Optimizing Nitrogen Fertilization to Maximize Yield and Bioactive Compounds in Ziziphora clinopodioides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Shoot Nitrogen Content
2.2. Extraction of EO
2.3. Gas Chromatography (GC) and Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
2.4. Data Analysis
3. Results
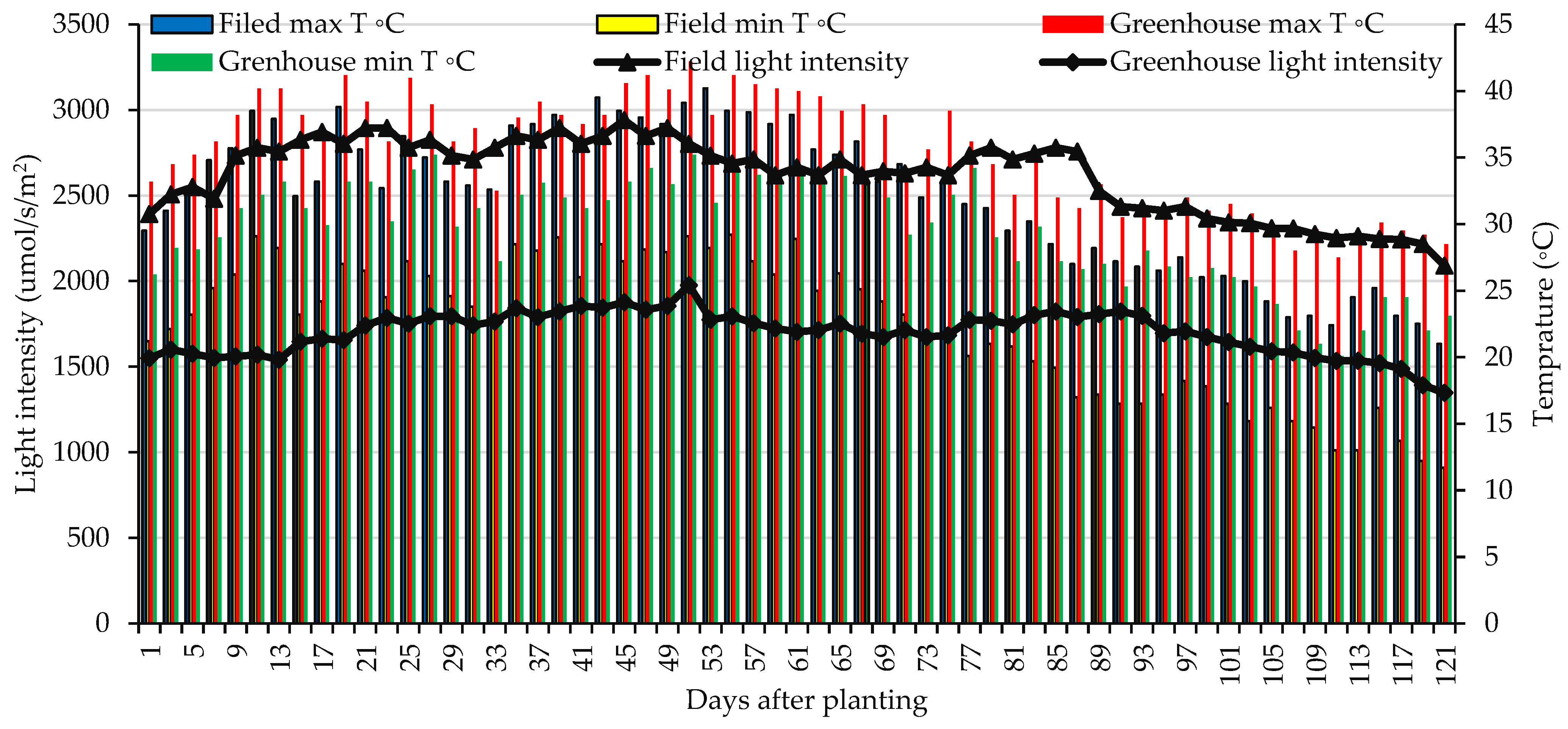
3.1. Plant Height in the Field and Greenhouse Settings
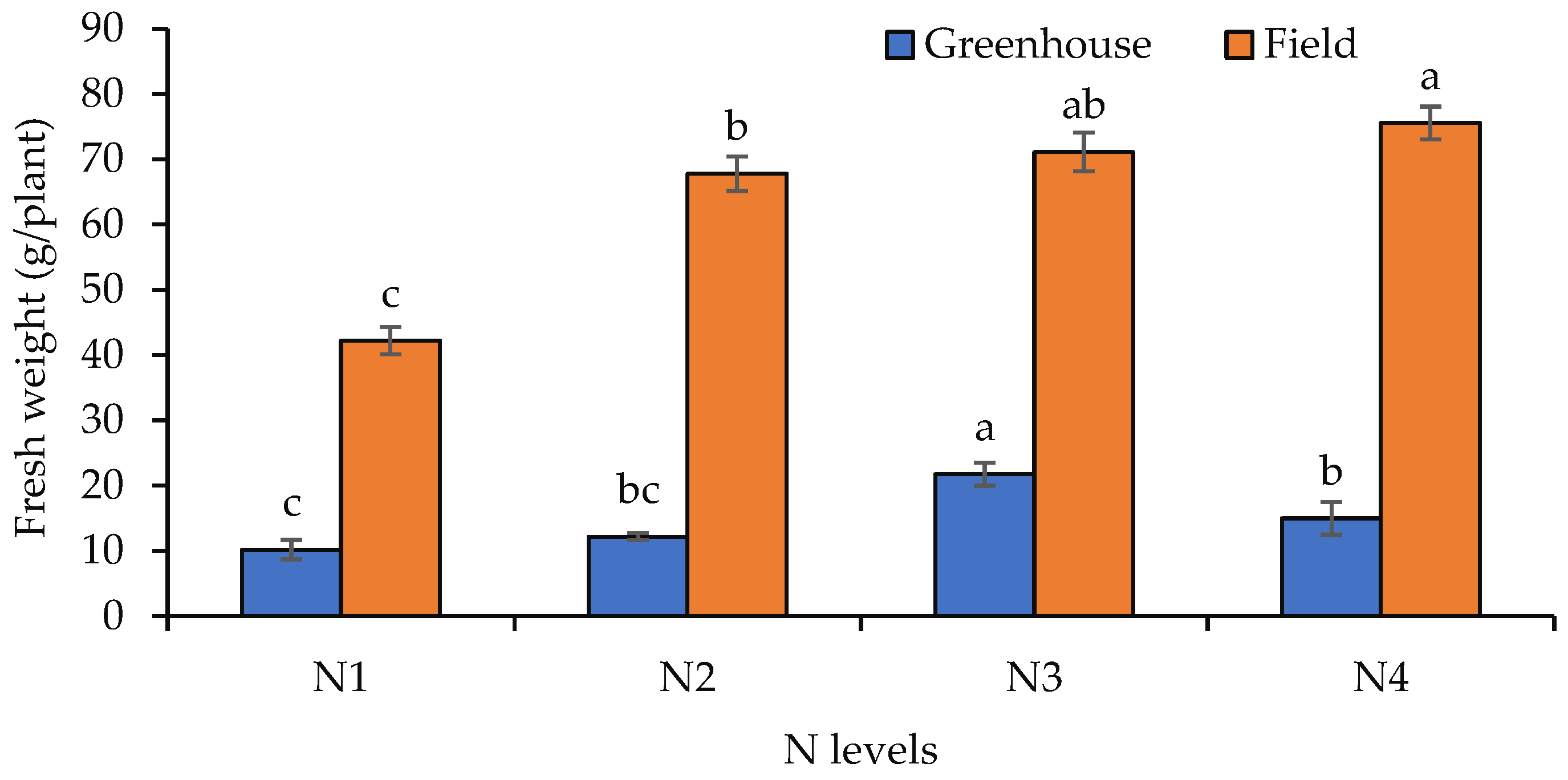
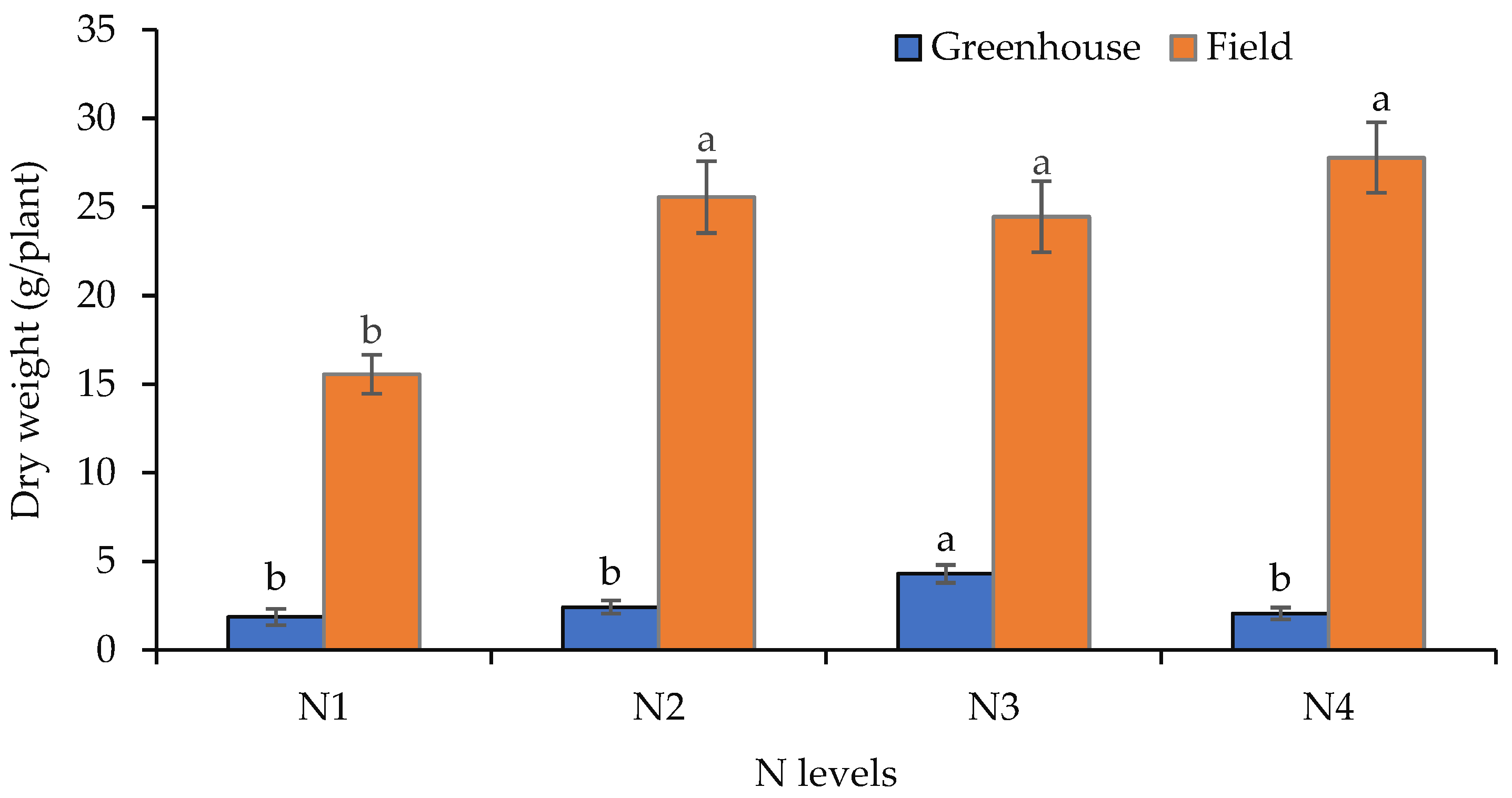
3.2. Fresh and Dry Biomass Production of Shoots
3.3. Shoot Nitrogen Concentration
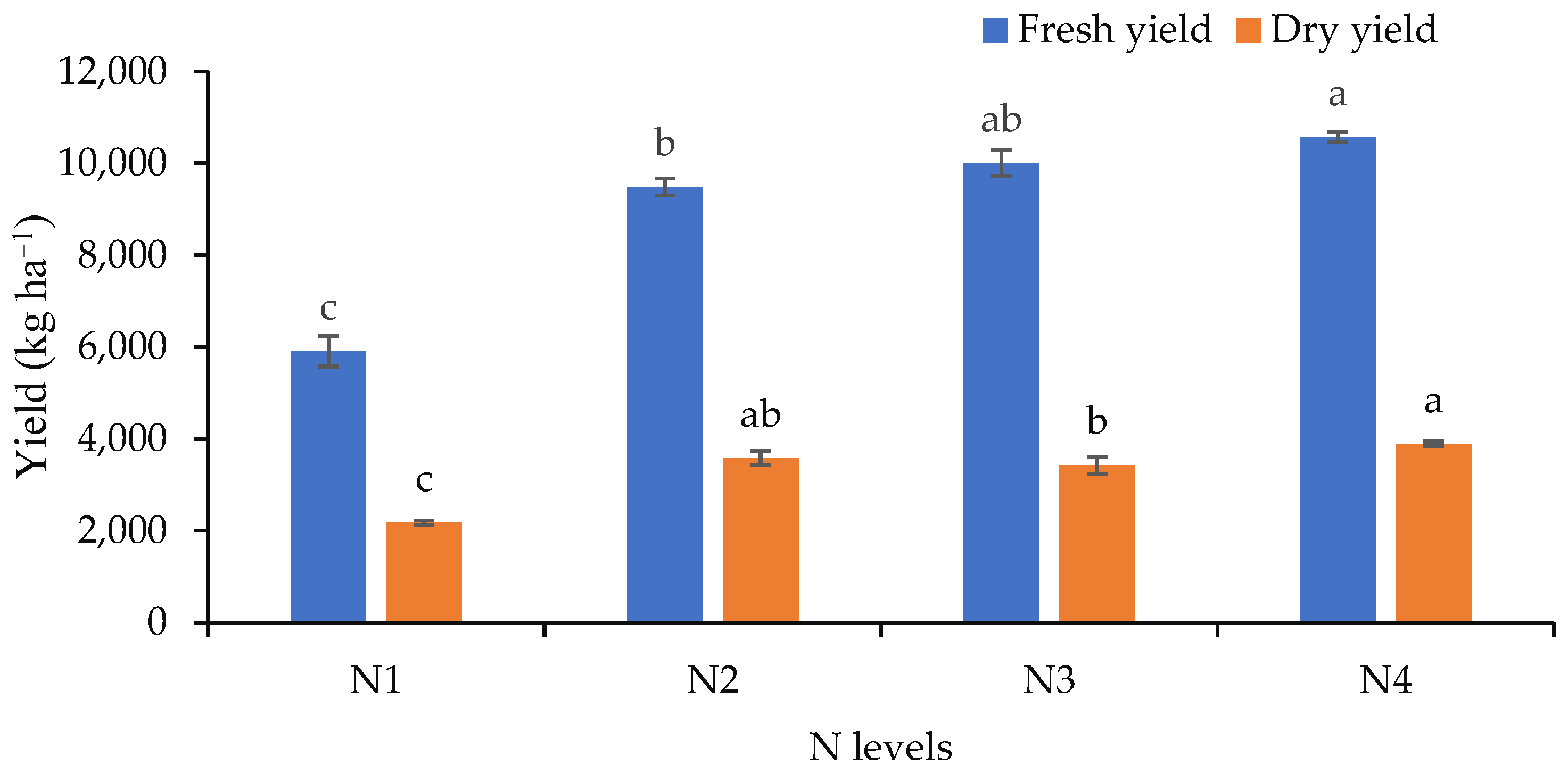
3.4. Essential Oil Yield of Dry Matter in the Field
3.5. Essential Oils Content of Dry Matter in the Field and Greenhouse
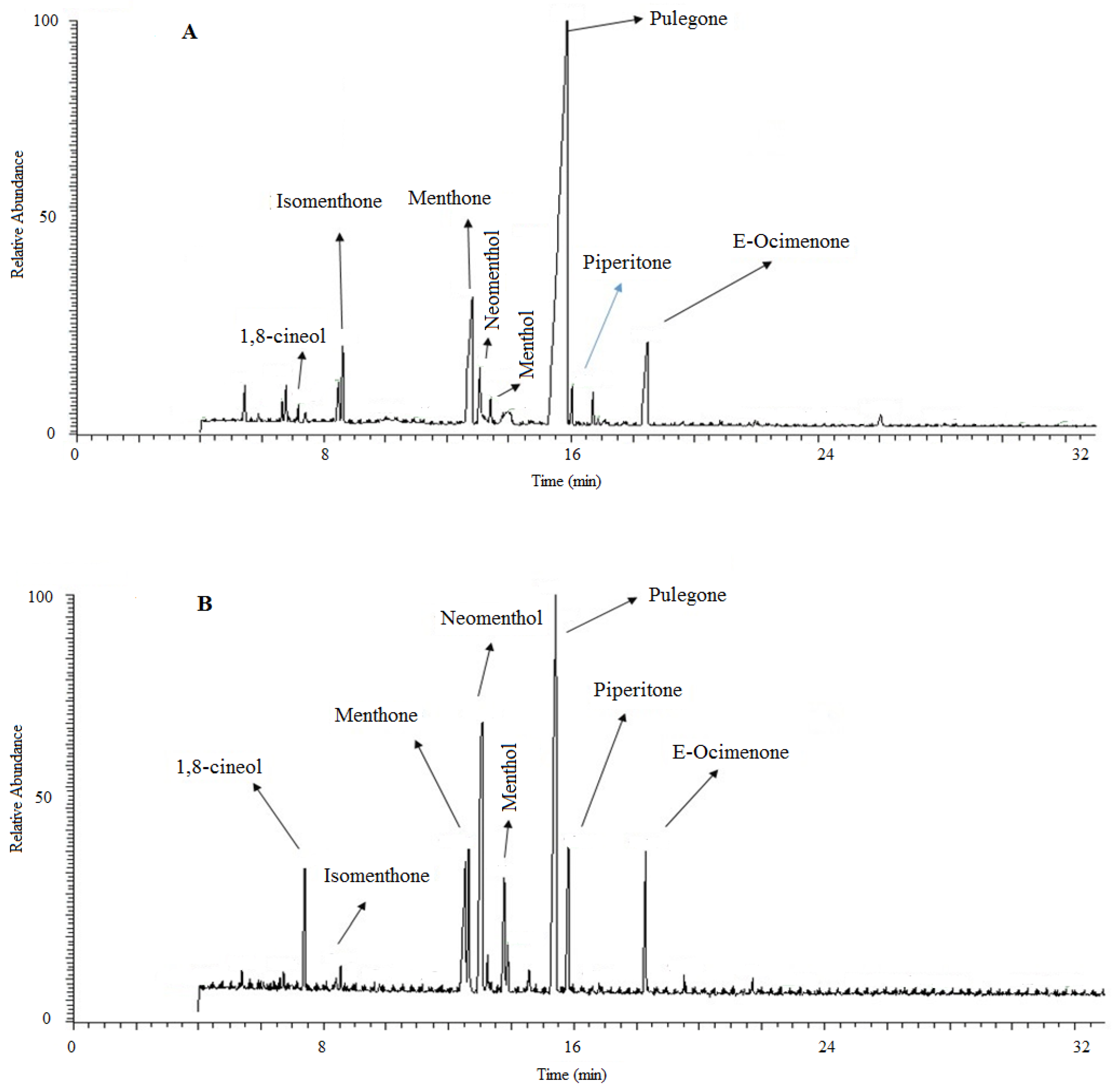
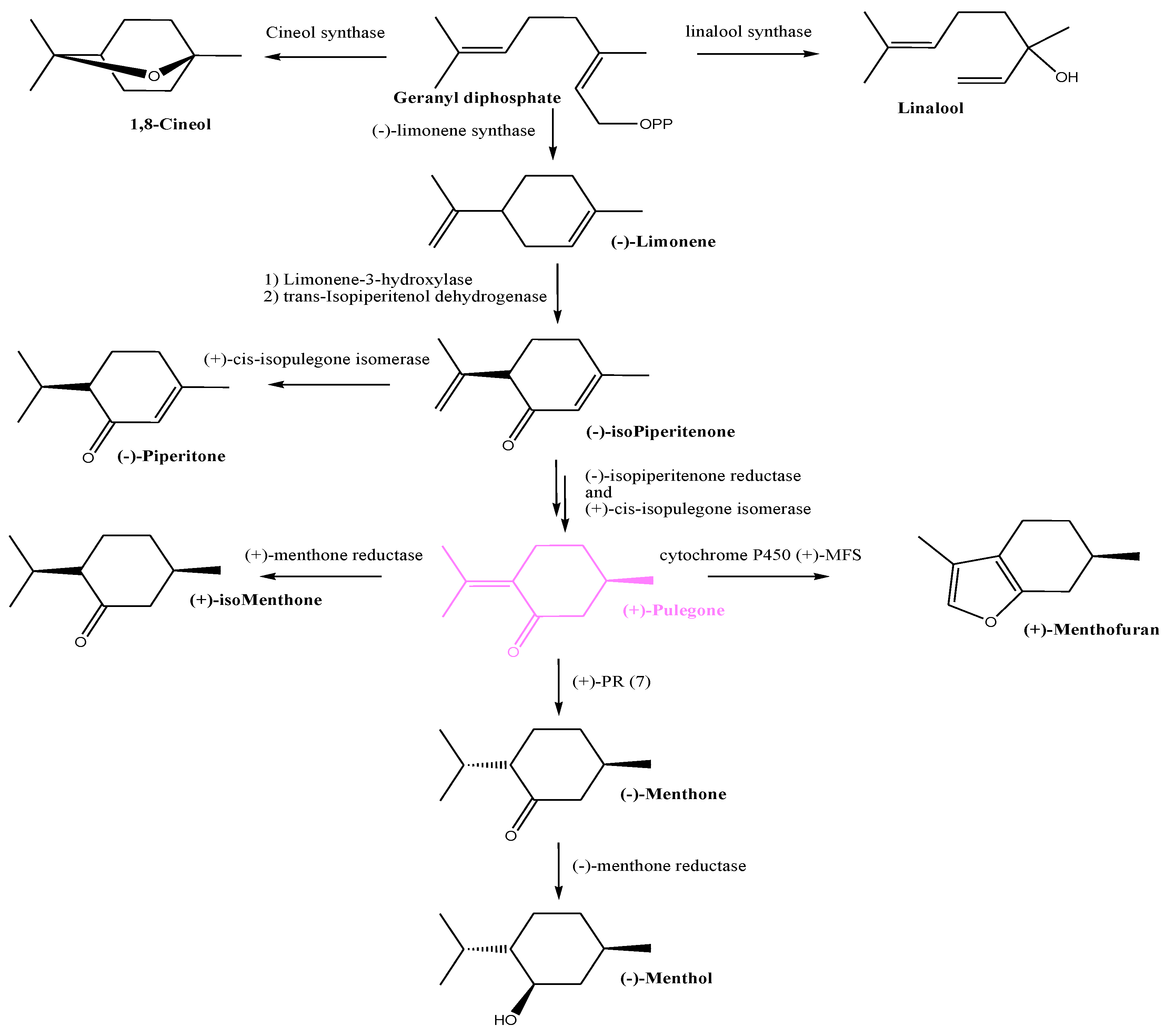
3.6. Essential Oil Compositions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Selwal, N.; Rahayu, F.; Herwati, A.; Latifah, E.; Suhara, C.; Suastika, I.B.K.; Mahayu, W.M.; Wani, A.K. Enhancing secondary metabolite production in plants: Exploring traditional and modern strategies. J. Agric. Food Res. 2023, 14, 100702. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Twaij, B.M.; Hasan, M.N. Bioactive secondary metabolites from plant sources: Types, synthesis, and their therapeutic uses. Int. J. Plant Biol. 2022, 13, 4–14. [Google Scholar] [CrossRef]
- Abdul, R.B. Medicinal plants (Importance and uses). Pharm. Anal. Acta 2012, 3, e139. [Google Scholar]
- Ochatt, S.; Alan, A.R.; Bhattacharya, A.; Hano, C.; Kiselev, K.V.; Marconi, P.L.; Otoni, W.C.; Park, S.Y.; Tang, K.X.; Weathers, P.J. Secondary metabolites: A boon from plants, the best chemist in nature: Preface from the editors. Plant Cell Tissue Organ Cult. 2022, 149, 1–6. [Google Scholar] [CrossRef]
- Jan, R.; Asaf, S.; Numan, M.; Kim, K.M. Plant secondary metabolite biosynthesis and transcriptional regulation in response to biotic and abiotic stress conditions. Agronomy 2021, 11, 968. [Google Scholar] [CrossRef]
- Lombardo, S.; Restuccia, C.; Muratore, G.; Barbagallo, R.N.; Licciardello, F.; Pandino, G.; Scifò, G.O.; Mazzaglia, A.; Ragonese, F.; Mauromicale, G.O. Effect of nitrogen fertilisation on the overall quality of minimally processed globe artichoke heads. J. Sci. Food Agric. 2017, 97, 650–658. [Google Scholar] [CrossRef]
- Hazrati, S.; Govahi, M.; Sedaghat, M.; Kashkooli, A.B. A comparative study of essential oil profile, antibacterial and antioxidant activities of two cultivated Ziziphora species (Z. clinopodioides and Z. tenuior). Ind. Crops Prod. 2020, 157, 112942. [Google Scholar] [CrossRef]
- Mohammad-hosseini, M. The ethnobotanical, phytochemical, and pharmacological properties and medicinal applications of essential oils and extracts of different Ziziphora species. Ind. Crops Prod. 2017, 105, 164–192. [Google Scholar] [CrossRef]
- Salehi, P.; Sonboli, A.; Eftekhar, F.; Nejad-Ebrahimi, S.; Yousefzadi, M. Essential oil composition, antibacterial and antioxidant activity of the oil and various extracts of Ziziphora clinopodioides subsp. rigida (BOISS.) RECH. f. from Iran. Biol. Pharm. Bull. 2005, 28, 1892–1896. [Google Scholar] [CrossRef]
- Ozturk, S.; Ercisli, S. Antibacterial activity and chemical constitutions of Ziziphora clinopodioides. Food Control 2007, 18, 535–540. [Google Scholar] [CrossRef]
- Šmejkal, K.; Malaník, M.; Zhaparkulova, K.; Sakipova, Z.; Ibragimova, L.; Ibadullaeva, G.; Žemlička, M. Kazakh Ziziphora species as sources of bioactive substances. Molecules 2016, 21, 826. [Google Scholar] [CrossRef]
- Ahmadi, A.; Gandomi, H.; Derakhshandeh, A.; Misaghi, A.; Noori, N. Phytochemical composition and in vitro safety evaluation of Ziziphora clinopodioides Lam. ethanolic extract: Cytotoxicity, genotoxicity and mutagenicity assessment. J. Ethnopharmacol. 2021, 266, 113428. [Google Scholar] [CrossRef]
- Asadi, A.; Cheniany, M. Enhancing effect of titanium dioxide nanoparticles on growth, phenolic metabolites production, and antioxidant potential of Ziziphora clinopodioides Lam. Russ. J. Plant Physiol. 2022, 69, 74. [Google Scholar]
- Feizi, H.; Tahan, V.; Kariman, K. In vitro, antibacterial activity of essential oils from Carum copticum and Ziziphora clinopodioides plants against the phytopathogen Pseudomonas syringae pv. syringae. Plant Biosyst. 2023, 157, 487–492. [Google Scholar] [CrossRef]
- Senejoux, F.; Demougeot, C.; Kerram, P.; Aisa, H.A.; Berthelot, A.; Bévalot, F.; Girard-Thernier, C. Bioassay-guided isolation of vasorelaxant compounds from Ziziphora clinopodioides Lam. (Lamiaceae). Fitoterapia 2012, 83, 377–382. [Google Scholar] [CrossRef]
- Dhingra, A.K.; Chopra, B. Pulegone: An emerging oxygenated cyclic monoterpene ketone scaffold delineating synthesis, chemical reactivity, and biological potential. Recent Adv. Anti-Infect. Drug Discov. 2023, 18, 16–28. [Google Scholar]
- da Silveira, N.S.; De Oliveira-Silva, G.L.; Lamanes, B.F.; Prado, L.C.; Bispo-da-Silva, L.B. The aversive, anxiolytic-like, and verapamil-sensitive psychostimulant effects of pulegone. Biol. Pharm. Bull. 2014, 37, 771–778. [Google Scholar] [CrossRef]
- Hilfiger, L.; Triaux, Z.; Marcic, C.; Héberlé, E.; Emhemmed, F.; Darbon, P.; Marchioni, E.; Petitjean, H.; Charlet, A. Anti-hyperalgesic properties of menthol and pulegone. Front. Pharmacol. 2021, 12, 753873. [Google Scholar] [CrossRef]
- Strzemski, M.; Dzida, K.; Dresler, S.; Sowa, I.; Kurzepa, J.; Szymczak, G.; Wójciak, M. Nitrogen fertilisation decreases the yield of bioactive compounds in Carlina acaulis L. grown in the field. Ind. Crops Prod. 2021, 170, 113698. [Google Scholar] [CrossRef]
- Lombardo, S.; Pandino, G.; Mauromicale, G. Optimizing nitrogen fertilization to improve qualitative performances and physiological and yield responses of potato (Solanum tuberosum L.). Agronomy 2020, 10, 352. [Google Scholar] [CrossRef]
- Pahalvi, H.N.; Rafiya, L.; Rashid, S.; Nisar, B.; Kamili, A.N. Chemical fertilizers and their impact on soil health. In Microbiota and Biofertilizers; Ecofriendly Tools for Reclamation of Degraded; Springer: Berlin/Heidelberg, Germany, 2021; Volume 2, pp. 1–20. [Google Scholar]
- Muhammad, I.; Lv, J.Z.; Yang, L.; Ahmad, S.; Farooq, S.; Zeeshan, M.; Zhou, X.B. Low irrigation water minimizes the nitrate nitrogen losses without compromising the soil fertility, enzymatic activities and maize growth. BMC Plant Biol. 2022, 22, 159. [Google Scholar] [CrossRef]
- Ayub, M.; Naeem, M.; Nadeem, M.A.; Tanveer, A.; Tahir, M.; Alam, R. Effect of nitrogen application on growth, yield, and oil contents of Fennel (Foenoculum vulgare Mill.). J. Med. Plant Res. 2011, 5, 2274–2277. [Google Scholar]
- Pavela, R.; Žabka, M.; Vrchotová, N.; Tříska, J. Effect of foliar nutrition on the essential oil yield of Thyme (Thymus vulgaris L.). Ind. Crops Prod. 2018, 112, 762–765. [Google Scholar] [CrossRef]
- Liava, V.; Karkanis, A.; Tsiropoulos, N. Yield and silymarin content in milk thistle (Silybum marianum (L.) Gaertn.) fruits affected by the nitrogen fertilizers. Ind. Crops Prod. 2021, 171, 113955. [Google Scholar] [CrossRef]
- Baranauskienė, R.; Venskutonis, P.R.; Viškelis, P.; Dambrauskienė, E. Influence of nitrogen fertilizers on the yield and composition of thyme (Thymus vulgaris). J. Agric. Food Chem. 2003, 51, 7751–7758. [Google Scholar] [CrossRef]
- Seif Sahandi, M.; Naghdi Badi, H.; Mehrafarin, A.; Khalighi-Sigaroodi, F.; Sharifi, M. Changes in essential oil content and composition of peppermint (Mentha piperita L.) in responses to nitrogen application. J. Med. Plants 2019, 18, 81–97. [Google Scholar] [CrossRef]
- Amiri, F.; Gholipouri, A.; Kheirkhah, M.; Mirjalili, M.H. Eco-physiology of Ziziphora clinopodioides Lam. (Lamiaceae) from Iran. Ann. Bot. 2019, 9, 63–72. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Dry combustion method using medium temperature resistance furnace. Chem. Microbiol. Properties 1982, 2, 539–579. [Google Scholar]
- Chapman, H.D. Cation-exchange capacity. In Methods of Soil Analysis—Chemical and Microbiological Properties; Black, C.A., Ed.; American Society of Agronomy: Madison, WI, USA, 1965; Volume 9, pp. 891–901. [Google Scholar]
- Novamsky, I.; Van Eck, R.; Van Schouwenburg, J.C.H.; Walinga, I. Total nitrogen determination in plant material by means of the indophenol blue method. Neth. J. Agri. Sci. 1974, 22, 3–5. [Google Scholar] [CrossRef]
- Knudsen, D.; Peterson, G.A.; Pratt, P.F. Lithium, sodium, and potassium. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; Page, A.L., Ed.; American Society of Agronomy: Madison, WI, USA, 1982; Volume 1, pp. 225–246. [Google Scholar]
- Røtset, O. Determination of phosphate species in nutrient solutions and phosphorus in plant material as phosphovanadomolybdic acid by flow injection analysis. Anal. Chim. Acta 1985, 178, 217–221. [Google Scholar] [CrossRef]
- Chapman, H.D.; Pratt, P.F. Methods of analysis for soils, plants and waters. Soil. Sci. 1962, 93, 68. [Google Scholar] [CrossRef]
- Horváth, B.; Opara-Nadi, O.; Beese, F. A simple method for measuring the carbonate content of soils. Soil. Sci. Soc. Am. J. 2005, 69, 1066–1068. [Google Scholar] [CrossRef]
- British Pharmacopoeia. Published on the Recommendation of the Medicines Commission Pursuant to the Medicines Act; Her Majesty’s Stationary Office: London, UK, 1968. [Google Scholar]
- Adams, P.R. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy; Allured Publishing Corporation, Carol Stream: DuPage, IL, USA, 2007. [Google Scholar]
- Toor, M.D.; Adnan, M.; Rehman, F.U.; Tahir, R.; Saeed, M.S.; Khan, A.U.; Pareek, V. Nutrients and their importance in agriculture crop production; A review. Ind. J. Pure App. Biosci. 2021, 9, 1–6. [Google Scholar] [CrossRef]
- He, X.; Wang, Y. Effects of regulated deficit irrigation on soil nutrient, microorganism and enzyme activity of isatis indigotica. In Proceedings of the Asia Conference on Geological Research and Environmental Technology, Kamakura City, Japan, 10–11 October 2020; IOP Publishing: Bristol, UK, 2021. [Google Scholar]
- Gu, J.; Li, Z.; Mao, Y.; Struik, P.C.; Zhang, H.; Liu, L.; Yang, J. Roles of nitrogen and cytokinin signals in root and shoot communications in maximizing of plant productivity and their agronomic applications. Plant Sci. 2018, 274, 320–331. [Google Scholar] [CrossRef]
- Torres-Bazurto, J.; Magnitskiy, S.; Sánchez, J.D. Effect of fertilization with N on height, number of leaves, and leaf area in banana (Musa AAA Simmonds, cv. Williams). Rev. Colomb. Cienc. Hortic. 2019, 13, 9–17. [Google Scholar] [CrossRef]
- Ahmadi, F.; Samadi, A.; Rahimi, A. Improving growth properties and phytochemical compounds of Echinacea purpurea (L.) medicinal plant using novel nitrogen slow release fertilizer under greenhouse conditions. Sci. Rep. 2020, 10, 13842. [Google Scholar] [CrossRef]
- Yao, Z.; Zhang, W.; Wang, X.; Zhang, L.; Zhang, W.; Liu, D.; Chen, X. Agronomic, environmental, and ecosystem economic benefits of controlled-release nitrogen fertilizers for maize production in Southwest China. J. Clean. Prod. 2021, 312, 127611. [Google Scholar] [CrossRef]
- Walia, S.; Kumar, R. Nitrogen and sulfur fertilization modulates the yield, essential oil and quality traits of wild marigold (Tagetes minuta L.) in the Western Himalaya. Front. Plant Sci. 2021, 11, 631154. [Google Scholar] [CrossRef]
- Liang, Y.; Cossani, C.M.; Sadras, V.O.; Yang, Q.; Wang, Z. The interaction between nitrogen supply and light quality modulates plant growth and resource allocation. Front. Plant Sci. 2022, 13, 864090. [Google Scholar] [CrossRef]
- Sharma, P.K.; Kumar, S. Soil Temperature and Plant Growth. In Soil Physical Environment and Plant Growth; Springer: Cham, Switzerland, 2023. [Google Scholar]
- Hatfield, J.L.; Prueger, J.H. Temperature extremes: Effect on plant growth and development. Weather. Clim. Extrem. 2015, 10, 4–10. [Google Scholar] [CrossRef]
- Lawlor, D.W. Carbon and nitrogen assimilation in relation to yield: Mechanisms are the key to understanding production systems. J. Exp. Bot. 2002, 53, 773–787. [Google Scholar] [CrossRef]
- Zayed, O.; Hewedy, O.A.; Abdelmoteleb, A.; Ali, M.; Youssef, M.S.; Roumia, A.F.; Seymour, D.; Yuan, Z.-C. Nitrogen Journey in Plants: From Uptake to Metabolism, Stress Response, and Microbe Interaction. Biomolecules 2023, 13, 1443. [Google Scholar] [CrossRef]
- Ohyama, T. Nitrogen as a major essential element of plants. Nitrogen Assim. Plants 2010, 37, 1–17. [Google Scholar]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press. Inc.: New York, NY, USA, 2002. [Google Scholar]
- Lombardo, S.; Pandino, G.; Mauromicale, G. Minerals profile of two globe artichoke cultivars as affected by NPK fertilizer regimes. Food Res. Int. 2017, 100, 95–99. [Google Scholar] [CrossRef]
- Tehulie, N.S.; Eskezia, H. Effects of nitrogen fertilizer rates on growth, yield components and yield of food Barley (Hordeum vulgare L.): A Review. J. Plant Sci. Agric. Res. 2021, 5, 46. [Google Scholar]
- Wang, L.; Yang, L.; Xiong, F.; Nie, X.; Li, C.; Xiao, Y.; Zhou, G. Nitrogen fertilizer levels affect the growth and quality parameters of Astragalus mongolica. Molecules 2020, 25, 381. [Google Scholar] [CrossRef]
- Daneshian, A.; Gurbuz, B.; Cosge, B.; Ipek, A. Chemical Components of Essential Oils from Basil (Ocimum basilicum L.) Grown at Different Nitrogen Levels. Int. J. Nat. Eng. Sci. 2009, 3, 9–13. [Google Scholar]
- Rioba, N.B.; Itulya, F.M.; Saidi, M.; Dudai, N.; Bernstein, N. Effects of nitrogen, phosphorus and irrigation frequency on essential oil content and composition of sage (Salvia officinalis L.). J. Appl. Res. Med. Aromat. Plants 2015, 2, 21–29. [Google Scholar] [CrossRef]
- Azizi, A.; Yan, F.; Honermeier, B. Herbage yield, essential oil content, and composition of three Oregano (Origanum vulgare L.) populations as affected by soil moisture regimes and nitrogen supply. Ind. Crops Prod. 2009, 29, 554–561. [Google Scholar]
- Bielski, S. Milk thistle (Silybum marianum L. Gaertn.) achene yield had a positive response to nitrogen fertilization, row spacing, sowing date, and weed control methods. Ind. Crops Prod. 2021, 160, 113104. [Google Scholar] [CrossRef]
- Singh, M.; Khan, M.M.A.; Naeem, M. Effect of nitrogen on growth, nutrient assimilation, essential oil content, yield and quality attributes in Zingiber officinale Rosc. J. Saudi Soc. Agric. 2016, 15, 171–178. [Google Scholar] [CrossRef]
- Kishorekumar, R.; Bulle, M.; Wany, A.; Gupta, K.J. An overview of important enzymes involved in nitrogen assimilation of plants. Methods Mol. Biol. 2020, 2057, 1–13. [Google Scholar]
- Xin, C.; Yan, Q.-W.; Sun, J.-L.; Xiao, S.; Xie, F.-C.; Chen, Y.-J. Research progress on nitrogen use and plant growth. J. Northeast Agric. Univ. 2014, 21, 68–74. [Google Scholar] [CrossRef]
- Sun, J.; Jin, L.; Li, R.; Meng, X.; Jin, N.; Wang, S.; Xu, Z.; Liu, Z.; Lyu, J.; Yu, J. Effects of Different Forms and Proportions of Nitrogen on the Growth, Photosynthetic Characteristics, and Carbon and Nitrogen Metabolism in Tomato. Plants 2023, 12, 4175. [Google Scholar] [CrossRef]
- Yamashita, N.; Tanabata, S.; Ohtake, N.; Sueyoshi, K.; Sato, T.; Higuchi, K.; Saito, A.; Ohyama, T. Effects of Different Chem-ical Forms of Nitrogen on the Quick and Reversible Inhibition of Soybean Nodule Growth and Nitrogen Fixation Activity. Front. Plant Sci. 2019, 10, 131. [Google Scholar] [CrossRef]
- Mi, W.; Hong, Y.; Gao, F.; Ma, Y.; Sun, T.; Wu, L.; Wang, G.; Chen, S. Effect of Different Form of N Fertilization on Yield Sustainability and Soil Quality in Double Cropped Rice System in a Long-Term Experiment. J. Soil. Sci. Plant Nutr. 2024, 24, 2815–2824. [Google Scholar] [CrossRef]
- Ma, C.; Ban, T.T.; Gao, Y.N.; Yu, H.J.; Li, Q.; Li, X.H.; Deng, Y.; Jiang, W.J. Different nitrogen forms on the growth and nitrogen utilization of cucumber. J. Agric. Crop Res 2020, 8, 90–97. [Google Scholar] [CrossRef]
- Cui, H.; Luo, Y.; Li, C.; Chang, Y.; Jin, M.; Li, Y.; Wang, Z. Effects of nitrogen forms on nitrogen utilization, yield, and quality of two wheat varieties with different gluten characteristics. Eur. J. Agron. 2023, 149, 126919. [Google Scholar] [CrossRef]
- Shen, N.S. Effects of different nitrogen forms and water stress on water absorption, photosynthesis and growth of Oryza sativa seedlings. Chin. Bull. Bot. 2007, 24, 477. [Google Scholar]
- Ali, A.M.; Saudi, A.M.; El-Sadek, A.N. Developing a nitrogen fertilizer management model for wheat in calcareous soils using the critical nitrogen dilution curve. Nutr. Cycl. Agroecosyst. 2023, 125, 379–392. [Google Scholar] [CrossRef]
- Geisseler, D.; Ortiz, R.S.; Diaz, J. Nitrogen nutrition and fertilization of onions (Allium cepa L.)–A literature review. Sci. Hortic. 2022, 291, 110591. [Google Scholar] [CrossRef]
- Li, S.; Lei, Y.; Zhang, Y.; Liu, J.; Shi, X.; Jia, H.; Wang, C.; Chen, F.; Chu, Q. Rational trade-offs between yield increase and fertilizer inputs are essential for sustainable intensification: A case study in wheat–maize cropping systems in China. Sci. Total Environ. 2019, 679, 328–336. [Google Scholar] [CrossRef]
- Ninou, E.; Cook, C.M.; Papathanasiou, F.; Aschonitis, V.; Avdikos, I.; Tsivelikas, A.L.; Stefanou, S.; Ralli, P.; Mylonas, I. Nitrogen effects on the essential oil and biomass production of field grown Greek oregano (Origanum vulgare subsp. hirtum) populations. Agronomy 2021, 11, 1722. [Google Scholar] [CrossRef]
- Ninou, E.G.; Paschalidis, K.A.; Mylonas, I.G.; Vasilikiotis, C.; Mavromatis, A.G. The effect of genetic variation and nitrogen fertilization on productive characters of Greek oregano. Acta Agric. Scand B Soil Plant Sci. 2017, 67, 372–379. [Google Scholar] [CrossRef]
- Abbaszadeh, B.; Farahani, H.A.; Valadabadi, S.A.; Moaveni, P. Investigation of variations of the morphological values and flowering shoot yield in different mint species at Iran. J. Hortic. For. 2009, 1, 109–112. [Google Scholar]
- Arabaci, O.; Bayram, E. The effect of nitrogen fertilization and different plant densities on some agronomic and technologic characteristic of Ocimum basilicum L. (Basil). J. Agron. 2004, 3, 255–262. [Google Scholar] [CrossRef]
- Ashraf, M.; Ali, Q.; Iqbal, Z. Effect of nitrogen application rate on the content and composition of oil, essential oil and minerals in black cumin (Nigella sativa L.) seeds. J. Sci. Food Agric. 2006, 86, 871–876. [Google Scholar] [CrossRef]
- Şekeroğlu, N.; Özgüven, M. Effects of different nitrogen doses and row spacing applications on yield and quality of Oenothera biennis L. grown in irrigated lowland and unirrigated dryland conditions. Turk. J. Agric. For. 2006, 30, 125–135. [Google Scholar]
- Zheljazkov, V.; Margina, A. Effect of increasing doses of fertilizer application on quantitative and qualitative characters of mint. Int. Symp. Med. Aromat. Plants 1995, 426, 579–592. [Google Scholar] [CrossRef]
- Said-Al Ahl, H.A.; Hussien, M.S. Effect of nitrogen and phosphorus application on herb and essential oil composition of Satureja montana L. ‘carvacrol’chemotype. J. Chem. Pharm. Res. 2016, 8, 119–128. [Google Scholar]
- Ram, M.; Ram, D.; Roy, S.K. Influence of an organic mulching on fertilizer nitrogen use efficiency and herb and essential oil yields in geranium (Pelargonium graveolens). Bioresour. Technol. 2003, 87, 273–278. [Google Scholar] [CrossRef]
- Koeduka, T.; Fridman, E.; Gang, D.R.; Vassão, D.G.; Jackson, B.L.; Kish, C.M.; Pichersky, E. Eugenol and isoeugenol, characteristic aromatic constituents of spices, are biosynthesized via reduction of a coniferyl alcohol ester. Proc. Natl. Acad. Sci. USA 2006, 103, 10128–10133. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Ma, A.; Bao, Y.; Wang, M.; Sun, Z. In Vitro antiviral, anti-inflammatory, and antioxidant activities of the ethanol extract of Mentha piperita L. Food Sci. Biotechnol. 2017, 26, 1675–1683. [Google Scholar] [CrossRef]
- Mahmoud, S.S.; Croteau, R.B. Menthofuran regulates essential oil biosynthesis in peppermint by controlling a downstream monoterpene reductase. Proc. Natl. Acad. Sci. USA 2003, 100, 14481–14486. [Google Scholar] [CrossRef]
- Ormeño, E.; Baldy, V.; Ballini, C.; Fernandez, C. Production and diversity of volatile terpenes from plants on calcareous and siliceous soils: Effect of soil nutrients. J. Chem. Ecol. 2008, 34, 1219–1229. [Google Scholar] [CrossRef]
- Ormeno, E.; Fernandez, C. Effect of soil nutrient on production and diversity of volatile terpenoids from plants. Curr. Bioact. Compd. 2012, 8, 71–79. [Google Scholar]
- Bustamante, M.Á.; Michelozzi, M.; Barra Caracciolo, A.; Grenni, P.; Verbokkem, J.; Geerdink, P.; Safi, C.; Nogues, I. Effects of soil fertilization on terpenoids and other carbon-based secondary metabolites in Rosmarinus officinalis Plants: A Comparative Study. Plants 2020, 9, 830. [Google Scholar] [CrossRef]
- Lorio, P.L. Growth-differentiation balance—A basis for understanding southern pine-beetle tree interactions. For. Ecol. Manag. 1986, 14, 259–273. [Google Scholar] [CrossRef]
- Burney, O.T.; Davis, A.S.; Jacobs, D.F. Phenology of foliar and volatile terpenoid production for Thuja plicata families under differential nutrient availability. Environ. Exp. Bot. 2012, 77, 44–52. [Google Scholar] [CrossRef]




| Item | Unit | Soil |
|---|---|---|
| Texture | - | Sandy loam |
| Organic carbon | (%) | 0.51 |
| EC | (ds/m) | 1.36 |
| pH | - | 8.20 |
| Total N | (%) | 0.05 |
| Available P | (mg kg−1) | 7.80 |
| Available K | (mg kg−1) | 162 |
| CaCO3 | (%) | 4.50 |
| Available Mg | (mg kg−1) | 9.84 |
| Available Fe | (mg kg−1) | 8.10 |
| Available Mn | (mg kg−1) | 6.22 |
| Available Zn | (mg kg−1) | 1.12 |
| Available Cu | (mg kg−1) | 1.05 |
| Available B | (mg kg−1) | 0.48 |
| Cation-exchange capacity (CEC) | (cmol kg−1) | 9.5 |
| Bulk density | (g cm−3) | 1.35 |
| kg N ha−1 | mg N pot−1 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N. | Compound Name | RI Calculated | RI Reference | 0 | 40 | 80 | 120 | Field Condition | Significance | 0 | 200 | 400 | 600 | Green House Condition | Significance |
| 1 | α-Pinene | 928 | 932 | 3.42 ± 0.02 a | 3.66 ± 0.14 a | 1.87 ± 0.27 b | 0.3 ± 0.04 c | 2.31 | ** | 0.84 ± 0.06 | 1.10 ± 0.15 | 1.56 ± 0.26 | 0.94 ± 0.09 | 1.11 | ns |
| 2 | Sabinene | 967 | 969 | 2.54 ± 0.11 a | 2.03 ± 0.03 b | 1.63 ± 0.01 c | 0.11 ± 0.01 d | 1.58 | *** | 00 ± 0.00 b | 00 ± 0.00 b | 1.51 ± 0.19 a | 1.42 ± 0.07 a | 0.73 | ** |
| 3 | β-pinene | 972 | 974 | 3.84 ± 0.10 a | 3.58 ± 0.02 a | 2.48 ± 0.02 b | 0.33 ± 0.03 c | 2.56 | *** | 1.20 ± 0.15 | 0.80 ± 0.01 | 0.82 ± 0.01 | 0.97 ± 0.07 | 0.95 | ns |
| 4 | Limonene | 1024 | 1024 | 1.78 ± 0.02 a | 1.80 ± 0.10 a | 1.29 ± 0.19 ab | 1.01 ± 0.01 c | 1.47 | * | 00 ± 0.00 b | 00 ± 0.00 b | 00 ± 0.00 b | 0.60 ± 0.10 a | 0.15 | * |
| 5 | 1,8-Cineol | 1027 | 1026 | 6.91 ± 0.29 a | 1.02 ± 0.01 c | 1.33 ± 0.14 bc | 1.87 ± 0.12 b | 2.78 | *** | 24.4 ± 1.90 a | 8.51 ± 0.59 b | 6.57 ± 0.45 b | 5.98 ± 0.42 b | 11.37 | ** |
| 6 | iso-menthone | 1145 | 1158 | 9.32 ± 0.62 a | 7.66 ± 0.40 a | 7.56 ± 0.55 a | 2.03 ± 0.03 b | 6.64 | * | 2.75 ± 0.45 | 2.48 ± 0.48 | 2.64 ± 0.35 | 2.33 ± 0.22 | 2.55 | ns |
| 7 | Menthone | 1148 | 1148 | 10.84 ± 0.46 b | 12.78 ± 0.78 b | 12.36 ± 0.16 b | 15.09 ± 0.60 a | 12.77 | ** | 8.56 ± 0.43 c | 12.59 ± 1.41 b | 15.44 ± 0.25 ab | 16.62 ± 1.10 a | 13.30 | ** |
| 8 | neo-Menthol | 1160 | 1161 | 4.81 ± 0.49 c | 5.11 ± 0.21 c | 10.44 ± 0.56 b | 15.84 ± 0.84 a | 9.05 | *** | 10.26 ± 0.74 c | 16.26 ± 0.26 b | 18.36 ± 0.86 a | 15.41 ± 0.40 b | 15.07 | *** |
| 9 | Menthol | 1168 | 1167 | 2.53 ± 0.37 b | 2.45 ± 0.25 b | 4.04 ± 0.55 a | 4.92 ± 0.18a | 3.49 | ** | 14.53 ± 1.27 a | 9.15 ± 1.04 b | 3.46 ± 0.54 c | 8.48 ± 0.52 b | 8.91 | *** |
| 10 | trans-pulegol | 1215 | 1213 | 4.04 ± 0.16 a | 3.93 ± 0.01 a | 1.84 ± 0.06 b | 0.35 ± 0.00 c | 2.54 | *** | 1.52 ± 0.08 a | 1.62 ± 0.18 a | 1.36 ± 0.16 a | 0.44 ± 0.04 b | 1.24 | *** |
| 11 | Pulegone | 1227 | 1233 | 38.24 ± 0.78 | 40.55 ± 1.35 | 40.4 ± 1.95 | 40.01 ± 0.99 | 39.80 | ns | 14.06 ± 0.98 c | 22.44 ± 0.65 b | 25.11 ± 0.99 a | 23.14 ± 1.35 ab | 21.19 | *** |
| 12 | Piperitone | 1249 | 1249 | 3.48 ± 0.8 b | 3.74 ± 0.25 b | 3.91 ± 0.19 b | 5.16 ± 0.33 a | 4.07 | *** | 7.31 ± 0.41 c | 9.26 ± 0.26 a | 8.20 ± 0.57 ab | 7.64 ± 0.37 b | 8.10 | ** |
| 13 | cis- Piperitone Epoxide | 1250 | 1250 | 2.87 ± 0.23 a | 3.36 ± 0.04 a | 1.26 ± 0.08 b | 0.24 ± 0.04 c | 1.93 | *** | 0.21 ± 0.02 | 0.40 ± 0.10 | 0.27 ± 0.01 | 0.50 ± 0.10 | 0.35 a | ns |
| 14 | E-Ocimenone | 1255 | 1235 | 1.78 ± 0.12 c | 4.42 ± 0.22 b | 5.42 ± 0.42 b | 9.02 ± 0.97 a | 5.16 | *** | 8.40 ± 0.40 | 9.07 ± 0.06 | 9.95 ± 1.05 | 9.96 ± 0.96 | 9.35 a | ns |
| 15 | Caryophyllene | 1378 | 1408 | 0.42 ± 0.02 a | 0.34 ± 0.00 ab | 0.27 ± 0.03 c | 0.30 ± 0.05 b | 0.33 | * | 2.45 ± 0.25 ab | 2.84 ± 0.16 a | 1.67 ± 0.02 b | 1.60 ± 0.06 b | 2.14 | * |
| 16 | Spathulenol | 1576 | 1577 | 0.20 ± 0.02 c | 0.14 ± 0.02 c | 1.41 ± 0.09 b | 2.67 ± 0.17 a | 1.10 | ** | 1.52 ± 0.27 | 1.14 ± 0.06 | 1.33 ± 0.03 | 1.26 ± 0.16 | 1.31 | ns |
| Total | - | 97.02 | 96.57 | 97.51 | 99.25 | - | - | 98.01 | 97.66 | 98.25 | 97.29 | - | - | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hazrati, S.; Mousavi, Z.; Mollaei, S.; Sedaghat, M.; Mohammadi, M.; Pignata, G.; Nicola, S. Optimizing Nitrogen Fertilization to Maximize Yield and Bioactive Compounds in Ziziphora clinopodioides. Agriculture 2024, 14, 1690. https://doi.org/10.3390/agriculture14101690
Hazrati S, Mousavi Z, Mollaei S, Sedaghat M, Mohammadi M, Pignata G, Nicola S. Optimizing Nitrogen Fertilization to Maximize Yield and Bioactive Compounds in Ziziphora clinopodioides. Agriculture. 2024; 14(10):1690. https://doi.org/10.3390/agriculture14101690
Chicago/Turabian StyleHazrati, Saeid, Zahra Mousavi, Saeed Mollaei, Mojde Sedaghat, Marzieh Mohammadi, Giuseppe Pignata, and Silvana Nicola. 2024. "Optimizing Nitrogen Fertilization to Maximize Yield and Bioactive Compounds in Ziziphora clinopodioides" Agriculture 14, no. 10: 1690. https://doi.org/10.3390/agriculture14101690








