation
3.5. Analysis of Differentially Expressed Genes between Different Treatment Groups
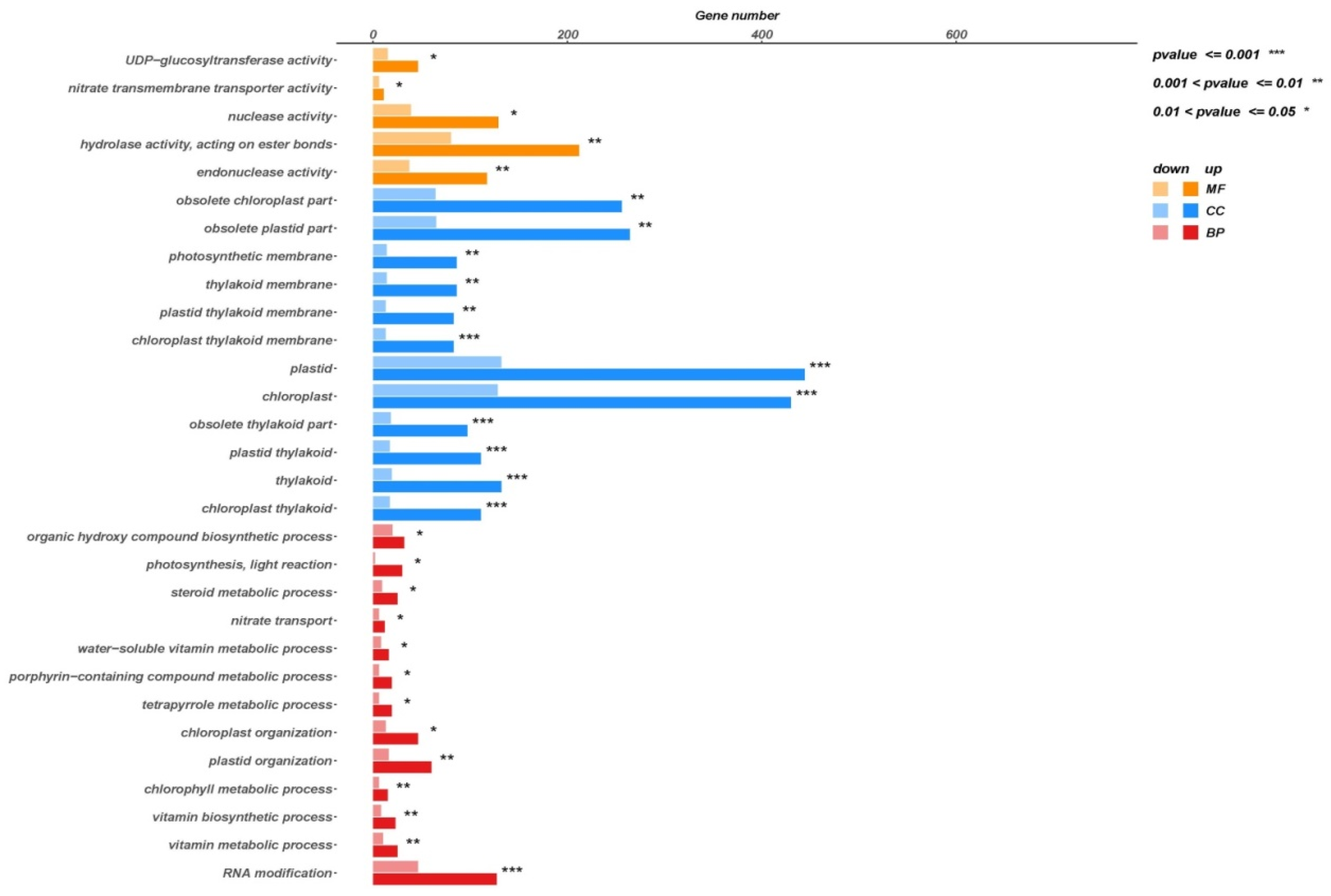
3.6. GO Function Classification of DEGs
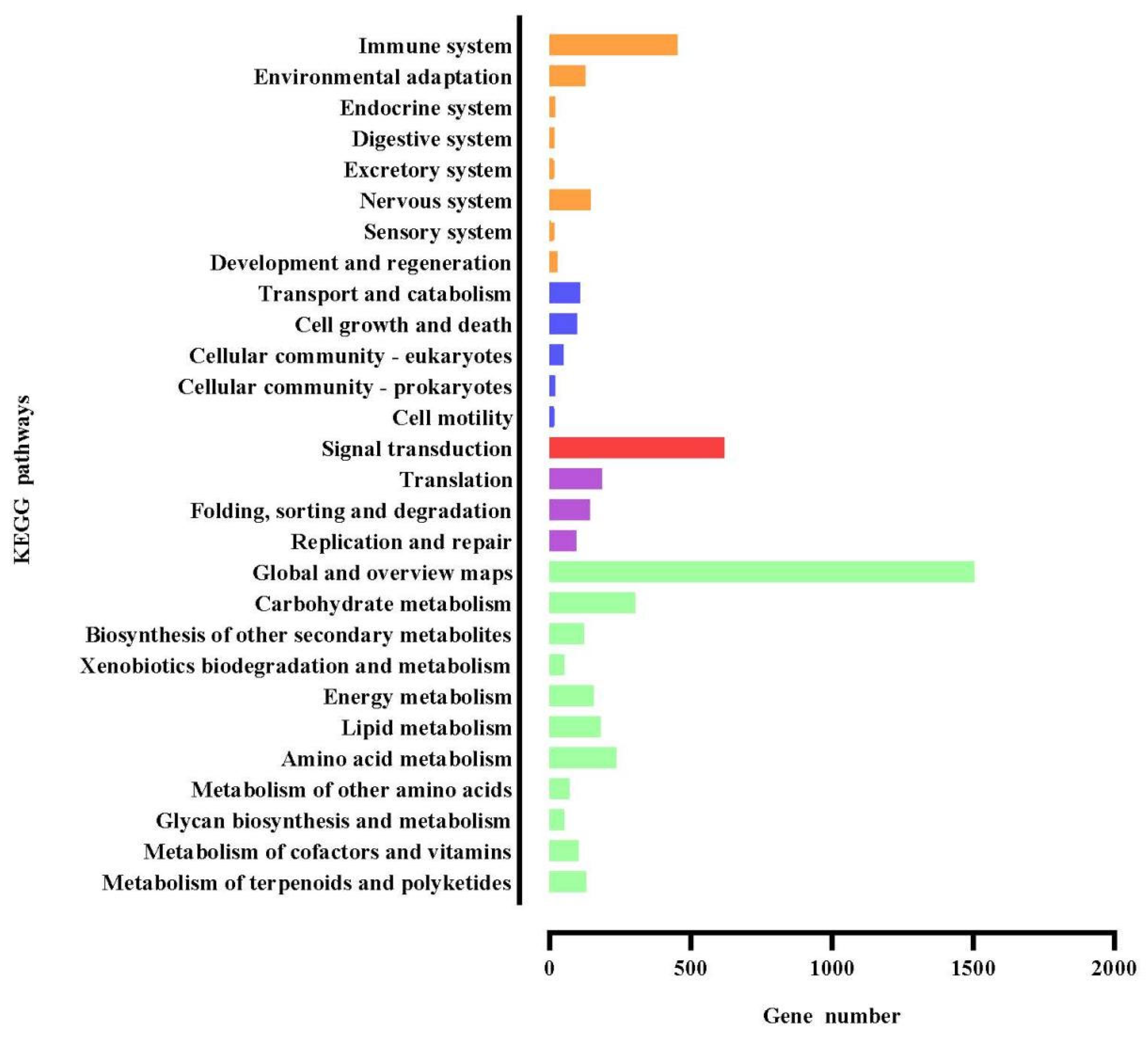
3.7. Functional Analysis of the KEGG Pathway of DEGs
3.8. DEGs Related to Heavy Metal Transport and Detoxification under Cd Stress
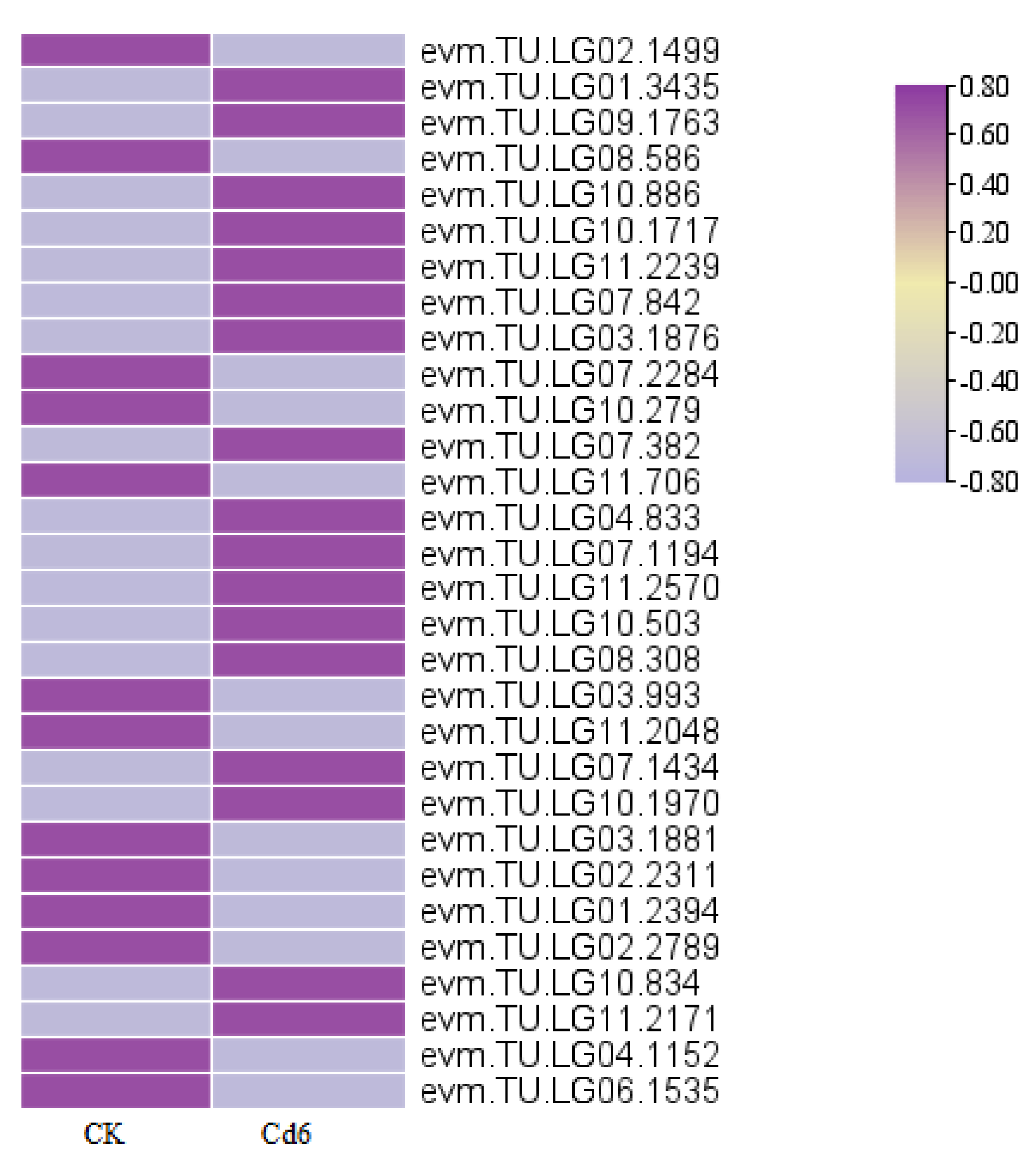
3.9. Cd-Tolerant, Uptake Enrichment-Related Gene Screening in S. aculeatissimum
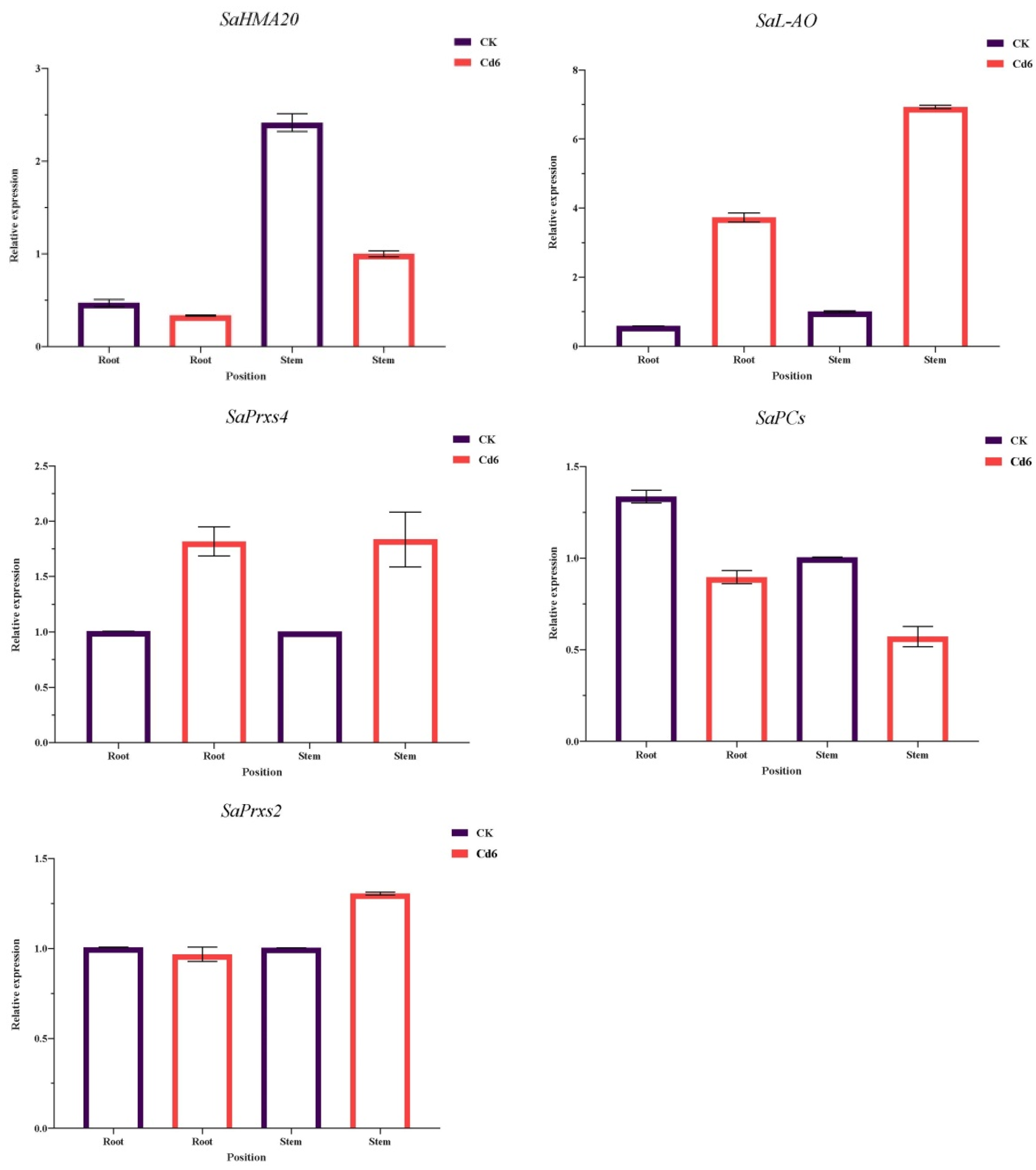
3.10. Validation of DEG Results by qRT-PCR Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, Y.; Wang, L.; Wang, W.; Li, T.; He, Z.; Yang, X. Current status of agricultural soil pollution by heavy metals in China: A meta-analysis. Sci. Total Environ. 2019, 651, 3034–3042. [Google Scholar] [CrossRef] [PubMed]
- Suwazono, Y.; Kido, T.; Nakagawa, H.; Nishijo, M.; Honda, R.; Kobayashi, E.; Dochi, M.; Nogawa, K. Biological half-life of cadmium in the urine of inhabitants after cessation of cadmium exposure. Biomarkers 2009, 14, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Namgyal, D.; Ali, S.; Mehta, R.; Sarwat, M. The neuroprotective effect of curcumin against Cd-induced neurotoxicity and hippocampal neurogenesis promotion through CREB-BDNF signaling pathway. Toxicology 2020, 442, 152542. [Google Scholar] [CrossRef]
- Xu, J.; Yang, J. Heavy Metals in Terrestrial Ecosystems; China Environmental Science Press: Beijing, China, 1995. [Google Scholar]
- China National Environment Minitoring Centre. Background Values of Soil Elements in China; China Environmental Science Press: Beijing, China, 1990. [Google Scholar]
- Teng, Y.; Wu, J.; Lu, S.; Wang, Y.; Jiao, X.; Song, L. Soil and soil environmental quality monitoring in China: A review. Environ. Int. 2014, 69, 177–199. [Google Scholar] [CrossRef]
- Johnson, M.D.; Kenney, N.; Stoica, A.; Hilakivi-Clarke, L.; Singh, B.; Chepko, G.; Clarke, R.; Sholler, P.F.; Lirio, A.A.; Foss, C.; et al. Cadmium mimics the in vivo effects of estrogen in the uterus and mammary gland. Nat. Med. 2003, 9, 1081–1084. [Google Scholar] [CrossRef]
- Sebastian, A.; Prasad, M.N.V. Cadmium minimization in rice. A review. Agron. Sustain. Dev. 2014, 34, 155–173. [Google Scholar] [CrossRef]
- Uraguchi, S.; Fujiwara, T. Cadmium transport and tolerance in rice: Perspectives for reducing grain cadmium accumulation. Rice 2012, 5, 5. [Google Scholar] [CrossRef]
- Chen, Q.Y.; DesMarais, T.; Costa, M. Metals and Mechanisms of Carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 537–554. [Google Scholar] [CrossRef] [PubMed]
- Zalups, R.K.; Ahmad, S. Molecular handling of cadmium in transporting epithelia. Toxicol. Appl. Pharmacol. 2003, 186, 163–188. [Google Scholar] [CrossRef]
- Viaene, M.K.; Masschelein, R.; Leenders, J.; De Groof, M.; Swerts, L.J.V.C.; Roels, H.A. Neurobehavioural effects of occupational exposure to cadmium: A cross sectional epidemiological study. Occup. Environ. Med. 2000, 57, 19. [Google Scholar] [CrossRef]
- Kubota, H.; Takenaka, C. Field Note: Arabis gemmifera is a Hyperaccumulator of Cd and Zn. Int. J. Phytoremediation 2003, 5, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Peng, W.; Jia, Y.; Lu, L.; Fan, W. Bioremediation of lead contaminated soil with Rhodobacter sphaeroides. Chemosphere 2016, 156, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Mao, X.; Xu, Y.; Li, Y.; Zhao, N.; Yao, J.; Dong, Y.; Tigabu, M.; Zhao, X.; Li, S. Comparative transcriptomic analysis reveals the coordinated mechanisms of Populus × canadensis ‘Neva’ leaves in response to cadmium stress. Ecotoxicol. Environ. Saf. 2021, 216, 112179. [Google Scholar] [CrossRef] [PubMed]
- Dai, F.; Luo, G.; Li, Z.; Wei, X.; Wang, Z.; Lin, S.; Tang, C. Physiological and transcriptomic analyses of mulberry (Morus atropurpurea) response to cadmium stress. Ecotoxicol. Environ. Saf. 2020, 205, 111298. [Google Scholar] [CrossRef]
- Ge, J.; Tao, J.; Zhao, J.; Wu, Z.; Zhang, H.; Gao, Y.; Tian, S.; Xie, R.; Xu, S.; Lu, L. Transcriptome analysis reveals candidate genes involved in multiple heavy metal tolerance in hyperaccumulator Sedum alfredii. Ecotoxicol. Environ. Saf. 2022, 241, 113795. [Google Scholar] [CrossRef]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef]
- Piechalak, A.; Tomaszewska, B.; Baralkiewicz, D.; Malecka, A. Accumulation and detoxification of lead ions in legumes. Phytochemistry 2002, 60, 153–162. [Google Scholar] [CrossRef]
- Nada, E.; Ferjani, B.A.; Ali, R.; Bechir, B.R.; Imed, M.; Makki, B. Cadmium-induced growth inhibition and alteration of biochemical parameters in almond seedlings grown in solution culture. Acta Physiol. Plant. 2007, 29, 57–62. [Google Scholar] [CrossRef]
- Rahoui, S.; Chaoui, A.; El Ferjani, E. Reserve Mobilization Disorder in Germinating Seeds of VICIA FABA L. Exposed to Cadmium. J. Plant Nutr. 2010, 33, 809–817. [Google Scholar] [CrossRef]
- Jun, Z.; Wenke, W.; Yani, G.; Zhoufeng, W.; Shumiao, C. Effect of Cd2+ Stress on Seed Germination Characteristics of Ryegrass, Indian Mustard and Grain Amaranth. Environ. Eng. Manag. J. 2019, 18, 1875–1884. [Google Scholar] [CrossRef]
- Ling, T.; Gao, Q.; Du, H.; Zhao, Q.; Ren, J. Growing, physiological responses and Cd uptake of Corn (Zea mays L.) under different Cd supply. Chem. Speciat. Bioavailab. 2017, 29, 216–221. [Google Scholar] [CrossRef]
- Hussain, I.; Ashraf, M.A.; Rasheed, R.; Iqbal, M.; Ibrahim, M.; Zahid, T.; Thind, S.; Saeed, F. Cadmium-induced Perturbations in Growth, Oxidative Defense System, Catalase Gene Expression and Fruit Quality in Tomato. Int. J. Agric. Biol. 2017, 19, 61–68. [Google Scholar] [CrossRef]
- Lin, L.; Zhou, T.; Tang, F.; Hu, H.; Fu, Q. Effects of Phosphorus on Growth and Uptake of Heavy Metals in Strawberry Grown in the Soil Contaminated by Cd and Pb. J. Agro-Environ. Sci. 2013, 32, 503–507. [Google Scholar]
- Hakla, H.R.; Sharma, S.; Urfan, M.; Yadav, N.S.; Rajput, P.; Kotwal, D.; Abdel Latef, A.A.H.; Pal, S. Gibberellins Target Shoot-Root Growth, Morpho-Physiological and Molecular Pathways to Induce Cadmium Tolerance in Vigna radiata L. Agronomy 2021, 11, 896. [Google Scholar] [CrossRef]
- Mozafarian, M.; Shekari, L.; Hawrylak-Nowak, B.; Kamelmanesh, M. Protective Role of Selenium on Pepper Exposed to Cadmium Stress during Reproductive Stage. Biol. Trace Elem. Res. 2014, 160, 97–107. [Google Scholar] [CrossRef]
- Zhang, X.; Du, Y.; Wang, L.; Zhou, Q.; Huang, X.; Sun, Z. Combined Effects of Lanthanum (III) and Acid Rain on Antioxidant Enzyme System in Soybean Roots. PLoS ONE 2015, 10, e0134546. [Google Scholar] [CrossRef]
- He, J.; Qin, J.; Long, L.; Ma, Y.; Li, H.; Li, K.; Jiang, X.; Liu, T.X.; Polle, A.; Liang, Z.; et al. Net cadmium flux and accumulation reveal tissue-specific oxidative stress and detoxification in Populus × canescens. Physiol. Plant. 2011, 143, 50–63. [Google Scholar] [CrossRef]
- Zeng, P.; Guo, Z.; Xiao, X.; Peng, C.; Liu, L.; Yan, D.; He, Y. Physiological stress responses, mineral element uptake and phytoremediation potential of Morus alba L. in cadmium-contaminated soil. Ecotoxicol. Environ. Saf. 2020, 189, 109973. [Google Scholar] [CrossRef]
- Xu, X.; Chen, Q.; Mo, S.; Qian, Y.; Wu, X.; Jin, Y.; Ding, H. Transcriptome-wide modulation combined with morpho-physiological analyses of Typha orientalis roots in response to lead challenge. J. Hazard. Mater. 2020, 384, 121405. [Google Scholar] [CrossRef]
- Zheng, S.; Ren, P.; Zhai, M.; Li, C.; Chen, Q. Identification of Genes Involved in Root Growth Inhibition under Lead Stress by Transcriptome Profiling in Arabidopsis. Plant Mol. Biol. Report. 2021, 39, 50–59. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, B.; Yuan, Y.; Xu, Q.; Chen, P. Transcriptome profiling of Fagopyrum tataricum leaves in response to lead stress. BMC Plant Biol. 2020, 20, 54. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hu, T.; Amombo, E.; Fu, J. Transcriptome profilings of two tall fescue (Festuca arundinacea) cultivars in response to lead (Pb) stress. BMC Genom. 2017, 18, 145. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, L.; Chen, Y.; Shen, H.; Gong, Y.; Limera, C.; Liu, L.W. Transcriptome Profiling of Radish (Raphanus sativus L.) Root and Identification of Genes Involved in Response to Lead (Pb) Stress with Next Generation Sequencing. PLoS ONE 2013, 8, e66539. [Google Scholar] [CrossRef]
- Gu, C.-S.; Liu, L.-Q.; Deng, Y.-M.; Zhang, Y.-X.; Wang, Z.-Q.; Yuan, H.-Y.; Huang, S.-Z. De novo characterization of the Iris lactea var. chinensis transcriptome and an analysis of genes under cadmium or lead exposure. Ecotoxicol. Environ. Saf. 2017, 144, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, M.; Shohag, M.J.I.; Tian, S.; Song, H.; Feng, Y.; Yang, X. Enhanced expression of SaHMA3 plays critical roles in Cd hyperaccumulation and hypertolerance in Cd hyperaccumulator Sedum alfredii Hance. Planta 2016, 243, 577–589. [Google Scholar] [CrossRef]
- Chen, S.; Yu, M.; Li, H.; Wang, Y.; Lu, Z.; Zhang, Y.; Liu, M.; Qiao, G.; Wu, L.; Han, X.; et al. SaHsfA4c from Sedum alfredii Hance Enhances Cadmium Tolerance by Regulating ROS-Scavenger Activities and Heat Shock Proteins Expression. Front. Plant Sci. 2020, 11, 142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sa, G.; Zhang, Y.; Hou, S.; Wu, X.; Zhao, N.; Zhang, Y.; Deng, S.; Deng, C.; Deng, J.; et al. Populus euphratica annexin1 facilitates cadmium enrichment in transgenic Arabidopsis. J. Hazard. Mater. 2021, 405, 124063. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cui, X.; Zhang, X.; Liu, W.; Cui, Z. Combined toxicity and detoxification of lead, cadmium and arsenic in Solanum nigrum L. J. Hazard. Mater. 2020, 389, 121874. [Google Scholar] [CrossRef]
- Tian, W.; Huang, Y.; Li, D.; Meng, L.; He, T.; He, G. Identification of StAP2/ERF genes of potato (Solanum tuberosum) and their multiple functions in detoxification and accumulation of cadmium in yest: Implication for Genetic-based phytoremediation. Sci. Total Environ. 2022, 810, 152322. [Google Scholar] [CrossRef]
- Su, L.; Xie, Y.; He, Z.; Zhang, J.; Tang, Y.; Zhou, X. Network response of two cherry tomato (Lycopersicon esculentum) cultivars to Cadmium stress as revealed by transcriptome analysis. Ecotoxicol. Environ. Saf. 2021, 222, 112473. [Google Scholar] [CrossRef]
- Deng, X.; Peng, K.; Chen, Y.; Shen, Z.; Xia, Y. Absorption and accumulation of heavy metals by four Solanaceae plants in mining contaminated soil. Environ. Pollut. Prev. 2011, 33, 46–51. [Google Scholar]
- Kohara, A.; Nakajima, C.; Yoshida, S.; Muranaka, T. Characterization and engineering of glycosyltransferases responsible for steroid saponin biosynthesis in Solanaceous plants. Phytochemistry 2007, 68, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Saijo, R.; Fuke, C.; Murakami, K.; Nohara, T.; Tomimatsu, T. Two steroidal glycosides, aculeatiside A and B from Solanum aculeatissimum. Phytochemistry 1983, 22, 733–736. [Google Scholar] [CrossRef]
- Rosangkima, G.; Jagetia, G. In vitro anticancer screening of medicinal plants of Mizoram State, India, against Dalton’s lymphoma, MCF-7 and HELA cells. Int. J. Recent Sci. Res. 2015, 6, 5648–5653. [Google Scholar]
- Kim, Y.C.; Che, Q.M.; Gunatilaka, A.A.L.; Kingston, D.G.I. Bioactive steroidal alkaloids from Solanum umbelliferum. J. Nat. Prod. 1996, 59, 283–285. [Google Scholar] [CrossRef]
- Ghimire, B.K.; Yu, C.Y.; Chung, I.M. Direct shoot organogenesis and assessment of genetic stability in regenerants of Solanum aculeatissimum Jacq. Plant Cell Tissue Organ Cult. 2012, 108, 455–464. [Google Scholar] [CrossRef]
- Chirumamilla, P.; Gopu, C.; Jogam, P.; Taduri, S. Highly efficient rapid micropropagation and assessment of genetic fidelity of regenerants by ISSR and SCoT markers of Solanum khasianum Clarke. Plant Cell Tissue Organ Cult. (PCTOC) 2021, 144, 397–407. [Google Scholar] [CrossRef]
- Yaniv, Z.; Palevitch, D.; Weissenberg, M. The Effect of Water Stress on Growth and Solasodine Content in Solanum khasianum. Planta Medica 1984, 50, 60–65. [Google Scholar] [CrossRef]
- Yang, X.; Liu, F.; Zhang, Y.; Wang, L.; Cheng, Y.F. Cold-responsive miRNAs and their target genes in the wild eggplant species Solanum aculeatissimum. BMC Genom. 2017, 18, 1000. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, J.; Bao, S.; Yang, Y.; Zhuang, Y. Molecular Cloning and Characterization of a Wild Eggplant Solanum aculeatissimum NBS-LRR Gene, Involved in Plant Resistance to Meloidogyne incognita. Int. J. Mol. Sci. 2018, 19, 583. [Google Scholar] [CrossRef]
- Zhou, X.H.; Bao, S.Y.; Liu, J.; Zhuang, Y. De Novo Sequencing and Analysis of the Transcriptome of the Wild Eggplant Species Solanum aculeatissimum in Response to Verticillium dahliae. Plant Mol. Biol. Rep. 2016, 34, 1193–1203. [Google Scholar] [CrossRef]
- Bao, S. Agrochemical Analysis of Soil, 3rd ed.; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- GB15618-2018; Soil Environmental Quality Agricultural Land Soil Pollution Risk Control Standard (Trial). Standards Press of China: Beijing, China, 2018.
- Li, H. Principles and Techniques of Plant Physiological and Biochemical Experiments, 3rd ed.; Higher Education Press: Beijing, China, 2000. [Google Scholar]
- Baker, A.; Reeves, R.; Hajar, A. Heavy metal accumulation and tolerance in British populations of the metallophyte Thlaspi caerulescens J. & C. Presl (Brassicaceae). New Phytol. 1994, 127, 61–68. [Google Scholar] [PubMed]
- Raskin, I.; Kumar, P.B.A.N.; Dushenkov, S.; Salt, D.E. Bioconcentration of heavy metals by plants. Curr. Opin. Biotechnol. 1994, 5, 285–290. [Google Scholar] [CrossRef]
- Yu, Z.; Zhou, Q. Growth responses and cadmium accumulation of Mirabilis jalapa L. under interaction between cadmium and phosphorus. J. Hazard. Mater. 2009, 167, 38–43. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, J.; Zhuang, Y. Selection of Appropriate Reference Genes in Solanum aculeatissimum for Quantitative Gene Expression Studies under Different Experimental Conditions. J. Hortic. 2014, 41, 1731–1738. [Google Scholar]
- Schrauzer, G.N. Trace elements in the environment biogeochemistry, biotechnology and bioremediation. Biol. Trace Elem. Res. 2006, 109, 301. [Google Scholar] [CrossRef]
- French, C.J.; Dickinson, N.M.; Putwain, P.D. Woody biomass phytoremediation of contaminated brownfield land. Environ. Pollut. 2006, 141, 387–395. [Google Scholar] [CrossRef]
- Zhang, L.D.; Zhang, L.X.; Sun, J.L.; Zhang, Z.X.; Ren, H.Z.; Sui, X.L. Rubisco gene expression and photosynthetic characteristics of cucumber seedlings in response to water deficit. Sci. Hortic. 2013, 161, 81–87. [Google Scholar] [CrossRef]
- Sun, Y.-b.; Zhou, Q.-x.; Liu, W.-t.; An, J.; Xu, Z.-Q.; Wang, L. Joint effects of arsenic and cadmium on plant growth and metal bioaccumulation: A potential Cd-hyperaccumulator and As-excluder Bidens pilosa L. J. Hazard. Mater. 2009, 165, 1023–1028. [Google Scholar] [CrossRef]
- Li, J.; Ju, L.; Zhang, L.; Sun, P.; Li, M.; Li, J.; Liu, Y. Research Progress on Lonicera japonica Thunb. Affected by Environmental Stress. Agric. Biotechnol. 2022, 11, 12–18. [Google Scholar]
- Abedin, M.; Meharg, A.A. Relative toxicity of arsenite and arsenate on germination and early seedling growth of rice (Oryza sativa L.). Plant Soil 2002, 243, 57–66. [Google Scholar] [CrossRef]
- Wang, X.J.; Song, Y.; Ma, Y.H.; Zhuo, R.Y.; Jin, L. Screening of Cd tolerant genotypes and isolation of metallothionein genes in alfalfa (Medicago sativa L.). Environ. Pollut. 2011, 159, 3627–3633. [Google Scholar] [CrossRef] [PubMed]
- Flores-Cáceres, M.L.; Hattab, S.; Hattab, S.; Boussetta, H.; Banni, M.; Hernández, L.E. Specific mechanisms of tolerance to copper and cadmium are compromised by a limited concentration of glutathione in alfalfa plants. Plant Sci. 2015, 233, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Peralta, J.R.; Gardea-torresdey, J.L.; Tiemann, K.J.; Gomez, E.; Arteaga, S.; Rascon, E.; Parsons, J.G. Uptake and Effects of Five Heavy Metals on Seed Germination and Plant Growth in Alfalfa (Medicago sativa L.). Bull. Environ. Contam. Toxicol. 2001, 66, 727–734. [Google Scholar] [CrossRef]
- Pardinho, R.B.; Vecchia, P.D.; Alves, C.M.A.C.; Pimentel, N.; Gazzana, D.; Bolzan, R.C.; Duarte, F.A.; Bisognin, D.A.; Flores, E.M.M. Ilex Paraguariensis exposition to As and Cd in a closed soilless system. Chemosphere 2020, 258, 127284. [Google Scholar] [CrossRef]
- Wang, X.; Gao, Y.; Feng, Y.; Li, X.; Wei, Q.; Sheng, X. Cadmium Stress Disrupts the Endomembrane Organelles and Endocytosis during Picea wilsonii Pollen Germination and Tube Growth. PLoS ONE 2014, 9, e94721. [Google Scholar] [CrossRef]
- Mohammadi, M.; Karr, A.L. Membrane lipid peroxidation, nitrogen fixation and leghemoglobin content in soybean root nodules. J. Plant Physiol. 2001, 158, 9–19. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, Z.; Guo, S. The impact of bacterial blight on scavengers of reactive oxygen species in hybrid rice seedlings. Agric. Sci. 1998, 49, 118–122. [Google Scholar]
- Cakmak, I.; Marschner, H. Magnesium deficiency enhances resistance to paraquat toxicity in bean leaves. Plant Cell Environ. 1992, 15, 955–960. [Google Scholar] [CrossRef]
- Mirzaee, M.; Moieni, A.; Ghanati, F. Effects of drought stress on the lipid peroxidation and antioxidant enzyme activities in two canola (Brassica napus L.) cultivars. J. Agric. Sci. Technol. 2013, 15, 593–602. [Google Scholar]
- Dai, H.; Wei, S.; Twardowska, I.; Hou, N.; Zhang, Q. Cosmopolitan cadmium hyperaccumulator Solanum nigrum: Exploring cadmium uptake, transport and physiological mechanisms of accumulation in different ecotypes as a way of enhancing its hyperaccumulative capacity. J. Environ. Manag. 2022, 320, 115878. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.; Abd_Allah, E.F.; Alqarawi, A.A.; Al Huqail, A.A.; Egamberdieva, D.; Wirth, S. Alleviation of cadmium stress in Solanum lycopersicum L. by arbuscular mycorrhizal fungi via induction of acquired systemic tolerance. Saudi J. Biol. Sci. 2016, 23, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Sun, W.; Jin, X.; Yu, L.; Chen, C.; Yue, Z.; Dong, Y. Analysis of 2,4-epibrassinolide created an enhancement tolerance on Cd toxicity in Solanum nigrum L. Environ. Sci. Pollut. Res. 2020, 27, 16784–16797. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Chen, Z.; Sun, K.; Yan, D.; Kang, M.; Zhao, Y. Effect of cadmium on the physiological parameters and the subcellular cadmium localization in the potato (Solanum tuberosum L.). Ecotoxicol. Environ. Saf. 2013, 97, 147–153. [Google Scholar] [CrossRef]
- Fidalgo, F.; Freitas, R.; Ferreira, R.; Pessoa, A.M.; Teixeira, J. Solanum nigrum L. antioxidant defence system isozymes are regulated transcriptionally and posttranslationally in Cd-induced stress. Environ. Exp. Bot. 2011, 72, 312–319. [Google Scholar] [CrossRef]
- Palace, V.; Brown, S.B.; Baron, C.; Fitzsimons, J.; Woodin, B.; Stegeman, J.; Klaverkamp, J.F. An evaluation of the relationships among oxidative stress, antioxidant vitamins and early mortality syndrome (EMS) of lake trout (Salvelinus namaycush) from Lake Ontario. Aquat. Toxicol. 1998, 43, 195–208. [Google Scholar] [CrossRef]
- Leng, B.; Jia, W.; Yan, X.; Yuan, F.; Dong, X.; Wang, B. Cadmium Stress in Halophyte Thellungiella halophila: Consequences on Growth, Cadmium Accumulation, Reactive Oxygen Species and Antioxidative Systems. IOP Conf. Ser. Earth Environ. Sci. 2018, 153, 062002. [Google Scholar] [CrossRef]
- Zhou, C.; Ma, Q.; Li, S.; Zhu, M.; Xia, Z.; Yu, W. Toxicological effects of single and joint sulfamethazine and cadmium stress in soil on pakchoi (Brassica chinensis L.). Chemosphere 2021, 263, 128296. [Google Scholar] [CrossRef]
- Jaffré, T. Detection of nickeliferous rocks by analysis of herbarium specimen of indicator plants. J. Geochem. Explor. 1977, 7, 49–57. [Google Scholar]
- Angle, J.S. Plants that Hyperaccumulate Heavy Metals: Their Role in Phytormediation, Microbiology, Archaeology, Mineral Exploration and Phytomining. J. Environ. Qual. 1999, 28, 1045. [Google Scholar] [CrossRef]
- Baker, A.; McGrath, S.; Reeves, R.; Smith, J.A.C. Metal hyperaccumulator plants: A review of the ecology and physiology of a biological resource for phytoremediation of metal-polluted soils. Phytoremediation Contamin. Soil Water 2000, 85, 85–107. [Google Scholar]
- Zhou, X.; Lou, X.; Larisa, D.; Elena, N.; Wang, H. Advances in Heavy Metal Accumulation Characteristics of Plants in Soil. J. Ecotoxicol. 2022, 17, 400–410. [Google Scholar]
- Ashrafzadeh, S.; Gaw, S.; Genet, R.; Glover, C.N.; Leung, D.W.M. Natural variation in correlations between cadmium and micronutrients in potato tubers. J. Food Compos. Anal. 2017, 59, 55–60. [Google Scholar] [CrossRef]
- Lima, C.; Pestana, I.; Azevedo, L.; Ribeiro, D.; Almeida, M.; Prins, C.; Marciano, C.; Souza, C. Bioconcentration and translocation of Cd and Hg in a tomato (Solanum lycopersicum) from cultivated soils in southeastern Brazil. Environ. Monit. Assess. 2019, 191, 103. [Google Scholar] [CrossRef]
- Li, M. The Stuty of Regulation Mechanism of Gene Expression for Populus × euramericana (Dode) Guineir ‘Neva’ under Cd Stress. Master’s Thesis, Chinese Academy of Forestry Sciences, Beijing, China, 2015. [Google Scholar]
- Deng, Y. Function Analysis of Hydrogen Sulfide-Mediated Alleviation of Cadmium Stress in Kenaf (Hibiscus cannabinus L.) Seedlings. Ph.D. Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2019. [Google Scholar]
- He, Q.; Zhou, T.; Sun, J.; Wang, P.; Bai, L.; Liu, M. Effect of cadmium stress on transcriptome differences in roots and leaves of Koelreuteria Paniculata seedlings. Acta Sci. Circumstantiae 2022, 42, 467–481. [Google Scholar]
- Wang, S. Study on the Genotvpic Variations in Responses to Pb, Cd and the Tolerance Mechanisms in Salix spp. Ph.D. Thesis, Zhejiang Uniersity, Hangzhou, China, 2015. [Google Scholar]
- Migeon, A.; Blaudez, D.; Wilkins, O.; Montanini, B.; Campbell, M.M.; Richaud, P.; Thomine, S.; Chalot, M. Genome-wide analysis of plant metal transporters, with an emphasis on poplar. Cell. Mol. Life Sci. 2010, 67, 3763–3784. [Google Scholar] [CrossRef]
- Hu, X.; Li, T.; Xu, W.; Chai, Y.-R. Distribution of cadmium in subcellular fraction and expression difference of its transport genes among three cultivars of pepper. Ecotoxicol. Environ. Saf. 2021, 216, 112182. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Wenmin, Q.; Yu, M.; Li, S.; Lu, Z.; Zhu, Y.; Kan, X.-z.; Renying, Z. Genome-Wide Characterization of Sedum plumbizincicola HMA Gene Family Provides Functional Implications in Cadmium Response. Plants 2022, 11, 215. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xu, X.; Hu, X.; Liu, Q.; Wang, Z.; Zhang, H.; Wang, H.; Wei, M.; Wang, H.; Liu, H.; et al. Genome-Wide Analysis and Heavy Metal-Induced Expression Profiling of the HMA Gene Family in Populus trichocarpa. Front. Plant Sci. 2015, 6, 1149. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Liu, J.; Niu, Y.; Chen, Y.; Hao, Y.; Zhao, J.; Sun, l.; Wang, H.; Xiao, J.; et al. Characterization of the Heavy-Metal-Associated Isoprenylated Plant Protein (HIPP) Gene Family from Triticeae Species. Int. J. Mol. Sci. 2020, 21, 6191. [Google Scholar] [CrossRef]
- Bari, M.A.; El-Shehawi, A.M.; Elseehy, M.M.; Naheen, N.N.; Rahman, M.M.; Kabir, A.H. Molecular characterization and bioinformatics analysis of transporter genes associated with Cd-induced phytotoxicity in rice (Oryza sativa L.). Plant Physiol. Biochem. 2021, 167, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y. Cloning and Function of SoHMA3 Gene in Sorghum dochna Snowden. Master’s Thesis, Central South University of Forestry and Technology, Changsha, China, 2020. [Google Scholar]
- De Rienzo, F.; Gabdoulline, R.R.; Menziani, M.C.; Wade, R.C. Blue copper proteins: A comparative analysis of their molecular interaction properties. Protein Sci. 2000, 9, 1439–1454. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.-M.; Luo, F.; Li, D.-D.; Zhang, J.; Liu, Z.-H.; Xu, W.-L.; Huang, G.-Q.; Li, X.-B. Cotton BCP genes encoding putative blue copper-binding proteins are functionally expressed in fiber development and involved in response to high-salinity and heavy metal stresses. Physiol. Plant. 2011, 141, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Mollet, J.-C.; Dong, J.; Zhang, K.; Park, S.-Y.; Lord, E.M. Chemocyanin, a small basic protein from the lily stigma, induces pollen tube chemotropism. Proc. Natl. Acad. Sci. USA 2003, 100, 16125–16130. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Kim, S.T.; Lord, E.M. Plantacyanin Plays a Role in Reproduction in Arabidopsis1. Plant Physiol. 2005, 138, 778–789. [Google Scholar] [CrossRef]
- Yoshizaki, M.; Furumoto, T.; Hata, S.; Shinozaki, M.; Izui, K. Characterization of a Novel Gene Encoding a Phytocyanin-Related Protein in Morning Glory (Pharbitis nil). Biochem. Biophys. Res. Commun. 2000, 268, 466–470. [Google Scholar] [CrossRef]
- Ma, H.; Zhao, H.; Liu, Z.; Zhao, J. The Phytocyanin Gene Family in Rice (Oryza sativa L.): Genome-Wide Identification, Classification and Transcriptional Analysis. PLoS ONE 2011, 6, e25184. [Google Scholar] [CrossRef]
- Ozturk, Z.N.; Talamé, V.; Deyholos, M.; Michalowski, C.B.; Galbraith, D.W.; Gozukirmizi, N.; Tuberosa, R.; Bohnert, H.J. Monitoring large-scale changes in transcript abundance in drought- and salt-stressed barley. Plant Mol. Biol. 2002, 48, 551–573. [Google Scholar] [CrossRef]
- Mascher, M.; Jost, M.; Kuon, J.-E.; Himmelbach, A.; Aßfalg, A.; Beier, S.; Scholz, U.; Graner, A.; Stein, N. Mapping-by-sequencing accelerates forward genetics in barley. Genome Biol. 2014, 15, R78. [Google Scholar] [CrossRef]
- Ezaki, B.; Sasaki, K.; Matsumoto, H.; Nakashima, S. Functions of two genes in aluminium (Al) stress resistance: Repression of oxidative damage by the AtBCB gene and promotion of efflux of Al ions by the NtGDI1gene. J. Exp. Bot. 2005, 56, 2661–2671. [Google Scholar] [CrossRef]
- Wu, H.; Shen, Y.; Hu, Y.; Tan, S.; Lin, Z. A phytocyanin-related early nodulin-like gene, BcBCP1, cloned from Boea crassifolia enhances osmotic tolerance in transgenic tobacco. J. Plant Physiol. 2011, 168, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, X.; Chu, S.; You, Y.; Chi, Y.; Wang, R.; Yang, X.; Hayat, K.; Zhang, D.; Zhou, P. Comparative cytology combined with transcriptomic and metabolomic analyses of Solanum nigrum L. in response to Cd toxicity. J. Hazard. Mater. 2022, 423, 127168. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xiao, Y.; Chen, W.-S.; Tang, K.; Zhang, L. Increased Vitamin C Content Accompanied by an Enhanced Recycling Pathway Confers Oxidative Stress Tolerance in Arabidopsis. J. Integr. Plant Biol. 2010, 52, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Garchery, C.; Gest, N.; Do, P.; Alhagdow, M.; Baldet, P.; Menard, G.; Rothan, C.; Massot, C.; Gautier, H.; Aarrouf, J.; et al. A diminution in ascorbate oxidase activity affects carbon allocation and improves yield in tomato under water deficit. Plant Cell Environ. 2012, 36, 159–175. [Google Scholar] [CrossRef]
- Batth, R.; Singh, K.; Kumari, S.; Mustafiz, A. Transcript Profiling Reveals the Presence of Abiotic Stress and Developmental Stage Specific Ascorbate Oxidase Genes in Plants. Front. Plant Sci. 2017, 8, 198. [Google Scholar] [CrossRef]



| Soil Type | Organic Matter (g kg−1) | Total Nitrogen (g kg−1) | Total Phosphorus (g kg−1) | Total Potassium (g kg−1) | Nitrate Nitrogen (g kg−1) | Ammoniacal Nitrogen (g kg−1) | Fast-Acting Potassium (mg kg−1) | pH |
|---|---|---|---|---|---|---|---|---|
| Topsoil | 58.19 | 6.14 | 6.03 | 2.29 | 0.14 | 0.009 | 12.24 | 6.99 |
| Time (Day) | Plant Height (cm) | ||||
|---|---|---|---|---|---|
| Treatment Group | 15 | 30 | 45 | 60 | |
| CK | 10.23 Cd | 13.63 Cf | 28.90 Bf | 40.33 Ac | |
| Cd1 | 17.30 Cab | 17.97 Ce | 43.17 Ba | 52.37 Ab | |
| Cd2 | 13.07 Dc | 19.30 Cde | 34.90 Bde | 53.13 Ab | |
| Cd3 | 13.93 Dc | 23.83 Cc | 36.43 Bcd | 52.57 Ab | |
| Cd4 | 14.93 Dc | 26.07 Cb | 37.77 Bc | 52.67 Ab | |
| Cd5 | 19.63 Da | 32.07 Ca | 40.50 Bb | 57.07 Aa | |
| Cd6 | 17.07 Cb | 20.37 Cd | 32.83 Be | 51.20 Ac | |
| Time (Day) | Stem Diameter (mm) | ||||
|---|---|---|---|---|---|
| Treatment Group | 15 | 30 | 45 | 60 | |
| CK | 2.86 Bb | 3.72 Bab | 5.27 Ac | 5.40 Ab | |
| Cd1 | 3.35 Cb | 4.11 Bab | 5.92 Aab | 6.36 Aa | |
| Cd2 | 3.23 Db | 4.09 Cab | 5.14 Bc | 5.53 Ab | |
| Cd3 | 3.09 Cb | 3.61 Bb | 5.17 Ac | 5.25 Ab | |
| Cd4 | 3.33 Cb | 4.21 Ba | 5.47 Abc | 5.47 Ab | |
| Cd5 | 4.91 Ca | 4.15 Dab | 5.92 Bab | 6.69 Aa | |
| Cd6 | 4.31 Ba | 4.09 Bab | 6.47 Aa | 6.57 Aa | |
| Time (Day) | Morphological Changes | ||||
|---|---|---|---|---|---|
| Treatment Group | 30 | 50 | 90 | 111 | |
| CK | Normal leaves | Normal leaves, 2 flowers, 1 open, with immature green fruit | 6 flowers, 3 open, 2 fruit, 1.88 cm in diameter | 16 flowers, 10 open, 9 fruit, 1.5 cm in diameter; 6 yellow fruit | |
| Cd1 | Normal leaves | Normal leaves, 5 flowers, 1 open | 14 flowers, 4 open, 2 fruit, 1.72 cm in diameter | 20 flowers, 5 open, 6 fruit, 1.6 cm in diameter; 2 yellow fruit | |
| Cd2 | Normal leaves | Normal leaves, 1 flower, 0~1 open, 1 fruit | 15 flowers, 5~6 open, 6 fruit, 1.1 cm in diameter | 19 flowers, 6~7 open, 9 fruit, 1.25 cm in diameter; 1 yellow fruit | |
| Cd3 | Normal leaves | Normal leaves, 1 flower, 0~1 open | 9 flowers, 1~2 open, 2 fruit, 0.43 cm in diameter | 14 flowers, 2~3 open, 4 fruit, 0.3 cm in diameter; no yellow fruit | |
| Cd4 | Normal leaves | Normal leaves, 7 flowers, 0~2 open | 14 flowers, 3~5 open, 3 fruit, fruit diameter 1.16 cm | 17 flowers, 3~5 open, 6 fruit, 1.90 cm in diameter; 1 yellow fruit | |
| Cd5 | Normal leaves | Normal leaves, 2 flowers, 0~2 open, 1 fruit | 9 flowers, 3~5 open, 4 fruit, fruit diameter 1.28 cm | 13 flowers, 3~5 open, 7 fruit, 1.85 cm in diameter; 1 yellow fruit | |
| Cd6 | Normal leaves | Normal leaves, 2 flowers, 1~2 open | 14 flowers, 9~11 open, 4 fruit, fruit diameter 0.87 cm | 19 flowers, 9~11 open, 9 fruit, 0.86 cm in diameter; 1 yellow fruit | |
| Treatment Group | Enrichment Factor | Translocation Factor |
|---|---|---|
| CK | 2.33 d | 10.77 a |
| Cd1 | 4.12 ab | 7.42 b |
| Cd2 | 4.27 ab | 3.05 c |
| Cd3 | 3.80 bc | 1.51 c |
| Cd4 | 4.58 a | 1.13 c |
| Cd5 | 3.26 c | 1.14 c |
| Cd6 | 2.49 d | 1.53 c |
| Sample | Total Reads | Clean Reads | Total Bases | Error Rate (%) | Q20 (%) | Q30 (%) | GC Content |
|---|---|---|---|---|---|---|---|
| CK | 34,513,646 | 22,329,418 | 3,305,425,613 | 0.04 | 97.46 | 91.58 | 47.87 |
| Cd6 | 31,622,016 | 19,480,982 | 2,895,836,234 | 0.04 | 97.95 | 93.09 | 45.92 |
| Average | 33,067,831 | 20,905,200 | 3,100,630,924 | 0.04 | 97.71 | 92.34 | 46.90 |
| Sample | Clean Reads | Mapped Reads | Mapped Rate (%) |
|---|---|---|---|
| ck | 22,329,418 | 11,870,318 | 53.16 |
| Cd6 | 19,480,982 | 13,675,649 | 70.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Sun, Z.; Li, L. Transcriptome Analysis Reveals Key Genes and Pathways Associated with Cadmium Stress Tolerance in Solanum aculeatissimum C. B. Clarke. Agriculture 2024, 14, 1686. https://doi.org/10.3390/agriculture14101686
Wu S, Sun Z, Li L. Transcriptome Analysis Reveals Key Genes and Pathways Associated with Cadmium Stress Tolerance in Solanum aculeatissimum C. B. Clarke. Agriculture. 2024; 14(10):1686. https://doi.org/10.3390/agriculture14101686
Chicago/Turabian StyleWu, Suying, Zhenghai Sun, and Liping Li. 2024. "Transcriptome Analysis Reveals Key Genes and Pathways Associated with Cadmium Stress Tolerance in Solanum aculeatissimum C. B. Clarke" Agriculture 14, no. 10: 1686. https://doi.org/10.3390/agriculture14101686






