Festuca ovina L. As a Monitor Plant Species of Traffic Air Along the Highway in of the City of Warsaw (Poland)
Abstract
:1. Introduction
2. Material and Methods
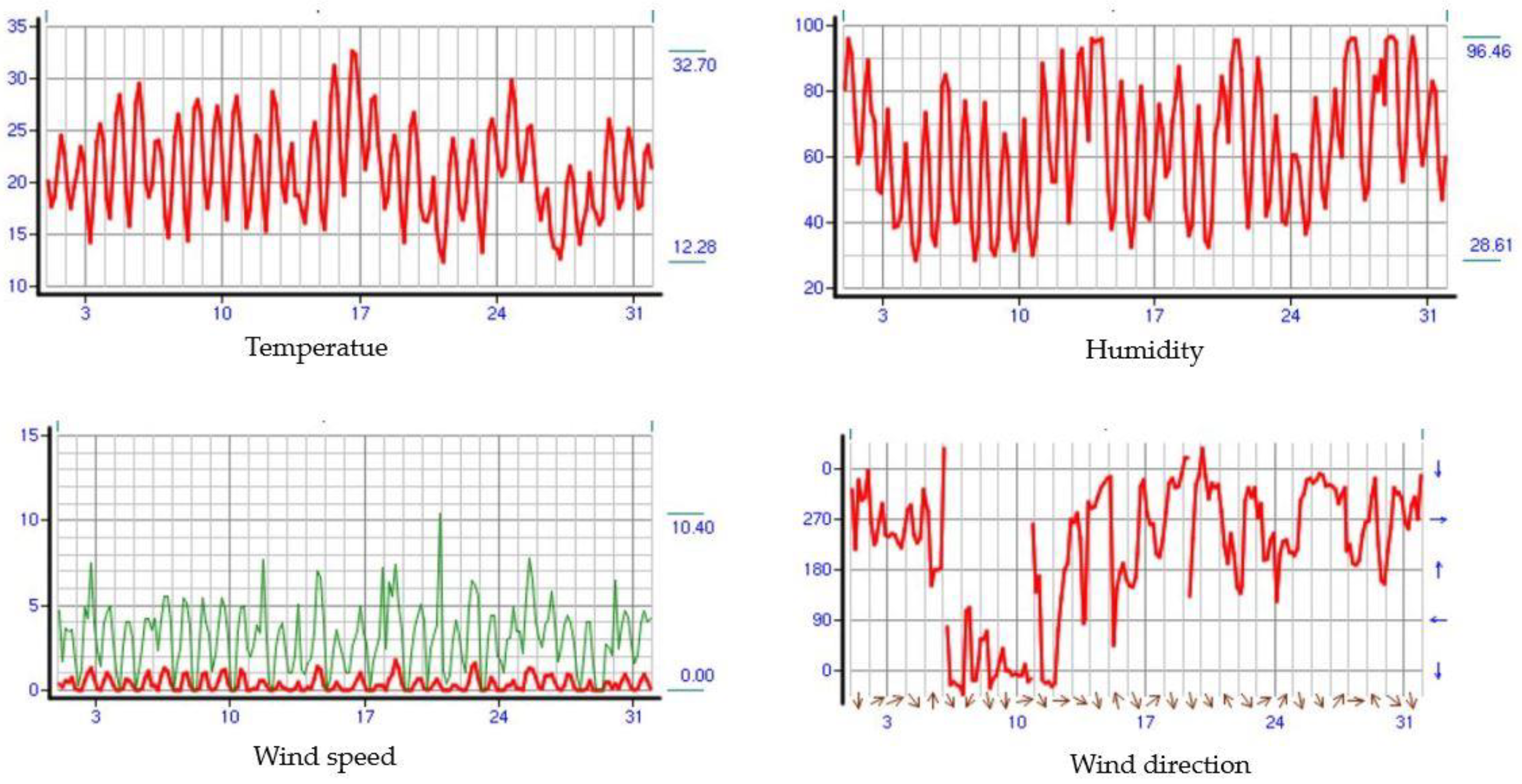
2.1. Study Area
2.2. Plant Sample Collection and Biochemical Analyses
2.3. Determination of Flavonoid Content
2.4. Determination of Ammonia Content
2.5. Determination of Free Proline Content
2.6. SEM Analyses
2.7. Statistical Analysis
2.8. Data Availability
3. Results
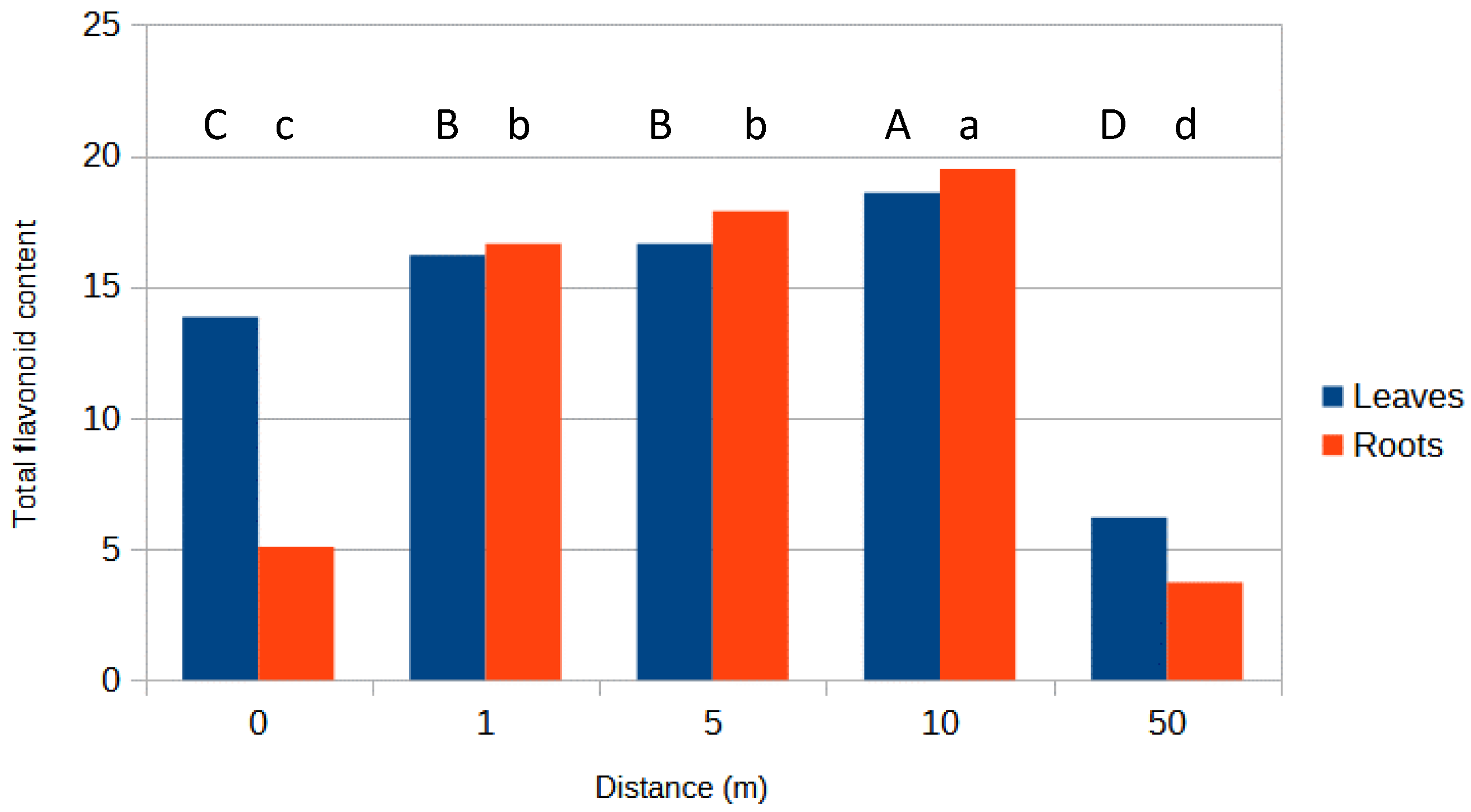
3.1. Total Flavonoid Content
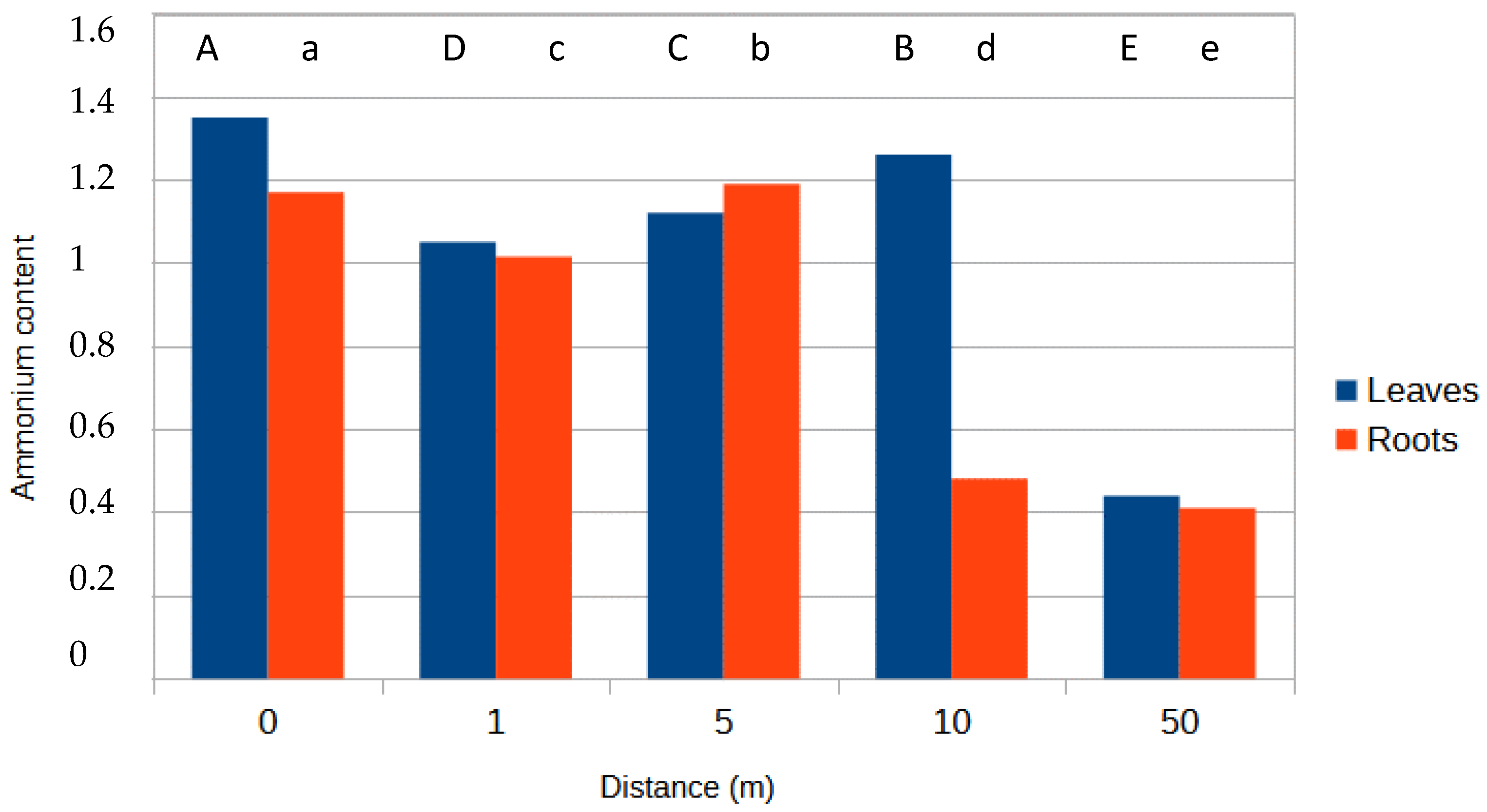
3.2. Ammonia Content
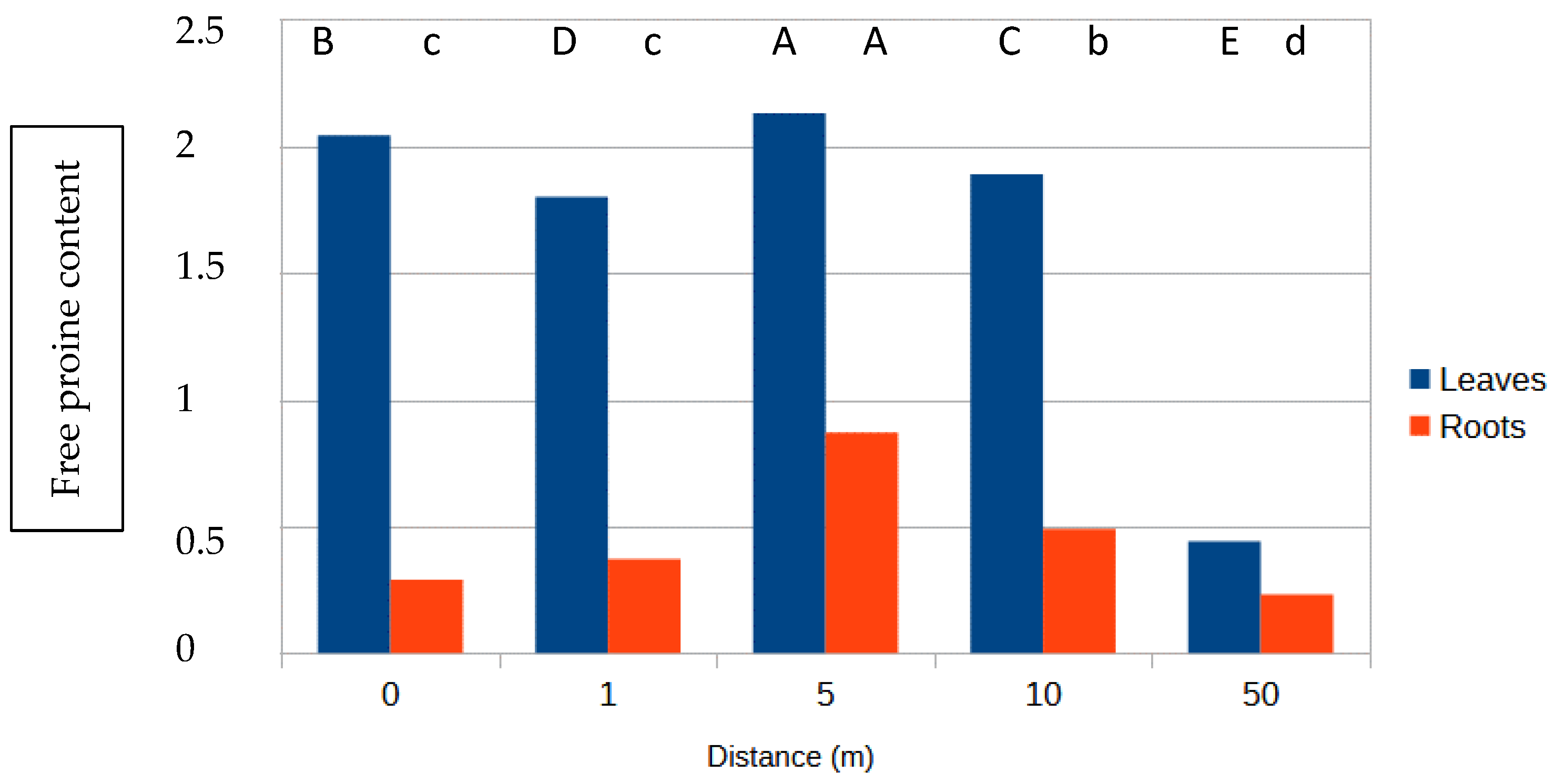
3.3. Free Proline Content
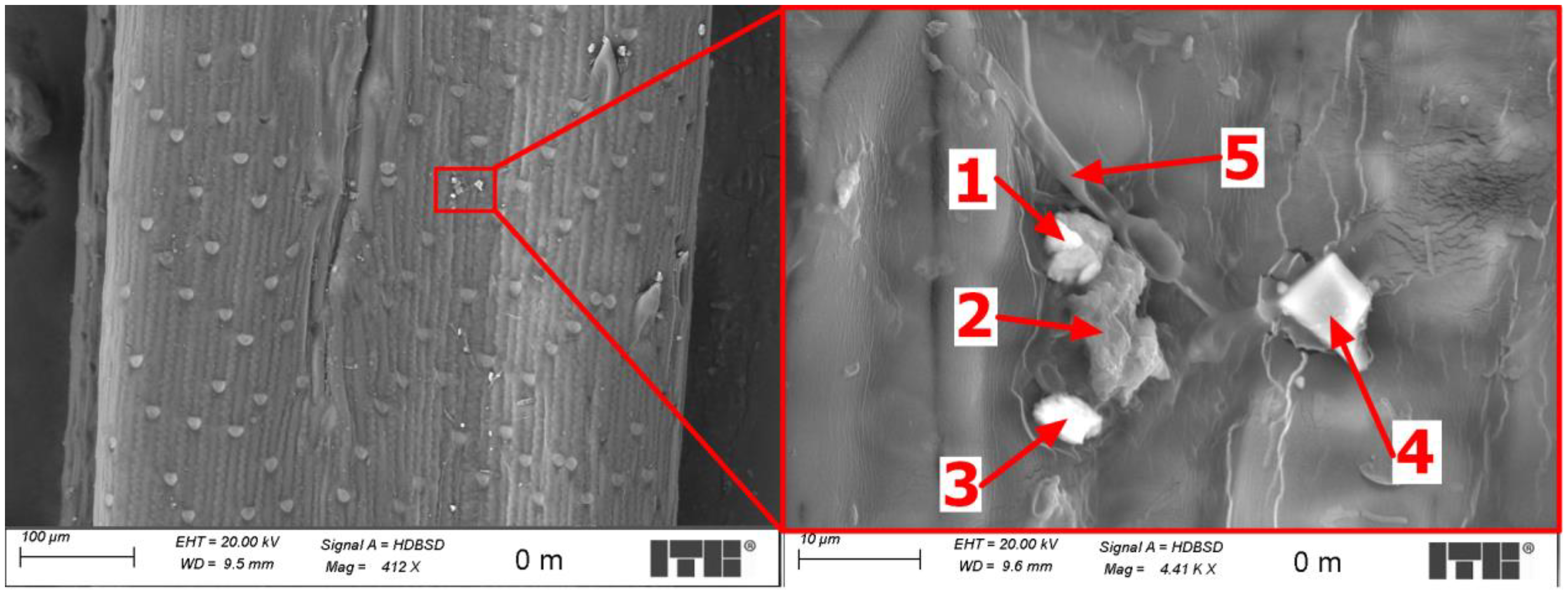
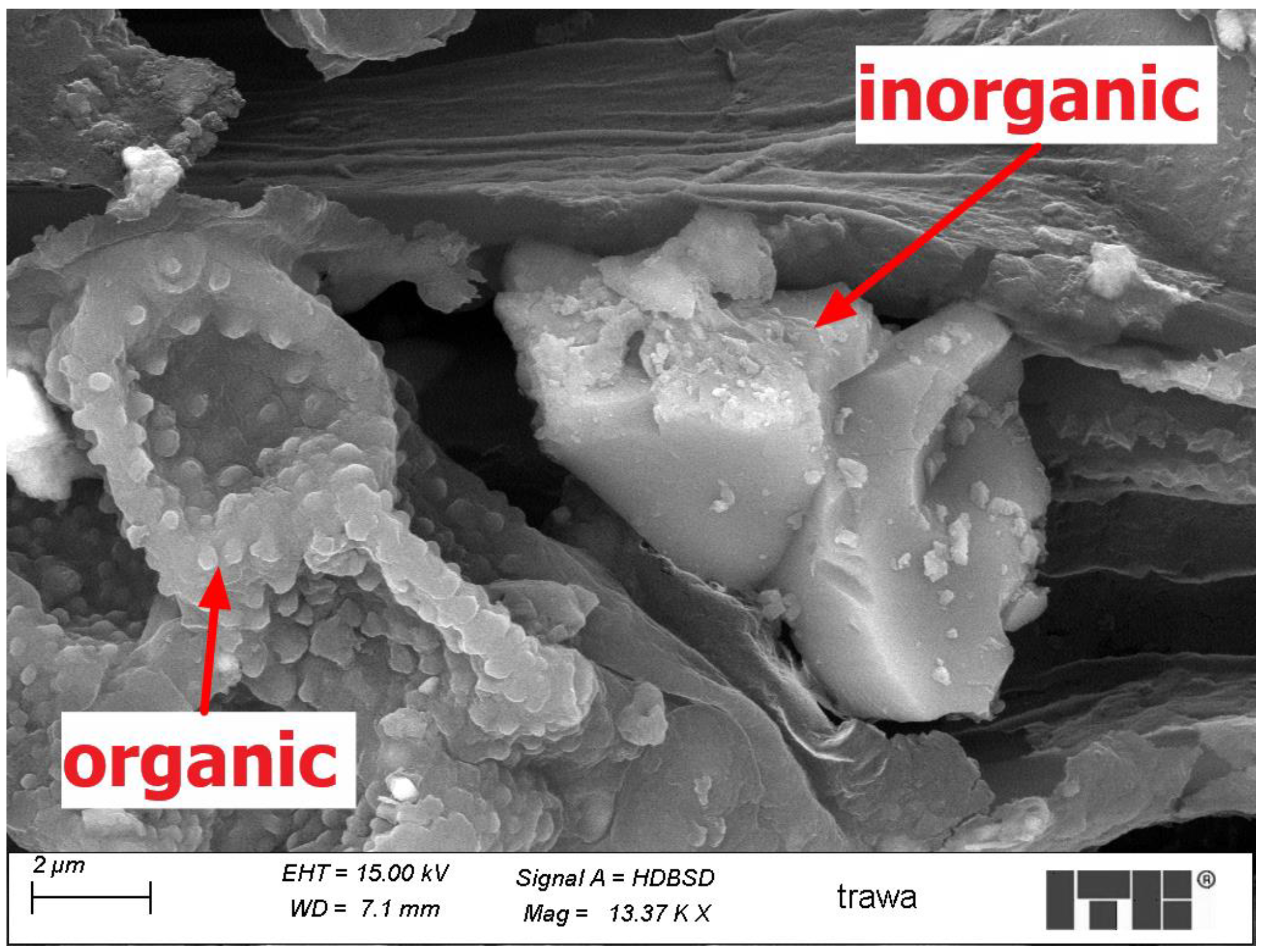
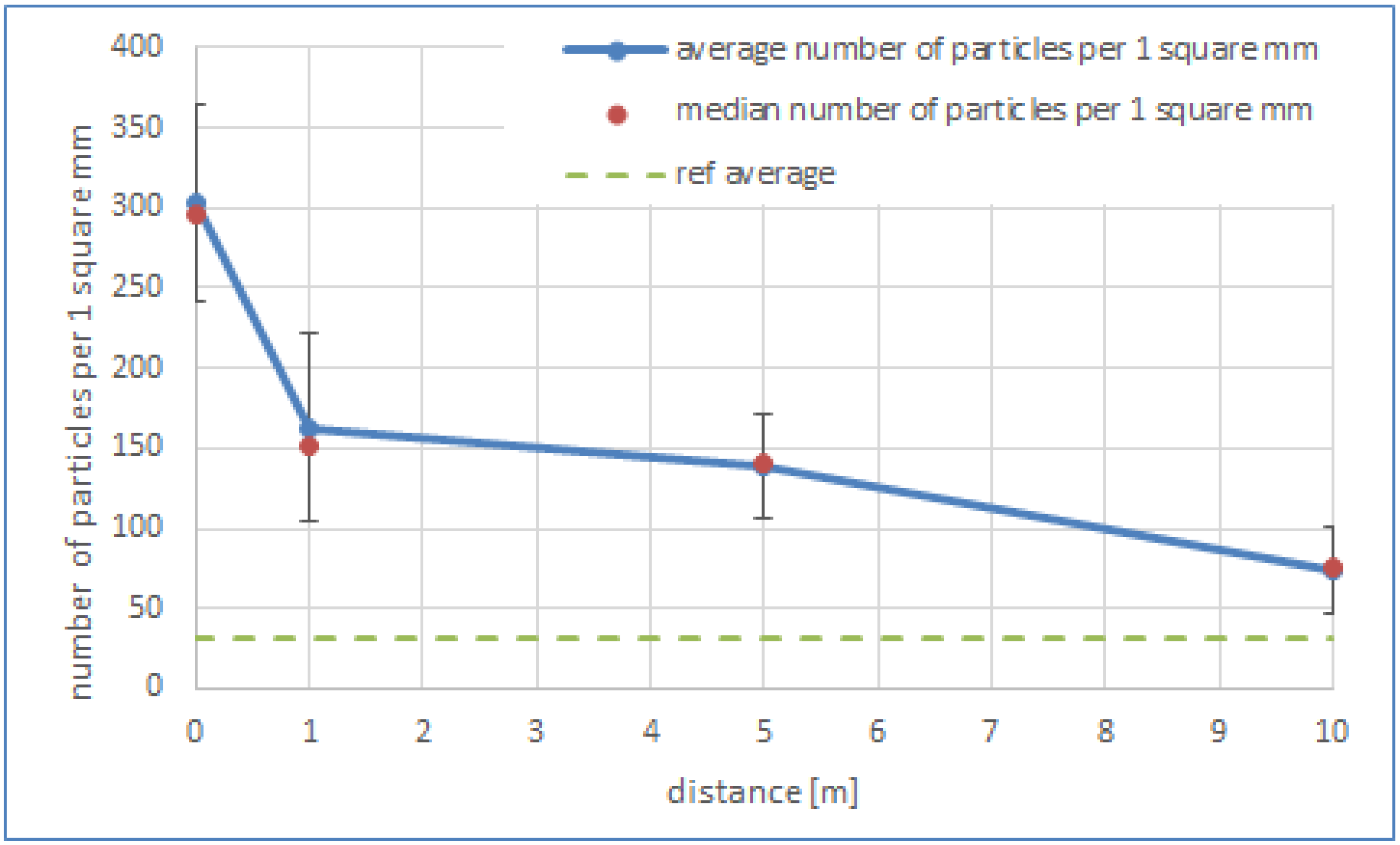
3.4. Microscopic Examinations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Barakat-Haddad, C.; Zhang, S.; Siddiqua, A.; Dghaim, R. Air Quality and Respiratory Health among Adolescents from the United Arab Emirates. J. Environ. Public Health 2015, 2015, 284595. [Google Scholar] [CrossRef] [PubMed]
- Referowska-Chodak, E. Pressures and Threats to Nature Related to Human Activities in European Urban and Suburban Forests. Forests 2019, 10, 765. [Google Scholar] [CrossRef]
- World Health Report 2013: Research for Universal Health Coverage; WHO: Geneva, Switzerland, 2013.
- Tong, D.; Pan, L.; Chen, W.; Lamsal, L.; Lee, P.; Tang, Y.; Kim, H.; Kondragunta, S.; Stajner, I. Impact of the 2008 Global Recession on air quality over the United States: Implications for surface ozone levels from changes in NOx emissions. Geophys. Res. Lett. 2016, 43, 9280–9288. [Google Scholar] [CrossRef]
- Hime, N.; Marks, G.; Cowie, C. A Comparison of the Health Effects of Ambient Particulate Matter Air Pollution from Five Emission Sources. Int. J. Environ. Res. Public Health 2018, 15, 1206. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, T.; Shen, G.; Esmaeili, N.; Zhang, H. Plants’ Response Mechanisms to Salinity Stress. Plants 2023, 12, 2253. [Google Scholar] [CrossRef]
- Isayenkov, S.V.; Maathuis, F.J.M. Plant Salinity Stress: Many Unanswered Questions Remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.M.; Pandey, V.; Yunus, M.; Ahmad, K.J. Plants as dust scavengers: A case study. Indian For. 1989, 115, 670–672. [Google Scholar]
- Lohr, V.I.; Pearson-Mims, C.H. Particulate matter accumulation on horizontal surfaces in interiors: Influence of foliage plants. Atmos. Environ. 1996, 30, 2565–2568. [Google Scholar] [CrossRef]
- Harrison, R.M.; Yin, J. Particulate matter in the atmosphere: Which particle properties are important for its effects on health? Sci. Total Environ. 2000, 249, 85–101. [Google Scholar] [CrossRef]
- Pal, A.; Kulshreshtha, K.; Ahmad, K.J.; Behl, H.M. Do leaf surface characters play a role in plant resistance to auto-exhaust pollution? Flora-Morphol. Distrib. Funct. Ecol. Plants 2002, 197, 47–55. [Google Scholar] [CrossRef]
- Tomasevic, M.; Anicic, M. Trace element content in urban tree leaves and SEM-EDAX characterization of deposited particles. Facta Univ.-Ser. Phys. Chem. Technol. 2010, 8, 1–13. [Google Scholar] [CrossRef]
- Samara, C.; Voutsa, D. Size distribution of airborne particulate matter and associated heavy metals in the roadside environment. Chemosphere 2005, 59, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Bućko, M.S.; Magiera, T.; Johanson, B.; Petrovský, E.; Pesonen, L.J. Identification of magnetic particulates in road dust accumulated on roadside snow using magnetic, geochemical and micro-morphological analyses. Environ. Pollut. 2011, 159, 1266–1276. [Google Scholar] [CrossRef] [PubMed]
- Chatson, K.; Doley, D. Mineral particulates and vegetation: Effect of coal dust, overburden and fly ash on light interception and leaf temperature. Clean Air Environ. Qual. 2006, 40, 40–44. [Google Scholar]
- Nadal, M.; Schuhmaher, M.; Domingo, J. Metal pollution of soils and vegetation in an area with petrochemical industry. Sci. Total Environ. 2004, 321, 59–69.17. [Google Scholar] [CrossRef]
- Guenat, S.; Dallimer, M. A global meta-analysis reveals contrasting impacts of air, light, and noise pollution on pollination. Ecol. Evol. 2023, 13, e9990. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, Z.; Yang, H.-H.; Awan, M.U.F.; Ahmed, U.; Hasnain, A.; Luqman, M.; Muhammad, S.; Sardar, A.A.; Chan, T.-Y.; Sharjeel, A. Effects of Air Pollution on Morphological, Biochemical, DNA, and Tolerance Ability of Roadside Plant Species. Sustainability 2024, 16, 3427. [Google Scholar] [CrossRef]
- Giri, S.; Shrivastava, D.; Deshmukh, K.; Dubey, P. Effect of Air Pollution on Chlorophyll Content of Leaves. Curr. Agric. Res. J. 2013, 1, 93–98. [Google Scholar] [CrossRef]
- Iqbal, M.; Breivik, K.; Syed, J.H.; Malik, R.N.; Li, J.; Zhang, G.; Jones, K.C. Emerging issue of e-waste in Pakistan: A review of status, research needs and data gaps. Environ. Pollut. 2015, 207, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Uka, U.N.; Hogarh, J.; Belford, E.J.D. Morpho-Anatomical and Biochemical Responses of Plants to Air Pollution. Int. J. Mod. Bot. 2017, 7, 1–11. [Google Scholar]
- Rubio, V.; Valverde, M.; Rojas, E. Effects of atmospheric pollutants on the Nrf2 survival pathway. Environ. Sci. Pollut. Res. 2010, 17, 369–382. [Google Scholar] [CrossRef]
- Husted, S.; Hebbern, C.A.; Mattsson, M.; Schjoerring, J.K. A critical experimental evaluation of methods for determination of NH4+ in plant tissue, xylem sap and apoplastic fluid. Physiol. Plant. 2000, 109, 167–179. [Google Scholar] [CrossRef]
- Britto, D.T.; Kronzucker, H.J. NH4+ toxicity in higher plants: A critical review. J. Plant Physiol. 2002, 159, 567–584. [Google Scholar] [CrossRef]
- Seifi, H.S.; Van Bockhaven, J.; Angenon, G.; Höfte, M. Glutamate metabolism in plant disease and defense: Friend or foe? Mol. Plant-Microbe Interact. 2013, 26, 475–485. [Google Scholar] [CrossRef]
- Lea, P.J.; Miflin, B.J. Glutamate synthase and the synthesis of glutamate in plants. Plant Physiol. Biochem. 2003, 41, 555–564. [Google Scholar] [CrossRef]
- Harborne, J.B.; Williams, C.A. Advances in Flavonoid Research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Biosynthesis of flavonoids and effects of stress. Curr. Opin. Plant Biol. 2002, 5, 218–223. [Google Scholar] [CrossRef] [PubMed]
- D’Auria, J.C.; Gershenzon, J. The secondary metabolism of Arabidopsis thaliana: Growing like a weed. Curr. Opin. Plant Biol. 2005, 8, 308–316. [Google Scholar] [CrossRef]
- Agati, G.; Biricolti, S.; Guidi, L.; Ferrini, F.; Fini, A.; Tattini, M. The biosynthesis of flavonoids is enhanced similarly by UV radiation and root zone salinity in L. vulgare leaves. J. Plant Physiol. 2011, 168, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Tattini, M.; Galardi, C.; Pinelli, P.; Massai, R.; Remorini, D.; Agati, G. Differential accumulation of flavonoids and hydroxycinnamates in leaves of Ligustrum vulgare under excess light and drought stress. N. Phytol. 2004, 163, 547–561. [Google Scholar] [CrossRef]
- Tattini, M.; Guidi, L.; Morassi-Bonzi, L.; Pinelli, P.; Remorini, D.; Degl’Innocenti, E.; Giordano, C.; Massai, R.; Agati, G. On the role of flavonoids in the integrated mechanisms of response of Ligustrum vulgare and Phillyrea latifolia to high solar radiation. N. Phytol. 2005, 167, 457–470. [Google Scholar] [CrossRef]
- Lillo, C.; Lea, U.S.; Ruoff, P. Nutrient depletion as a key factor for manipulating gene expression and product formation in different branches of the flavonoid pathway. Plant Cell Environ. 2008, 31, 587–601. [Google Scholar] [CrossRef] [PubMed]
- Ollson, L.C.; Veit, M.; Bornman, J.F. Epidermal transmittance and phenolic composition of atrazine-tolerant and atrazine-sensitive cultivars of Brassica napus grown under enhanced UV-B radiation. Physiol. Plant. 1999, 107, 259–266. [Google Scholar] [CrossRef]
- Agati, G.; Galardi, C.; Gravano, E.; Romani, A.; Tattini, M. Flavonoid distribution in tissues of Phillyrea latifolia as estimated by microspectrofluorometry and multispectral fluorescence microimaging. Photochem. Photobiol. 2002, 76, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Matteini, P.; Goti, A.; Tattini, M. Chloroplast-located flavonoids can scavenge singlet oxygen. N. Phytol. 2007, 174, 77–89. [Google Scholar] [CrossRef]
- Brown, I.; Trethowan, J.; Kerry, M.; Mansfield, J.W.; Bolwell, G.P. Location of components of the oxidative cross-linking of glycoproteins and callose synthesis in papillae formed during the interaction between non-pathogenic strains of Xanthomonas campestris and French bean mesophyll cells. Plant J. 1998, 15, 333–343. [Google Scholar] [CrossRef]
- Melidou, M.; Riganakos, K.; Galaris, D. Protection against nuclear DNA damage offered by flavonoids in cells exposed to hydrogen peroxide: The role of iron chelation. Free Radic. Biol. Med. 2005, 39, 1591–1600. [Google Scholar] [CrossRef]
- Hernandez, I.; Alegre, L.; Van Breusegem, F.; Munne-Bosch, S. How relevant are flavonoids as antioxidants in plants? Trends Plant Sci. 2009, 14, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.A.; Bell, R.M.; Davidson, J.M.; Oconnor, G.A. Plant Uptake of Non-ionic organic chemicals from soils. Chemosphere 1988, 17, 2299–2323. [Google Scholar] [CrossRef]
- Babu, A.G.; Kim, J.D.; Oh, B.T. Enhancement of heavy metal phytoremediation by Alnus firma with endophytic Bacillus thuringiensis GDB-1. J. Hazard. Mater. 2013, 250–251, 477–483. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Mandziuk, A.; Fornal-Pieniak, B.; Stangierska, D.; Parzych, S.; Widera, K. Social Preferences of Young Adults Regarding Urban Forest Recreation Management in Warsaw, Poland. Forests 2021, 12, 1524. [Google Scholar] [CrossRef]
- García-Romero, L. Biogeomorphological processes in an arid transgressive dunefield as monitor plant of air pollutions of human impact by urbanization. Sci. Total Environ. 2019, 650, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Eom, S.; Jin, C.W.; Park, H.J.; Kim, E.H.; Chung, I.M.; Kim, M.J.; Yu, C.Y.; Cho, D.H. Far infrared ray irradiation stimulates antioxidant activity in Vitis flexuosa THUNB. Berries. Korean J. Med. Crop Sci. 2007, 15, 319–323. [Google Scholar]
- Weatherburn, M.W. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 1967, 39, 971–974. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Ram, S.S. SEMEDS: An important tool for air pollution bio-monitoring. Micron 2012, 43, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Ram, S.S. Nuclear Microscopy for Air-Pollutant Characterization and Its Advantages over Traditional Techniques. J. Appl. Spectrosc. 2014, 81, 145–150. [Google Scholar] [CrossRef]
- Ram, S.S. Plant canopies: Bio-monitor and trap for re-suspended dust particulates contaminated with heavy metals. Mitig. Adapt. Strateg. Glob. Chang. 2014, 19, 499–508. [Google Scholar] [CrossRef]
- Tomašević, M. Heavy metals accumulation in tree leaves from urban areas. Environ. Chem. Lett. 2004, 2, 151–154. [Google Scholar] [CrossRef]
- Kar, S. Metallic components of traffic-induced urban aerosol, their spatial variation, and source apportionment. Environ. Monit. Assess. 2010, 168, 561–574. [Google Scholar] [CrossRef]
- Neill, S.J. Hydrogen peroxide and nitric oxide as signalling molecules in plants. J. Exp. Bot. 2002, 53, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Younis, U.Y. Dust Interception Capacity and Alteration of Various Biometric and Biochemical Attributes in Cultivated Population of Ficus carica L. IOSR J. Pharm. Biol. Sci. 2013, 6, 35–42. [Google Scholar]
- Bandhu, H. Elemental composition and sources of air pollution in the city of Chandigarh, India, using EDXRF and PIXE techniques. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2000, 160, 126–138. [Google Scholar] [CrossRef]
- Dadkhah-Aghdash, H. Detection of urban trees sensitivity to air pollution using physiological and biochemical leaf traits in Tehran, Iran. Sci. Rep. 2022, 12, 15398. [Google Scholar] [CrossRef] [PubMed]
- Maryum, Z. An overview of salinity stress, mechanism of salinity tolerance and strategies for its management in cotton. Front. Plant Sci. 2022, 13, 935543. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T. Absorption of Formaldehyde by Oleander (Nerium indicum). Environ. Sci. Technol. 1995, 29, 2901–2903. [Google Scholar] [CrossRef] [PubMed]
- Landi, M.; Tattini, M.; Gould, K.S. Multiple functional roles of anthocyanins in plant-environment interactions. Environ. Exp. Bot. 2015, 119, 4–17. [Google Scholar] [CrossRef]
- Clemens, S. Tolerance to toxic metals by a gene family of phytochelatin synthases from plants and yeast. EMBO J. 1999, 18, 3325–3333. [Google Scholar] [CrossRef]
- Jozefczak, M. Glutathione Is a Key Player in Metal-Induced Oxidative Stress Defenses. Int. J. Mol. Sci. 2012, 13, 3145–3175. [Google Scholar] [CrossRef]
- Hare, P.D. Proline synthesis and degradation: A model system for elucidating stress-related signal transduction. J. Exp. Bot. 1999, 50, 413–434. [Google Scholar] [CrossRef]
- Kavi Kishor, P.B. Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: Its implications in plant growth and abiotic stress tolerance. Curr. Sci. 2005, 88, 424–438. [Google Scholar]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Yancey, P.H. Living with Water Stress: Evolution of Osmolyte Systems. Science 1982, 217, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Jaleel, C.A.; Manivannan, P.; Sankar, B.; Kishorekumar, A.; Gopi, R.; Somasundaram, R.; Panneerselvam, R. Changes in biochemical constituents and induction of early sprouting by triadimefon treatment in white yam (Dioscorea rotundata Poir.) tubers during storage. J. Zhejiang Univ. Sci. B 2007, 8, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, N.; Hermans, C. Proline accumulation in plants: A review. Amino Acids 2008, 35, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.K. Impacts of particulate matter pollution on plants: Implications for environmental biomonitoring. Ecotoxicol. Environ. Saf. 2016, 129, 120–136. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Karmakar, R.; Padhy, P.K. Antioxidant defense and heavy metal accumulation in plants grown on contaminated soil by automobile exhaust. Environ. Sci. Pollut. Res. 2019, 26, 28327–28341. [Google Scholar]
- Kelemen, D.; Tóth, B.; Kovács, B. Effect of heavy metals on the root growth and oxidative stress of oak species. Cent. Eur. For. J. 2015, 61, 178–184. [Google Scholar]
- Havaux, M.; Kloppstech, K. The protective functions of carotenoid and flavonoid pigments against excess visible radiation at chilling temperature investigated in Arabidopsis npq and tt mutants. Planta 2001, 213, 953–966. [Google Scholar] [CrossRef]
- Popek, R.; Łukowski, A.; Bates, C.; Oleksyn, J. Particulate matter, heavy metals and polycyclic aromatic hydrocarbons accumulation on the leaves of Tilia cordata Mill. in five Polish cities with different level of air pollution. Int. J. Phytoremediat. 2017, 19, 1134–1141. [Google Scholar] [CrossRef]
- Mandal, M.; Popek, R.; Przybysz, A.; Roy, A.; Das, S.; Sarkar, A. Breathing fresh air in the city: Implementing avenue trees as a sustainable solution to reduce particulate pollution in urban agglomerations. Plants 2023, 12, 1545. [Google Scholar] [CrossRef] [PubMed]
- Łukowski, A.; Popek, R.; Karolewski, P. Particulate matter on foliage of Betula pendula, Quercus robur, and Tilia cordata: Deposition and ecophysiology. Environ. Sci. Pollut. Res. Int. 2020, 27, 10296–10307. [Google Scholar] [CrossRef] [PubMed]
- Hrotkó, K.; Gyeviki, M.; Sütöriné, D.M.; Magyar, L.; Mészáros, R.; Honfi, P.; Kardos, L. Foliar dust and heavy metal deposit on leaves of urban trees in Budapest (Hungary). Environ. Geochem. Health 2021, 43, 1927–1940. [Google Scholar] [CrossRef] [PubMed]
- Simon, E.; Baranyai, E.; Braun, M.; Cserháti, C.; Fábián, I.; Tóthmérész, B. Elemental concentrations in deposited dust on leaves along an urbanization gradient. Sci. Total Environ. 2014, 490, 514–520. [Google Scholar] [CrossRef] [PubMed]
- González, J.A.; Prado, F.E.; Piacentini, R.D. Atmospheric dust accumulation on native and non-native species: Effects on gas exchange parameters. J. Environ. Qual. 2014, 43, 801–808. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jędrzejuk, A.; Chyliński, F.; Fornal-Pieniak, B. Festuca ovina L. As a Monitor Plant Species of Traffic Air Along the Highway in of the City of Warsaw (Poland). Agriculture 2024, 14, 1750. https://doi.org/10.3390/agriculture14101750
Jędrzejuk A, Chyliński F, Fornal-Pieniak B. Festuca ovina L. As a Monitor Plant Species of Traffic Air Along the Highway in of the City of Warsaw (Poland). Agriculture. 2024; 14(10):1750. https://doi.org/10.3390/agriculture14101750
Chicago/Turabian StyleJędrzejuk, Agata, Filip Chyliński, and Beata Fornal-Pieniak. 2024. "Festuca ovina L. As a Monitor Plant Species of Traffic Air Along the Highway in of the City of Warsaw (Poland)" Agriculture 14, no. 10: 1750. https://doi.org/10.3390/agriculture14101750






