Effect of Harvest Maturity and Lactiplantibacillus plantarum Inoculant on Dynamics of Fermentation Characteristics and Bacterial and Fungal Community of Triticale Silage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Triticale Sowing and Overview
2.2. Silage Material and Ensiling
2.3. Fermentation Profile and Chemical Composition
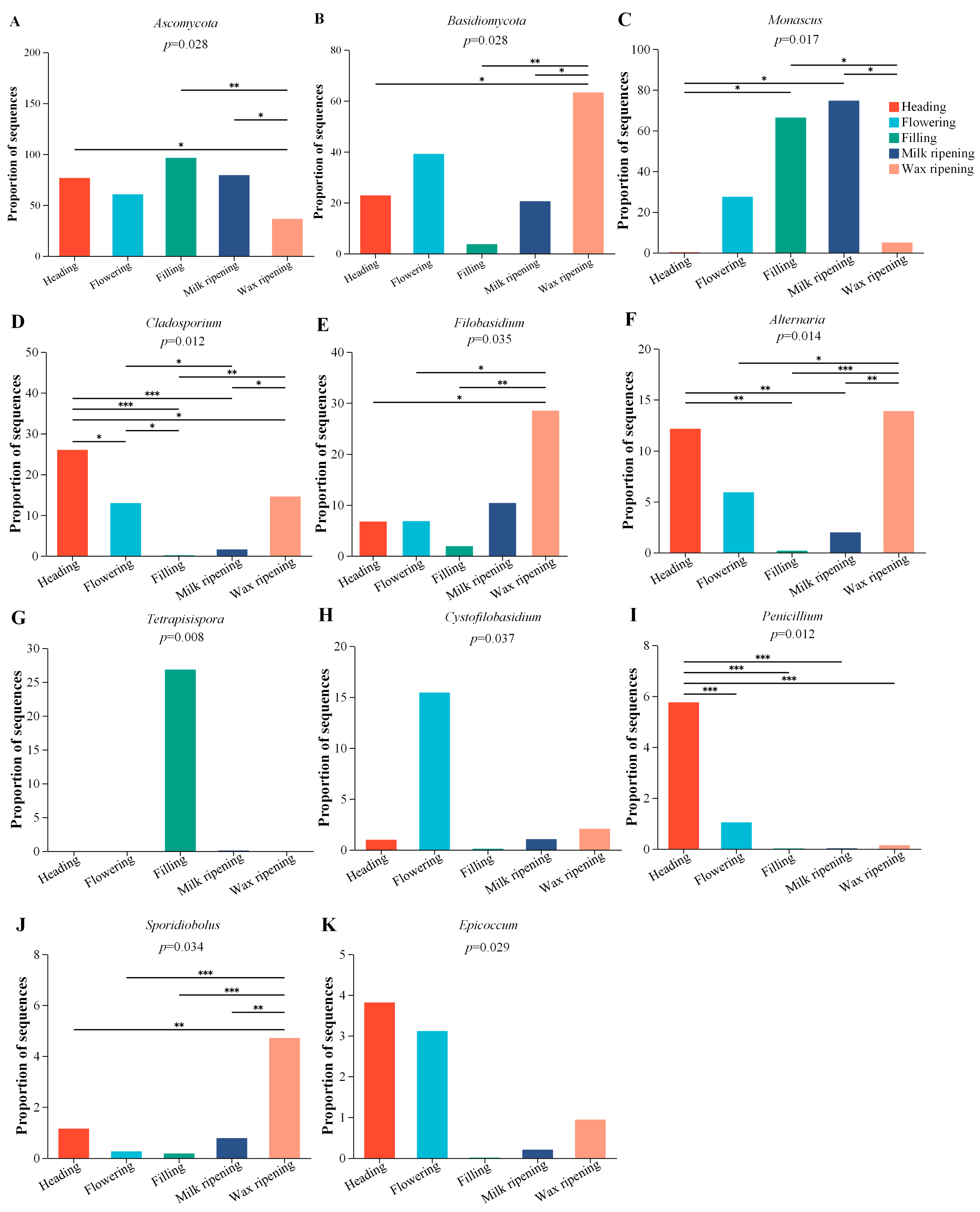
2.4. Microbial Community Composition
2.5. Statistical Analyses
3. Results
3.1. Chemical Compositions of Triticale before Ensiling
3.2. Fermentation Characteristics of Triticale after Ensiling
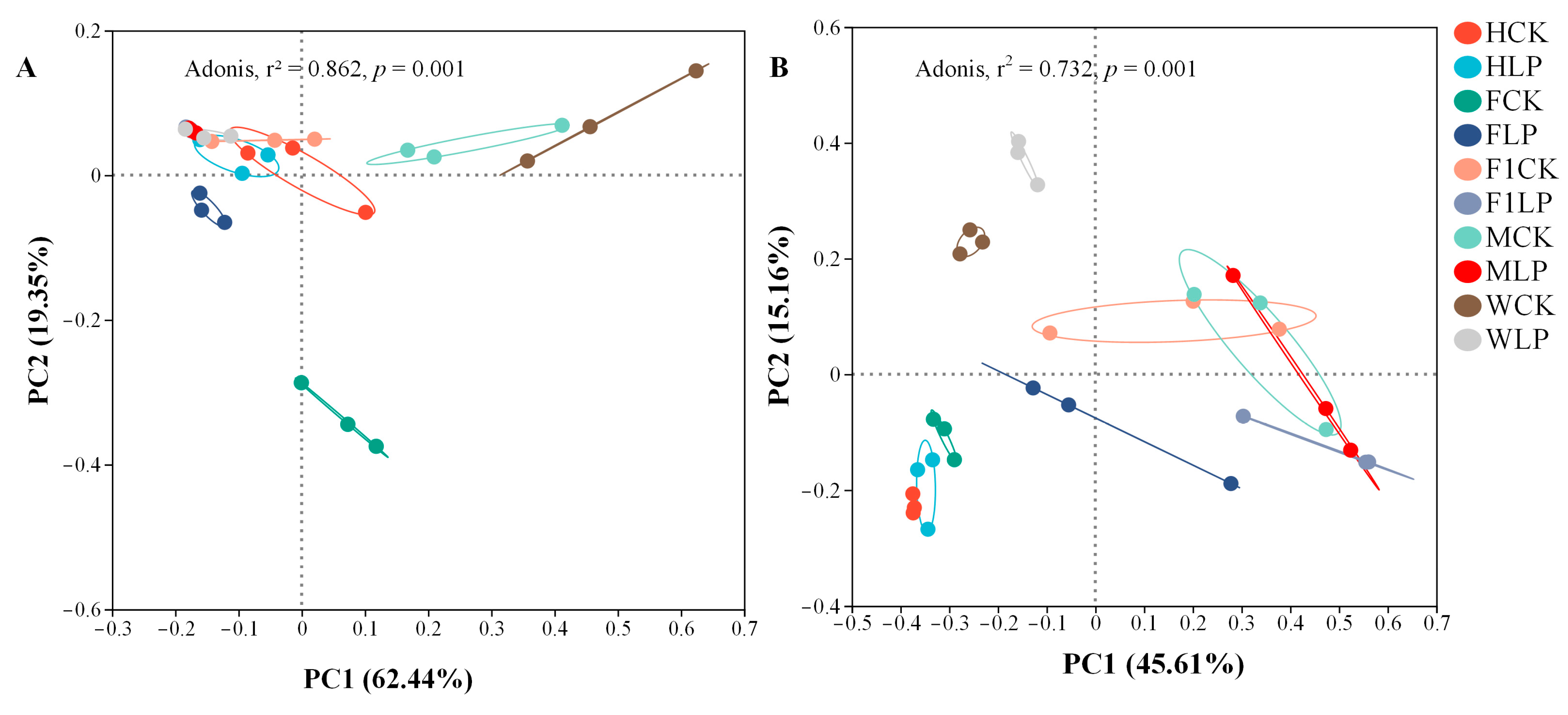
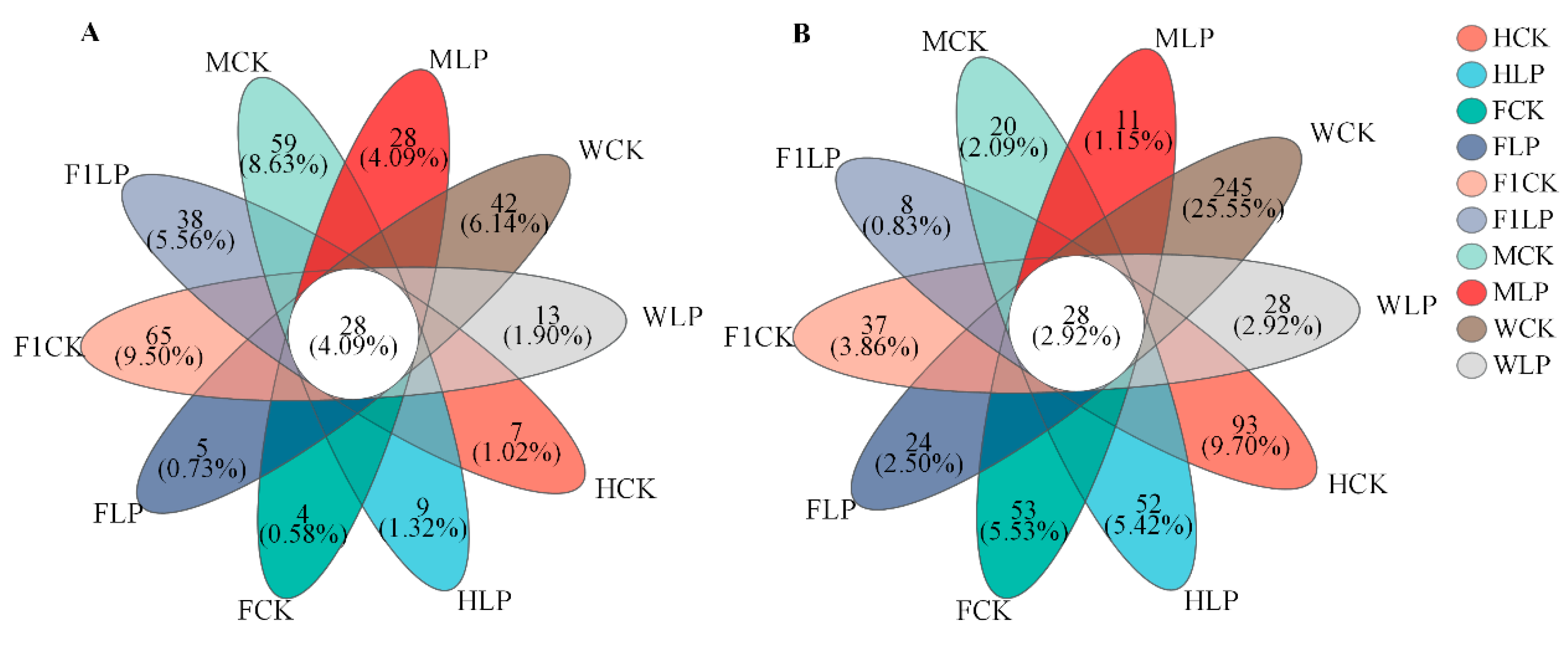
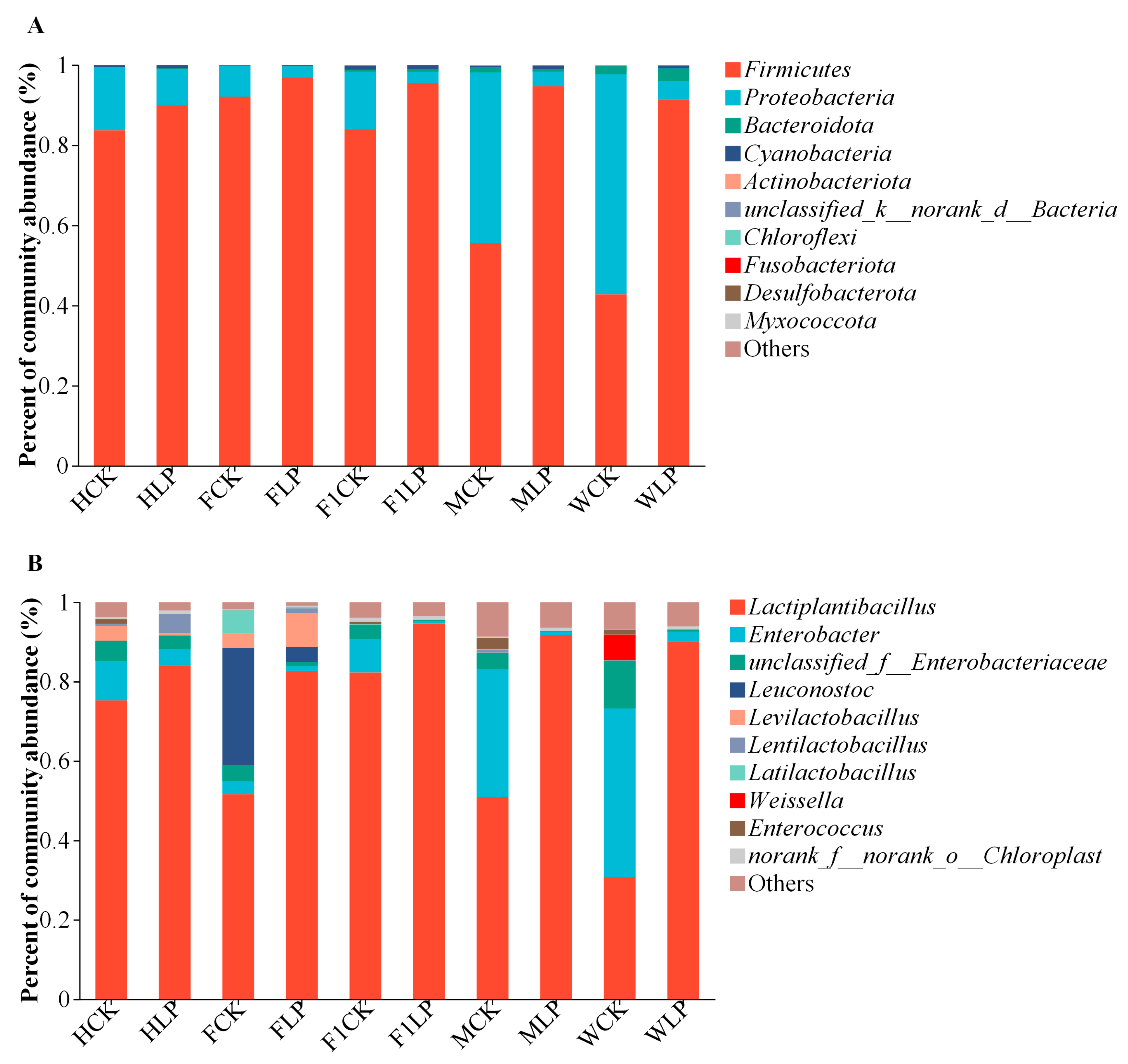
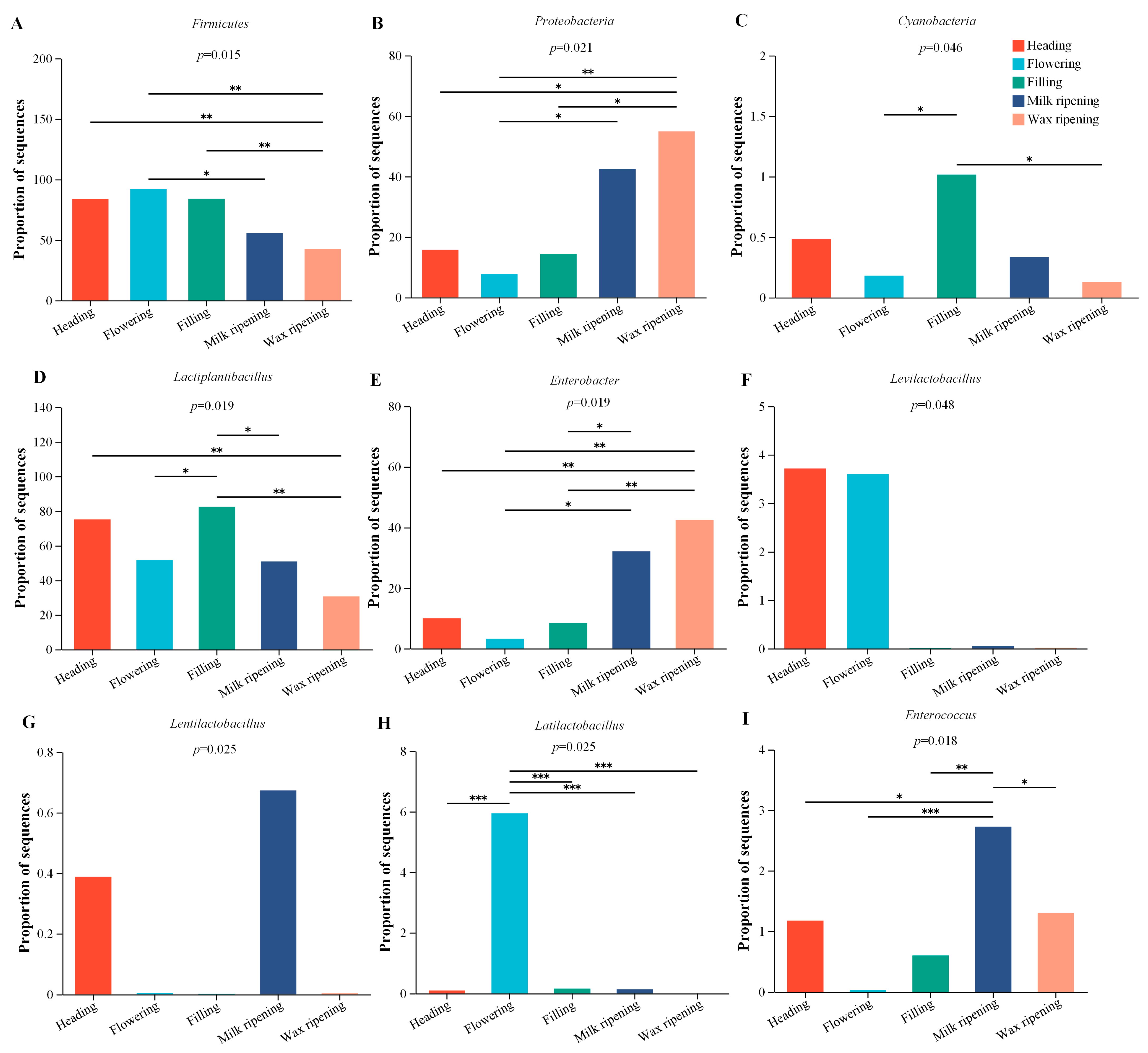
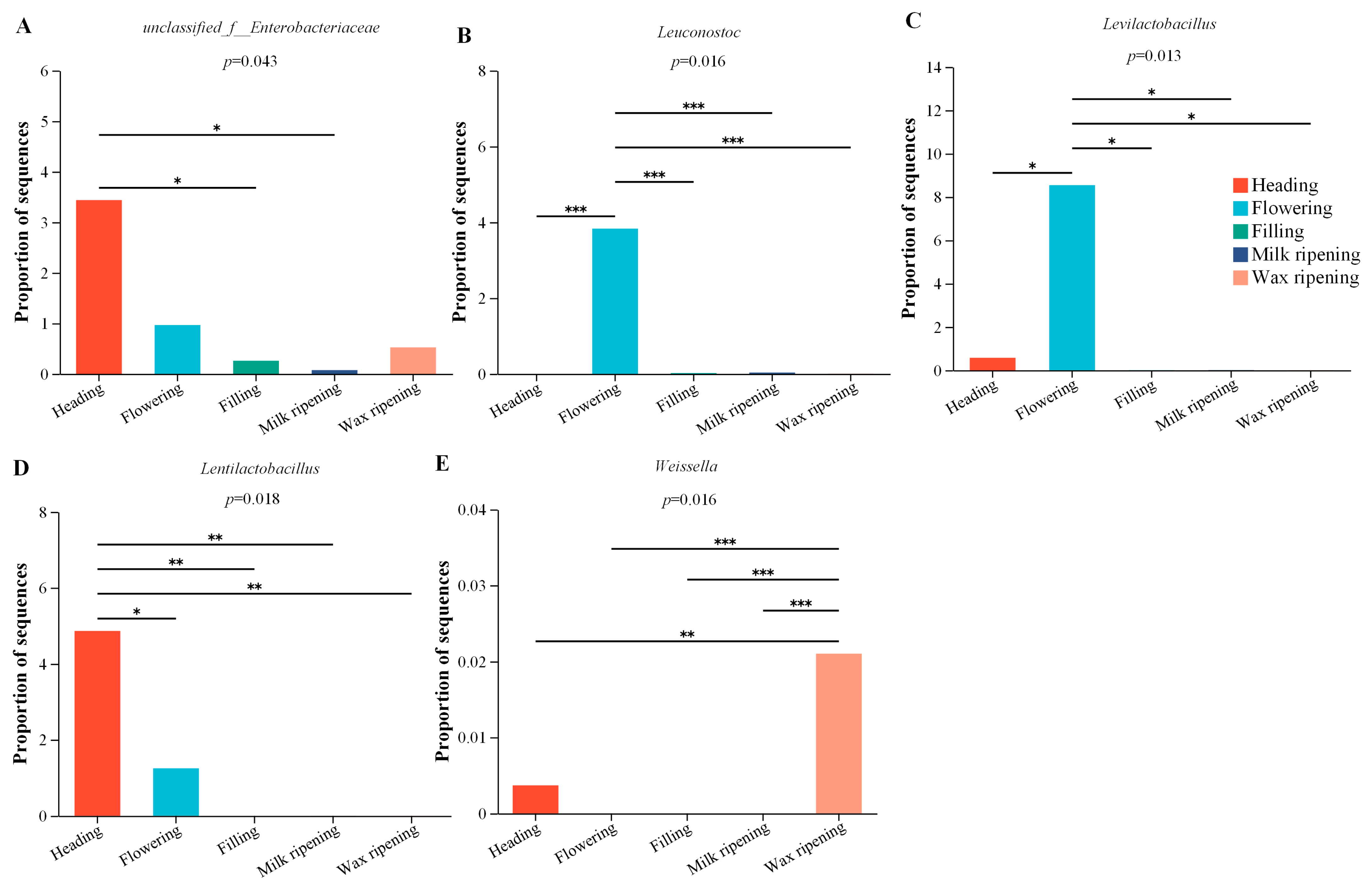
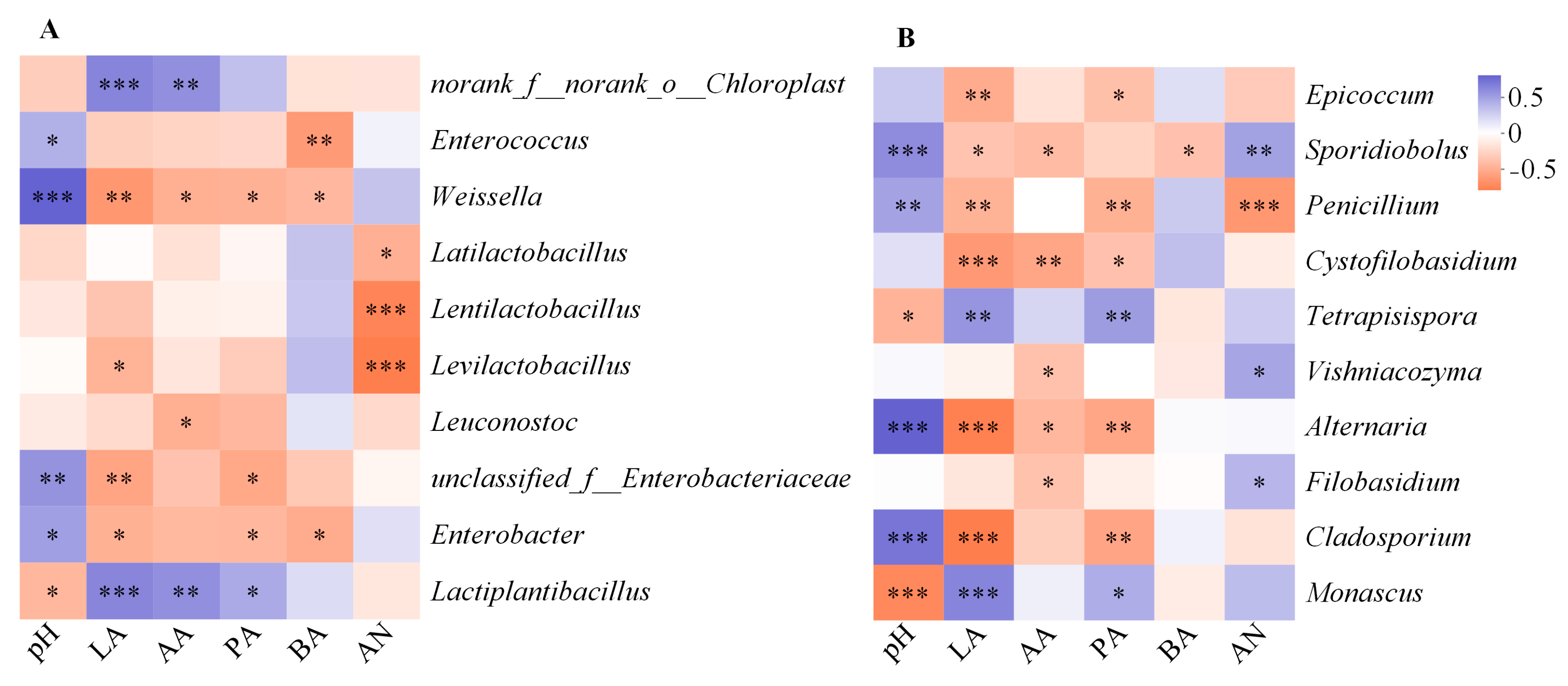
3.3. Bacterial Changes in Triticale after Ensiling
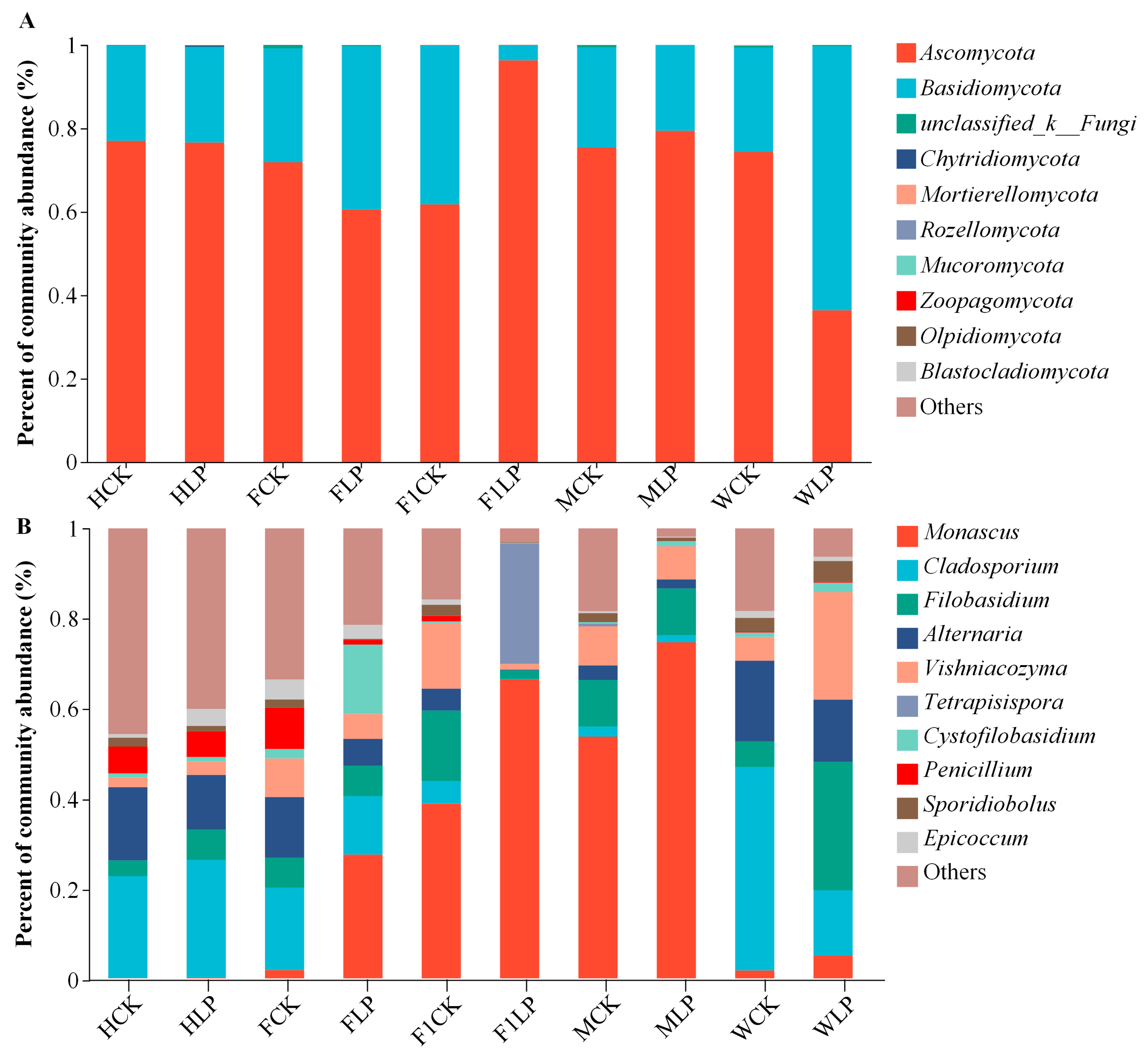
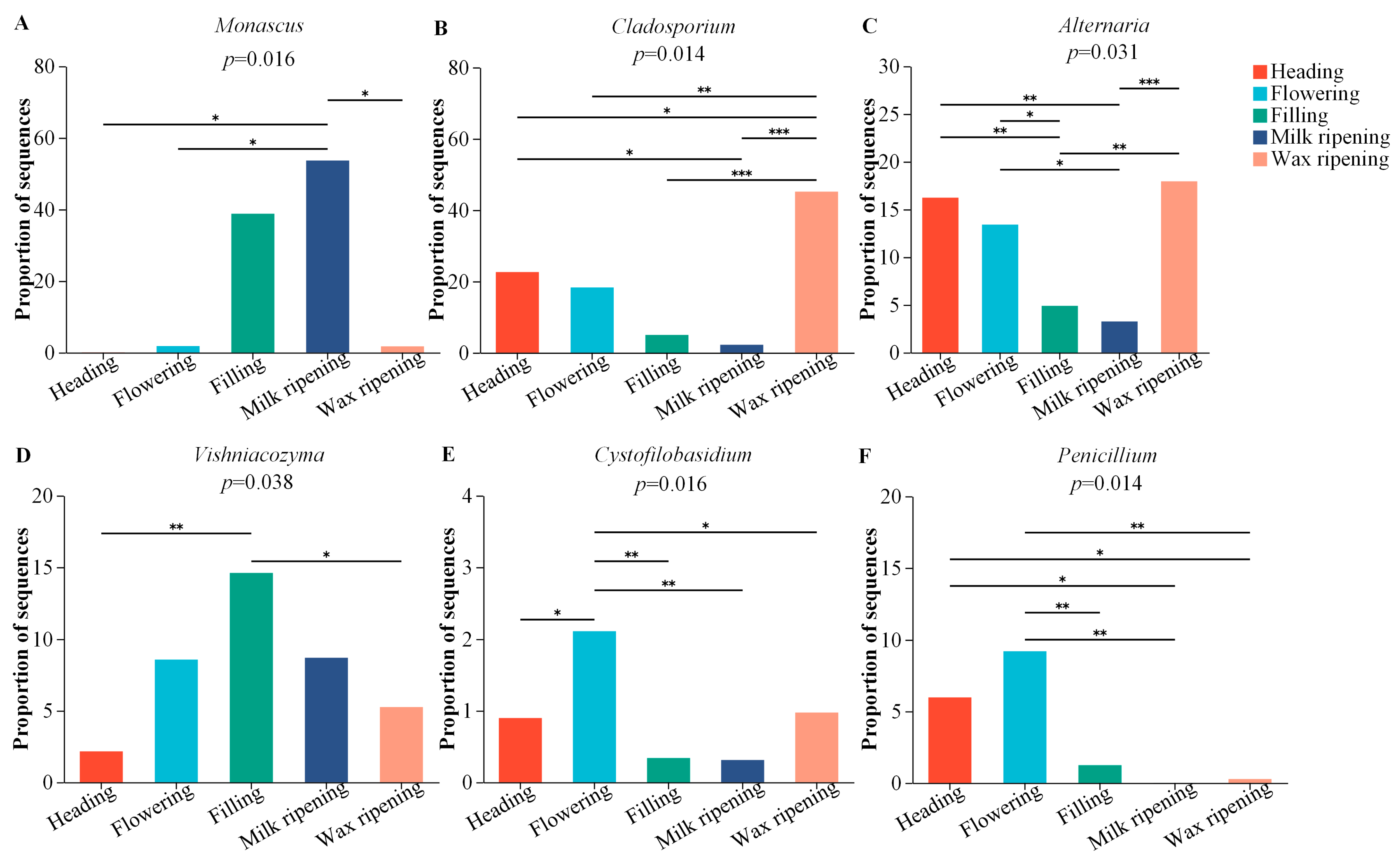
3.4. Fungal Changes in Triticale after Ensiling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zillinsky, F.J. The development of Triticale. Adv. Agron. 1974, 26, 315–348. [Google Scholar] [CrossRef]
- Harper, M.T.; Oh, J.; Giallongo, F.; Roth, G.W.; Hristov, A.N. Inclusion of wheat and triticale silage in the diet of lactating dairy cows. J. Dairy. Sci. 2017, 100, 6151–6163. [Google Scholar] [CrossRef] [PubMed]
- Jr, V.; Guimares, V.; Ribeiro, E.; Silva, L.; Zanin, E. Productive performance of lambs fed with high-moisture triticale grain ensiled with different additives. Can. J. Anim. Sci. 2019, 100, 323. [Google Scholar] [CrossRef]
- Soundharrajan, I.; Kim, D.; Kuppusamy, P.; Muthusamy, K.; Lee, H.J.; Choi, K.C. Probiotic and triticale silage fermentation potential of Pediococcus pentosaceus and Lactobacillus brevis and their impacts on pathogenic bacteria. Microorganisms 2019, 7, 318. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Liu, H.C.; Tian, X.H.; Du, W.H. Lodging resistance and feeding quality of triticale and cereal rye lines in an alpine pastoral area of P. R. China. Agron. J. 2022, 114, 1284–1297. [Google Scholar] [CrossRef]
- Wu, B.; Ai, J.; Li, T.; Qin, W.; Hu, Z.; Siqin, T.; Wu, T.; Wang, C.; Niu, H. Fermentation quality, aerobic stability, and microbiome structure and function of Caragana korshinskii silage inoculated with/without Lactobacillus rhamnosus or Lactobacillus buchneri. Front. Sustain. Food Syst. 2023, 7, 1255936. [Google Scholar] [CrossRef]
- Penagos-Tabares, F.; Khiaosa-Ard, R.; Schmidt, M.; Pacífico, C.; Faas, J.; Jenkins, T.; Nagl, V.; Sulyok, M.; Labuda, R.; Zebeli, Q. Fungal species and mycotoxins in mouldy spots of grass and maize silages in Austria. Mycotoxin Res. 2022, 38, 117–136. [Google Scholar] [CrossRef]
- Smith, D.; Lynch, G. Aspergillus fumigatus in samples of moldy silage. J. Dairy. Sci. 1973, 56, 828–829. [Google Scholar] [CrossRef]
- Maxin, G.; Andueza, D.; Le Morvan, A.; Baumont, R. Effect of intercropping vetch (Vicia sativa L.), field pea (Pisum sativum L.) and triticale (X Triticosecale) on dry-matter yield, nutritive and ensiling characteristics when harvested at two growth stages. Grass Forage Sci. 2017, 72, 777–784. [Google Scholar] [CrossRef]
- Jung, J.S.; Ravindran, B.; Soundharrajan, I.; Awasthi, M.K.; Choi, K.C. Improved performance and microbial community dynamics in anaerobic fermentation of triticale silages at different stages. Bioresour. Technol. 2022, 345, 126485. [Google Scholar] [CrossRef]
- Wan, J.C.; Xie, K.Y.; Wang, Y.X.; Liu, L.; Yu, Z.; Wang, B. Effects of wilting and additives on the ensiling quality and in vitro rumen fermentation characteristics of sudangrass silage. Asian-Australas. J. Anim. Sci. 2020, 34, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Yun, Y.; Yu, Z. Propionic acid and sodium benzoate affected biogenic amine formation, microbial community, and quality of oat silage. Front. Microbiol. 2021, 12, 750920. [Google Scholar] [CrossRef] [PubMed]
- Pieper, R.; Hackl, W.; Korn, U.; Zeyner, A.; Souffrant, W.B.; Pieper, B. Effect of ensiling triticale, barley and wheat grains at different moisture content and addition of Lactobacillus plantarum (DSMZ 8866 and 8862) on fermentation characteristics and nutrient digestibility in pigs. Anim. Feed Sci. Technol. 2011, 164, 96–105. [Google Scholar] [CrossRef]
- Vissers, M.M.M.; Driehuis, F.; Giffel, M.C.T.; De Jong, P.; Lankveld, J.M.G. Concentrations of butyric acid bacteria spores in silage and relationships with aerobic deterioration. J. Dairy. Sci. 2007, 90, 928–936. [Google Scholar] [CrossRef]
- Mu, L.; Xie, Z.; Hu, L.; Chen, G.; Zhang, Z. Cellulase interacts with Lactobacillus plantarum to affect chemical composition, bacterial communities, and aerobic stability in mixed silage of high-moisture amaranth and rice straw. Bioresour. Technol. 2020, 315, 123772. [Google Scholar] [CrossRef]
- Bai, J.; Xu, D.; Xie, D.; Wang, M.; Li, Z.; Guo, X. Effects of antibacterial peptide-producing Bacillus subtilis and Lactobacillus buchneri on fermentation, aerobic stability, and microbial community of alfalfa silage. Bioresour. Technol. 2020, 315, 123881. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Luo, Y.; Xu, S.; Wang, M.; Sun, Z.; Wang, L.; Yu, Z. Effects of replacing ensiled-alfalfa with fresh-alfalfa on dynamic fermentation characteristics, chemical compositions, and protein fractions in fermented total mixed ration with different additives. Animals 2021, 11, 572. [Google Scholar] [CrossRef]
- Broderick, G.A.; Kang, J.H. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J. Dairy. Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef]
- He, L.; Zhou, W.; Wang, Y.; Wang, C.; Chen, X.; Zhang, Q. Effect of applying lactic acid bacteria and cellulase on the fermentation quality, nutritive value, tannins profile and in vitro digestibility of Neolamarckia cadamba leaves silage. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1429–1436. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy. Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Murphy, R.P. A method for the extraction of plant samples and the determination of total soluble carbohydrates. J. Food Agric. 2010, 9, 714–717. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, D.; Ma, W.; Guo, Y.; Wang, A.; Wang, Q.; Lee, D.-J. Denitrifying sulfide removal process on high-salinity wastewaters in the presence of Halomonas sp. Appl. Microbiol. Biotechnol. 2016, 100, 1421–1426. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Jia, Y.; Ge, G.; Wang, Z.; Liu, M.; Bao, J.; Zhao, M.; Si, Q.; Liu, Y.; Zhao, W. Microbiomics and volatile metabolomics-based investigation of changes in quality and flavor of oat (Avena sativa L.) silage at different stages. Front. Plant Sci. 2023, 14, 1278715. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, F.; Lee, Y.H.; Lee, W.H.; Oh, Y.-K.; Park, K.; Kwak, W.S. Long-term anaerobic conservation of fruit and vegetable discards without or with moisture adjustment after aerobic preservation with sodium metabisulfite. Waste Manag. 2019, 87, 258–267. [Google Scholar] [CrossRef]
- He, L.; Wang, C.; Xing, Y.; Zhou, W.; Pian, R.; Chen, X.; Zhang, Q. Ensiling characteristics, proteolysis and bacterial community of high-moisture corn stalk and stylo silage prepared with Bauhinia variegate flower. Bioresour. Technol. 2020, 296, 122336. [Google Scholar] [CrossRef]
- Ren, H.; Feng, Y.; Pei, J.; Li, J.; Wang, Z.; Fu, S.; Zheng, Y.; Li, Z.; Peng, Z. Effects of Lactobacillus plantarum additive and temperature on the ensiling quality and microbial community dynamics of cauliflower leaf silages. Bioresour. Technol. 2020, 307, 123238. [Google Scholar] [CrossRef]
- Zhao, J.; Dong, Z.; Li, J.; Chen, L.; Bai, Y.; Jia, Y.; Shao, T. Evaluation of Lactobacillus plantarum MTD1 and waste molasses as fermentation modifier to increase silage quality and reduce ruminal greenhouse gas emissions of rice straw. Sci. Total Environ. 2019, 688, 143–152. [Google Scholar] [CrossRef]
- Keshri, J.; Chen, Y.; Pinto, R.; Kroupitski, Y.; Weinberg, Z.G.; Sela, S. Microbiome dynamics during ensiling of corn with and without Lactobacillus plantarum inoculant. Appl. Microbiol. Biotechnol. 2018, 102, 4025–4037. [Google Scholar] [CrossRef]
- Queiroz, O.C.M.; Ogunade, I.M.; Weinberg, Z.; Adesogan, A.T. Silage review: Foodborne pathogens in silage and their mitigation by silage additives. J. Dairy. Sci. 2018, 101, 4132–4142. [Google Scholar] [CrossRef]
- Xiao, Y.; Sun, L.; Xin, X.; Xu, L.; Du, S. Physicochemical characteristics and microbial community succession during oat silage prepared without or with Lactiplantibacillus plantarum or Lentilactobacillus buchneri. Microbiol. Spectr. 2023, 11, e0222823. [Google Scholar] [CrossRef]
- Zeng, T.; Li, X.; Guan, H.; Yang, W.; Liu, W.; Liu, J.; Du, Z.; Li, X.; Xiao, Q.; Wang, X.; et al. Dynamic microbial diversity and fermentation quality of the mixed silage of corn and soybean grown in strip intercropping system. Bioresour. Technol. 2020, 313, 123655. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Wang, C.; Sun, L.; Xu, H.; Jiang, Y.; Na, N.; Yin, G.; Liu, S.; Xue, Y. Dynamics of bacterial and fungal communities and metabolites during aerobic exposure in whole-plant corn silages with two different moisture levels. Front. Microbiol. 2021, 12, 663895. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Teng, K.; Cao, Y.; Shi, W.; Xuan, Z.; Zhou, J.; Zhang, J.; Zhong, J. Effects of Lactobacillus hilgardii 60TS-2, with or without homofermentative Lactobacillus plantarum B90, on the aerobic stability, fermentation quality and microbial community dynamics in sugarcane top silage. Bioresour. Technol. 2020, 312, 123600. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Gao, R.; Wu, Z.; Yu, Z. Functional analysis of sugars in modulating bacterial communities and metabolomics profiles of Medicago sativa silage. Front. Microbiol. 2020, 11, 641. [Google Scholar] [CrossRef] [PubMed]
- Lian, T.; Zhang, W.; Cao, Q.; Wang, S.; Dong, H.; Yin, F. Improving production of lactic acid and volatile fatty acids from dairy cattle manure and corn straw silage: Effects of mixing ratios and temperature. Bioresour. Technol. 2020, 359, 127449. [Google Scholar] [CrossRef]
- Wang, M.; Gao, R.; Franco, M.; Hannaway, D.B.; Ke, W.; Ding, Z.; Yu, Z.; Guo, X. Effect of mixing alfalfa with whole-plant corn in different proportions on fermentation characteristics and bacterial community of silage. Agriculture 2021, 11, 174. [Google Scholar] [CrossRef]
- Srianta, I.; Ristiarini, S.; Nugerahani, I.; Sen, S.K.; Zhang, B.B.; Xu, G.R.; Blanc, P. Recent research and development of Monascus fermentation products. Int. Food Res. J. 2014, 21, 1–12. [Google Scholar]
- Suraiya, S.; Kim, J.-H.; Tak, J.Y.; Siddique, M.P.; Young, C.J.; Kim, J.K.; Kong, I.-S. Influences of fermentation parameters on lovastatin production by Monascus purpureus using Saccharina japonica as solid fermented substrate. Lwt-Food Sci. Technol. 2018, 92, 1–9. [Google Scholar] [CrossRef]
- Silva, L.J.G.; Pereira, A.M.P.T.; Pena, A.; Lino, C.M. Citrinin in foods and supplements: A review of occurrence and analytical methodologies. Foods 2021, 10, 14. [Google Scholar] [CrossRef]
| Harvest Maturity Stages | DM | CP | αNDF | ADF | WSC |
|---|---|---|---|---|---|
| % | %DM | ||||
| Heading | 20.59 d | 11.88 a | 63.45 | 36.80 | 1.70 c |
| Flowering | 20.93 d | 12.40 a | 59.45 | 34.42 | 2.33 bc |
| Filling | 33.64 c | 7.91 b | 55.40 | 32.85 | 6.52 a |
| Milk ripening | 39.15 b | 6.74 bc | 56.41 | 33.81 | 3.46 b |
| Wax ripening | 59.96 a | 5.13 c | 60.61 | 37.06 | 2.05 bc |
| SEM | 3.869 | 0.793 | 1.039 | 0.655 | 0.501 |
| p-value | <0.001 | <0.001 | 0.079 | 0.163 | <0.001 |
| Items | Additives | Harvest Maturity Stages | SEM | p-Value | Interaction | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Heading | Flowering | Filling | Milk Ripening | Wax Ripening | S | A1 | S*A1 | ||||
| pH | CK | 4.17 bA | 3.99 cA | 3.89 cd | 3.79 dA | 4.81 aA | 0.098 | <0.001 | <0.001 | <0.001 | <0.001 |
| LP | 3.88 bB | 3.75 cB | 3.84 b | 3.71 cB | 4.04 aB | 0.031 | <0.001 | ||||
| SEM | 0.069 | 0.057 | 0.013 | 0.018 | 0.175 | ||||||
| p-value | 0.012 | 0.011 | 0.138 | 0.034 | 0.013 | ||||||
| LA | CK | 1.62 bB | 1.50 b | 2.99 a | 2.61 a | 0.47 c | 0.247 | <0.001 | <0.001 | <0.001 | 0.134 |
| %DM | LP | 2.16 bA | 1.52 b | 3.74 a | 3.16 a | 1.69 b | 0.255 | 0.002 | |||
| SEM | 0.141 | 0.085 | 0.254 | 0.238 | 0.317 | ||||||
| p-value | 0.026 | 0.825 | 0.034 | 0.097 | 0.111 | ||||||
| AA | CK | 0.73 abB | 0.50 cd | 0.77 aB | 0.57 bc | 0.36 d | 0.044 | 0.001 | <0.001 | <0.001 | 0.002 |
| %DM | LP | 1.38 aA | 0.44 b | 1.30 aA | 0.79 b | 0.52 b | 0.113 | 0.001 | |||
| SEM | 0.152 | 0.041 | 0.134 | 0.082 | 0.076 | ||||||
| p-value | 0.008 | 0.604 | 0.014 | 0.131 | 0.482 | ||||||
| PA | CK | 0.48 cB | 0.52 b | 0.51 bB | 1.22 a | 0.26 c | 0.091 | <0.001 | <0.001 | 0.006 | 0.357 |
| %DM | LP | 0.82 bA | 0.44 b | 0.83 bA | 1.45 a | 0.64 b | 0.109 | 0.015 | |||
| SEM | 0.088 | 0.025 | 0.081 | 0.146 | 0.126 | ||||||
| p-value | 0.048 | 0.257 | 0.036 | 0.481 | 0.246 | ||||||
| BA | CK | 0.00 | 0.02 | 0.00 | 0.00 | 0.00 | 0.002 | 0.053 | 0.003 | 0.032 | 0.971 |
| %DM | LP | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.002 | 0.156 | |||
| SEM | 0.001 | 0.004 | 0.001 | 0.001 | 0.001 | ||||||
| p-value | 0.184 | 0.423 | 0.184 | 0.423 | 0.184 | ||||||
| NH3-N | CK | 2.69 eA | 3.01 dA | 3.93 c | 4.17 b | 5.52 a | 0.267 | <0.001 | <0.001 | 0.005 | 0.449 |
| %TN | LP | 2.43 cB | 2.66 cB | 3.66 b | 4.17 b | 4.97 a | 0.262 | <0.001 | |||
| SEM | 0.060 | 0.088 | 0.093 | 0.036 | 0.218 | ||||||
| p-value | 0.009 | 0.015 | 0.091 | 1.000 | 0.231 | ||||||
| Items | Additives | Harvesting Stages | SEM | p-Value | Interaction | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Heading | Flowering | Filling | Milk Ripening | Wax Ripening | S | A1 | S*A1 | ||||
| Sobs | CK | 70.00 | 66.33 | 167.67 | 121.67 | 120.67 | 12.941 | 0.057 | 0.004 | 0.326 | 0.500 |
| LP | 80.67 | 73.33 | 118.33 | 110.67 | 105.67 | 6.394 | 0.098 | ||||
| SEM | 3.896 | 2.197 | 16.878 | 10.248 | 18.265 | ||||||
| p-value | 0.162 | 0.277 | 0.242 | 0.471 | 0.809 | ||||||
| Shannon | CK | 0.78 b | 1.38 abA | 0.82 b | 1.45 a | 1.54 a | 0.119 | 0.049 | 0.073 | <0.001 | 0.086 |
| LP | 0.73 a | 0.74 aB | 0.36 b | 0.49 ab | 0.53 ab | 0.059 | 0.036 | ||||
| SEM | 0.173 | 0.148 | 0.116 | 0.234 | 0.259 | ||||||
| p-value | 0.910 | 0.026 | 0.070 | 0.069 | 0.120 | ||||||
| Simpson | CK | 0.60 a | 0.36 bB | 0.69 a | 0.38 bB | 0.33 bB | 0.045 | 0.006 | 0.002 | <0.001 | 0.017 |
| LP | 0.72 b | 0.70 bA | 0.90 a | 0.85 aA | 0.82 aA | 0.027 | 0.003 | ||||
| SEM | 0.055 | 0.079 | 0.054 | 0.107 | 0.116 | ||||||
| p-value | 0.347 | 0.028 | 0.090 | 0.030 | 0.044 | ||||||
| Ace | CK | 136.39 | 132.00 | 202.37 | 148.52 | 191.03 | 12.601 | 0.357 | 0.077 | 0.091 | 0.557 |
| LP | 145.01 a | 108.30 b | 142.79 a | 139.38 a | 158.45 a | 5.484 | 0.030 | ||||
| SEM | 11.482 | 7.530 | 20.923 | 13.341 | 15.037 | ||||||
| p-value | 0.794 | 0.246 | 0.233 | 0.546 | 0.490 | ||||||
| Chao | CK | 110.79 | 99.23 | 195.34 | 147.46 | 161.92 | 13.119 | 0.170 | 0.024 | 0.352 | 0.649 |
| LP | 120.84 | 102.35 | 145.85 | 139.42 | 143.32 | 6.831 | 0.281 | ||||
| SEM | 8.150 | 5.912 | 18.095 | 13.723 | 17.863 | ||||||
| p-value | 0.546 | 0.844 | 0.281 | 0.571 | 0.761 | ||||||
| Coverage | CK | 0.9993 | 0.9992 | 0.9987 | 0.9992 | 0.9989 | 0.000 | 0.226 | 0.078 | 0.694 | 0.396 |
| LP | 0.9991 | 0.9992 | 0.9991 | 0.9991 | 0.9990 | 0.000 | 0.441 | ||||
| SEM | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||||||
| p-value | 0.183 | 1.000 | 0.214 | 0.860 | 0.774 | ||||||
| Items | Additives | Harvesting Stages | SEM | p-Value | Interaction | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Heading | Flowering | Filling | Milk Ripening | Wax Ripening | S | A1 | S*A1 | ||||
| Sobs | CK | 172.33 | 126.33 | 131.00 | 70.67 | 212.00 | 21.586 | 0.369 | 0.133 | 0.026 | 0.711 |
| LP | 118.67 | 78.00 | 65.00 | 68.33 | 105.00 | 9.364 | 0.383 | ||||
| SEM | 25.599 | 15.435 | 21.323 | 8.531 | 46.417 | ||||||
| p-value | 0.375 | 0.243 | 0.161 | 0.926 | 0.336 | ||||||
| Shannon | CK | 3.54 a | 3.53 a | 2.41 ab | 1.88 bA | 2.49 ab | 0.215 | 0.039 | <0.001 | 0.001 | 0.009 |
| LP | 3.32 a | 2.59 a | 0.70 b | 1.03 bB | 2.38 a | 0.282 | 0.001 | ||||
| SEM | 0.099 | 0.255 | 0.452 | 0.329 | 0.144 | ||||||
| p-value | 0.409 | 0.090 | 0.053 | 0.005 | 0.706 | ||||||
| Simpson | CK | 0.07 | 0.06 | 0.24 B | 0.38 | 0.23 | 0.043 | 0.157 | <0.001 | 0.012 | 0.089 |
| LP | 0.09 b | 0.17 b | 0.70 aA | 0.49 a | 0.16 b | 0.077 | 0.007 | ||||
| SEM | 0.011 | 0.040 | 0.121 | 0.110 | 0.025 | ||||||
| p-value | 0.383 | 0.217 | 0.029 | 0.100 | 0.591 | ||||||
| Ace | CK | 179.30 | 145.28 | 139.34 | 77.99 | 217.73 | 21.514 | 0.408 | 0.188 | 0.051 | 0.704 |
| LP | 124.43 | 111.12 | 83.83 | 73.43 | 108.09 | 8.875 | 0.476 | ||||
| SEM | 25.166 | 10.837 | 20.563 | 8.231 | 47.151 | ||||||
| p-value | 0.365 | 0.357 | 0.269 | 0.856 | 0.326 | ||||||
| Chao | CK | 184.00 | 133.05 | 140.48 | 74.95 | 219.17 | 22.068 | 0.378 | 0.142 | 0.026 | 0.705 |
| LP | 121.50 | 86.83 | 76.29 | 72.72 | 109.58 | 8.864 | 0.458 | ||||
| SEM | 27.237 | 13.492 | 21.378 | 8.689 | 47.274 | ||||||
| p-value | 0.369 | 0.176 | 0.172 | 0.932 | 0.318 | ||||||
| Coverage | CK | 0.9998 | 0.9998 | 0.9997 | 0.9998 | 0.9998 | 0.000 | 0.506 | 0.054 | 0.351 | 0.271 |
| LP | 0.9999 a | 0.9999 a | 0.9996 b | 0.9999 a | 0.9999 a | 0.000 | 0.028 | ||||
| SEM | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||||||
| p-value | 0.194 | 0.774 | 0.436 | 0.860 | 0.173 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, R.; Liu, Y.; Wu, B.; Jia, C.; Yu, Z.; Wang, G. Effect of Harvest Maturity and Lactiplantibacillus plantarum Inoculant on Dynamics of Fermentation Characteristics and Bacterial and Fungal Community of Triticale Silage. Agriculture 2024, 14, 1707. https://doi.org/10.3390/agriculture14101707
Gao R, Liu Y, Wu B, Jia C, Yu Z, Wang G. Effect of Harvest Maturity and Lactiplantibacillus plantarum Inoculant on Dynamics of Fermentation Characteristics and Bacterial and Fungal Community of Triticale Silage. Agriculture. 2024; 14(10):1707. https://doi.org/10.3390/agriculture14101707
Chicago/Turabian StyleGao, Run, Yi Liu, Bo Wu, Chunlin Jia, Zhu Yu, and Guoliang Wang. 2024. "Effect of Harvest Maturity and Lactiplantibacillus plantarum Inoculant on Dynamics of Fermentation Characteristics and Bacterial and Fungal Community of Triticale Silage" Agriculture 14, no. 10: 1707. https://doi.org/10.3390/agriculture14101707






