Lasioptera rubi, a Pest of Rubus idaeus: Galls Morphology, Anatomy and Histochemistry
Abstract
:1. Introduction
2. Materials and Methods
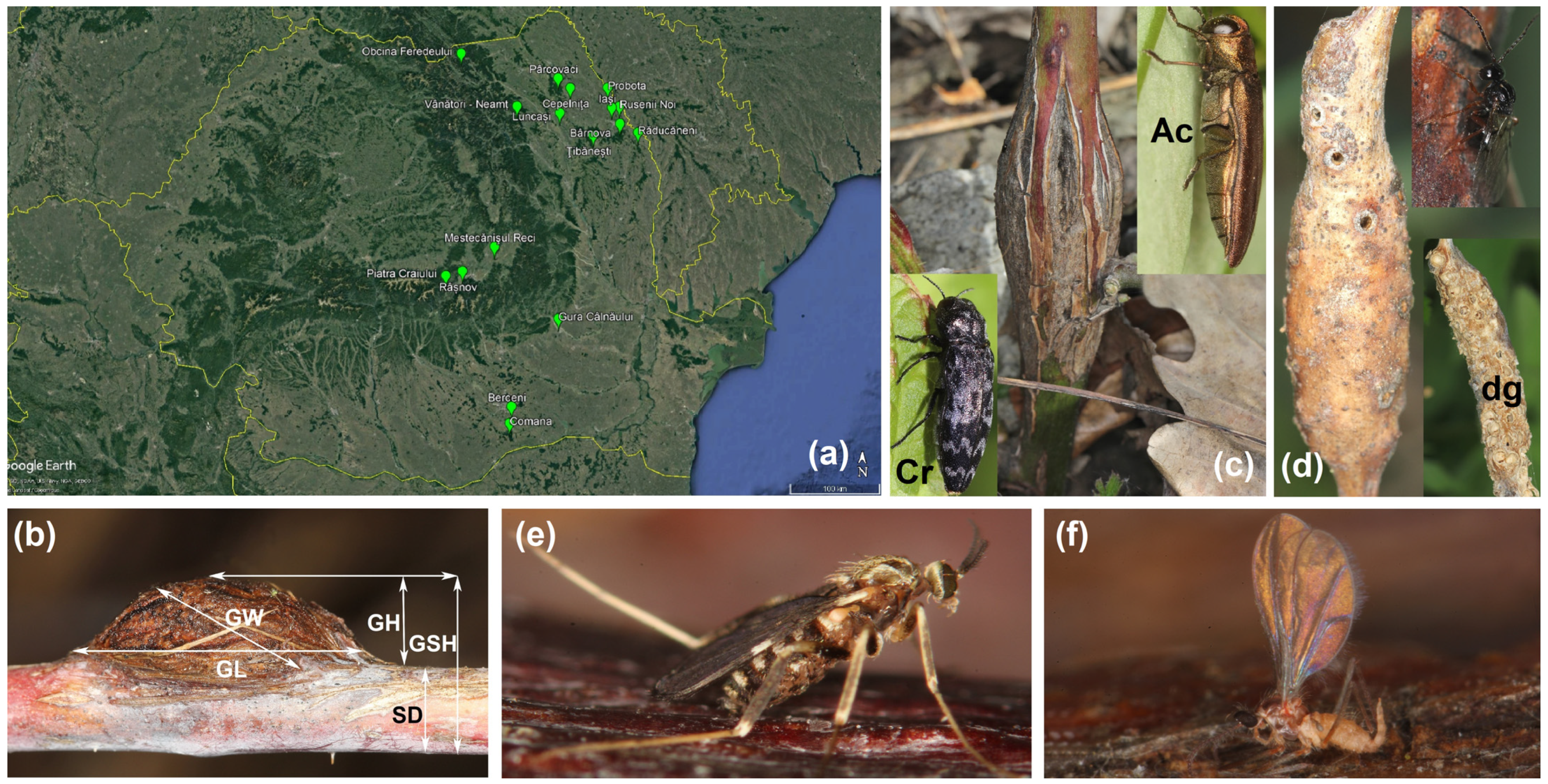
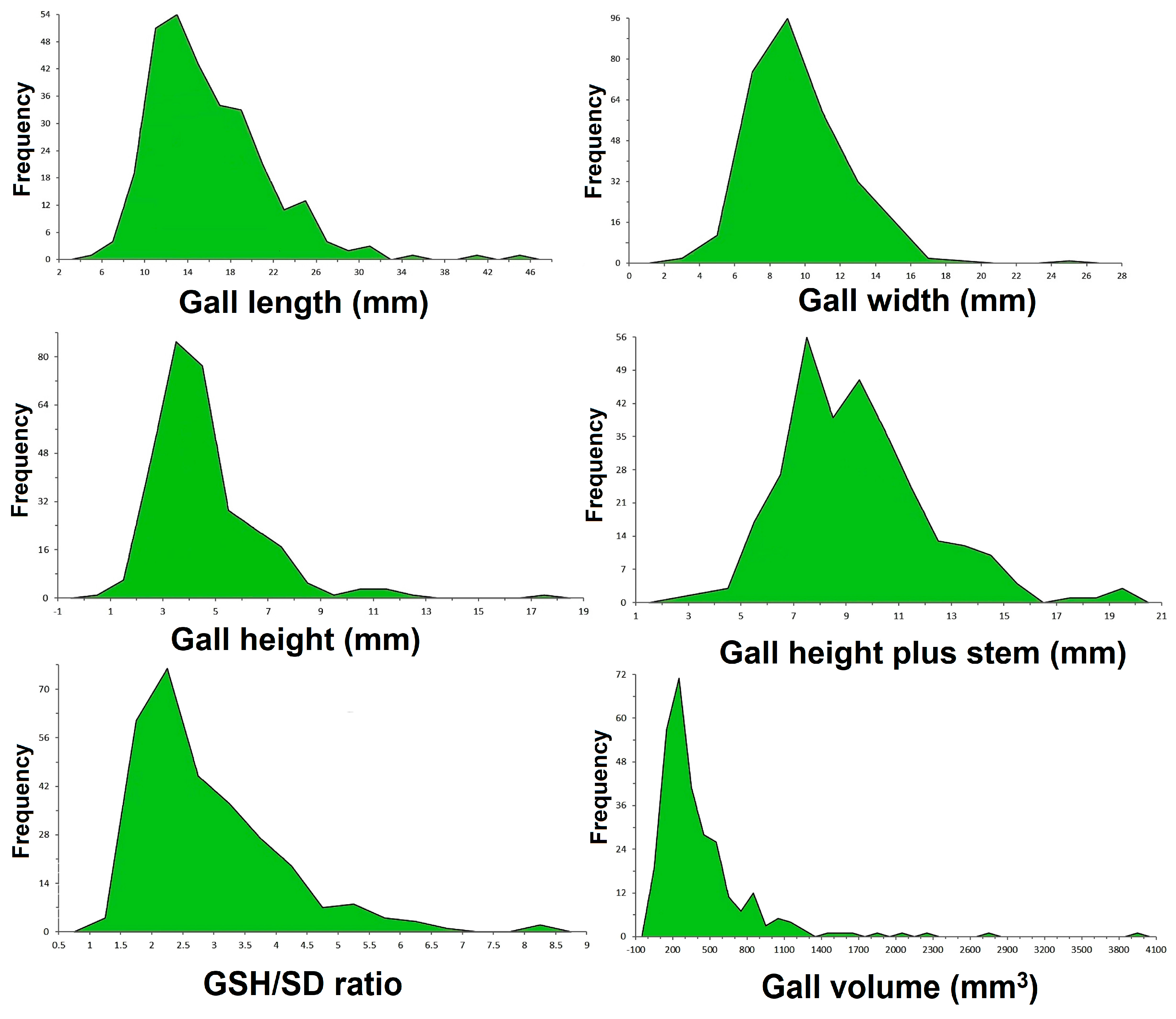
2.1. Gall Sampling, Measurement and Morphometric Analysis
2.2. Light Microscopy
2.3. Histo-Anatomical Analysis
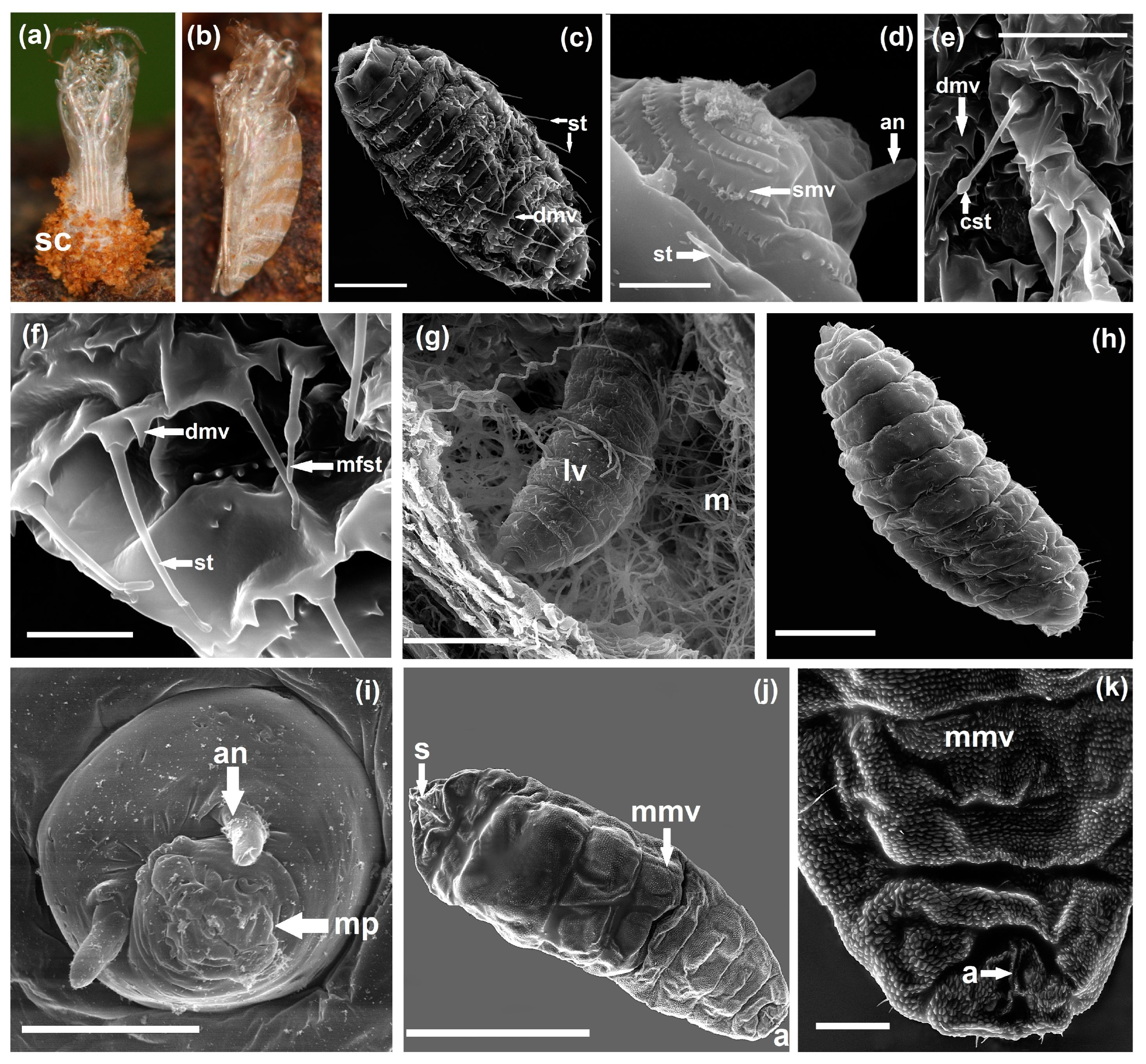
2.4. Scanning Electron Microscopy (SEM)
2.5. Histochemical Analysis
2.6. Statistical Analysis
3. Results
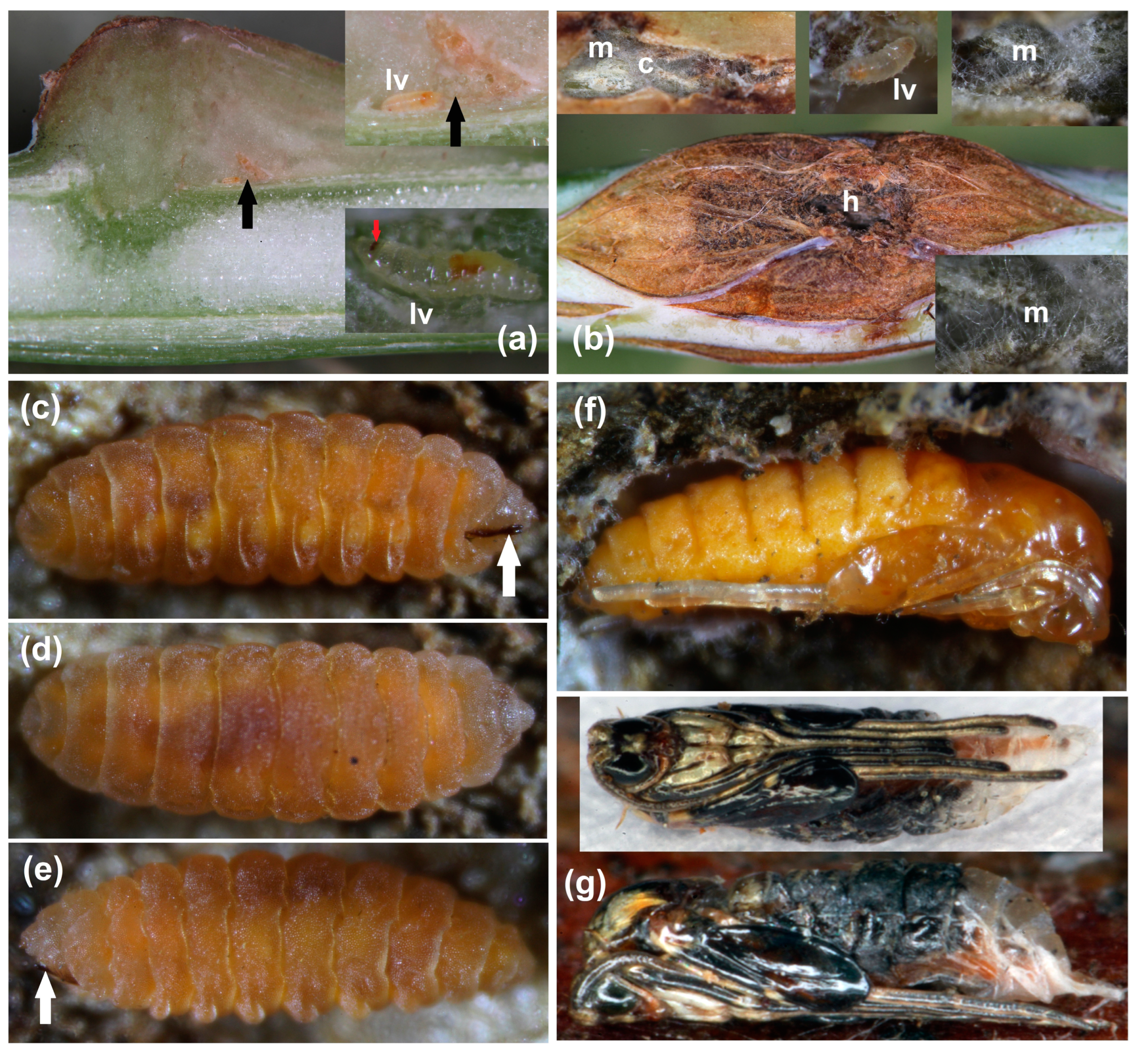
3.1. Gall Inducer—Lasioptera rubi
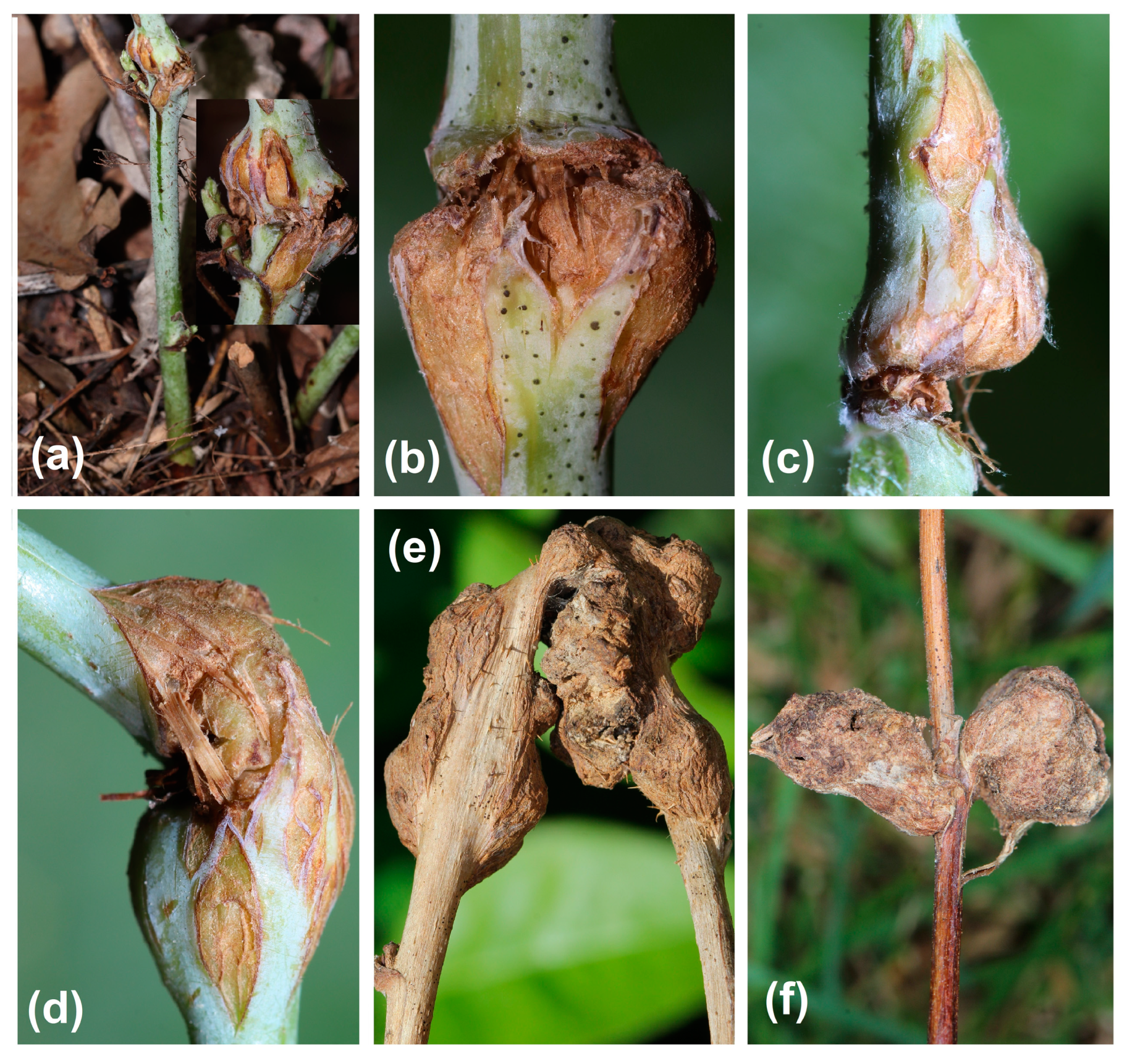
3.2. Stem Galls’ Morphology and Morphometry
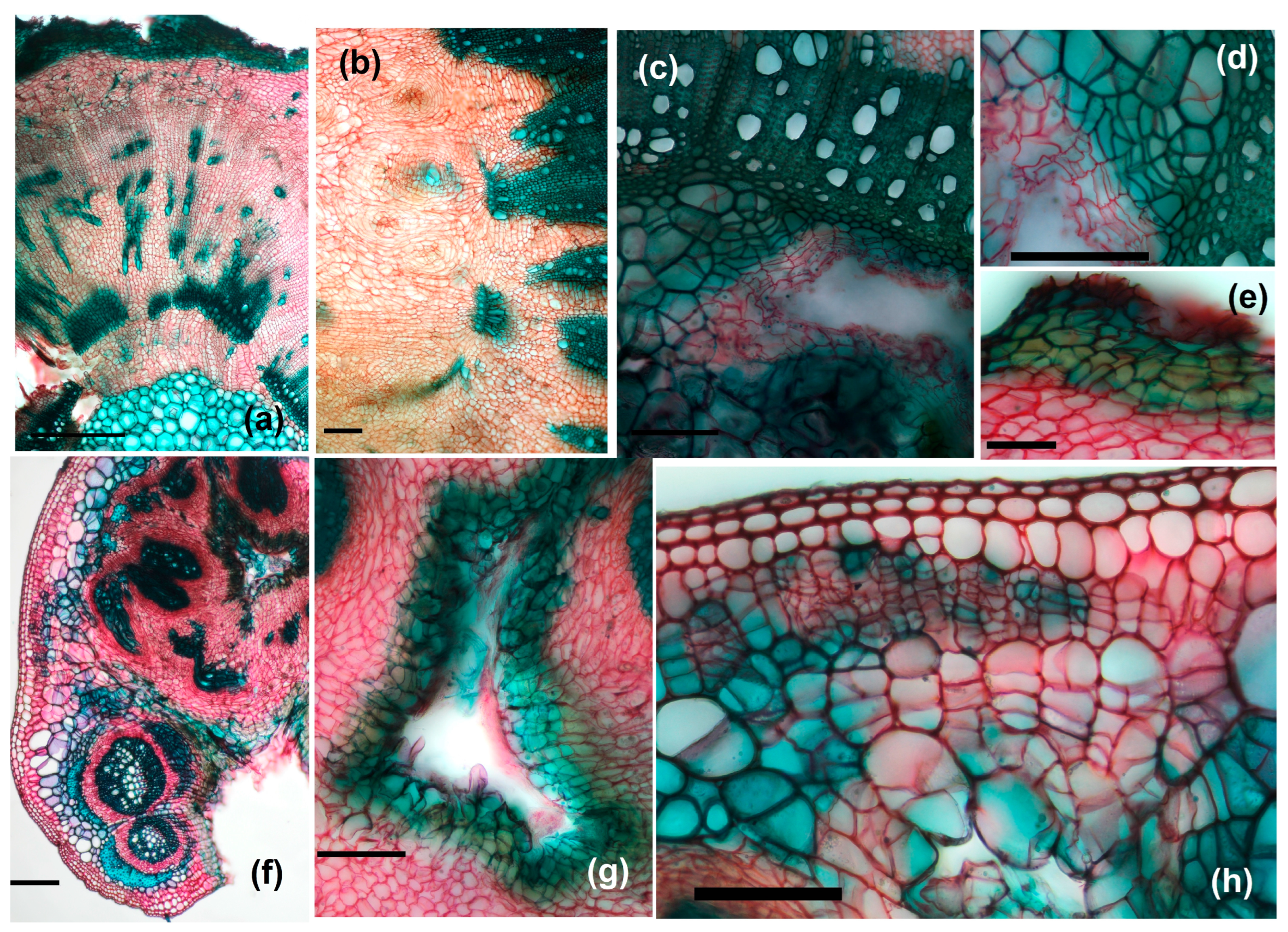
3.3. Galls’ Anatomy
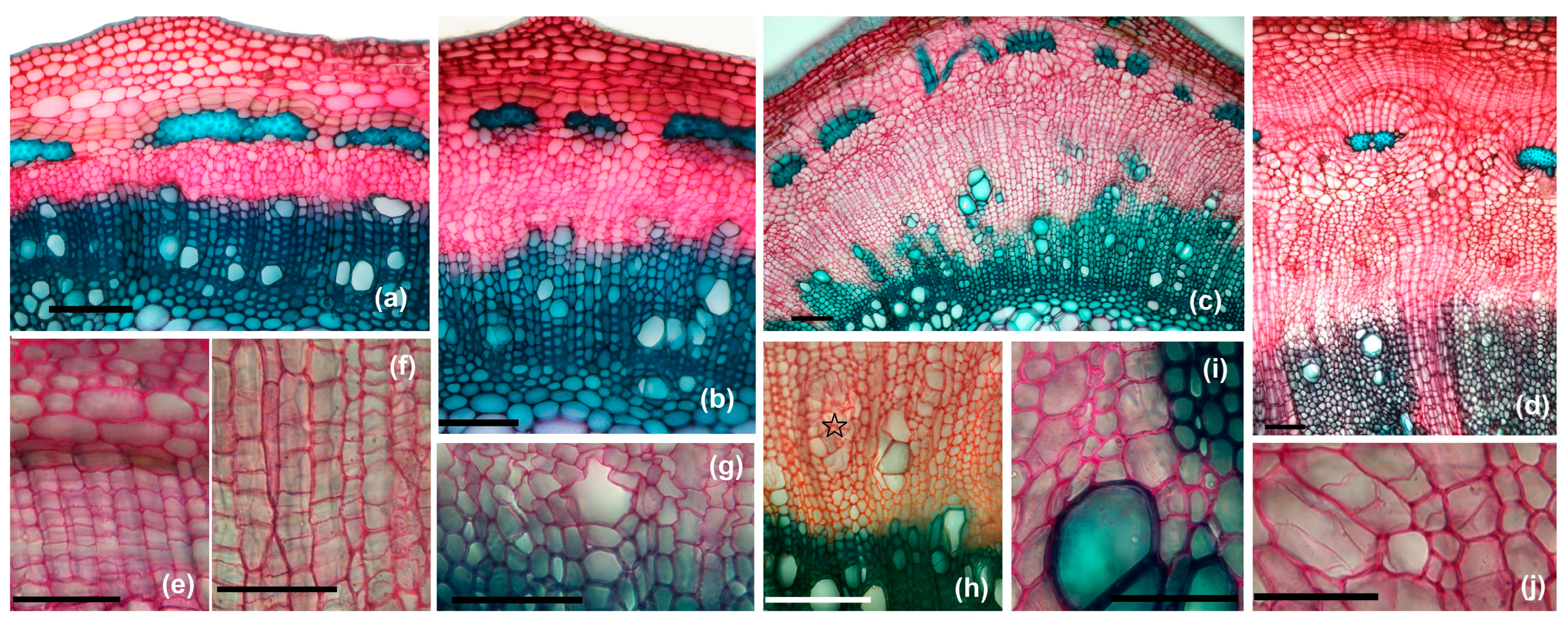
3.3.1. Non-Galled Stems Structure
3.3.2. Non-Galled Petiole Structure
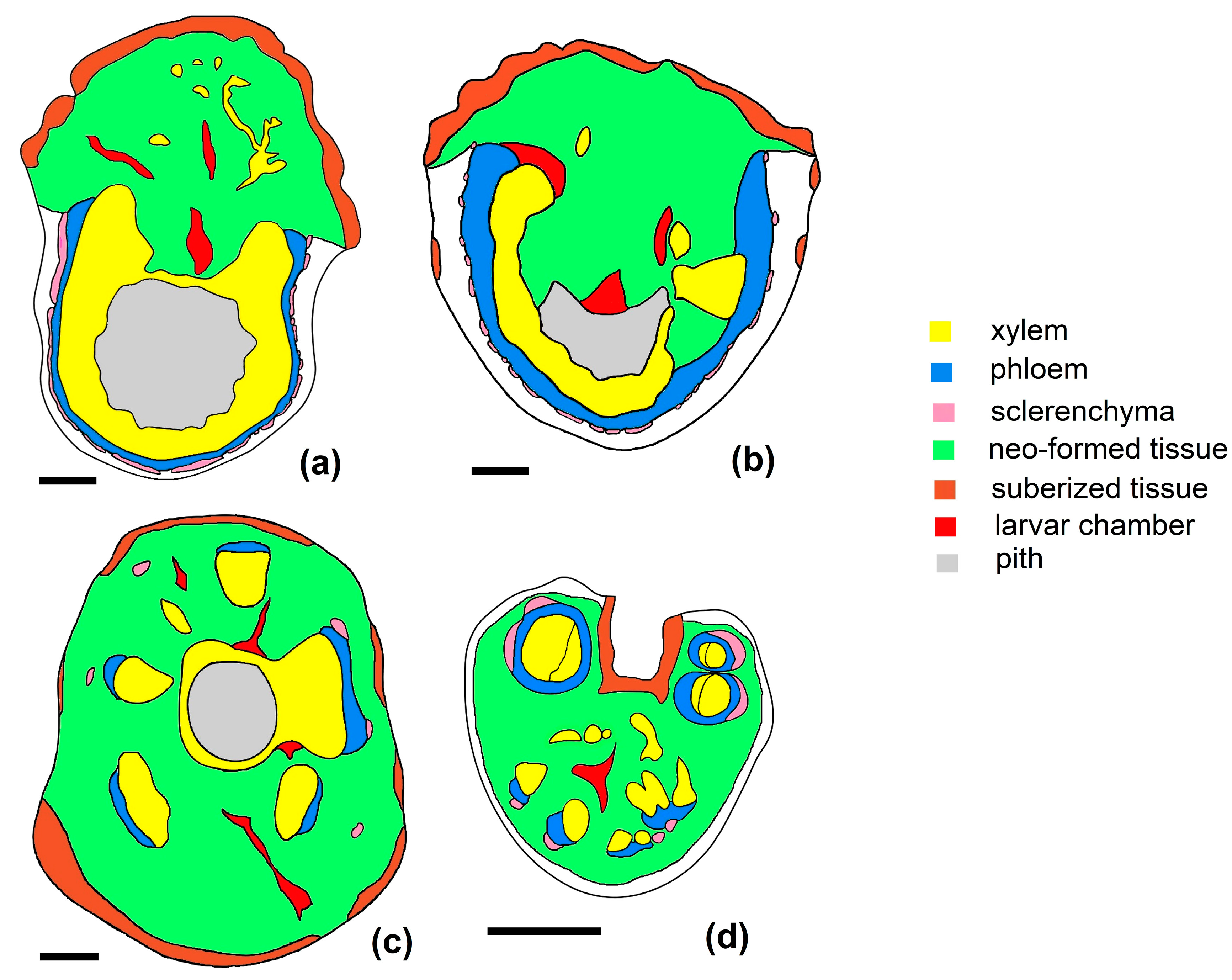
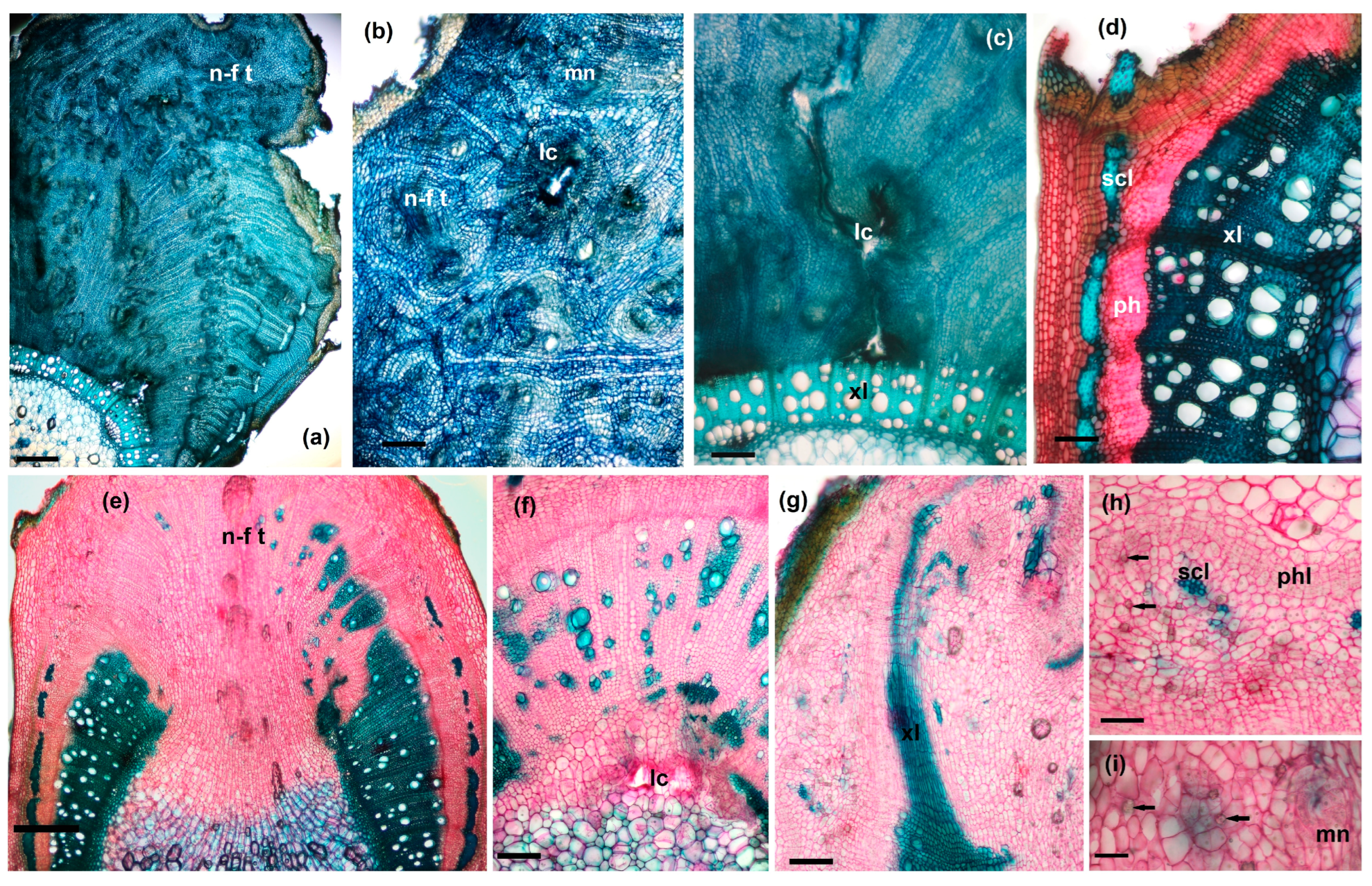
3.3.3. Galled Stems’ Structure
- Anatomical structure of GL 1 galls
- Anatomical structure of GL 2 galls
- Anatomical structure of GL 3 galls
3.3.4. Petiole Galls’ Structure
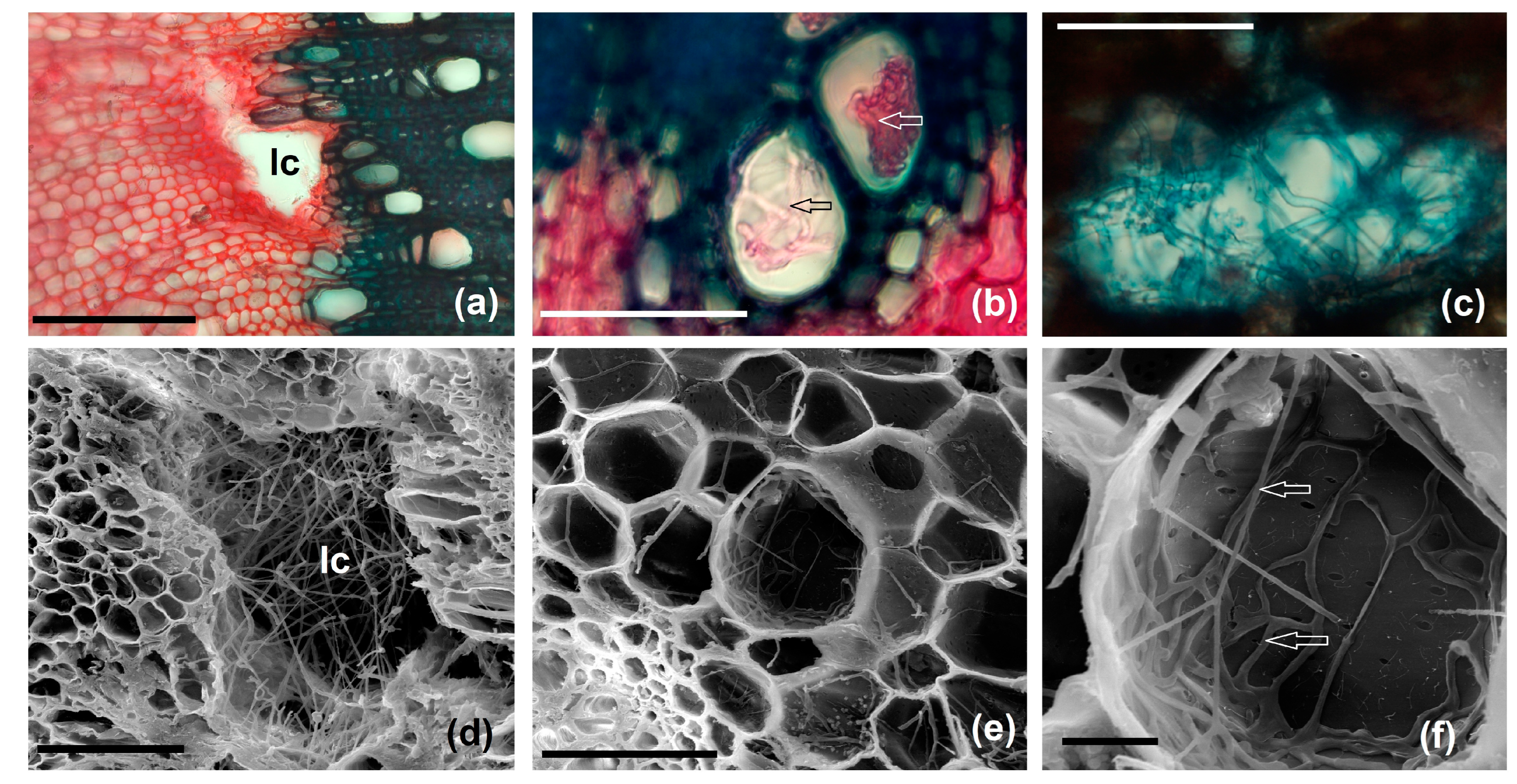
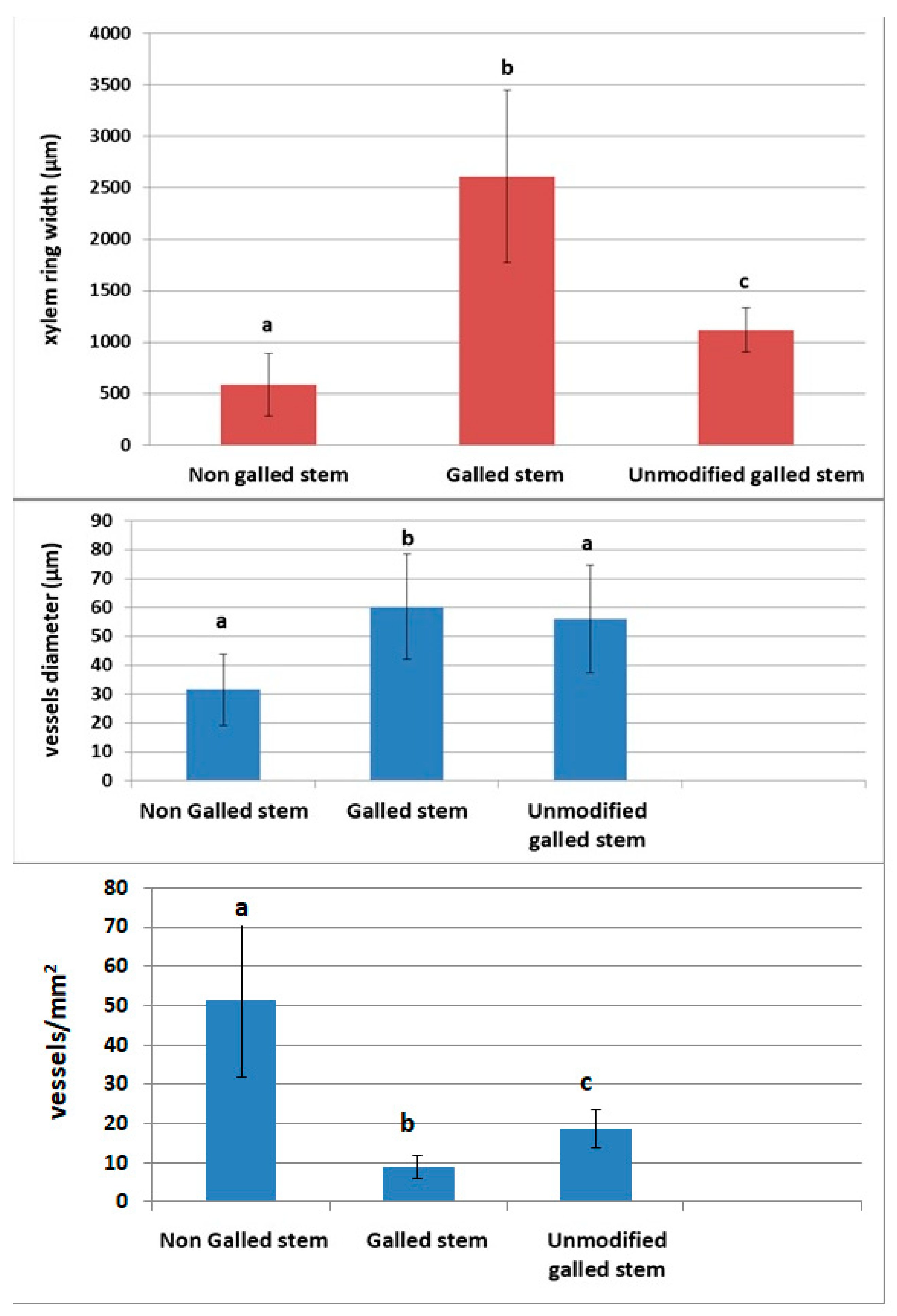
3.3.5. Changes in Vascular Tissue Morphogenesis Induced by Gall Formation
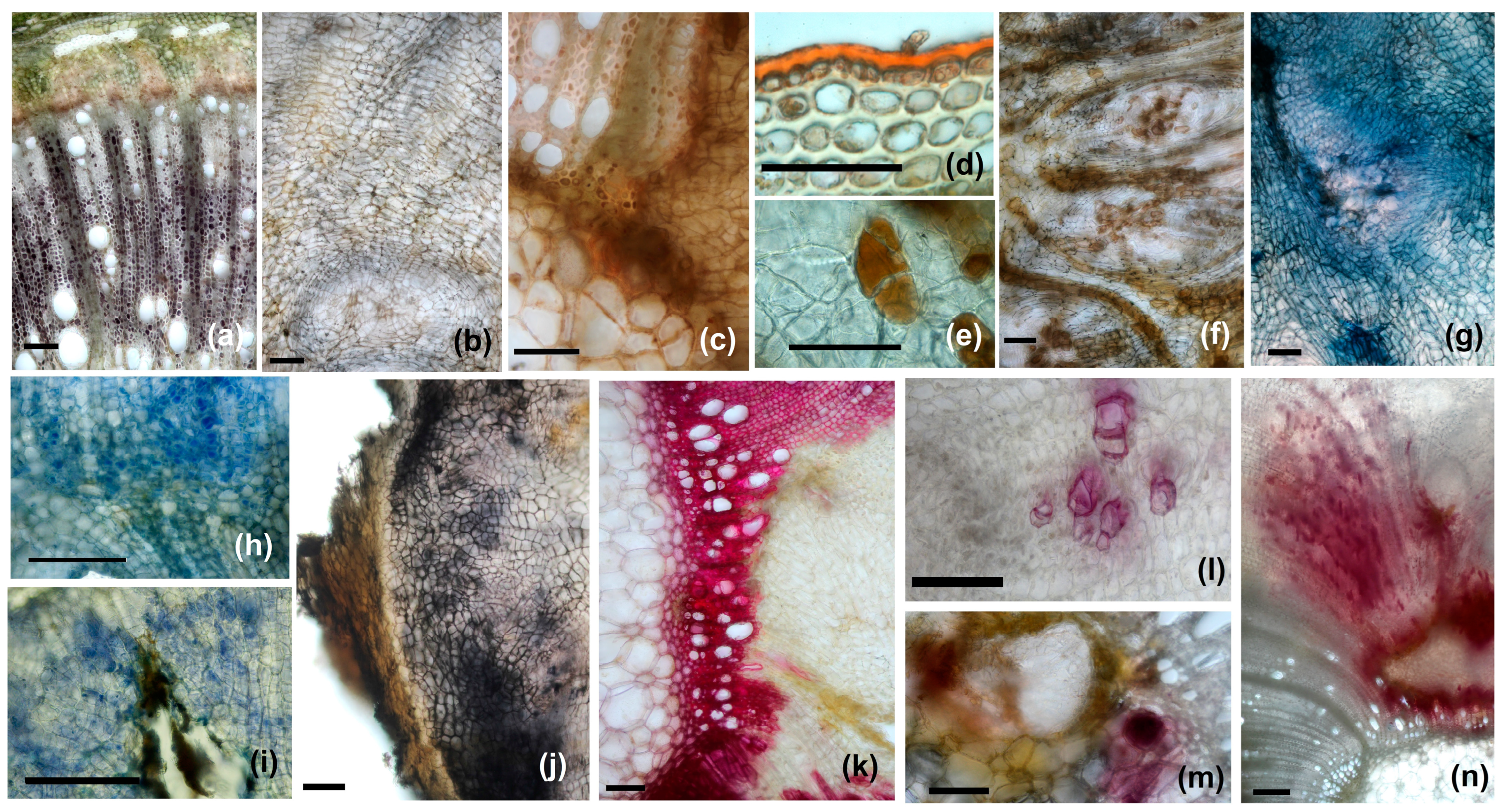
3.4. Histochemical Peculiarities of the Gall Tissues
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Weber, H.E.; Rubus, L. Illustrierte Flora von Mitteleuropa. Band IV. Teil 2A. Spermatophyta: Angiospermae: Dicotyledones; Conert, H.J., Jäger, E.J., Kadereit, J.W., Schultze-Motel, W., Wagenitz, G., Weber, H.E., Eds.; Blackwell: Berlin, Germany, 1995. [Google Scholar]
- POWO—Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Available online: https://powo.science.kew.org/ (accessed on 26 July 2024).
- WFO. World Flora Online. Version 2024.06. 2024. Available online: http://www.worldfloraonline.org (accessed on 29 July 2024).
- Lingdi, L.; Boufford, D.E.R.; Editorial Committee of Flora of China. Flora of China; Wu, Z., Raven, P.H., Eds.; Chinese Academy of Sciences, Science Press: Beijing, China, 2003. [Google Scholar]
- Beldie, A.; Buia, A.; Guşuleac, M.; Nyárády, E.I.; Prodan, I.; Răvăruţ, M. Flora Republicii Populare Române; Editura Academiei Republicii Populare Române: Bucharest, Romania, 1956; Volume 6. [Google Scholar]
- Totic, I. Raspberry Breeding and Protection against Disease and Pests. Bulg. J. Agric. Sci. 2014, 20, 391–404. [Google Scholar]
- IUCN. The IUCN Red List of Threatened Species. Version 2024-1. 2024. Available online: www.iucnredlist.org (accessed on 20 July 2024).
- Khela, S. Rubus idaeus (Europe Assessment). The IUCN Red List of Threatened Species 2012: e.T203456A2765767. Rubus idaeus (Raspberry). Available online: https://www.iucnredlist.org/ (accessed on 28 July 2024).
- Aronsson, M.; Bioret, F.; Bita-Nicolae, C.; Capelo, J.; Čarni, A.; Dimopoulos, P.; Janssen, J.; Loidi, J. F3.1b Temperate Rubus Scrub; European Red List of Habitats—Heathland Habitat Group: Gland, Switzerland, 2016. [Google Scholar]
- Tobyn, G.; Denham, A.; Whitelegg, M. Rubus idaeus, Raspberry. In Medical Herbs; Elsevier: Amsterdam, The Netherlands, 2011; pp. 271–282. ISBN 978-0-443-10344-5. [Google Scholar]
- Rao, A.V.; Snyder, D.M. Raspberries and Human Health: A Review. J. Agric. Food Chem. 2010, 58, 3871–3883. [Google Scholar] [CrossRef]
- Bojkovska, K.; Jankulovski, N.; Mihajlovski, G.; Momirceski, J. Analysis of Market Opportunities for Raspberry Production in The Republic of North Macedonia. Int. J. Res. GRANTHAALAYAH 2020, 8, 149–154. [Google Scholar] [CrossRef]
- Redfern, M. Plant Galls; Harper Collins Publishers: New York City, NY, USA, 2011. [Google Scholar]
- Gagné, R.J.; Jaschhof, M. A Catalog of the Cecidomyiidae (Diptera) of the World, 5th ed.; Systematic Entomology Laboratory, Agricultural Research Service, U.S. Department of Agriculture, U.S. National Museum: Washington, DC, USA, 2021. [Google Scholar]
- Skuhravá, M.; Skuhravý, V. Species Richness of Gall Midges (Diptera: Cecidomyiidae) in Europe (West Palaearctic): Biogeography and Coevolution with Host Plants. Acta Soc. Zool. Bohem. 2009, 73, 87–156. [Google Scholar]
- Hebert, P.D.N.; Ratnasingham, S.; Zakharov, E.V.; Telfer, A.C.; Levesque-Beaudin, V.; Milton, M.A.; Pedersen, S.; Jannetta, P.; deWaard, J.R. Counting Animal Species with DNA Barcodes: Canadian Insects. Philos. Trans. R. Soc. B 2016, 371, 20150333. [Google Scholar] [CrossRef] [PubMed]
- Bragança, G.P.P.; Freitas, M.D.S.C.; Isaias, R.M.D.S. The Influence of Gall Position over Xylem Features in Leaflets of Inga ingoides (Rich.) Willd. (Fabaceae: Caesalpinioideae). Trees 2021, 35, 199–209. [Google Scholar] [CrossRef]
- Guedes, L.M.; Aguilera, N.; Gavilán, E.; Péndola, J.A.; Villagrán, N.E. Vascular Implications of Dasineura sp. Galls’ Establishment on Peumus boldus Stems. Plant Biol. J. 2023, 25, 965–972. [Google Scholar] [CrossRef]
- Carneiro, R.G.; Burckhardt, D.; Isaias, R.M. Biology and systematics of gall-inducing triozids (Hemiptera: Psylloidea) associated with Psidium spp.(Myrtaceae). Zootaxa 2013, 3620, 129–146. [Google Scholar] [CrossRef]
- Arduin, M.; Kraus, J.E. Anatomia de Galhas de Ambrosia Em Folhas de Baccharis concinna e Baccharis dracunculifolia (Asteraceae). Braz. J. Bot. 2001, 24, 63–72. [Google Scholar] [CrossRef]
- Neger, F.W. Ambrosiapilze. Berichte Dtsch. Bot. Ges. 1908, 26A, 735–754. [Google Scholar]
- Tanasković, S.; Milenković, S.; Sretenović, D. Intensity of Attack of Raspberry Gall Midge Lasioptera rubi Schrank (Diptera, Cecidomyiidae) on Some Raspberry Genotypes. Acta Entomol. Serbica 2008, 13, 43–50. [Google Scholar]
- Möhn, E. Beiträge Zur Systematik Der Larven Itonididae (Cecidomyiidae, Diptera). 1. Teil: Porricondylinae Und Itonidinae Mitteleuropas. Lieferung 1. In Zoologica Original-Abhandlungen aus dem Gesamtgebiete der Zoologie; Forgotten Books: London, UK, 1955; Volume 105, pp. 1–247. [Google Scholar]
- Yukawa, J. A Revision of the Japanese Gall Midges (Diptera: Cecidomyiidae); Memoirs of the Faculty of Agriculture Kagoshima University: Kagoshima, Japan, 1971; Volume 8, pp. 1–203. [Google Scholar]
- Thomas, D.B.; Goolsby, J.A. Morphology of the Preimaginal Stages of Lasioptera donacis Coutin (Diptera: Cecidomyiidae), a Candidate Biocontrol Agent for Giant Arundo Cane. Psyche J. Entomol. 2015, 2015, 262678. [Google Scholar] [CrossRef]
- Jensen, W.A. Botanical Histochemistry: Principles and Practice; W. H. Freeman and Co: San Francisco, CA, USA, 1962. [Google Scholar]
- Şerbănescu-Jitariu, G.; Andrei, M.; Mitroiu-Rădulescu, N.; Petria, E. Practicum de Biologie Vegetală; CERES: Bucureşti, Romania, 1983. [Google Scholar]
- O’Brien, T.P.; Feder, N.; McCully, M.E. Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 1964, 59, 368–373. [Google Scholar] [CrossRef]
- Srebotnik, E.; Messner, K. A Simple Method That Uses Differential Staining and Light Microscopy to Assess the Selectivity of Wood Delignification by White Rot Fungi. Appl. Environ. Microbiol. 1994, 60, 1383–1386. [Google Scholar] [CrossRef] [PubMed]
- Johansen, D.A. Plant Microtechnique; McGrawHill: New York, NY, USA, 1940. [Google Scholar]
- Brundrett, M.C.; Kendrick, B.; Peterson, C.A. Efficient lipid staining in plant material with Sudan red 7B or fluoral yellow 088 in polyethylene glycol-glycerol. Biotech. Histochem. 1991, 66, 111–116. [Google Scholar] [CrossRef]
- Sass, J.E. Botanical Microtechnique, 2nd ed.; Iowa State College Press: Ames, IA, USA, 1951. [Google Scholar]
- David, R.; Carde, J.P. Histochimie-Coloration Differentielle Des Inclusions Lipidiques et Terpeniques des Pseudophylles du Pin Maritime au Moyen du Reactif NADI. Comptes Rendus Hebd. Seances Acad. Sci. 1964, 258, 1338. [Google Scholar]
- Gahan, P.B. Plant Histochemistry and Cytochemistry: An Introduction; Academic Press: Orlando, FL, USA, 1984. [Google Scholar]
- Bedetti, C.S.; Modolo, L.V.; Isaias, R.M.D.S. The Role of Phenolics in the Control of Auxin in Galls of Piptadenia gonoacantha (Mart.) MacBr (Fabaceae: Mimosoideae). Biochem. Syst. Ecol. 2014, 55, 53–59. [Google Scholar] [CrossRef]
- Tastás-Duque, R.; Sylvén, E. Sensilla and Cuticular Appendages on the Female Abdomen of Lasioptera rubi (Schrank) (Diptera, Cecidomyiidae). Acta Zool. 1989, 70, 163–174. [Google Scholar] [CrossRef]
- Rohfritsch, O. Plants, Gall Midges, and Fungi: A Three-component System. Entomol. Exp. Appl. 2008, 128, 208–216. [Google Scholar] [CrossRef]
- Rohfritsch, O. A fungus associated gall midge, Lasioptera arundinis (Schiner), on Phragmites australis (Cav.) Trin. Bull. Soc. Bot. Fr. Lett. Bot. 1992, 139, 45–59. [Google Scholar] [CrossRef]
- Rohfritsch, O. Morphological and Behavioural Adaptations of the Gall Midge Lasioptera arundinis (Schiner) (Diptera, Cecidomyiidae) to Collect and Transport Conidia of Its Fungal Symbiont. Tijdschr. Voor Entomol. 1997, 140, 59–66. [Google Scholar]
- Barnes, H.F. On Some Factors Governing the Emergence of Gall Midges (Cecidomyidæ: Diptera). Proc. Zool. Soc. Lond. 1930, 100, 381–393. [Google Scholar] [CrossRef]
- Gordon, S.C.; Woodford, J.A.T.; Birch, A.N.E. Arthropod Pests of Rubus in Europe: Pest Status, Current and Future Control Strategies. J. Hortic. Sci. 1997, 72, 831–862. [Google Scholar] [CrossRef]
- Barnes, H.F. The Gall Midges of Blackberries and Raspberries. J. Pomol. Hortic. Sci. 1926, 5, 137–140. [Google Scholar] [CrossRef]
- Barnes, H.F. Gall Midges of Economic Importance, Volume 3, Fruit; Crosby Lockwood & Son Ltd.: London, UK, 1948. [Google Scholar]
- Viggiani, G.; Mazzone, P. Infestazioni di Lasioptera rubi (Schrank) (Diptera, Cecidomyiidae) in Lamponeti Dell’ltalia Meridionale. Inf. Fitopatol. 1978, 2, 3–4. [Google Scholar]
- Darvas, B.; Skuhravá, M.; Andersen, A. Agricultural Dipteran Pests of the Palaearctic Region. In Contributions to a Manual of Palaearctic Diptera; Papp, L., Darvas, B., Eds.; General and Applied Dipterology; Science Herald: Budapest, Romania, 2000; Volume 1, pp. 565–650. [Google Scholar]
- Skuhravá, M.; Skuhravý, V.; Meyer, H. Gall Midges (Diptera: Cecidomyiidae: Cecidomyiinae) of Germany; Druckzentrum, Neumünster: Kiel, Germany, 2014. [Google Scholar]
- Simova, D.; Dobrivojević, K. Raspberry Gall Midge (Lasioptera rubi Heeg.) A Little Known Pests of Raspberry. Plant Prot. 1966, 91, 323–330. [Google Scholar]
- Dobrivojević, K. Ekonomski Značajne Štetočine Maline u Proizvodnim Područjima Valjeva i Čačka. Zaštita Bilja 1968, 100–101, 253–271. [Google Scholar]
- Simova-Tošić, D A Little Known Pests of Raspberry from Family Cecidomyiidae (Diptera). J. Yugosl. Pomol. 1970, 11–12, 193–197.
- Milenković, S.; Tanasković, S. Harmfulness of Raspberry Gall Midge, Lasioptera rubi Schrank (Diptera, Cecidomyiidae), to Some Raspberry Cultivars. IOBC/WPRS Bull. 2008, 39, 71–75. [Google Scholar]
- Gordon, S.C.; Milenković, S.; Cross, J.V.; Stanisavljevic, M. Raspberry Pests in Serbia. Bull. OILB/SROP 2003, 26, 23–27. [Google Scholar]
- Tanasković, S.; Milenković, S. Occurrence of Raspberry Gall Midge Lasioptera rubi Schrank (Diptera, Cecidomyiidae) in Some Raspberry Cultivars. Acta Agric. Serbica 2009, 14, 79–85. [Google Scholar]
- Tanasković, S.T.; Milenković, S.N. Open Field Surveys to Evaluate the Susceptibility of Red Raspberry Genotypes to Raspberry Gall Midge, Lasioptera rubi Schrank (Diptera, Cecidomyiidae)—4 Year Results. Acta Hortic. 2012, 946, 247–251. [Google Scholar] [CrossRef]
- Milenković, S.; Ranković, M. Integral Raspberry Protection. Proc. Winter Sch. Agron. Fac. Agron. 2001, 5, 59–65. [Google Scholar]
- Yegorenkova, E.; Yefremova, Z. Notes on Lasioptera rubi (Schrank) (Diptera: Cecidomyiidae) and Its Larval Parasitoids (Hymenoptera) on Raspberries in Russia. Entomol. Fenn. 2019, 27, 15–22. [Google Scholar] [CrossRef]
- OEPP/EPPO Recommendations made by EPPO Council in 1992: Scheme for the production of classified vegetatively propagated ornamental plants to satisfy health standards. Bull. OEPP/EPPO Bull. 1993, 23, 735–736.
- Teodorescu, G. Contribuții La Studiul Biologiei și Ecologiei Musculiței Galicole a Lăstarilor de Zmeur și Mur, Lasioptera rubi Heeger. Bul. Prot. Plantelor 1989, 2, 55–57. [Google Scholar]
- Popescu, I.E. Torymid Wasps (Hymenoptera, Chalcidoidea, Torymidae) New for Romanian Fauna. Analele Ştiinţifice Univ. “Al. I. Cuza” Iaşi Ser. Biol. Anim. 2003, 49, 83–86. [Google Scholar]
- Neacşu, P. Galls from Romania; Victor Bortaş Publishing House: Bucureşti, Romania, 2006. [Google Scholar]
- Chiriceanu, C.; Chiriloaie, A.; Teodoru, A.; Sivu, C. Contribution to Knowledge of the Gall Insects and Mites Associated with Plants in Southern Romania. Sci. Pap. Ser. B Hortic. 2015, 59, 27–36. [Google Scholar]
- National Phytosanitary Agency Guide for Identification of the Disease and Pests of Fruit Shrubs. Bucharest 2020. Available online: https://www.anfdf.ro/sanatate/ghid/ghid_arbusti.pdf (accessed on 10 August 2024).
- Krishnamurthy, K.V. Plant-Insect-Fungus Association in Some Plant Galls. Proc. Anim. Sci. 1984, 93, 265–273. [Google Scholar] [CrossRef]
- Pyszko, P.; Šigutová, H.; Kolařík, M.; Kostovčík, M.; Ševčík, J.; Šigut, M.; Višňovská, D.; Drozd, P. Mycobiomes of Two Distinct Clades of Ambrosia Gall Midges (Diptera: Cecidomyiidae) Are Species-Specific in Larvae but Similar in Nutritive Mycelia. Microbiol. Spectr. 2024, 12, e02830-23. [Google Scholar] [CrossRef]
- Kaiser, P. Importance of Fungal Hyphae in Mycotic Galls. Studies on Lasioptera rubi (Schrank, 1803) (Diptera, Cecidomyiidae). Entomol. Mitteilungen Zool. Mus. Hambg. 1978, 6, 41–48. [Google Scholar]
- Tan, J.L.; Trandem, N.; Fránová, J.; Hamborg, Z.; Blystad, D.-R.; Zemek, R. Known and Potential Invertebrate Vectors of Raspberry Viruses. Viruses 2022, 14, 571. [Google Scholar] [CrossRef]
- Meyer, J. Cécidogenèse de La Galla de Lasioptera rubi Heeger et Rôle Nourricier d’un Mycélium Symbiotique. Comptes Rendus Hebd. Seances Acad. Des Sci. 1952, 234, 2256–2558. [Google Scholar]
- Mani, M.S. Ecology of Plant Galls; Springer-Science+Business Media, B.V.: Dordrecht, The Netherlands, 1964. [Google Scholar]
- Carneiro, R.G.S.; Isaias, R.M.S.; Moreira, A.S.F.P.; Oliveira, D.C. Reacquisition of New Meristematic Sites Determines the Development of a New Organ, the Cecidomyiidae Gall on Copaifera langsdorffii Desf. (Fabaceae). Front. Plant Sci. 2017, 8, 1622. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Isaias, R.M.; De Oliveira, D.C.; Da Silva Carneiro, R.G.; Kraus, J.E. Developmental Anatomy of Galls in the Neotropics: Arthropods Stimuli Versus Host Plant Constraints. In Neotropical Insect Galls; Fernandes, G.W., Santos, J.C., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 15–34. ISBN 978-94-017-8782-6. [Google Scholar]
- Jorge, N.D.C.; Freitas, M.D.S.C.; Caffaro, R.M.; Vale, F.H.A.; Lemos-Filho, J.P.; Isaias, R.M.D.S. Vascular Traits of Stem Galls: Cell Increment versus Morphogenetic Constraints in Wood Anatomy. Plant Biol. J. 2022, 24, 450–457. [Google Scholar] [CrossRef]
- Marini-Filho, O.J.; Fernandes, G.W. Stem Galls Drain Nutrients and Decrease Shoot Performance in Diplusodon orbicularis (Lythraceae). Arthropod-Plant Interact. 2012, 6, 121–128. [Google Scholar] [CrossRef]
- Coelho Kuster, V.; Costa Rezende, U.; Fernandes Cardoso, J.C.; Dos Santos Isaias, R.M.; Coelho De Oliveira, D. How Galling Organisms Manipulate the Secondary Metabolites in the Host Plant Tissues? A Histochemical Overview in Neotropical Gall Systems. In Co-Evolution of Secondary Metabolites; Mérillon, J.-M., Ramawat, K.G., Eds.; Reference Series in Phytochemistry; Springer International Publishing: Cham, Switzerland, 2020; pp. 823–842. ISBN 978-3-319-96396-9. [Google Scholar]
- Chao, J.-F.; Liao, G.-I. Histocytological Aspects of Four Types of Ambrosia Galls on Machilus zuihoensis Hayata (Lauraceae). Flora—Morphol. Distrib. Funct. Ecol. Plants 2013, 208, 157–164. [Google Scholar] [CrossRef]
- Bragança, G.P.P.; Ferreira, B.G.; Isaias, R.M.D.S. Distinct Cytological Mechanisms for Food Availability in Three Inga ingoides (Fabaceae)—Cecidomyiidae Gall Systems. Protoplasma 2022, 259, 155–162. [Google Scholar] [CrossRef]
- Bronner, R. The role of nutritive cells in the nutrition of cynipids and cecidomyiids. In Biology of Insect-Induced Galls; Shorthouse, J.D., Rohfritsch, O., Eds.; Oxford University Press: New York, NY, USA, 1992; pp. 118–140. [Google Scholar]
- Costa, E.C.; Oliveira, D.C.; Ferreira, D.K.L.; Isaias, R.M.S. Structural and Nutritional Peculiarities Related to Lifespan Differences on Four Lopesia Induced Bivalve-Shaped Galls on the Single Super-Host Mimosa gemmulata. Front. Plant Sci. 2021, 12, 660557. [Google Scholar] [CrossRef]
- Sá, C.E.M.D.; Silveira, F.A.O.; Santos, J.C.; Isaias, R.M.D.S.; Fernandes, G.W. Anatomical and Developmental Aspects of Leaf Galls Induced by Schizomyia macrocapillata Maia (Diptera: Cecidomyiidae) on Bauhinia brevipes Vogel (Fabaceae). Braz. J. Bot. 2009, 32, 319–327. [Google Scholar] [CrossRef]
- Zhao, Y. Auxin Biosynthesis and Its Role in Plant Development. Annu. Rev. Plant Biol. 2010, 61, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Sorce, C.; Giovannelli, A.; Sebastiani, L.; Anfodillo, T. Hormonal Signals Involved in the Regulation of Cambial Activity, Xylogenesis and Vessel Patterning in Trees. Plant Cell Rep. 2013, 32, 885–898. [Google Scholar] [CrossRef] [PubMed]
- Gatjens-Boniche, O. The Mechanism of Plant Gall Induction by Insects: Revealing Clues, Facts, and Consequences in a Cross-Kingdom Complex Interaction. Rev. Biol. Trop. 2019, 67, 1359–1382. [Google Scholar] [CrossRef]
- Bailey, S.; Percy, D.M.; Hefer, C.A.; Cronk, Q.C.B. The Transcriptional Landscape of Insect Galls: Psyllid (Hemiptera) Gall Formation in Hawaiian Metrosideros polymorpha (Myrtaceae). BMC Genom. 2015, 16, 943. [Google Scholar] [CrossRef] [PubMed]
- Bhalerao, R.P.; Bennett, M.J. The Case for Morphogens in Plants. Nat. Cell Biol. 2003, 5, 939–943. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Sood, P.; Citovsky, V. The Roles of Plant Phenolics in Defence and Communication during Agrobacterium and Rhizobium Infection. Mol. Plant Pathol. 2010, 11, 705–719. [Google Scholar] [CrossRef]
- Hori, K. Insect secretions and their effect on plant growth, with special references to Hemipteran. In Biology of Insect-Induced Galls; Shorthouse, J.D., Rohfritsch, O., Eds.; Oxford University Press: New York, NY, USA, 1992; pp. 157–170. [Google Scholar]
- Łozowicka, B.; Kaczyński, P.; Jankowska, M.; Rutkowska, E.; Hrynko, I. Pesticide Residues in Raspberries (Rubus idaeus L.) and Dietary Risk Assessment. Food Addit. Contam. Part B 2012, 5, 165–171. [Google Scholar] [CrossRef]
- Thompson, D.A.; Lehmler, H.-J.; Kolpin, D.W.; Hladik, M.L.; Vargo, J.D.; Schilling, K.E.; LeFevre, G.H.; Peeples, T.L.; Poch, M.C.; LaDuca, L.E.; et al. A Critical Review on the Potential Impacts of Neonicotinoid Insecticide Use: Current Knowledge of Environmental Fate, Toxicity, and Implications for Human Health. Environ. Sci. Process. Impacts 2020, 22, 1315–1346. [Google Scholar] [CrossRef] [PubMed]
- Alsafran, M.; Rizwan, M.; Usman, K.; Saleem, M.H.; Jabri, H.A. Neonicotinoid Insecticides in the Environment: A Critical Review of Their Distribution, Transport, Fate, and Toxic Effects. J. Environ. Chem. Eng. 2022, 10, 108485. [Google Scholar] [CrossRef]
- Gostin, I.N.; Popescu, I.E. Evaluation of the Essential Oils Used in the Production of Biopesticides: Assessing Their Toxicity toward Both Arthropod Target Species and Beneficial Pollinators. Agriculture 2023, 14, 81. [Google Scholar] [CrossRef]
- Popescu, I.E.; Gostin, I.N.; Blidar, C.F. An Overview of the Mechanisms of Action and Administration Technologies of the Essential Oils Used as Green Insecticides. AgriEngineering 2024, 6, 1195–1217. [Google Scholar] [CrossRef]

| Reagent/Tested Substances | Anatomical Sites for Positive Reaction | |||
|---|---|---|---|---|
| Non-Galled Stems | Galled Stems | Non-Galled Petiole | Galled Petiole | |
| Lugol/Starch | xylem parenchyma cells, pith cells, cortex | xylem parenchyma cells | xylem parenchyma cells | xylem parenchyma cells |
| Sudan III/lipids | cuticle, cork cells | cuticle, external suberized cells | cuticle | cuticle |
| Fehling’s reagent/reducing sugars | inner cortex cells layers, external pith cells, vascular tissues (parenchyma cells) | neo-formed tissue, external part of the gall, vascular parenchyma, pith | vascular tissues (parenchyma cells), parenchyma (isolate cells) | vascular tissues (parenchyma cells), parenchyma, neo-formed issues |
| Coomassie brilliant blue/Proteins | vascular tissues (parenchyma cells) | meristematic nodules, around larval chamber, vascular tissues | vascular tissues (parenchyma cells) | meristematic nodules, around larval chamber, vascular tissues |
| NADI reagent/terpenoids | negative | neo-formed tissue, vascular parenchyma, around larval chamber | negative | neo-formed tissue, vascular parenchyma, around larval chamber |
| Ferric chloride/phenolic compounds | negative | neo-formed tissue, around larval chamber | negative | neo-formed tissue, around larval chamber |
| Phloroglucinol/lignins | xylem, sclerenchyma | xylem, sclerenchyma, neo-formed xylem elements | xylem, sclerenchyma | xylem, sclerenchyma, neo-formed xylem elements |
| Ehrlich reagent/IAA | negative | neo-formed tissue, around larval chamber | negative | neo-formed tissue, around larval chamber |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popescu, I.E.; Gostin, I.N. Lasioptera rubi, a Pest of Rubus idaeus: Galls Morphology, Anatomy and Histochemistry. Agriculture 2024, 14, 1761. https://doi.org/10.3390/agriculture14101761
Popescu IE, Gostin IN. Lasioptera rubi, a Pest of Rubus idaeus: Galls Morphology, Anatomy and Histochemistry. Agriculture. 2024; 14(10):1761. https://doi.org/10.3390/agriculture14101761
Chicago/Turabian StylePopescu, Irinel Eugen, and Irina Neta Gostin. 2024. "Lasioptera rubi, a Pest of Rubus idaeus: Galls Morphology, Anatomy and Histochemistry" Agriculture 14, no. 10: 1761. https://doi.org/10.3390/agriculture14101761







