Abstract
Canola (Brassica napus L.) is an important oilseed crop that provides essential vegetable oil but faces significant competition from weeds that are influenced by various agronomic practices and environmental conditions. This study examines the complex interactions between canola stand density and weed intensity over three growing seasons, identifying a total of 27 weed species. It is important to establish a connection between the density of winter canola stands, the intensity of weeding and the response of individual weed species in real conditions. The case study was executed on plots located in the Přerov district (Olomouc region, Czech Republic). The assessment was carried out during two periods—autumn in October and spring in April. Canola plants (plant density) were counted in each evaluated area, weed species were identified, and the number of plants for each weed species was determined. Half of the plots were covered with foil before herbicide application to prevent these areas from being treated with herbicides. We used redundancy analysis (RDA) to evaluate the relationships between canola density and weed dynamics, both with and without herbicide treatment. The results show the ability of canola to compete with weeds; however, that is factored by the density of the canola stand. In dense stands (over 60 plants/m²), canola is able to suppress Galium aparine L., Geranium pusillum L., Lamium purpureum L., Papaver rhoeas L. and Chamomilla suaveolens (Pursh) Rydb. Nevertheless, there are weed species that grow well even in dense canola stands (Echinochloa crus-galli (L.) P. Beauv., Phragmites australis (Cav.) Steud., Tripleurospermum inodorum (L.) Sch. Bip. and Triticum aestivum L.). These findings highlight the potential for using canola stand density as a strategic component of integrated weed management to reduce herbicide reliance and address the growing challenge of herbicide-resistant weed populations. This research contributes significantly to our understanding of the dynamics of weed competition in canola systems and informs sustainable agricultural practices for improved crop yield and environmental stewardship.
1. Introduction
Canola (Brassica napus L.), a major source of vegetable oil, is the third largest oilseed crop in the world and is used to produce edible oil, feed protein and biodiesel [,]. Canola stands are generally less competitive than cereal stands, but their competitiveness is influenced by the interaction of optimal planting date with several agronomic factors, including seed size, quality, variety, seeding rate, row spacing, fertilisation and pest management, as well as environmental conditions such as weather and soil quality [,,]. These interactions affect not only canola stands but also weed vegetation.
The growth and development of canola is strongly influenced by the date of sowing. Above all, later sowing dates usually shorten the growth period, change the stand structure and reduce canola yields [,,,,,]. Most studies show that increasing the canola cover rate suppresses weed emergence and growth substantially; however, it negatively affects canola seed production and has diverse effects on canola grain quality [,,]. Based on the monitoring carried out by the Central Institute for Supervising and Testing in Agriculture in the Czech Republic, the most common weeds found in canola were Capsella bursa-pastoris, Elymus repens, Galium aparine, Papaver rhoea, Thlaspi arvense, Tripleurospermum inodorum and Viola arvensi. These weed species are considered to be the main causes of yield loss in rape [].
Greater competitiveness of canola cultivars against weeds can be enhanced by increasing seeding rates, optimising row spacing, promoting canola stand cover and height and utilising allelopathy [,,,,].
Nevertheless, canola yields start to decline from a certain seeding density due to intense competition between crop plants for limited light, nutrient and water resources [,,]. Lemerle et al. [] devised a canola ideotype that should feature strong competitiveness and fast and early growth; further, it should quickly reach final plant height and early flowering stage, have sufficiently large leaves to effectively shade weeds at all stages of canola growth. It should also evince a high degree of allelopathy to form a root system quickly to allow an intake of nutrients (N, P, K, S) and water. However, according to Clements et al. [], crops are not bred for weed competitiveness but for crop yield, which is the primary priority of breeders. Crop yield is often achieved under weed-free conditions. Weeds inhibit canola, leave rosette development, increase terminal bud height excessively [], reduce seed yields [] and reduce oilseed quality [].
Interactions between canola cultivars and other agronomic techniques, such as seeding rate and herbicide application timing, also affect the ability of weeds to compete with canola []. The species composition of weeds is modified to varying degrees by tillage systems; however, the results of field trials are not unambiguous and largely depend on the type of crop []. Previous research has shown that weed growth rate and species composition are markedly influenced by the depth of tillage and tillage method used [,]. According to Małecko-Jankowiak et al. [], weed pressure is lowest in conventional tillage systems and highest in reduced tillage systems. The increase in weeding of various crops in the conditions of no-tillage systems was also found in studies by other authors [,,,,]. In contrast, Santín-Montanyá et al. [] showed that weed numbers in winter wheat stands were not affected by conventional or no-tillage systems.
In a study by Romaneckas et al. [], reduced tillage intensity resulted in a slight increase in weed density and biomass. The tillage system adjusts the number and distribution of weed seeds in the soil profile. It, therefore, affects both the species composition of weeds and the intensity of weeding in crop stands [,,]. Higher weeding in a no-tillage system may result in the accumulation of freshly sown weed seeds and vegetative reproductive organs in the topsoil, from where they germinate and appear abundantly in the crop stand [,,,,]. Weeds were most effectively controlled with foliar herbicides in a strip-till system, preemergence or sequential herbicides in a low-tillage system and preemergence herbicides in a conventional tillage system []. According to Franek [], Hamzei et al. [], and Pacanoski [], the timing of herbicide application can cause substantial differences in canola yield. Pesticide use is one of the most debated aspects of agricultural intensification due to its potential direct and indirect consequences at the level of individuals, populations and ecosystems [,].
Integrated weed control strategies also use crop rotation. Crop rotation is widely recognised for its benefits in protecting crop production against pathogens, diseases, pests and weeds [,]. Varied crop rotations in more biodiverse agricultural systems provide important environmental benefits []. A number of studies have shown that crop rotation systems and the selection of suitable crops can effectively regulate weed intensity [,]. Ramsdale et al. [] state that, according to their results, it is possible to reduce weed density and limit the application of herbicides by using a suitable combination of crop rotation and tillage. However, the use of herbicides to control dominant weeds is necessary if heavy weeding and high-yield losses are to be avoided.
Weeds employ successful life strategies that involve the synchronisation of weed and crop life cycles, including the timing of crop management []. However, climate change may disrupt timing patterns for weed and crop phenology, possibly selecting for an altered weed life strategy and also altering weed selection outcomes []. For example, the population of Sinapis arvensis, according to Franks et al. [], was able to accelerate and extend their flowering time over several generations, which affected the amount of new weed seeds produced. Cultivation technology, soil and meteorological conditions affect the evenness of the canola stand and co-create heterogeneity in the density of the canola stand in operating conditions. Weed species composition and density are influenced by the health and structure of the canola crop. Optimal seeding rates and row spacing enhance canola’s ability to outcompete weeds for light, nutrients and water. Environmental factors such as soil fertility and moisture also influence weed establishment [,,,]. For example, early planting can allow canola to create a canopy that limits light to weeds and reduces their growth. Understanding these dynamics is critical to developing integrated weed management strategies that focus not only on herbicide use but also on the ecological interactions between canola and weed species []. These factors create a denser and more vigorous canola stand that competes effectively for light, nutrients and water []. This competition reduces the resources available to weeds, thereby reducing their growth and establishment. In addition, integrated weed management practices that focus on these agronomic adaptations not only enhance canola competitiveness but also contribute to more sustainable cropping systems [,].
The practical implementation of cultivation technology brings certain inaccuracies in real conditions caused by the imperfection of machinery, human error, etc. The result of practical implementation is not a homogeneous stand but a stand with a certain degree of heterogeneity. Under practical conditions, the heterogeneity of canola stands induces a response in the weed vegetation. The weed response to stand heterogeneity is not fully understood. The relationship between canola stand density and weed vegetation is still very important and has a tendency to change. The aim of this work is (i) to establish the connection between the density of the winter canola stand and the intensity of weeding, (ii) to determine the effect of the density of the winter canola stand on the species composition of weeds, (iii) to determine the importance of the application of herbicides on the relationship between the canola stand and weeds and (iv) to define competitive weed species and species that winter canola can suppress.
2. Materials and Methods
2.1. Study Area
The plots used for the field experiment are located in the Přerov district (Olomouc region, Czech Republic, GPS 49.5703928 N, 17.0211019 E). The altitude is between 201 and 205 m. The soil on the plots is deep to medium deep, and loamy–sandy to clay–loamy soil types predominate Phaeozem (PH) and Fluvisol (FL) []. The landscape is mostly flat with omnidirectional exposure; the land is not significantly oriented to any side of the world. The area has an average annual temperature of 8.1 °C, the average daily minimum air temperature is 4.1 °C, and the average daily maximum air temperature is 13.1 °C. The hottest month of the year is July; the coldest is January. The average annual rainfall is 530 mm. Rainfall is unevenly distributed during the growing season [,,]. Canola stands were evaluated in the 2017/2018, 2018/2019 and 2019/2020 seasons. In each year, 2 plots were selected, on which an evaluation of canola density and an evaluation of weeding were carried out.
2.2. Canola Cultivation Technology
Winter wheat and spring barley have always been pre-crops for winter canola. In July, the harvest of the pre-crop was followed by stubble-tillage using a compact disc harrow Horsch 3CT disc cultivator. Loosening to a depth of 0.30 m followed 1 to 2 weeks after the stubble-tillage with a Horsch Terrano 3FX compact cultivator. The canola sowing dates were 20 August 2017, 25 August 2018 and 30 August 2019. The sowing bed was prepared just before sowing with a combined cultivator OPaLL-AGRI Saturn IV 5. Sowing with a row spacing of 0.15 m was carried out with a Horsch Pronto 3DC machine. The “ARABELLAcpg” variety was grown on the plots with a sowing rate of 3.9 kg.ha−1.
The area in the 2017/2018 season was 7.22 ha; in the 2018/2019 season, it was 8.54 ha; in the 2019/2020 season, it was 7.89 ha.
Fertilisation was carried out before sowing with NPK mineral fertiliser (nutrient ratio 6:26:30) at a dose of 200 kg.ha−1 and DAM 390 fertiliser at a dose of 100 L.ha−1. During the winter months, the fertiliser Urea Stabil was applied at a dose of 100 kg.ha−1. Spring regeneration fertilisation was carried out in two doses, namely at a dose of 200 kg.ha−1 with the fertiliser LAD 27 and at a dose of 200 kg.ha−1 with the nitrogen fertiliser with sulphur admixture DASA 26 + 13 S. Further fertilisation was carried out with the fertiliser DAM 390 at a dose of 155 L.ha−1 and the last with the fertiliser SAM 240 at a dose of 135 L.ha−1.
Immediately after sowing, the plot was treated with the soil herbicide Butisan® Complete (metazachlor, dimethenamid-P, chinmerak) at a dose of 2.50 L.ha−1. This was followed by the application of a fungicide Caryx (mepiquat chlorid, metkonazol) at 0.60 L.ha−1 in a tank mix with a graminicide Garland Forte (propachizafop) at 0.60 L.ha−1 and an insecticide Nexide (gamma-cyhalothrin) at 0.08 L.ha−1. The next application was a second application of growth regulator (Caryx at 0.60 L.ha−1) together with foliar nutrition Borosan Forte (boron) at 2.00 L.ha−1. The first spring treatment consisted of an insecticide Proteus 110 OD (thiacloprid, deltamethrin) at 0.60 L.ha−1 and a fungicide Efilor (metkonazol, boskalid) at 0.80 L.ha−1 together with foliar nutrition SULFUR 165 (sulfur, nitrogen) at 2.00 L.ha−1 and Borosan Forte at 2.00 L.ha−1. This was followed by the final fungicide PICTOR ACTIVE (boscalid, pyraclostrobin) at 0.45 L.ha−1 and insecticide Biscaya 240 OD (thiakloprid) at 0.30 L.ha−1 for protection during flowering.
2.3. Evaluation of Weed Infestation
In each plot and in each season, 20 evaluation plots were selected. Ten out of the twenty plots were covered with foil before herbicide application to prevent them from being treated with herbicide. All plots were treated with herbicides in autumn.
The areas for evaluation were delimited evenly on the selected plots to capture the heterogeneity of the vegetation cover. Each evaluated area had a size of 1 m2. Canola plants (plant density) were counted within the area, weed species were identified, and the number of plants for each weed species was determined. Taxonomic nomenclature of plants follows Kaplan et al. []. The assessment was carried out in two periods—in autumn in October and in spring in April.
The obtained data on the density of canola stands and the intensity of weeding were processed into graphs for each evaluated area.
2.4. Statistical Data Processing
Baseline data from individual observations were used for statistical processing. We divided the obtained data into 4 sets according to the date of evaluation and herbicide application. Data from different seasons (first evaluation, second evaluation) and weed control methods (treated with herbicides, untreated) were evaluated separately. In the analyses, the number of canola plants was used as a factor, and the number of individuals of the weed species found was used as a variable.
Multivariate analysis of ecological data was used to analyse the results of the evaluation of the number of canola plants and the representation of the weed species found. The first analysis was detrended correspondence analysis (DCA), which influences the selection of the most appropriate analysis. The result of the DCA is the length of the gradient. For the data from the first assessment without treatment (in autumn), response data are compositional and have a gradient of 2.7 SD units long, so a linear method is recommended. For the data from the second evaluation without treatment (in spring), response data are compositional and have a gradient of 3.1 SD units long, so a linear method is recommended. For the data from the first evaluation with herbicide treatment (in autumn), response data are compositional and have a gradient of 3.1 SD units long, so a linear method is recommended. For data from the second assessment (in spring) with herbicide treatment, response data are compositional and have a gradient of 3.4 SD units long, so a linear method is recommended. Based on the output from the Canoco 5.0 software and the methodological procedure, redundancy analysis (RDA) was chosen. Computed axes 4, Detrending None, Hybrid analysis not performed, Response data (species) transformation log (Log transformation formula: Y’ = 1 × Y + 1), Center and standardise by species yes.
Statistical significance was assessed using the Monte Carlo test with 999 permutations. All multivariate analyses and necessary calculations were performed using Canoco 5.0 software [].
3. Results
A total of 27 weed species were identified during the three-year-long evaluation (Alsinula media, Brassica rapa, Cirsium arvense, Echinochloa crus-galli, Euphorbia helioscopia, Fagopyrum convolvulus, Fumaria officinalis, Galium aparine, Geranium pusillum, Hordeum vulgare, Chamomilla suaveolens, Chenopodium album, Lamium purpureum, Papaver rhoeas, Phacelia tanacetifolia, Phragmites australis, Poa annua, Polygonum aviculare, Polygonum lapathifolia, Raphanus raphanistrum, Sinapis alba, Stellaria media, Thlaspi arvense, Tripleurospermum inodorum, Triticum aestivum, Veronica persica, Veronica polita and Viola arvensis).
On the monitored plots, the average yield of rapeseed was 3.52 t.ha−1 in the 2017/2018 season, 3.31 t.ha−1 in the 2018/2019 season, and 3.12 t.ha−1 in the 2019/2020 season.
The relationship between canola density and weeding intensity in the first evaluation without herbicide treatment is depicted in Figure 1. Furthermore, the results of the RDA analysis, which assessed the relationship between canola density and weed species found in conditions without herbicide treatment, are also demonstrated in Figure 1.
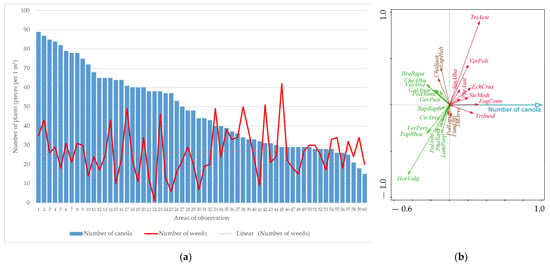
Figure 1.
The relationship between canola density, weed intensity (a) and occurrence of weed species (b) found in the first evaluation term without herbicide treatment (RDA result; figure: total explained variability = 6.3%, F-ratio = 3.9, p-value = 0.001). Explanatory notes of abbreviations weeds: Cirsium arvense (CirArve), Echinochloa crus-galli (EchCrus), Euphorbia helioscopia (EupHeli), Fagopyrum convolvulus (FagConv), Fumaria officinalis (FumOffi), Galium aparine (GalApar), Geranium pusillum (GerPusi), Chamomilla suaveolens (ChaSuav), Chenopodium album (CheAlbu), Lamium purpureum (LamPurp), Papaver rhoeas (PapRhoe), Phragmites australis (PhrAust), Poa annua (PoaAnnu), Polygonum aviculare (PolAvic), Polygonum lapathifolia (PolLapa), Raphanus raphanistrum (RapRaph), Sinapis alba (SinAlba), Stellaria media (SteMedi), Thlaspi arvense (ThlArve), Tripleurospermum inodorum (TriInod), Veronica persica (VerPers), Veronica polita (VerPoli), Viola arvensis (VioArve). Explanation of unwanted crop abbreviations: Brassica rapa (BraRapa), Hordeum vulgare (HorVulg), Phacelia tanacetifolia (PhaTana), Triticum aestivum (TriAest).
The relationship between canola density and weed intensity in the second evaluation without herbicide treatment (in spring) is shown in Figure 2. Furthermore, the results of the RDA analysis, which evaluated the relationship between rapeseed density and weed species found in conditions without herbicides, are presented.
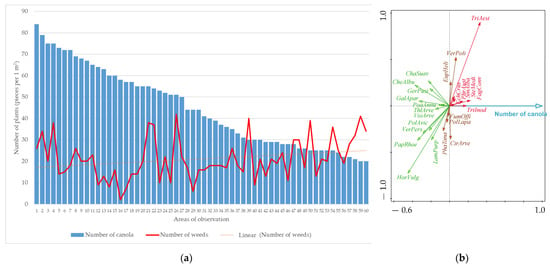
Figure 2.
The relationship between canola density, weed intensity (a) and occurrence of weed species (b) found in the second term of evaluation without herbicide treatment (RDA result; figure: total explained variability = 7.2%, F-ratio = 4.5, p-value = 0.001). Explanatory notes of abbreviations: Cirsium arvense (CirArve), Echinochloa crus-galli (EchCrus), Euphorbia helioscopia (EupHeli), Fagopyrum convolvulus (FagConv), Fumaria officinalis (FumOffi), Galium aparine (GalApar), Geranium pusillum (GerPusi), Chamomilla suaveolens (ChaSuav), Chenopodium album (CheAlbu), Lamium purpureum (LamPurp), Papaver rhoeas (PapRhoe), Phragmites australis (PhrAust), Poa annua (PoaAnnu), Polygonum aviculare (PolAvic), Polygonum lapathifolia (PolLapa), Sinapis alba (SinAlba), Stellaria media (SteMedi), Thlaspi arvense (ThlArve), Tripleurospermum inodorum (TriInod), Veronica persica (VerPers), Veronica polita (VerPoli), Viola arvensis (VioArve). Explanation of unwanted crop abbreviations: Hordeum vulgare (HorVulg), Phacelia tanacetifolia (PhaTana), Triticum aestivum (TriAest).
The relationship between the canola density and the weed intensity in the first evaluation with herbicide application (in autumn) is shown in Figure 3. Furthermore, the results of the RDA analysis, which assessed the relationship between canola density and weed species found under herbicide conditions, are demonstrated.
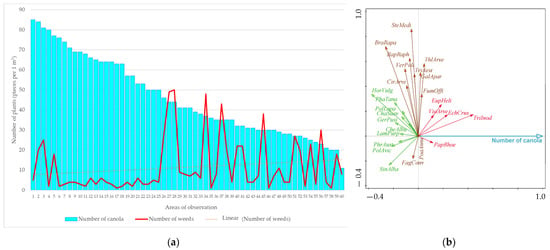
Figure 3.
The relationship between canola density, weed intensity (a) and occurrence of weed species (b) found in the first term of evaluation with the application of herbicides (RDA result; figure: total explained variability = 2.9%, F-ratio = 1.7, p-value = 0.0114). Explanatory notes of abbreviations: Alsinula media (AlsMedi), Cirsium arvense (CirArve), Echinochloa crus-galli (EchCrus), Euphorbia helioscopia (EupHeli), Fagopyrum convolvulus (FagConv), Fumaria officinalis (FumOffi), Galium aparine (GalApar), Geranium pusillum (GerPusi), Chamomilla suaveolens (ChaSuav), Chenopodium album (CheAlbu), Lamium purpureum (LamPurp), Papaver rhoeas (PapRhoe), Phragmites australis (PhrAust), Poa annua (PoaAnnu), Polygonum aviculare (PolAvic), Polygonum lapathifolia (PolLapa), Raphanus raphanistrum (RapRaph), Sinapis alba (SinAlba), Stellaria media (SteMedi), Thlaspi arvense (ThlArve), Tripleurospermum inodorum (TriInod), Veronica polita (VerPoli), Viola arvensis (VioArve). Explanation of unwanted crop abbreviations: Brassica rapa (BraRapa), Hordeum vulgare (HorVulg), Phacelia tanacetifolia (PhaTana), Triticum aestivum (TriAest).
The relationship between canola density and weed intensity in the second evaluation with herbicide application (in spring) is shown in Figure 4. Furthermore, the results of the RDA analysis are presented here, which evaluated the relationship between canola density and weed species found under the conditions of herbicide application.
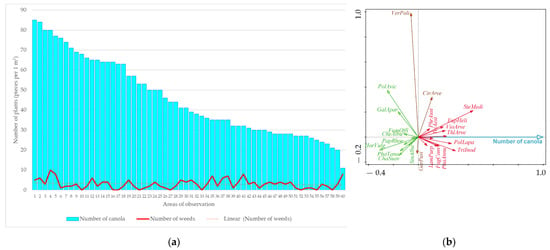
Figure 4.
The relationship between canola density, weed intensity (a) and occurrence of weed species (b) found in the second term of evaluation with the application of herbicides (RDA result; figure: total explained variability = 4.0%, F-ratio = 2.3, p-value = 0.008). Explanatory notes of abbreviations: Cirsium arvense (CirArve), Euphorbia helioscopia (EupHeli), Fagopyrum convolvulus (FagConv), Fumaria officinalis (FumOffi), Galium aparine (GalApar), Geranium pusillum (GerPusi), Chamomilla suaveolens (ChaSuav), Chenopodium album (CheAlbu), Lamium purpureum (LamPurp), Papaver rhoeas (PapRhoe), Phragmites australis (PhrAust), Poa annua (PoaAnnu), Polygonum aviculare (PolAvic), Polygonum lapathifolia (PolLapa), Stellaria media (SteMedi), Thlaspi arvense (ThlArve), Tripleurospermum inodorum (TriInod), Veronica polita (VerPoli), Viola arvensis (VioArve). Explanation of unwanted crop abbreviations: Hordeum vulgare (HorVulg), Phacelia tanacetifolia (PhaTana), Triticum aestivum (TriAest).
The RDA results showed that the response of the identified weed species to herbicide treatment is different. In Table 1, the weed species are divided into groups according to their response to canola density in different environmental conditions. The first group consists of species capable of growing only in sparse canola stands. The second group consists of weed species on which the density of the stand did not have a clear effect, and their occurrence was influenced more by other factors. The third group includes weed species capable of growing even in dense canola stands.

Table 1.
Response of weed species to stand density on two evaluation dates with different weed control conditions.
4. Discussion
A number of studies focusing on canola cultivar competitiveness and weed suppression have focused on higher seeding rates, optimal row spacing, crop biomass formation, crop height and allelopathy [,,,]. Our results evaluate the relationships between weed vegetation and stand density under real operating conditions. The results (Figure 1a, Figure 2a and Figure 3a) show a statistically significant trend of an increase in the number of weeds with a decreasing number of canola plants (Monte Carlo test). This matches the results of Daugovish et al. [], who evaluated the relationships of the competitive ability of canola against Avena fatua. Weed suppression increased with increasing canola stand density. Similar findings were reported by Harker et al. [] from a trial in Canada and Lemerle et al. [] from Australia, who state that the recommended rate is 40 canola plants per m2. This density of the canola plant suppresses weeds to the maximum and minimises the loss of canola yield. However, Blackshaw et al. [] recommend a stand density of up to 100 canola plants per m2 (1.5 times higher than the standard seeding rate). High seeding reduced weed dry biomass by 60%, and there was also a drop in the soil weed seed bank by up to 38%. The yield of canola crops was more influenced by the course of the weather in a given year.
This trend is not evident in plots from the spring evaluation that have been treated with herbicides (Figure 4a). The result is statistically highly significant according to the Monte Carlo test. The weeding rate is similar regardless of the number of canola plants and is relatively low. The lower intensity of weeding is a consequence of the effectiveness of herbicides. The application of herbicides changes the relationship between crop stand density and weeding. This effect was also reported by Andreasen et al. [] and Mehrtens et al. [], according to which herbicide effects in current crop stands overlap all other factors.
Strongly competitive canola stands are considered a low-cost and relatively simple tactic in integrated weed management. This can be used to reduce reliance on herbicides, decelerate the spread of herbicide-resistant weeds, and lower farm production costs through reduced herbicide use []. Integrated management, which uses a combination of weed control methods, is considered the most effective approach to weed control [] and contributes to reducing weed resistance to herbicides. Weed resistance is the biggest threat to sustainable agricultural production []. A new factor that affects weed vegetation in canola stands is the Clearfield® (CL) system, which allows successful control of weeds from the Brassicaceae family. It can be assumed that the CL system will work more efficiently than conventional weed control methods []. CL technology can raise certain questions from the point of view of self-sufficiency in plant production and dependence on the supply of seeds and herbicides, and it can limit the choice of alternative solutions.
The weed species found in canola are common weed species. According to Hanzlik and Gerowitt [], these species include Chenopodium spp., Galium aparine, Lamium spp., Matricaria spp., Stellaria media, Thlaspi arvense, Veronica spp., and Viola arvensis, which were found during extensive surveys from similar locations. Schröder et al. [] expect an increase in the proportion of weeds from the Brassicaceae family in canola stands. This trend is not apparent from our results; only the Brassica rapa, Raphanus raphanistrum, Sinapis alba and Thlaspi arvense species were found from the Brassicaceae family.
After sowing, the unwanted pre-crop threshed seeds, especially cereals (barley and wheat), begin to grow in the canola stand. Subsequently, species from the group of winter weeds appear. In this period, weeds from the group of spring and summer species can also be encountered. These weeds produce only small plants in the stand, which bloom and, in some cases, produce a small amount of new seeds. The growth of these species is limited by shortening days. First, frosts destroy the spring weed species. According to Chao et al. [], longer frost conditions can lead to weed suppression in canola. In spring, weeds that germinated during the winter or weeds that survived weed control measures continue to grow. However, species from the group of winter weeds also germinate in spring, especially in places with sparse canola growth or in places where the canola is overwintered poorly. In this period, entomophilous weeds are problematic for canola cultivation. The flowering of entomophilous weeds prevents the application of insecticides, which are dangerous for bees.
Canola cultivation technology is decisive for the heterogeneity of density and for the competitiveness of canola against weeds in operational conditions. Weed species react differently to canola density. In sparse canola stands (with less than 30 canola plants per 1 m2), the following species Galium aparine, Geranium pusillum, Hordeum vulgare, Chamomilla suaveolens, Chenopodium album, Lamium purpureum, Papaver rhoeas, Phacelia tanacetifolia, and Polygonum aviculare grow easily (statistically highly significant, Figure 1b, Figure 2b and Figure 4b; statistically significant, Figure 3b). Based on our results, it can be stated that sparse canola stands with a low density of canola plants will be weeded mainly by species from the group of winter weeds (Galium aparine, Geranium pusillum, Lamium purpureum, Papaver rhoeas, Chamomilla suaveolens), also by species from the group of spring weeds (Polygonum aviculare), and by species from the group of summer weeds (Chenopodium album). Unwanted threshed seeds of pre-crops (Hordeum vulgare) and green manure crops (Phacelia tanacetifolia), which were cultivated in previous years, will also sprout there.
Unwanted threshed seeds of barley (Hordeum vulgare), which germinate parallelly with canola, can, therefore, compete directly at the germination stage with canola and limit its emergence. Because of this, places with a lower number of canola plants and a higher representation of barley can be formed. Based on our evaluation, which was carried out in October, the barley root was gaining ground mainly in sparse stands. It can be assumed that the unwanted threshed seeds of barley create these places.
Certain types of weeds can be considered competitively very strong, as they are able to grow even in dense canola stands (with a density of over 30 canola plants per 1 m2). Stellaria media, Echinochloa crus-galli, Fagopyrum convolvulus, Phragmites australis, Tripleurospermum inodorum and Triticum aestivum can be included in this group (statistically highly significant, Figure 1b, Figure 2b and Figure 4b; statistically significant, Figure 3b).
Our results bring attention to the types of weeds that are not suppressed, even in dense canola stands. These species include short-height weeds (Stellaria media), which limit canola plants, especially at the beginning of their growing season. The group also includes weeds with a winding stem (Fagopyrum convolvulus) and species of the same height or taller than canola (Echinochloa crus-galli, Phragmites australis, Tripleurospermum inodorum, Triticum aestivum). The species Echinochloa crus-galli does not have a similar growing season to canola and is not capable of surviving the winter season in winter crop stands. The species Phragmites australis is very competitive and difficult to regulate, and its occurrence is tied to places with high groundwater levels []. The occurrence of the species Triticum aestivum is determined by the harvest losses of the pre-crop and the success of the regulation before canola sowing. If the regulation is not sufficient, Triticum aestivum has great harmful potential. Tripleurospermum inodorum can be described as a very dangerous weed; its growing season overlaps with canola, and the height of both plants is similar, which creates a large harmful potential.
Canola ripening is associated with a decrease in its competitive ability. Weed development at this stage depends on sufficient rainfall. In case of sufficient water supplies, late spring species (Chenopodium album, Echinochloa crus-galli) start to germinate. Their harmfulness is manifested by increasing the humidity of the canola seeds. The fleshy and fragile parts of the weeds remain part of the seeds when passing through the harvester and increase the demands for post-harvest treatment. During this period, regeneration of overwintering weed species also occurs. Primarily, the stems of the bedstraw plant (Galium verum), which reach a length of several meters, create a tangle of canola stems and siliquas. When the thresher passes through, the stalks of bedstraw may be pulled from neighbouring parts of the stand, the siliquas crack, and harvest losses increase. Canola seeds lost in this way turn into undesired weeds that affect subsequent crops.
Successful field weed life strategies include, but are not limited to, synchronisation between the weed life cycle and crop life cycle and crop management. Weed seed production needs to be formed before or after crop harvest so that it does not become destroyed during the crop harvest []. The specific conditions of arable land and the effect of cultivation technologies lead to the creation of a new anthropogenic life strategy [].
Crops and weeds are affected by climate change, which may be the reason for the failure of effective weed control. Therefore, it is essential to employ different agronomic tactics, including crop competitiveness, which keeps weed populations and crop production in balance []. The use of competitive canola varieties, higher canola seeding rate and optimisation of row spacing are some of the simple interventions currently available to farmers to control weeds. New canola varieties need to be assessed from the point of view of competitiveness against weeds, mainly due to the increase in resistance of weeds to herbicides [].
5. Conclusions
Weed vegetation is a very dynamically changing plant community. Weed control is carried out with the application of herbicides; however, due to the increase in resistant populations and the environmental impact of herbicides, it is necessary to search for new ways of weed control. A modification of the density of winter canola rate results in alterations to the conditions within the stand, which in turn elicits a response in the species composition and abundance of weed populations.
Crops, including canola, are able to compete with weeds. The main role is played by crop density and the different responses of individual weed species. Canola is able to suppress Galium aparine, Geranium pusillum, Lamium purpureum, Papaver rhoeas and Chamomilla suaveolens in dense stands. Nevertheless, there are weed species that will grow well even in dense canola stands (Echinochloa crus-galli, Phragmites australis, Tripleurospermum inodorum and Triticum aestivum).
High weed control efficiency creates a vacant living space for weeds, allowing new weed species to grow. These processes can be characterised as microevolution on agricultural land. The result of the microevolution is a change in the spectrum of weeds in canola stands, which places new demands on the correct identification of weed species and changes in their regulation.
Author Contributions
Conceptualisation, L.V. and J.W.; methodology, L.V., T.J.K. and J.W.; validation, L.V. and J.W.; formal analysis, J.W.; investigation, L.V. and P.M.B.; resources, L.V. and J.W.; data curation, L.V. and J.W.; writing—original draft preparation, L.V. and J.W.; writing—review and editing, P.M.B. and I.D.; visualisation, L.V. and J.W.; supervision, I.D.; project administration, J.W.; funding acquisition, J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable for studies not involving humans or animals.
Data Availability Statement
The data are not available to the public in order to preserve the originality of the data, which must be preserved for the successful completion of Lucie Vykydalová’s doctoral studies.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, H.; Yin, Y. Analysis and strategy for oil crop industry in China. Chin. J. Oil Crop Sci. 2014, 36, 414–421. [Google Scholar] [CrossRef]
- Ji, C.; Zhai, Y.; Zhang, T.; Shen, X.; Bai, Y.; Hong, J. Carbon, energy and water footprints analysis of rapeseed oil production: A case study in China. J. Environ. Manag. 2021, 287, 112359. [Google Scholar] [CrossRef]
- Lemerle, D.; Luckett, D.J.; Wu, H.; Widderick, M.J. Agronomic interventions for weed management in canola (Brassica napus L.)—A review. Crop Prot. 2017, 95, 69–73. [Google Scholar] [CrossRef]
- Nelson, M.N.; Nesi, N.; Barrero, J.M.; Fletcher, A.L.; Greaves, I.K.; Hughes, T.; Laperche, A.; Snowdon, R.; Rebetzke, G.J.; Kirkegaard, J.A. Strategies to improve field establishment of canola: A review. Adv. Agron. 2022, 175, 133–177. [Google Scholar] [CrossRef]
- Galon, L.; Concenço, G.; Agazzi, L.R.; Nonemacher, F.; Melo, T.S.; da Silva, L.B.X.; Melo, S.T.; Perin, G.F.; Aspiazú, I. Competitive ability of canola (Brassica napus var. oleifera) hybrids with black oat (Avena strigosa) in a subtropical environment. Rev. Fac. Cienc. Agrar. UNCuyo 2021, 53, 119–131. [Google Scholar] [CrossRef]
- Ozer, H. Sowing date and nitrogen rate effects on growth, yield and yield components of two summer rapeseed cultivars. Eur. J. Agron. 2003, 19, 453–463. [Google Scholar] [CrossRef]
- Weymann, W.; Böttcher, U.; Sieling, K.; Kage, H. Effects of weather conditions during different growth phases on yield formation of winter oilseed rape. Field Crops Res. 2015, 173, 41–48. [Google Scholar] [CrossRef]
- Kirkegaard, J.; Lilley, J.; Brill, R.; Sprague, S.; Fettell, N.; Pengilley, G. Re-evaluating sowing time of spring canola (Brassica napus L.) in south-eastern Australia—How early is too early? Crop Pasture Sci. 2016, 67, 381–396. [Google Scholar] [CrossRef]
- Al-Doori, S.A.M. A study of the importance of sowing dates and plant density affecting some rapeseed cultivars (Brassica napus L.). Coll. Basic Educ. Res. J. 2011, 11, 615–632. [Google Scholar]
- Vincze, E. The effect of sowing date and plant density on yield elements of different winter oilseed rape (Brassica napus var. napus f. biennis L.) genotypes. Columella J. Agric. Environ. Sci. 2017, 4, 21–25. [Google Scholar] [CrossRef]
- Gusta, L.; Johnson, E.; Nesbitt, N.; Kirkland, K. Effect of seeding date on canola seed quality and seed vigour. Can. J. Plant Sci. 2004, 84, 463–471. [Google Scholar] [CrossRef]
- Kirkegaard, J.A.; Lilley, J.M.; Brill, R.D.; Ware, A.H.; Walela, C.K. The critical period for yield and quality determination in canola (Brassica napus L.). Field Crops Res. 2018, 222, 180–188. [Google Scholar] [CrossRef]
- Sonjeková, M. Survey of the Occurrence and Distribution of Weeds in the Czech Republic in 2012 [Průzkum Výskytu a Rozšíření Plevelů v ČR v Roce 2012], 1st ed.; State Plant Medicinal Administration: Brno, Czech Republic, 2013; p. 31. (In Czech) [Google Scholar]
- Zand, E.; Beckie, H.J. Competitive ability of hybrid and open-pollinated canola (Brassica napus) with wild oat (Avena fatua). Can. J. Plant Sci. 2002, 82, 473–480. [Google Scholar] [CrossRef]
- Harker, K.N.; Clayton, G.W.; Blackshaw, R.E.; O’Donovan, J.T.; Stevenson, F.C. Seeding rate, herbicide timing and competitive hybrids contribute to integrated weed management in canola (Brassica napus). Can. J. Plant Sci. 2003, 83, 433–440. [Google Scholar] [CrossRef]
- Beckie, H.J.; Johnson, E.N.; Blackshaw, R.E.; Gan, Y. Weed suppression by canola and mustard cultivars. Weed Technol. 2008, 22, 182–185. [Google Scholar] [CrossRef]
- Asaduzzaman, M.; Pratley, J.E.; An, M.; Luckett, D.J.; Lemerle, D. Canola interference for weed control. Springer Sci. Rev. 2014, 2, 63–74. [Google Scholar] [CrossRef]
- Vann, R.A.; Reberg-Horton, S.C.; Brinton, C.M. Row spacing and seeding rate effects on canola population, weed competition, and yield in winter organic canola production. Agron. J. 2016, 108, 2425–2432. [Google Scholar] [CrossRef]
- Griesh, M.; Yakout, G. Effect of plant population density and nitrogen fertilization on yield and yield components of some white and yellow maize hybrids under drip irrigation system in sandy soil. In Plant Nutrition; Springer: Berlin/Heidelberg, Germany, 2001; pp. 810–811. [Google Scholar] [CrossRef]
- Guan, C.; Chen, S. Investigation on planting density of double low rapeseed “Xiangyou 15”. Crop Res. 2003, 17, 136–137. [Google Scholar] [CrossRef]
- Sandhu, R.; Irmak, S. Assessment of AquaCrop model in simulating maize canopy cover, soil-water, evapotranspiration, yield, and water productivity for different planting dates and densities under irrigated and rainfed conditions. Agric. Water Manag. 2019, 224, 105753. [Google Scholar] [CrossRef]
- Lemerle, D.; Luckett, D.J.; Lockley, P.; Koetz, E.; Wu, H. Competitive ability of Australian canola (Brassica napus) genotypes for weed management. Crop Pasture Sci. 2014, 65, 1300–1310. [Google Scholar] [CrossRef]
- Clements, D.R.; Jones, V.L. Ten Ways That Weed Evolution Defies Human Management Efforts Amidst a Changing Climate. Agronomy 2021, 11, 284. [Google Scholar] [CrossRef]
- Tonev, T.; Mitkov, A. Chemical control of weeds in major field crops. Farming Plus 2015, 2, 33–44. [Google Scholar]
- Deligios, P.A.; Carboni, G.; Farci, R.; Solinas, S.; Ledda, L. Low-input herbicide management: Effects on rapeseed production and profitability. Sustainability 2018, 10, 2258. [Google Scholar] [CrossRef]
- Jankowski, K.J. Winter and spring oilseed rape. In Crop Production; Kotecki, A., Ed.; Wrocław University of Environmental and Life Sciences: Wrocław, Poland, 2020; Volume 3, pp. 305–383. (In Polish) [Google Scholar]
- Lemerle, D.; Luckett, D.J.; Koetz, E.A.; Potter, T.; Wu, H. Seeding rate and cultivar effects on canola (Brassica napus) competition with volunteer wheat (Triticum aestivum). Crop Pasture Sci. 2016, 67, 857–863. [Google Scholar] [CrossRef]
- Gawęda, D.; Haliniarz, M. The Yield and Weed Infestation of Winter Oilseed Rape (Brassica napus L. ssp. oleifera Metzg) in Two Tillage Systems. Agriculture 2022, 12, 563. [Google Scholar] [CrossRef]
- Plaza, E.H.; Navarrete, L.; González-Andújar, J.L. Intensity of soil disturbance shapes response trait diversity of weed communities: The long-term effects of different tillage systems. Agric. Ecosys. Environ. 2015, 207, 101–108. [Google Scholar] [CrossRef]
- Nichols, V.; Verhulst, N.; Cox, R.; Govaerts, B. Weed dynamics and conservation agriculture principles: A review. Field Crops Res. 2015, 183, 56–68. [Google Scholar] [CrossRef]
- Małecka-Jankowiak, I.; Blecharczyk, A.; Sawinska, Z.; Piechota, T.; Waniorek, B. Impact of crop sequence and tillage system on weed infestation of winter wheat. Fragm. Agronom. 2015, 32, 54–63. [Google Scholar]
- Gawęda, D.; Haliniarz, M.; Cierpiała, R.; Klusek, I. Yield, Weed infestation and seed quality of soybean (Glycine max (L.) Merr.) under different tillage systems. Tarim Bilim. Derg. 2017, 23, 268–275. [Google Scholar]
- Gawęda, D.; Haliniarz, M.; Bronowicka-Mielniczuk, U.; Łukasz, J. Weed Infestation and Health of the Soybean Crop Depending on Cropping System and Tillage System. Agriculture 2020, 10, 208. [Google Scholar] [CrossRef]
- Chovancová, S.; Neudert, L.; Winkler, J. The efect of three soil tillage treatments on weed infestation in forage maize. Acta Agrobot. 2019, 72, 1756. [Google Scholar] [CrossRef]
- Brozović, B.; Jug, I.; Ðurdevi’c, B.; Ravli’c, M.; Vukadinovi´c, V.; Rojnica, I.; Jug, D. Initial Weed and Maize Response to Conservation Tillage and Liming in Different Agroecological Conditions. Agronomy 2023, 13, 1116. [Google Scholar] [CrossRef]
- Winkler, J.; Dvořák, J.; Hosa, J.; Martínez Barroso, P.; Vaverková, M.D. Impact of Conservation Tillage Technologies on the Biological Relevance of Weeds. Land 2023, 12, 121. [Google Scholar] [CrossRef]
- Santín-Montanyá, M.I.; Martín-Lammerding, D.; Walter, I.; Zambrana, E.; Tenorio, J.L. Effects of tillage, crop systems and fertilization on weed abundance and diversity in 4-year dry land winter wheat. Eur. J Agron. 2013, 48, 43–49. [Google Scholar] [CrossRef]
- Romaneckas, K.; Kimbirauskienė, R.; Sinkevičienė, A.; Jaskulska, I.; Buragienė, S.; Adamavičienė, A.; Šarauskis, E. Weed Diversity, Abundance, and Seedbank in Differently Tilled Faba Bean (Vicia faba L.) Cultivations. Agronomy 2021, 11, 529. [Google Scholar] [CrossRef]
- Ruisi, P.; Frangipane, B.; Amato, G.; Badagliacca, G.; di Miceli, G.; Plaia, A.; Giambalvo, D. Weed seedbank size and composition in a long-term tillage and crop sequence experiment. Weed Res. 2015, 55, 320–328. [Google Scholar] [CrossRef]
- Chovancová, S.; Illek, F.; Winkler, J. The effect of three tillage treatments on weed infestation in maize monoculture. Pak. J. Bot. 2020, 52, 697–701. [Google Scholar] [CrossRef]
- Kotlánová, B.; Hledík, P.; Hudec, S.; Martínez Barroso, P.; Vaverková, M.D.; Jiroušek, M.; Winkler, J. The Influence of Sugar Beet Cultivation Technologies on the Intensity and Species Biodiversity of Weeds. Agronomy 2024, 14, 390. [Google Scholar] [CrossRef]
- Mahmoodi, S.; Rahimi, A. The critical period of weed control in corn in Birjand region, Iran. Int. J. Plant Prod. 2009, 3, 91–96. [Google Scholar]
- Mohler, C.L.; Frisch, J.C.; McCulloch, C.E. Vertical movement of weed seed surrogates by tillage implements and natural processes. Soil Tillage Res. 2006, 86, 110–122. [Google Scholar] [CrossRef]
- Peigné, J.; Ball, B.C.; Roger-Estrade, J.; David, C. Is conservation tillage suitable for organic farming? Rev. Soil Use Manag. 2007, 23, 129–144. [Google Scholar] [CrossRef]
- Stancevičius, A.; Špokienė, N.; Jodaugienė, D.; Trečiokas, K.; Raudonius, S. Impact of reduced soil tillage on crop weedinesss. Proc. Lith. Acad. Agric. 2002, 55, 50–58. (In Lithuanian) [Google Scholar]
- Winkler, J.; Kopta, T.; Ferby, V.; Neudert, L.; Vaverková, M.D. Effect of Tillage Technology Systems for Seed Germination Rate in a Laboratory Tests. Environments 2022, 9, 13. [Google Scholar] [CrossRef]
- Jankowski, K.J.; Sokólski, M.; Szatkowski, A.; Załuski, D. The Effects of Tillage Systems on the Management of Agronomic Factors in Winter Oilseed Rape Cultivation: A Case Study in North-Eastern Poland. Agronomy 2024, 14, 437. [Google Scholar] [CrossRef]
- Franek, M. Reacting of six winter oilseed rape cultivars to herbicides applied post sowing and post emergence. Rośliny Oleiste Oilseed Crops 2001, 22, 91–96. (In Polish) [Google Scholar]
- Hamzei, J.; Nasab, A.D.M.; Khoie, F.R.; Javanshir, A.; Moghaddam, M. Critical period of weed control in three winter oilseed rape (Brassica napus L.) cultivars. Turk. J. Agric. For. 2007, 31, 83–90. [Google Scholar]
- Pacanoski, Z. Application time and herbicide rate effects on weeds in oilseed rape (Brassica napus var. oleifera). Herbologia 2014, 14, 33–45. [Google Scholar] [CrossRef]
- Guerrero, I.; Morales, M.B.; Onate, J.J.; Geiger, F.; Berendse, F.; de Snoo, G.; Eggers, S.; Pärt, T.; Bengtsson, J.; Clement, L.W.; et al. Response of ground-nesting farmland birds to agricultural intensificationacross Europe: Landscape and field level management factors. Biol. Conserv. 2012, 152, 74–80. [Google Scholar] [CrossRef]
- Mitra, A.; Chatterjee, C.; Mandal, F.B. Synthetic chemical pesticides and theireffects on birds. Res. J. Environ. Toxicol. 2011, 5, 81–96. [Google Scholar] [CrossRef]
- Cook, R.J. Toward cropping systems that enhance productivity and sustainability. Proc. Natl. Acad. Sci. USA 2006, 103, 18389–18394. [Google Scholar] [CrossRef]
- Winkler, J.; Hledík, P.; Procházková, B. Influence of Crop Rotation on Actual Weed Infestation of Sugar Beet. Listy Cukrov. Řepařské: Odb. Časopis Obor Cukrovka-Cukr-Líh 2015, 131, 162–166. (In Czech) [Google Scholar]
- Bowles, T.M.; Mooshammer, M.; Socolar, Y.; Calderón, F.; Cavigelli, M.A.; Culman, S.W.; Deen, W.; Drury, C.F.; Garcia, A.G.Y.; Gaudin, A.C.M.; et al. Long-term evidence shows that crop-rotation diversification increases agricultural resilience to adverse growing conditions in North America. One Earth 2020, 2, 284–293. [Google Scholar] [CrossRef]
- Minhas, W.A.; Mumtaz, N.; Ur-Rehman, H.; Farooq, S.; Farooq, M.; Ali, H.M.; Hussain, M. Weed infestation and productivity of wheat crop sown in various cropping systems under conventional and conservation tillage. Front. Plant Sci. 2023, 14, 1176738. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, M.; Hussain, M.; Jabran, K.; Farooq, M.; Farooq, S.; Gašparovič, K.; Barboricova, M.; Aljuaid, B.S.; El-Shehawi, A.M.; Zuan, A.T.K. The Impact of Different Crop Rotations by Weed Management Strategies’ Interactions on Weed Infestation and Productivity of Wheat (Triticum aestivum L.). Agronomy 2021, 11, 2088. [Google Scholar] [CrossRef]
- Ramsdale, B.K.; Kegode, G.O.; Messersmith, C.G.; Nalewaja, J.D.; Nord, C.A. Long-term effects of spring wheat–soybean cropping systems on weed populations. Field Crops Res. 2006, 97, 197–208. [Google Scholar] [CrossRef]
- Neve, P.; Vila-Aiub, M.; Roux, F. Evolutionary-thinking in agricultural weed management. New Phytol. 2009, 184, 783–793. [Google Scholar] [CrossRef]
- Cleland, E.E.; Chiariello, N.R.; Loarie, S.R.; Mooney, H.A.; Field, C.B. Diverse responses of phenology to global changes in a grassland ecosystem. Proc. Natl. Acad. Sci. USA 2006, 103, 13740–13744. [Google Scholar] [CrossRef] [PubMed]
- Franks, S.J.; Sim, S.; Weis, A.E. Rapid evolution of flowering time by an annual plant in response to a climate fluctuation. Proc. Natl. Acad. Sci. USA 2007, 104, 1278–1282. [Google Scholar] [CrossRef]
- Asaduzzaman, M.; Pratley, J.E.; Luckett, D.; Lemerle, D.; Wu, H. Weed management in canola (Brassica napus L.): A review of current constraints and future strategies for Australia. Arch. Agron. Soil Sci. 2020, 66, 427–444. [Google Scholar] [CrossRef]
- Travlos, I.; Gazoulis, I.; Simić, M.; Kanatas, P. The Underestimated Role of Cultural Practices in Ecologically Based Weed Management Approaches. In Ecologically-Based Weed Management: Concepts, Challenges, and Limitations; John Wiley & Sons, Inc.: New York, NY, USA, 2023; pp. 75–92. [Google Scholar] [CrossRef]
- Iboyi, J.E.; Mulvaney, M.J.; Balkcom, K.S.; Seepaul, R.; Bashyal, M.; Perondi, D.; Leon, R.G.; Devkota, P.; Small, I.M.; George, S.; et al. Tillage system and seeding rate effects on the performance of Brassica carinata. GCB Bioenergy 2021, 13, 600–617. [Google Scholar] [CrossRef]
- Nosratti, I.; Chauhan, B.S. The Ecological Base of Nonchemical Weed Control. In Ecologically-Based Weed Management: Concepts, Challenges, and Limitations; John Wiley & Sons, Inc.: New York, NY, USA, 2023; pp. 49–74. [Google Scholar] [CrossRef]
- Mwendwa, J.M.; Brown, W.B.; Weston, P.A.; Haque, K.S.; Preston, C.; Weston, L.A. Evaluation of selected commercial oilseed rape cultivars for early vigour, weed suppression and yield in southern New South Wales. Weed Res. 2020, 60, 450–463. [Google Scholar] [CrossRef]
- Lin, G.; Li, H.; Yang, Z.; Ruan, Y.; Liu, C. Pod canopy staggered-layer cultivation increases rapeseed (Brassica napus L.) yield by improving population canopy structure and fully utilizing light-energy resources. Eur. J. Agron. 2024, 158, 127229. [Google Scholar] [CrossRef]
- Scavo, A.; Mauromicale, G. Integrated weed management in herbaceous field crops. Agronomy 2020, 10, 466. [Google Scholar] [CrossRef]
- Anderson, W.K.; Brennan, R.F.; Jayasena, K.W.; Micic, S.; Moore, J.H.; Nordblom, T. Tactical crop management for improved productivity in winter-dominant rainfall regions: A review. Crop Pasture Sci. 2020, 71, 621–644. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022; 236p, Available online: https://www.isric.org/sites/default/files/WRB_fourth_edition_2022-12-18.pdf (accessed on 12 June 2024).
- CGS. Geological Map of the Czech Republic, 1:50,000; Czech Geological Society: Prague, Czech Republic, 2018; Available online: https://mapy.geology.cz/geocr50 (accessed on 8 November 2023).
- CGS. Map of Soil Types of the Czech Republic, 1:50,000; Czech Geological Society: Prague, Czech Republic, 2017; Available online: https://mapy.geology.cz/pudy (accessed on 8 November 2023).
- Culek, M. (Ed.) Biogeographical Division of the Czech Republic (Biogeografické členění České Republiky), 1st ed.; Enigma: Prague, Czech Republic, 1996; p. 347. (In Czech) [Google Scholar]
- Kaplan, Z.; Danihelka, J.; Chrtek, J.; Kirschner, J.; Kubát, K.; Štech, M.; Štěpánek, J. (Eds.) Key to the Flora of the Czech Republic [Klíč ke Květeně České Republiky], 2nd ed.; Academia: Prague, Czech Republic, 2019; p. 1168. (In Czech) [Google Scholar]
- Ter Braak, C.J.F.; Šmilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination, Version 5.0; Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar]
- Vykydalová, L.; Martínez Barroso, P.; Děkanovský, I.; Neoralová, M.; Lumbantobing, Y.R.; Winkler, J. Interactions between Weeds, Pathogen Symptoms and Winter Rapeseed Stand Structure. Agronomy 2024, 14, 2273. [Google Scholar] [CrossRef]
- Vykydalová, L.; Martínez Barroso, P.; Děkanovský, I.; Hrudová, E.; Lumbantobing, Y.R.; Michutová, M.; Winkler, J. The Response of Insects and Weeds within the Crop to Variation in Sowing Density of Canola. Land 2024, 13, 1509. [Google Scholar] [CrossRef]
- Daugovish, O.; Thill, D.C.; Shafii, B. Modeling competition between wild oat (Avena fatua L.) and yellow mustard or canola. Weed Sci. 2003, 51, 102–109. [Google Scholar] [CrossRef]
- Blackshaw, R.E.; Beckie, H.J.; Molnar, L.J.; Entz, T.; Moyer, J.R. Combining agronomic practices and herbicides improves weed management in wheat–canola rotations within zero-tillage production systems. Weed Sci. 2005, 53, 528–535. [Google Scholar] [CrossRef]
- Andreasen, C.; Streibig, J.; Haas, H. Soil properties affecting the distribution of 37 weed species in Danish fields. Weed Res. 1991, 31, 181–187. [Google Scholar] [CrossRef]
- Mehrtens, J.; Schulte, M.; Hurle, K. Unkrautflora in Mais—Ergebnisse eines Monitorings in Deutschland. Ges Pfl. 2005, 57, 206–218. [Google Scholar] [CrossRef]
- Myers, R.; Weber, A.; Tellatin, S. Cover Crop Economics: Opportunities to Improve Your Bottom Line in Row Crops; SARE Technical Bulletin; US Department of Agriculture, National Institute of Food and Agriculture: Washington, DC, USA, 2019. Available online: https://www.sare.org/learning-center/bulletins/cover-crop-economics (accessed on 31 January 2023).
- Shaner, D.L.; Beckie, H.J. The future for weed control and technology. Pest Manag. Sci. 2014, 70, 1329–1339. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, S.; Gruber, S.; Claupein, W. Oilseed Rape Yield Performance in the Clearfield® System under Varying Management Intensities. Agronomy 2021, 11, 2551. [Google Scholar] [CrossRef]
- Hanzlik, K.; Gerowitt, B. Occurrence and distribution of important weed species in German winter oilseed rape fields. J Plant Dis Prot. 2012, 119, 107–120. [Google Scholar] [CrossRef]
- Schröder, G. Die Ausbreitung der Raukenarten von den Ruderalstandorten auf die Agrarflächen bereitet im Winterraps, aber zunehmend auch in anderen Kulturen, Probleme bei der Unkrautbekämpfung. Mitt. Biol. Bundesanst. LandForstwirtsch. 1998, 357, 228. [Google Scholar]
- Chao, W.S.; Anderson, J.V.; Li, X.; Gesch, R.W.; Berti, M.T.; Horvath, D.P. Overwintering Camelina and Canola/Rapeseed Show Promise for Improving Integrated Weed Management Approaches in the Upper Midwestern U.S. Plants 2023, 12, 1329. [Google Scholar] [CrossRef]
- Tyler, T.; Herbertsson, L.; Olofsson, J.; Olsson, P.A. Ecological indicator and traits values for swedish vascular plants. Ecol. Indic. 2021, 120, 106923. [Google Scholar] [CrossRef]
- Winkler, J.; Vaverková, M.D.; Havel, L. Anthropogenic life strategy of plants. Anthr. Rev. 2023, 10, 455–462. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).