The Influence of Planting Density on the Flowering Pattern and Seed Yield in Peanut (Arachis hypogea L.) Grown in the Northern Region of Japan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture Condition and Experimental Design
2.2. Counting of Flower Number
2.3. Yield Measurement
2.4. Statistical Analysis
3. Results
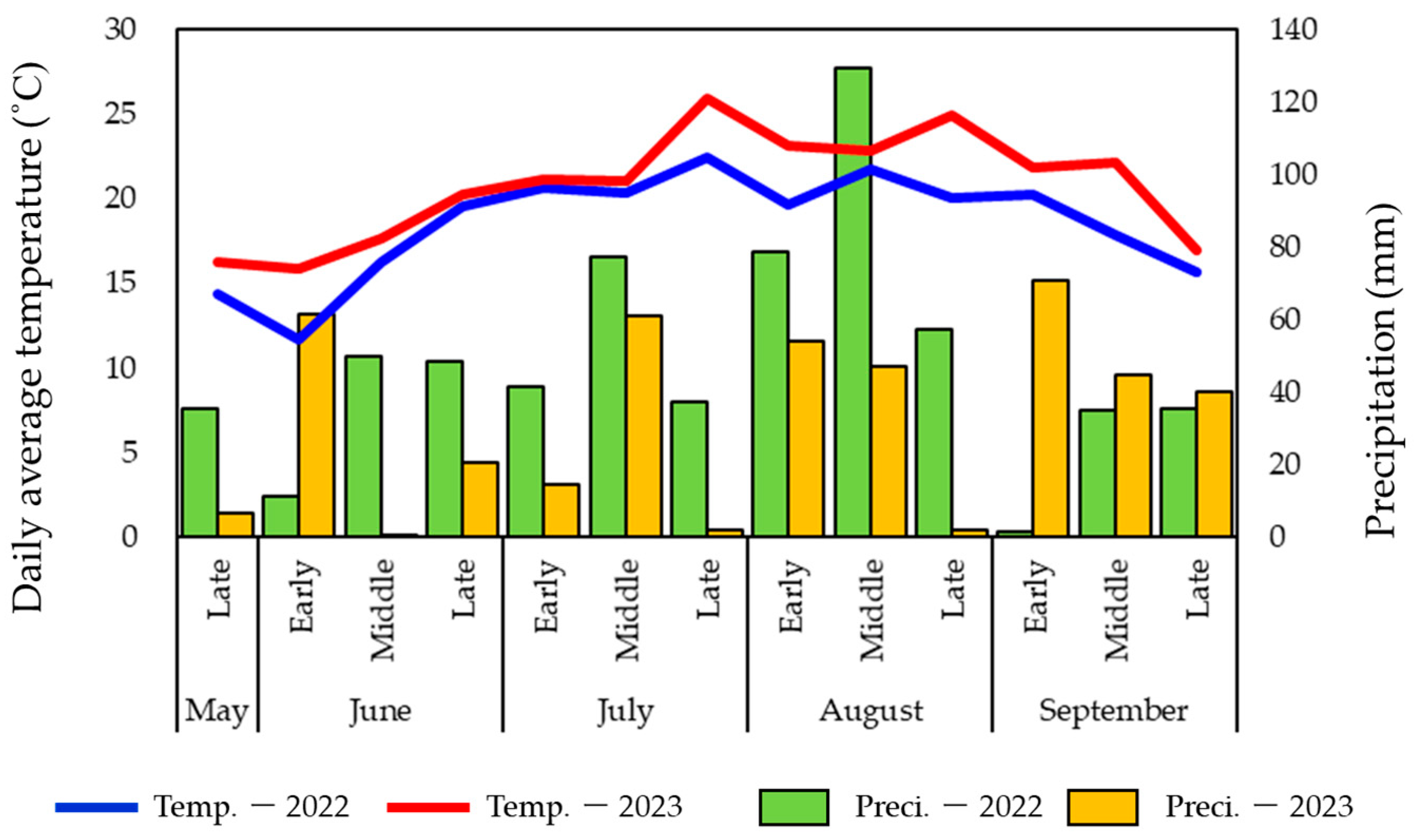
3.1. Weather Conditions of Two Years
3.2. Flowering Pattern
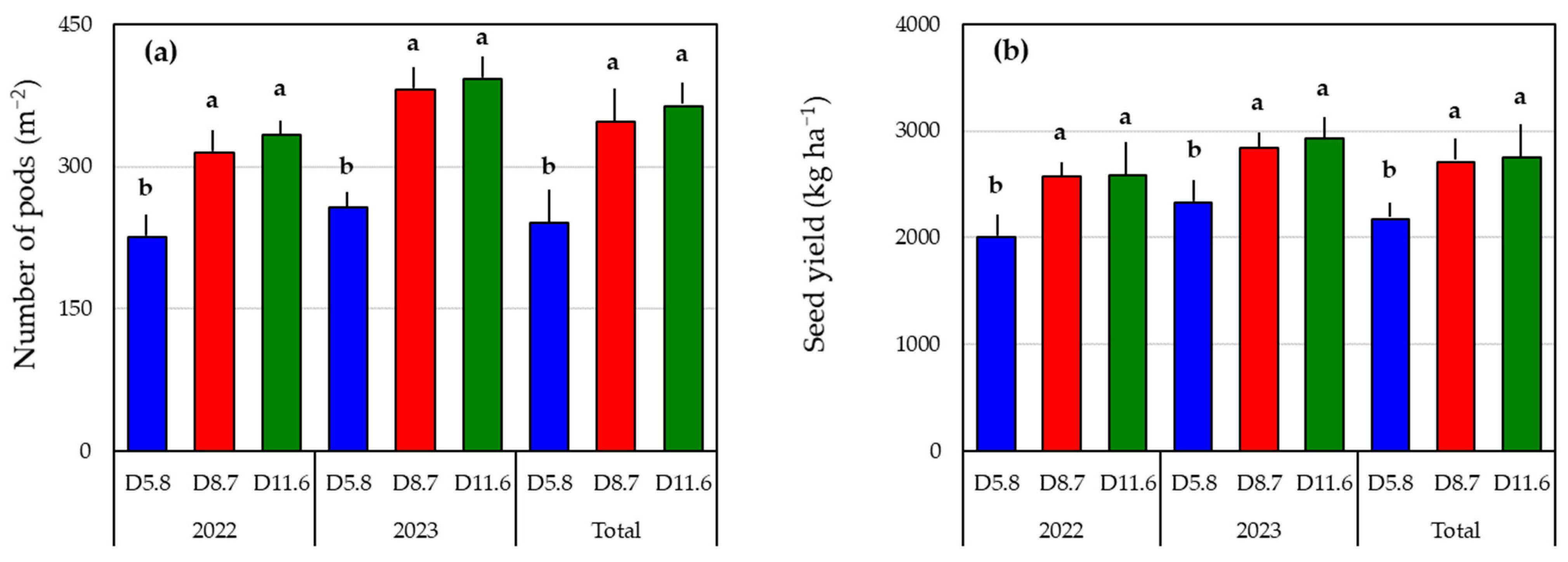
3.3. Seed Yield and Yield Components
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 2 July 2024).
- Official Statistics of Japan. Available online: https://www.e-stat.go.jp/en/stat-search/files?page=1&layout=datalist&toukei=00500215&tstat=000001013427&cycle=0&tclass1=000001032288&tclass2=000001034728&tclass3val=0 (accessed on 24 June 2024).
- Nojima, N. Economic globalization and management strategies for the peanut processing industry. J. Comput. Soc. Sci. 2012, 10, 11–24. [Google Scholar]
- Akimoto, M.; Sato, K.; Kumada, F.; Tsujimoto, H.; Kobayashi, N.; Hirato, S.; Tanaka, I. Basic study for the appropriate cultivation method of groundnut (Arachis hypogaea L.) in the Tokachi region II. Selection of the suitable varieties for the production in the Tokachi region. Res. Bull. Obihiro Univ. 2018, 39, 15–23. [Google Scholar]
- Akimoto, M.; Nakata, S.; Kumada, F.; Tsujimoto, H.; Kobayashi, N.; Hirato, S.; Tanaka, I. Basic study for the appropriate cultivation method of groundnut (Arachis hypogaea L.) in the Tokachi region. Res. Bull. Obihiro Univ. 2017, 38, 13–24. [Google Scholar]
- Takahashi, Y.; Takeuchi, S.; Kamekura, H.; Saito, S.; Ishii, R.; Ishida, Y.; Nagasawa, J.; Katsura, H. On the new peanut variety “Nakateyutaka”. Bull. Chiba Agric. Exp. Stn. 1981, 22, 57–69. [Google Scholar]
- Takeuchi, S.; Kamekura, H.; Saito, S.; Ishii, R.; Ishida, Y. On the new peanut variety “Tachimasari”. Bull. Chiba Agric. Exp. Stn. 1975, 16, 135–146. [Google Scholar]
- Suzuki, K.; Nakanishi, T.; Takahashi, Y.; Matsuda, T.; Iwata, Y.; Suzuki, S.; Ishii, R.; Kajiro, M.; Katsura, H.; Yashiki, T. On ’Satonoka’. a new variety of peanut (Arachis hypogaea L.). Bull. Chiba Agric. Exp. Stn. 1997, 38, 55–66. [Google Scholar]
- Liew, X.Y.; Sinniah, U.R.; Yusoff, M.M.; Witty, U.A. Flowering pattern and seed development in ideterminate peanut cv. ‘Margenta’ and its influence on seed quality. Seed Sci. Technol. 2021, 49, 45–62. [Google Scholar] [CrossRef]
- Stalker, H.T. Peanut (Arachis hypogaea L). Field Crops Res. 1997, 53, 205–217. [Google Scholar] [CrossRef]
- Cattan, P.; Fleury, A. Flower production and growth in groundnut plants. Eur. J. Agron. 1998, 8, 13–27. [Google Scholar] [CrossRef]
- Jonishi, T.; Fujii, Y.; Nakamura, C.; Omichi, M. Effect of sowing date on growth and yield of peanuts (Arachis hypogaea L.) in Hokkaido. Res. Bull. Takushoku Jr. Univ. 2023, 3, 1–8. [Google Scholar] [CrossRef]
- Demir, İ. Inter and intra row competition effects on growth and yield components of sunflower (Helianthus annuus L.) under rainfed conditions. J. Anim. Plant Sci. 2020, 30, 147–153. [Google Scholar]
- Geleta, B.; Atak, M.; Baenziger, P.S.; Nelson, L.A.; Baltenesperger, D.D.; Eskridge, K.M.; Shipman, M.J.; Shelton, D.R. Seeding rate and genotype effect on agronomic performance and end-use quality of winter wheat. Crop Sci. 2002, 42, 827–832. [Google Scholar] [CrossRef]
- Harker, K.N.; O’Donovan, J.T.; Smith, E.G.; Johnson, E.N.; Peng, G.; Willenborg, C.J.; Gulden, R.H.; Mohr, R.; Gill, K.S.; Grenkow, L.A. Seed size and seeding rate effects on canola emergence, development, yield and seed weight. Can. J. Plant Sci. 2015, 95, 1–8. [Google Scholar] [CrossRef]
- Ismail, S.M.; Mousa, M.A.A. Optimizing tomato productivity and water use efficiency using water regimes, plant density and row spacing under arid land conditions. Irrig. Drain. 2014, 63, 640–650. [Google Scholar] [CrossRef]
- Haro, R.J.; Carrega, W.C.; Otegui, M.E. Row spacing and growth habit in peanut crops: Effects on seed yield determination across environments. Field Crops Res. 2022, 275, 108363. [Google Scholar] [CrossRef]
- Sato, S.; Ishiyama, S.; Tanaka, I.; Akimoto, M. Study on the optimal planting density for the cultivation of peanut in Tokachi Region. Jpn. J. Crop Sci. 2024, 93, 122–131. [Google Scholar] [CrossRef]
- Domínguez-May, R.; Gasca-Leyva, E.; Robledo, D. Harvesting time optimization and risk analysis for the mariculture of Kappaphycus alvarezii (Rhodophyta). Rev. Aquac. 2017, 9, 227–237. [Google Scholar] [CrossRef]
- Tjørve, K.M.C.; Tjørve, E. The use of Gompertz models in growth analyses, and new Gompertz-model approach: An addition to the Unified-Richards family. PLoS ONE 2017, 12, e0178691. [Google Scholar] [CrossRef]
- Oshima, K.; Hofuku, I.; Inagawa, A. Two-step cumulative curve and modified Gompertz curve. Jpn. J. Ind. Appl. Math. 1994, 4, 259–274. [Google Scholar] [CrossRef]
- Isobe, K.; Sato, R.; Sakamoto, S.; Arai, T.; Miyamoto, M.; Higo, M.; Torigoe, Y. Studies on optimum planting density of quinoa (Chenopodium quinoa Willd.) Variety NL-6 considering efficiency for light energy utilization, matter production and yield. Jpn. J. Crop Sci. 2015, 84, 369–377. [Google Scholar] [CrossRef]
- Jain, R.; Singh, M.K.; Swaroop, K.; Reddy, M.V.; Janakiram, T.; Kumar, P.; Pinder, R. Optimization of spacing and nitrogen dose for growth and flowering of statice (Limonium sinuatum). Indian J. Agric. Sci. 2018, 88, 1108–1114. [Google Scholar] [CrossRef]
- Bezu, T.; Kassa, N. Planting density and corm size effects on flower yield and quality of cut-freesia (Freesia hybrid) in Ethiopia. J. Appl. Hortic. 2014, 16, 76–79. [Google Scholar] [CrossRef]
- Barišić, N.; Stojković, B.; Tarasjev, A. Plastic responses to light intensity and planting density in three Lamium species. Plant Syst. Evol. 2006, 262, 25–36. [Google Scholar] [CrossRef]
- Donald, C.M. Competition among crop and pasture plants. Adv. Agron. 1963, 15, 1–118. [Google Scholar] [CrossRef]
- Postma, J.A.; Hecht, V.L.; Hikosaka, K.; Nord, E.A.; Pons, T.L.; Poorter, H. Dividing the pie: A quantitative review on plant density responses. Plant Cell Environ. 2021, 44, 1072–1094. [Google Scholar] [CrossRef]
- Minh, T.X.; Thanh, N.C.; Thin, T.H.; Tieng, N.T.; Giang, N.T.H. Effects of plant density and row spacing on yield and yield components of peanut (Arachis hypogaea L.) on the coastal sandy land area in Nghe An Province, Vietnam. Indian J. Agric. Res. 2021, 55, 468–472. [Google Scholar] [CrossRef]
- Kharel, P.; Devkota, P.; Macdonald, G.E.; Tillman, B.L.; Mulvaney, M.J. Influence of planting date, row spacing, and reduced herbicide inputs on peanut canopy and sicklepod growth. Agron. J. 2022, 114, 717–726. [Google Scholar] [CrossRef]
- Cordeiro, C.F.D.S.; Pilon, C.; Echer, F.R.; Albas, R.; Tubbs, R.S.; Harris, G.H.; Rosolem, C.A. Adjusting peanut plant density and potassium fertilization for different production environments. Agron. J. 2023, 115, 817–832. [Google Scholar] [CrossRef]
- Steberl, K.; Hartung, J.; Munz, S.; Graeff-Hönninger, S. Effect of row spacing, sowing density, and harvest time on floret yield and yield components of two safflower cultivars grown in southwestern Germany. Agronomy 2020, 10, 664. [Google Scholar] [CrossRef]
- Almekinders, C.J.M. Effect of plant density on the inflorescence production of stems and the distribution of flower production in potato. Potato Res. 1993, 36, 97–105. [Google Scholar] [CrossRef]
- Hayashi, S. Growth, grain yield and grain quality of sparsely planted rice (Oryza sativa L.) cultivar “Nanatsuboshi” in Hokkaido. Jpn. J. Crop Sci. 2017, 86, 129–138. [Google Scholar] [CrossRef]
- Hu, Q.; Jiang, W.Q.; Qiu, S.; Xing, Z.P.; Hu, Y.J.; Guo, B.W.; Liu, G.D.; Gao, H.; Zhang, H.C.; Wei, H.Y. Effect of wide-narrow row arrangement in mechanical pot-seedling transplanting and plant density on yield formation and grain quality of japonica rice. J. Integr. Agric. 2020, 19, 1197–1214. [Google Scholar] [CrossRef]
- Takeuchi, S.; Ashiya, O.; Kamekura, H. On the flowering and fruiting habits of peanut “Chiba Handachi”. Bull. Chiba Agric. Exp. Stn. 1964, 5, 113–121. [Google Scholar]
- Inoue, Y. Studies on the flowering habit in peanut (Arachis hypogaea L.). Jpn. J. Hortic. 1958, 27, 245–248. [Google Scholar] [CrossRef]
- Gan, Y.; Harker, K.N.; Kutcher, H.R.; Gulden, R.H.; Irvine, B.; May, W.E.; O’Donovan, J.T. Canola seed yield and phenological responses to plant density. Can. J. Plant Sci. 2016, 96, 151–159. [Google Scholar] [CrossRef]
- Chassaigne-Ricciulli, A.A.; Mendoza-Onofre, L.E.; Córdova-Téllez, L.; Carballo-Carballo, A.; San Vicente-García, F.M.; Dhliwayo, T. Effective seed yield and flowering synchrony of parents of cimmyt three-way-cross tropical maize hybrids. Agriculture 2021, 11, 161. [Google Scholar] [CrossRef]
- Cao, Y.; Xiao, Y.; Huang, H.; Xu, J.; Hu, W.; Wang, N. Simulated warming shifts the flowering phenology and sexual reproduction of Cardamine hirsuta under different planting densities. Sci. Rep. 2016, 6, 27835. [Google Scholar] [CrossRef]
- Marcelis, L.F.M.; Heuvelink, E.; Baan Hofman-Eijer, L.R.; Den Bakker, J.; Xue, L.B. Flower and fruit abortion in sweet pepper in relation to source and sink strength. J. Exp. Bot. 2004, 55, 2261–2268. [Google Scholar] [CrossRef]
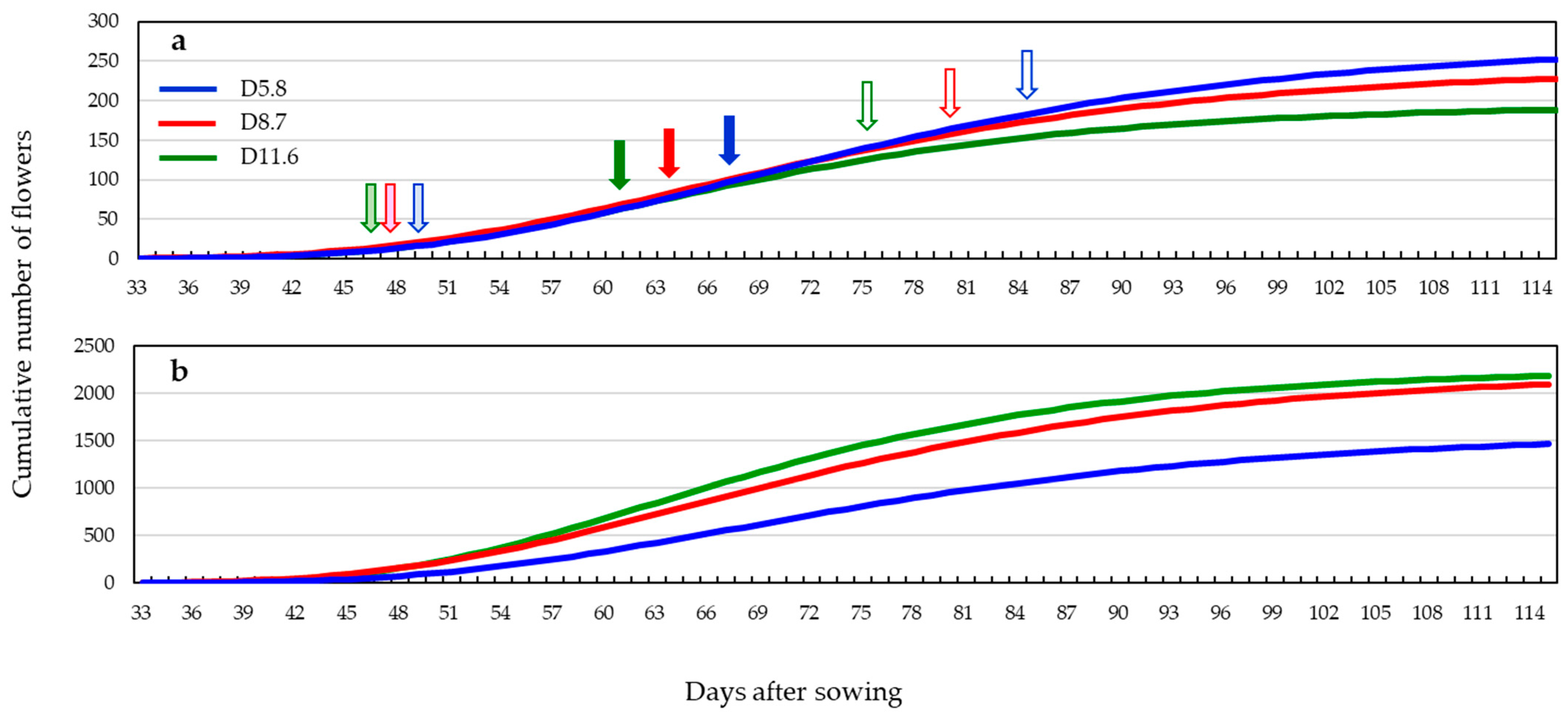
| Gompertzian Parameters | Maximum | Days after Sowing | Flowering Period | |||||
|---|---|---|---|---|---|---|---|---|
| Plot | K | a | b | ∆f(x) | IF | PF | TF | (Days) |
| D5.8 | 270.8 | 33.0 | 0.056 | 5.6 | 49.1 | 66.7 | 84.2 | 35.1 |
| D8.7 | 241.3 | 33.1 | 0.057 | 5.1 | 47.2 | 63.9 | 80.5 | 33.3 |
| D11.6 | 194.4 | 33.0 | 0.066 | 4.7 | 46.8 | 61.6 | 76.2 | 29.4 |
| No. of | No. of | No. of | Pod Setting | Pod | 100-Seed | ||
|---|---|---|---|---|---|---|---|
| Year | Plot | Pegs | Pods | Fertile Pods | Rate (%) | Fertility (%) | Weight (g) |
| D5.8 | 155.0 ± 3.7 a | 91.7 ± 3.3 a | 52.1 ± 2.7 a | 59.5 ± 2.6 a | 56.6 ± 1.5 b | 47.3 ± 1.2 | |
| 2022 | D8.7 | 117.2 ± 5.7 b | 59.8 ± 3.2 b | 40.5 ± 2.2 b | 52.0 ± 3.0 b | 67.8 ± 0.8 a | 48.6 ± 1.1 |
| D11.6 | 87.8 ± 4.0 c | 41.3 ± 2.3 c | 28.8 ± 1.7 c | 48.0 ± 3.2 c | 69.8 ± 1.7 a | 47.6 ± 1.7 | |
| D5.8 | 147.8 ± 7.6 a | 94.7 ± 1.8 a | 58.5 ± 2.6 a | 65.7 ± 3.2 a | 61.8 ± 2.5 b | 52.3 ± 1.8 | |
| 2023 | D8.7 | 112.9 ± 5.7 b | 66.3 ± 2.4 b | 48.9 ± 3.1 b | 59.3 ± 1.6 b | 734. ± 2.5 a | 52.8 ± 2.0 |
| D11.6 | 93.3 ± 3.9 c | 54.6 ± 2.6 c | 39.7 ± 2.2 c | 59.1 ± 2.8 b | 72.6 ± 1.4 a | 53.4 ± 2.8 | |
| D5.8 | 151.4 ± 4.2 a | 93.2 ± 1.9 a | 55.3 ± 1.9 a | 62.6 ± 2.1 a | 59.2 ± 1.5 b | 49.8 ± 1.2 | |
| Total | D8.7 | 115.0 ± 4.0 b | 63.0 ± 2.1 b | 44.7 ± 2.0 b | 55.7 ± 1.8 b | 70.6 ± 1.4 a | 50.7 ± 1.2 |
| D11.6 | 90.6 ± 2.8 c | 48.0 ± 2.2 c | 34.3 ± 1.8 c | 53.5 ± 2.4 c | 71.2 ± 1.1 a | 50.5 ± 1.7 | |
| ANOVA | Year | * | ** | ** | ** | ** | ** |
| Density | ** | ** | ** | ** | ** | ns | |
| Year × Density | ns | ns | ns | ns | ns | ns | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akimoto, M.; Sato, S.; Tanaka, I. The Influence of Planting Density on the Flowering Pattern and Seed Yield in Peanut (Arachis hypogea L.) Grown in the Northern Region of Japan. Agriculture 2024, 14, 1736. https://doi.org/10.3390/agriculture14101736
Akimoto M, Sato S, Tanaka I. The Influence of Planting Density on the Flowering Pattern and Seed Yield in Peanut (Arachis hypogea L.) Grown in the Northern Region of Japan. Agriculture. 2024; 14(10):1736. https://doi.org/10.3390/agriculture14101736
Chicago/Turabian StyleAkimoto, Masahiro, Sota Sato, and Ichiro Tanaka. 2024. "The Influence of Planting Density on the Flowering Pattern and Seed Yield in Peanut (Arachis hypogea L.) Grown in the Northern Region of Japan" Agriculture 14, no. 10: 1736. https://doi.org/10.3390/agriculture14101736






