Effect of Sod Production on Physical, Chemical, and Biological Properties of Soils in North and South China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Overview of the Sampling Sites
2.2. Soil Sampling and Analysis
2.3. Statistical Analysis
3. Results
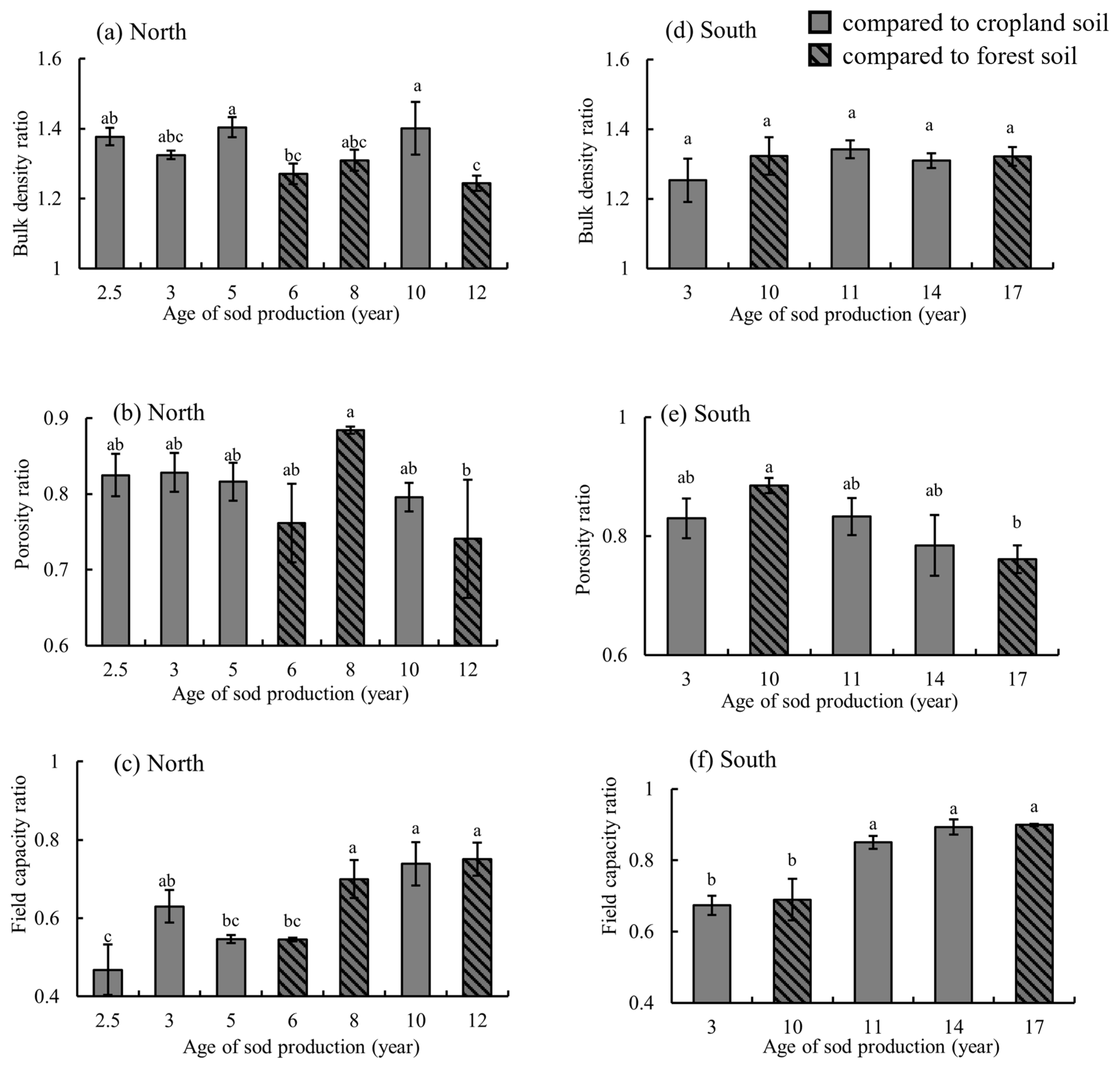
3.1. Effects of Sod Production on Soil Physical Properties
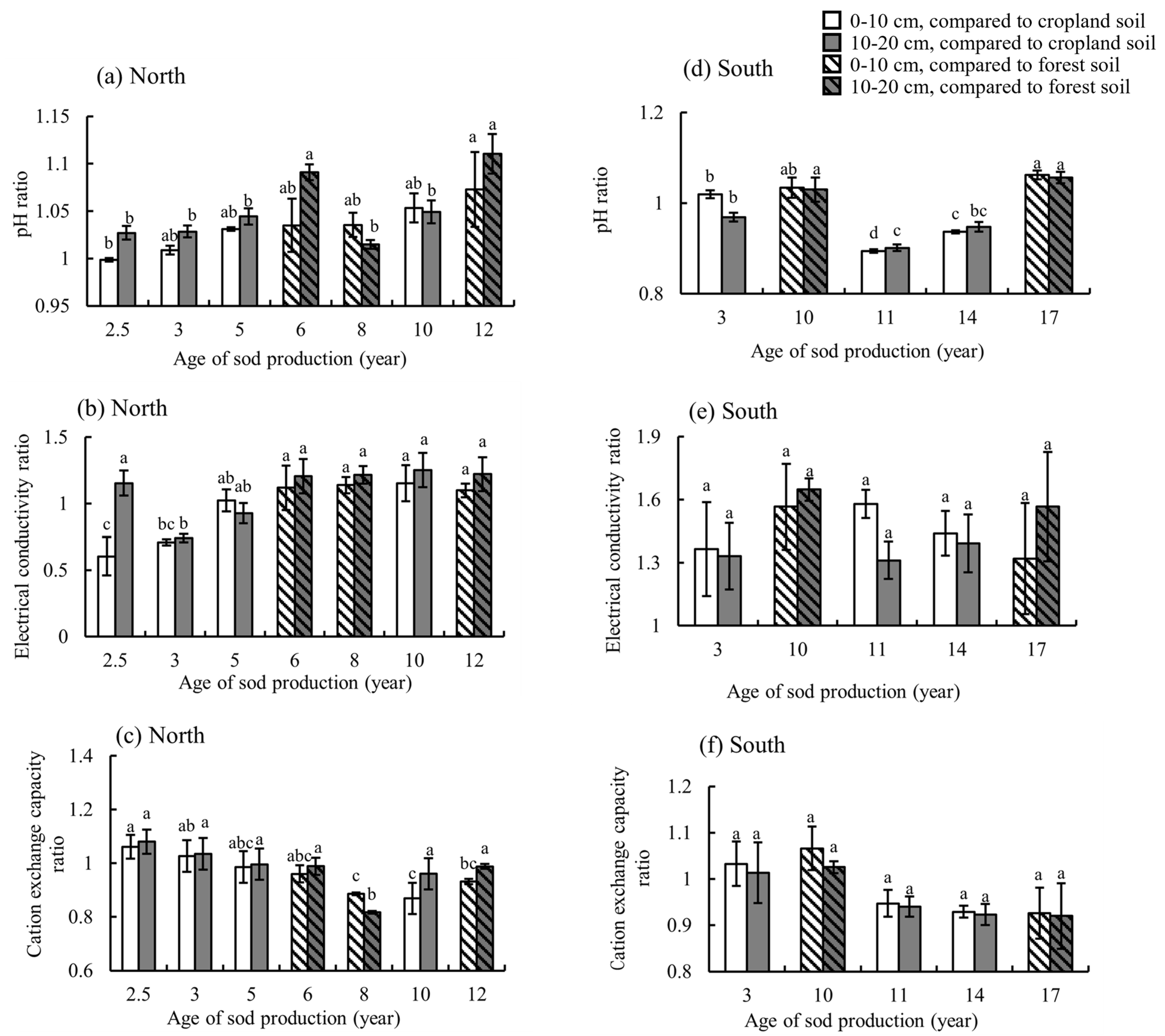
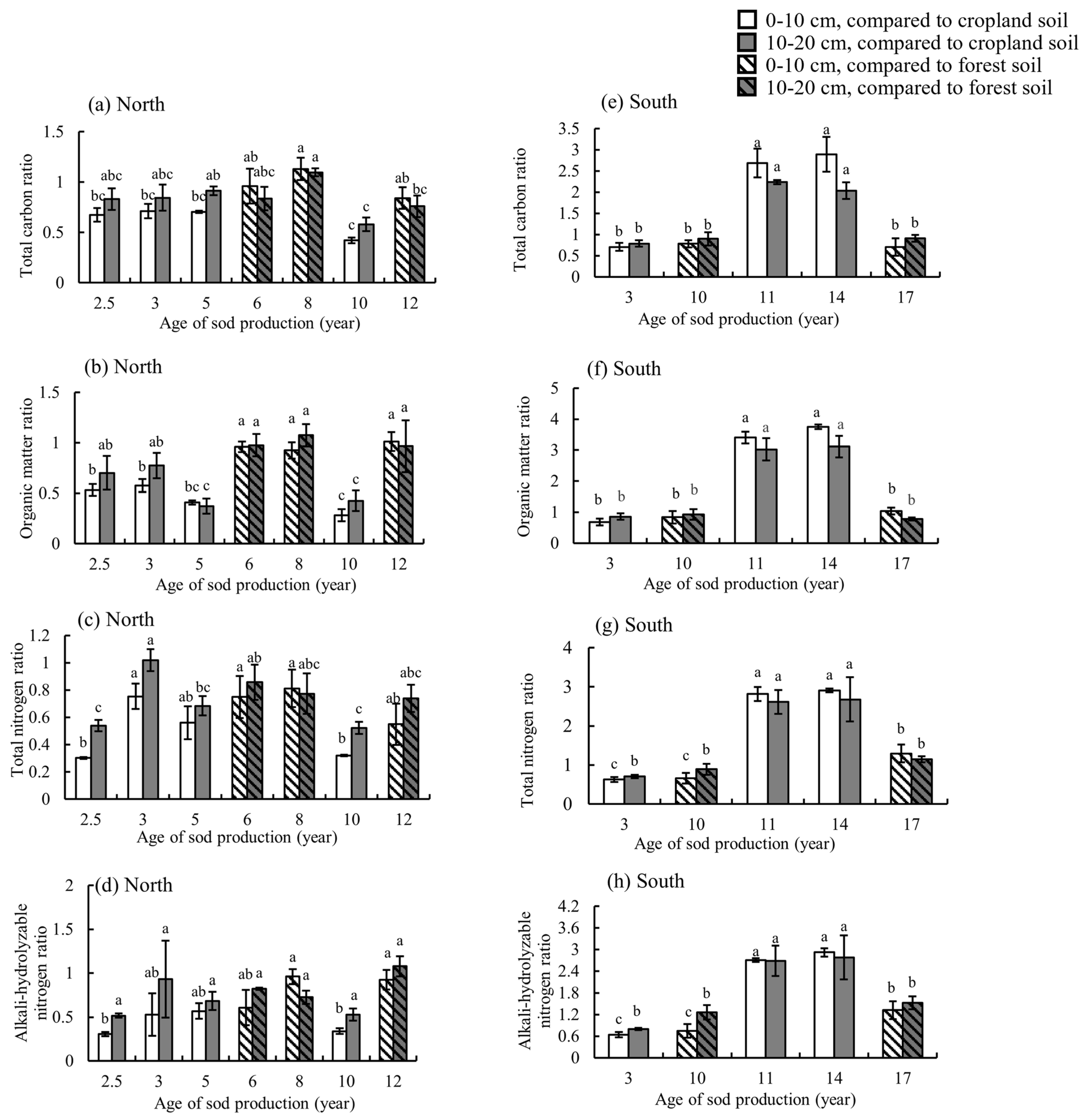
3.2. Effects of Sod Production on Soil Chemical Properties
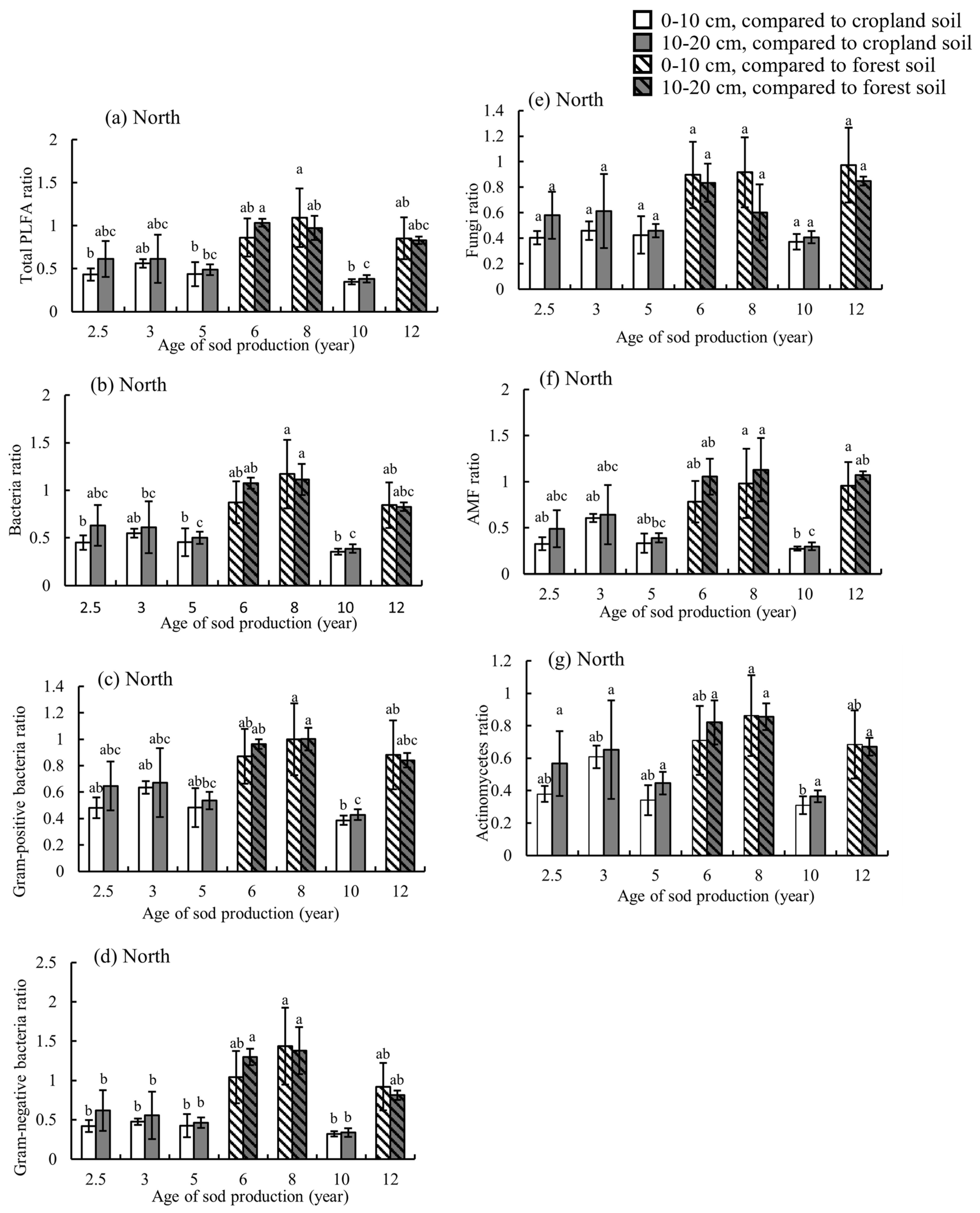
3.3. Effects of Sod Production on Soil Biological Properties
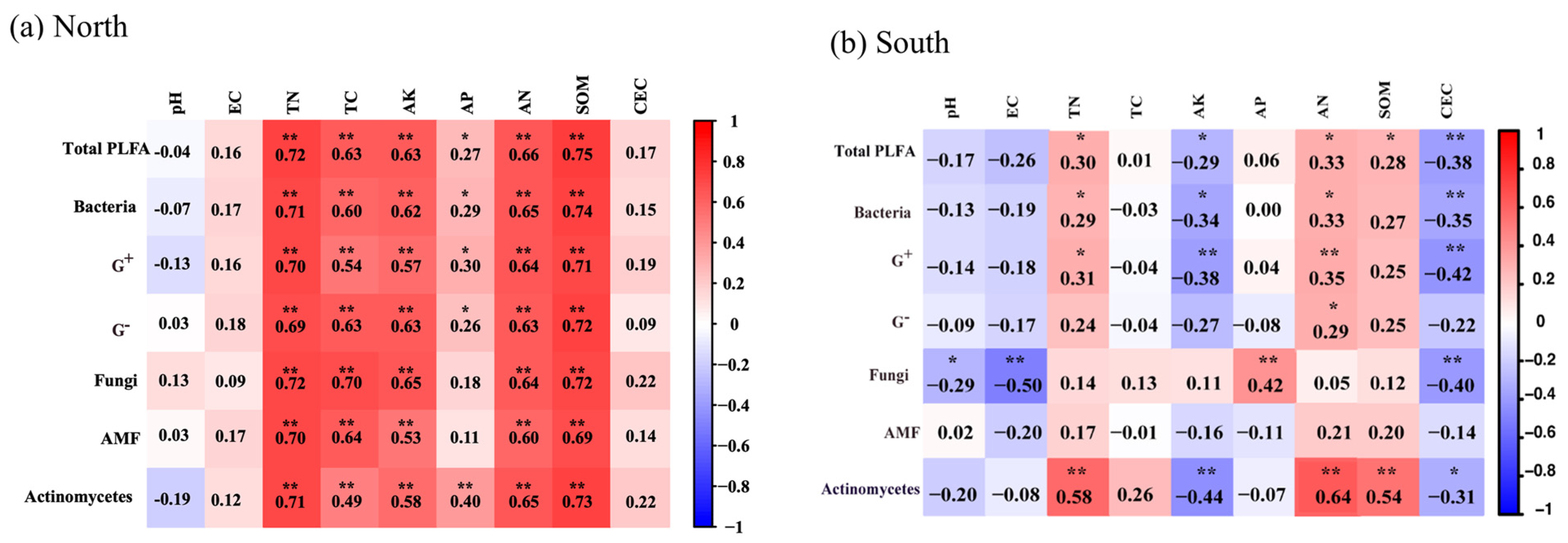
3.4. Correlation and Redundancy Analysis
4. Discussion
4.1. Soil Physical Properties
4.2. Soil Chemical Properties
4.3. Soil Biological Properties
4.4. Correlation and Redundancy Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Measurement Indexes | Cropland (10, 5, 2.5 Years Ground Comparison) | Kentucky Bluegrass (2.5 Years) | Kentucky Bluegrass (5 Years) | Tall Fescue (10 Years) | Cropland (3 Years Ground Comparison) | Tall Fescue: Kentucky Bluegrass (9:1) (3 Years) | Woodland (6, 12 Years Ground Comparison) | Tall Fescue (6 Years) | Tall Fescue: Kentucky Bluegrass (15:8) (12 Years) | Woodland (8 Years Ground Comparison) | Tall Fescue (8 Years) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bulk density (g/cm3) | 1.16 ± 0.05 | 1.6 ± 0.02 ** | 1.63 ± 0.05 ** | 1.62 ± 0.09 ** | 1.28 ± 0.03 | 1.69 ± 0.07 ** | 1.39 ± 0.07 | 1.77 ± 0.04 ** | 1.73 ± 0.10 ** | 1.21 ± 0.02 | 1.59 ± 0.05 ** |
| Porosity (%) | 47.17 ± 1.69 | 38.84 ± 0.87 ** | 38.45 ± 1.29 ** | 37.55 ± 2.55 ** | 45.61 ± 1.13 | 37.80 ± 2.93 * | 45.42 ± 0.25 | 34.58 ± 3.91 ** | 33.64 ± 5.96 * | 44.98 ± 0.39 | 39.77 ± 0.60 ** |
| Field water holding capacity (%) | 39.22 ± 0.48 | 18.38 ± 4.54 ** | 21.45 ± 0.96 ** | 28.94 ± 3.45 ** | 33.77 ± 1.23 | 21.29 ± 2.64 ** | 29.26 ± 0.99 | 15.97 ± 0.67 ** | 21.93 ± 1.66 ** | 33.75 ± 3.18 | 23.44 ± 0.49 ** |
| Measurement Indexes | Rice (3 Years Ground Comparison) | Bermudagrass (3 Years) | Woodland (10 Years Ground Comparison) | Bermudagrass (10 Years) | Rice (11, 14 Years Ground Comparison) | Creeping Bentgrass (11 Years) | Bermudagrass (14 Years) | Woodland (17 Years Ground Comparison) | Bermudagrass (17 Years) |
| Bulk density (g/cm3) | 1.25 ± 0.04 | 1.56 ± 0.09 ** | 1.23 ± 0.07 | 1.62 ± 0.06 ** | 1.16 ± 0.02 | 1.56 ± 0.03 ** | 1.52 ± 0.01 ** | 1.16 ± 0.05 | 1.54 ± 0.01 ** |
| Porosity (%) | 46.17 ± 0.62 | 38.31 ± 2.60 ** | 43.65 ± 0.66 | 38.63 ± 1.55 ** | 41.79 ± 0.94 | 34.77 ± 1.55 ** | 32.74 ± 3.39 ** | 42.98 ± 0.09 | 32.70 ± 1.73 ** |
| Field water holding capacity (%) | 33.67 ± 2.68 | 22.75 ± 3.24 * | 32.19 ± 2.19 | 22.05 ± 1.89 ** | 25.42 ± 1.81 | 21.63 ± 2.13 | 22.75 ± 2.56 | 32.86 ± 2.23 | 29.55 ± 2.12 |
| Measurement Indexes | Soil Depth (cm) | Cropland (10, 5, 2.5 Years Ground Comparison) | Kentucky Bluegrass (2.5 Years) | Kentucky Bluegrass (5 Years) | Tall Fescue (10 Years) | Cropland (3 Years Ground Comparison) | Tall Fescue: Kentucky Bluegrass (9:1) (3 Years) | Woodland (6, 12 Years Ground Comparison) | Tall Fescue (6 Years) | Tall Fescue: Kentucky Bluegrass (15:8) (12 Years) | Woodland (8 Years Ground Comparison) | Tall Fescue (8 Years) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 0–10 cm | 7.63 ± 0.05 | 7.62 ± 0.07 | 7.86 ± 0.06 ** | 8.03 ± 0.18 * | 8.03 ± 0.05 | 8.10 ± 0.03 | 7.51 ± 0.39 | 7.76 ± 0.07 | 8.04 ± 0.08 | 8.14 ± 0.13 | 8.43 ± 0.06 * |
| 10–20 cm | 7.76 ± 0.07 | 7.97 ± 0.06 * | 8.10 ± 0.05 ** | 8.14 ± 0.10 ** | 7.97 ± 0.02 | 8.19 ± 0.08 * | 7.03 ± 0.10 | 7.67 ± 0.01 ** | 7.81 ± 0.16 ** | 8.30 ± 0.05 | 8.42 ± 0.05 * | |
| Electrical conductivity (μs/cm) | 0–10 cm | 232.67 ± 23.71 | 143.67 ± 72.53 | 240.03 ± 52.66 | 264.67 ± 29.57 | 231.33 ± 18.58 | 163.63 ± 3.99 ** | 89.43 ± 23.41 | 95.63 ± 4.81 | 98.30 ± 27.63 | 149.40 ± 12.89 | 169.33 ± 1.15 |
| 10–20 cm | 232.67 ± 29.94 | 265.67 ± 16.50 | 214.00 ± 19.29 | 287.67 ± 28.29 | 211.40 ± 25.34 | 155.83 ± 7.14 * | 97.73 ± 23.35 | 115.07 ± 16.75 | 116.00 ± 9.04 | 151.70 ± 3.75 | 184.33 ± 17.24 * | |
| Cation exchange capacity (cmol/kg) | 0–10 cm | 17.88 ± 0.31 | 18.98 ± 0.88 | 17.63 ± 0.31 | 15.54 ± 1.53 | 16.94 ± 0.63 | 17.37 ± 0.77 | 17.54 ± 0.73 | 16.84 ± 1.00 | 16.31 ± 0.47 | 17.90 ± 0.79 | 15.85 ± 1.26 |
| 10–20 cm | 16.59 ± 0.82 | 17.89 ± 0.53 | 16.47 ± 0.90 | 15.90 ± 1.30 | 18.09 ± 0.79 | 18.68 ± 1.05 | 16.58 ± 0.24 | 16.38 ± 0.74 | 16.38 ± 0.08 | 19.38 ± 0.46 | 15.83 ± 0.47 ** | |
| Total carbon (g/kg) | 0–10 cm | 22.10 ± 0.46 | 14.89 ± 2.43 ** | 15.57 ± 0.34 ** | 9.30 ± 1.04 ** | 30.69 ± 5.64 | 21.40 ± 0.72 * | 7.50 ± 1.55 | 6.93 ± 1.33 | 6.12 ± 0.07 | 20.56 ± 1.82 | 23.08 ± 2.87 |
| 10–20 cm | 15.84 ± 1.93 | 12.91 ± 1.64 | 14.38 ± 0.55 | 9.05 ± 1.03 ** | 21.16 ± 1.16 | 17.71 ± 3.84 | 7.83 ± 2.26 | 6.24 ± 0.60 | 5.67 ± 0.51 | 19.59 ± 1.24 | 21.44 ± 0.82 | |
| Organic matter (g/kg) | 0–10 cm | 23.23 ± 2.02 | 12.25 ± 1.75 ** | 9.45 ± 0.04 ** | 6.46 ± 2.13 ** | 25.27 ± 3.17 | 14.30 ± 1.22 ** | 9.74 ± 2.01 | 9.46 ± 2.66 | 9.80 ± 2.37 | 13.18 ± 1.57 | 12.08 ± 1.13 |
| 10–20 cm | 12.63 ± 2.96 | 8.29 ± 1.21 | 4.98 ± 2.56 * | 5.09 ± 1.32 * | 14.74 ± 4.20 | 10.88 ± 1.31 | 9.85 ± 3.24 | 9.20 ± 0.92 | 8.57 ± 2.09 | 11.73 ± 2.02 | 12.35 ± 0.81 | |
| Total nitrogen (g/kg) | 0–10 cm | 1.59 ± 0.01 | 0.48 ± 0.03 ** | 0.87 ± 0.24 * | 0.51 ± 0.05 ** | 1.62 ± 0.32 | 1.18 ± 0.06 * | 0.69 ± 0.19 | 0.48 ± 0.03 | 0.36 ± 0.14 | 0.90 ± 0.20 | 0.73 ± 0.29 |
| 10–20 cm | 0.87 ± 0.08 | 0.46 ± 0.03 ** | 0.59 ± 0.05 ** | 0.45 ± 0.04 ** | 1.03 ± 0.13 | 1.04 ± 0.12 | 0.62 ± 0.19 | 0.51 ± 0.02 | 0.44 ± 0.04 | 0.85 ± 0.24 | 0.62 ± 0.03 | |
| Alkali-hydrolyzable nitrogen (mg/kg) | 0–10 cm | 122.73 ± 18.05 | 37.07 ± 3.3 ** | 70.03 ± 20.94 * | 41.17 ± 5.62 ** | 137.99 ± 18.41 | 69.32 ± 47.65 | 41.21 ± 5.88 | 24.29 ± 11.67 | 37.29 ± 2.90 | 62.53 ± 4.12 | 60.33 ± 12.20 |
| 10–20 cm | 64.69 ± 3.73 | 33.43 ± 3.61 ** | 43.97 ± 9.86 * | 33.94 ± 6.45 ** | 81.02 ± 20.43 | 70.46 ± 51.68 | 39.53 ± 7.98 | 32.49 ± 6.38 | 41.69 ± 3.72 | 68.59 ± 19.9 | 48.34 ± 9.4 | |
| Available phosphorus (mg/kg) | 0–10 cm | 25.45 ± 3.75 | 16.28 ± 0.57 * | 9.47 ± 0.66 ** | 20.07 ± 1.57 | 64.00 ± 7.21 | 11.60 ± 3.12 ** | 53.87 ± 3.22 | 40.41 ± 2.27 ** | 42.69 ± 0.88 ** | 3.52 ± 0.24 | 2.75 ± 0.24 * |
| 10–20 cm | 11.76 ± 0.24 | 11.33 ± 0.27 | 5.03 ± 0.66 ** | 8.39 ± 0.13 * | 19.07 ± 3.95 | 5.41 ± 2.37 ** | 36.31 ± 1.71 | 25.14 ± 2.30 ** | 26.57 ± 0.35 ** | 2.63 ± 0.18 | 1.89 ± 0.29 * | |
| Available potassium (mg/kg) | 0–10 cm | 176.69 ± 38.41 | 158.47 ± 24.42 | 94.44 ± 26.85 * | 139.06 ± 3.94 | 777.44 ± 162.28 | 172.82 ± 4.55 ** | 72.51 ± 3.85 | 52.95 ± 3.91 ** | 60.17 ± 11.5 | 182.05 ± 15.79 | 140.31 ± 16.32 * |
| 10–20 cm | 115.55 ± 14.10 | 127.32 ± 10.29 | 103.84 ± 6.7 | 97.28 ± 8.21 | 378.57 ± 70.31 | 149.37 ± 9.84 ** | 77.72 ± 8.09 | 71.22 ± 6.03 | 75.18 ± 8.15 | 155.85 ± 14.79 | 146.63 ± 13.53 |
| Measurement Indexes | Soil Depth (cm) | Rice (3 Years Ground Comparison) | Bermudagrass (3 Years) | Woodland (10 Years Ground Comparison) | Bermudagrass (10 Years) | Rice (11, 14 Years Ground Comparison) | Creeping Bentgrass (11 Years) | Bermudagrass (14 Years) | Woodland (17 Years Ground Comparison) | Bermudagrass (17 Years) |
|---|---|---|---|---|---|---|---|---|---|---|
| pH | 0–10 cm | 6.93 ± 0.21 | 7.07 ± 0.18 | 6.59 ± 0.31 | 6.81 ± 0.19 | 7.80 ± 0.16 | 6.97 ± 0.15 ** | 7.30 ± 0.11 * | 7.17 ± 0.08 | 7.62 ± 0.05 ** |
| 10–20 cm | 7.03 ± 0.23 | 6.82 ± 0.34 | 6.64 ± 0.13 | 6.84 ± 0.23 | 7.82 ± 0.14 | 7.05 ± 0.17 * | 7.41 ± 0.02 ** | 7.22 ± 0.08 | 7.63 ± 0.08 ** | |
| Electrical conductivity (μs/cm) | 0–10 cm | 140.87 ± 18.18 | 187.67 ± 34.61 | 174.11 ± 21.16 | 268.00 ± 33.85 * | 193 ± 2.43 | 304.87 ± 22.20 * | 278.23 ± 39.25 | 166.77 ± 23.23 | 216.53 ± 71.04 |
| 10–20 cm | 149.73 ± 9.07 | 199.20 ± 42.78 | 167.51 ± 19.64 | 275.23 ± 24.49 ** | 184.30 ± 5.27 | 241.21 ± 12.60 | 257.43 ± 8.96 | 137.51 ± 16.39 | 218.57 ± 80.08 | |
| Cation exchange capacity (cmol/kg) | 0–10 cm | 15.25 ± 0.59 | 15.77 ± 1.56 | 15.58 ± 1.34 | 16.54 ± 1.67 | 19.97 ± 0.24 | 18.91 ± 0.96 | 18.56 ± 0.28 ** | 16.32 ± 1.00 | 15.07 ± 1.19 |
| 10–20 cm | 15.45 ± 0.57 | 15.65 ± 1.64 | 15.73 ± 0.58 | 16.14 ± 0.94 | 19.45 ± 0.25 | 18.28 ± 0.49 * | 17.95 ± 0.53 * | 16.45 ± 0.76 | 15.07 ± 1.35 | |
| Total carbon (g/kg) | 0–10 cm | 12.00 ± 2.25 | 8.27 ± 0.81 | 15.40 ± 2.27 | 12.20 ± 3.70 | 6.27 ± 1.01 | 17.43 ± 0.31 ** | 17.67 ± 0.31 ** | 16.40 ± 3.34 | 11.00 ± 0.42 |
| 10–20 cm | 11.70 ± 2.98 | 9.10 ± 2.25 | 13.87 ± 1.55 | 12.40 ± 3.64 | 7.57 ± 1.05 | 15.80 ± 0.96 ** | 15.30 ± 2.69 ** | 15.53 ± 2.10 | 14.00 ± 1.06 | |
| Organic matter (g/kg) | 0–10 cm | 19.42 ± 3.27 | 12.91 ± 2.32 * | 18.09 ± 2.07 | 15.43 ± 7.12 | 7.06 ± 0.58 | 23.93 ± 0.36 ** | 26.47 ± 1.59 ** | 18.76 ± 1.40 | 19.23 ± 2.14 |
| 10–20 cm | 18.8 ± 4.73 | 15.80 ± 3.69 | 19.85 ± 1.88 | 18.05 ± 4.60 | 7.18 ± 1.81 | 21.04 ± 2.30 ** | 21.65 ± 2.04 ** | 19.18 ± 0.72 | 15.00 ± 1.87 * | |
| Total nitrogen (g/kg) | 0–10 cm | 1.57 ± 0.21 | 0.97 ± 0.06 ** | 1.93 ± 0.38 | 1.33 ± 0.67 | 0.70 ± 0.00 | 1.87 ± 0.06 ** | 2.03 ± 0.06 ** | 1.60 ± 0.20 | 2.03 ± 0.47 |
| 10–20 cm | 1.80 ± 0.20 | 1.27 ± 0.21 * | 1.73 ± 0.15 | 1.53 ± 0.38 | 0.73 ± 0.23 | 1.77 ± 0.06 ** | 1.83 ± 0.32 ** | 1.63 ± 0.12 | 1.87 ± 0.15 | |
| Alkali-hydrolyzable nitrogen (mg/kg) | 0–10 cm | 120.91 ± 20.95 | 75.55 ± 4.55 * | 131.28 ± 12.43 | 100.49 ± 50.75 | 52.66 ± 1.64 | 142.37 ± 7.46 ** | 153.78 ± 10.97 ** | 125.65 ± 15.22 | 162.45 ± 37.26 |
| 10–20 cm | 122.50 ± 24.08 | 97.51 ± 18.74 | 89.68 ± 17.78 | 112.72 ± 32.91 | 54.65 ± 16.33 | 139.16 ± 6.11 * | 142.06 ± 30.45 * | 96.35 ± 13.32 | 145.03 ± 14.27 * | |
| Available phosphorus (mg/kg) | 0–10 cm | 25.19 ± 0.52 | 13.22 ± 2.89 ** | 30.03 ± 2.27 | 23.97 ± 2.07 * | 11.45 ± 2.74 | 7.67 ± 1.89 | 6.72 ± 3.22 | 33.36 ± 6.84 | 4.80 ± 0.10 ** |
| 10–20 cm | 13.61 ± 1.62 | 14.25 ± 1.73 | 31.30 ± 3.52 | 32.37 ± 3.03 | 10.16 ± 2.67 | 7.76 ± 3.18 | 5.15 ± 2.39 | 29.50 ± 3.85 | 6.65 ± 2.20 ** | |
| Available potassium (mg/kg) | 0–10 cm | 168.51 ± 35.09 | 127.33 ± 8.37 | 225.54 ± 23.45 | 101.23 ± 10.15 ** | 312.29 ± 97.07 | 126.72 ± 15.31 * | 115.09 ± 0.26 * | 235.54 ± 13.23 | 90.36 ± 15.53 ** |
| 10–20 cm | 152.40 ± 12.56 | 138.85 ± 12.97 | 174.22 ± 18.10 | 122.20 ± 14.25 * | 279.53 ± 24.52 | 126.04 ± 16.25 ** | 119.47 ± 22.34 ** | 194.22 ± 25.44 | 91.38 ± 9.11 ** |
| Measurement Indexes | Soil Depth (cm) | Cropland (10, 5, 2.5 Years Ground Comparison) | Kentucky Bluegrass (2.5 Years) | Kentucky Bluegrass (5 Years) | Tall Fescue (10 Years) | Cropland (3 Years Ground Comparison) | Tall Fescue: Kentucky Bluegrass (9:1) (3 Years) | Woodland (6, 12 Years Ground Comparison) | Tall Fescue (6 Years) | Tall Fescue: Kentucky Bluegrass (15:8) (12 Years) | Woodland (8 Years Ground Comparison) | Tall fescue (8 Years) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total phospholipid fatty acids (nmol/g) | 0–10 cm | 21.66 ± 4.18 | 9.11 ± 1.44 ** | 8.78 ± 3.30* | 7.48 ± 1.71 ** | 23.80 ± 6.23 | 13.23 ± 3.43 | 11.36 ± 4.16 | 8.80 ± 2.51 | 8.61 ± 1.07 ** | 12.81 ± 2.32 | 13.10 ± 4.90 |
| 10–20 cm | 14.40 ± 3.23 | 8.30 ± 3.63 | 6.79 ± 0.25 * | 5.34 ± 0.29 ** | 15.85 ± 7.62 | 7.45 ± 1.63 | 7.13 ± 0.50 | 7.36 ± 0.74 | 5.90 ± 0.12 * | 9.17 ± 0.28 | 8.96 ± 2.48 | |
| Bacteria (nmol/g) | 0–10 cm | 16.79 ± 3.54 | 7.32 ± 1.19 * | 7.02 ± 2.56 * | 5.16 ± 1.23 ** | 18.00 ± 4.49 | 9.82 ± 2.41 * | 8.88 ± 3.10 | 7.02 ± 2.00 * | 6.70 ± 0.90 ** | 8.85 ± 1.64 | 9.68 ± 3.66 |
| 10–20 cm | 11.28 ± 2.58 | 6.69 ± 2.92 | 5.47 ± 0.24 * | 4.23 ± 0.24 ** | 12.31 ± 5.94 | 5.77 ± 1.18 | 5.63 ± 0.37 | 6.05 ± 0.67 | 4.63 ± 0.15 * | 6.51 ± 0.30 | 7.22 ± 1.75 | |
| Gram-positive bacteria (nmol/g) | 0–10 cm | 8.59 ± 1.98 | 3.98 ± 0.59 * | 3.81 ± 1.21 * | 2.91 ± 0.54 * | 8.29 ± 1.83 | 5.24 ± 1.13 | 4.79 ± 1.43 | 3.84 ± 0.77 | 3.80 ± 0.61 * | 4.35 ± 1.18 | 4.12 ± 1.63 |
| 10–20 cm | 5.99 ± 1.14 | 3.73 ± 1.50 | 3.12 ± 0.18 * | 2.51 ± 0.09 ** | 6.28 ± 2.54 | 3.49 ± 0.70 | 3.30 ± 0.23 | 3.17 ± 0.19 | 2.76 ± 0.14 * | 3.63 ± 0.32 | 3.64 ± 0.66 | |
| Gram-negative bacteria (nmol/g) | 0–10 cm | 7.10 ± 1.37 | 2.87 ± 0.52 ** | 2.76 ± 1.17 * | 1.87 ± 0.59 ** | 8.32 ± 2.32 | 3.91 ± 1.10 * | 3.21 ± 1.70 | 2.75 ± 1.16 | 2.42 ± 0.27 * | 3.74 ± 0.80 | 5.00 ± 2.30 |
| 10–20 cm | 4.57 ± 1.29 | 2.54 ± 1.26 | 2.02 ± 0.12 * | 1.47 ± 0.15 * | 5.05 ± 2.97 | 1.20 ± 0.49 | 1.92 ± 0.09 | 2.50 ± 0.41 | 1.56 ± 0.17 * | 2.33 ± 0.23 | 3.13 ± 0.83 | |
| Fungi (nmol/g) | 0–10 cm | 2.45 ± 0.32 | 0.97 ± 0.10 ** | 0.99 ± 0.46 * | 0.79 ± 0.30 ** | 3.41 ± 1.06 | 1.98 ± 0.64 | 1.38 ± 0.66 | 1.05 ± 0.35 | 1.14 ± 0.12 | 2.38 ± 0.52 | 2.08 ± 0.77 |
| 10–20 cm | 1.62 ± 0.35 | 0.88 ± 0.33 | 0.72 ± 0.03 * | 0.64 ± 0.03 ** | 1.97 ± 0.90 | 0.93 ± 0.26 | 0.85 ± 0.08 | 0.72 ± 0.28 | 0.72 ± 0.03 | 1.91 ± 0.24 | 1.12 ± 0.64 | |
| arbuscular mycorrhizal fungi (nmol/g) | 0–10 cm | 1.41 ± 0.28 | 0.44 ± 0.11 ** | 0.44 ± 0.16 ** | 0.36 ± 0.10 ** | 1.37 ± 0.40 | 0.82 ± 0.22 | 0.49 ± 0.26 | 0.31 ± 0.02 | 0.43 ± 0.05 | 1.15 ± 0.49 | 1.00 ± 0.58 |
| 10–20 cm | 0.86 ± 0.23 | 0.38 ± 0.19 * | 0.32 ± 0.02 * | 0.25 ± 0.03 ** | 0.87 ± 0.50 | 0.39 ± 0.08 | 0.28 ± 0.01 | 0.29 ± 0.09 | 0.30 ± 0.01 | 0.38 ± 0.34 | 0.30 ± 0.08 | |
| Actinomycetes (nmol/g) | 0–10 cm | 1.00 ± 0.13 | 0.37 ± 0.05 ** | 0.33 ± 0.13 ** | 0.22 ± 0.09 ** | 1.01 ± 0.30 | 0.61 ± 0.18 | 0.62 ± 0.17 | 0.40 ± 0.16 | 0.38 ± 0.09 | 0.43 ± 0.15 | 0.35 ± 0.16 |
| 10–20 cm | 0.64 ± 0.10 | 0.35 ± 0.19 | 0.28 ± 0.03 ** | 0.23 ± 0.01 ** | 0.70 ± 0.29 | 0.36 ± 0.12 | 0.37 ± 0.04 | 0.30 ± 0.05 | 0.25 ± 0.03 * | 0.37 ± 0.04 | 0.32 ± 0.08 |
| Measurement Indexes | Soil Depth (cm) | Rice (3 Years Ground Comparison) | Bermudagrass (3 Years) | Woodland (10 Years Ground Comparison) | Bermudagrass (10 Years) | Rice (11, 14 Years Ground Comparison) | Creeping Bentgrass (11 Years) | Bermudagrass (14 Years) | Woodland (17 Years Ground Comparison) | Bermudagrass (17 Years) |
|---|---|---|---|---|---|---|---|---|---|---|
| Total phospholipid fatty acids (nmol/g) | 0–10 cm | 14.63 ± 3.70 | 8.63 ± 1.98 | 8.26 ± 1.17 | 9.06 ± 1.23 | 6.02 ± 1.44 | 7.10 ± 2.20 | 9.66 ± 2.73 | 8.40 ± 0.92 | 10.72 ± 1.63 |
| 10–20 cm | 14.00 ± 6.70 | 9.57 ± 2.55 | 7.29 ± 1.52 | 8.76 ± 1.03 | 4.35 ± 1.06 | 7.13 ± 1.56 | 8.47 ± 1.12 ** | 7.25 ± 1.72 | 7.33 ± 0.46 | |
| Bacteria (nmol/g) | 0–10 cm | 11.83 ± 3.15 | 7.01 ± 1.51 | 6.05 ± 1.11 | 7.35 ± 0.91 | 4.63 ± 1.13 | 5.63 ± 1.79 | 7.76 ± 2.22 | 6.10 ± 1.21 | 8.59 ± 1.48 |
| 10–20 cm | 11.02 ± 5.39 | 7.67 ± 1.78 | 5.40 ± 0.93 | 7.32 ± 0.86 | 3.50 ± 0.84 | 5.77 ± 1.42 | 7.11 ± 1.00 ** | 5.34 ± 0.90 | 5.95 ± 0.37 | |
| Gram-positive bacteria (nmol/g) | 0–10 cm | 6.66 ± 1.52 | 4.41 ± 0.91 | 3.80 ± 0.78 | 4.75 ± 0.66 | 2.76 ± 0.63 | 3.27 ± 0.92 | 4.48 ± 1.35 | 3.80 ± 0.85 | 5.24 ± 0.71 |
| 10–20 cm | 6.42 ± 2.56 | 4.71 ± 0.87 | 3.46 ± 0.61 | 4.81 ± 0.48 * | 2.39 ± 0.35 | 3.37 ± 0.93 | 4.55 ± 0.82 * | 3.52 ± 0.56 | 4.04 ± 0.27 | |
| Gram-negative bacteria (nmol/g) | 0–10 cm | 4.22 ± 1.43 | 2.00 ± 0.45 | 1.64 ± 0.39 | 2.01 ± 0.23 | 1.54 ± 0.42 | 2.02 ± 0.76 | 2.51 ± 0.65 | 1.68 ± 0.39 | 2.66 ± 0.70 |
| 10–20 cm | 3.77 ± 2.43 | 2.40 ± 0.95 | 1.43 ± 0.21 | 1.89 ± 0.36 | 0.83 ± 0.38 | 2.37 ± 0.38 * | 1.98 ± 0.21 ** | 1.32 ± 0.24 | 1.44 ± 0.12 | |
| Fungi (nmol/g) | 0–10 cm | 1.43 ± 0.26 | 1.13 ± 0.50 | 1.93 ± 0.38 | 1.64 ± 0.75 | 1.04 ± 0.23 | 0.83 ± 0.24 | 0.99 ± 0.23 | 1.71 ± 0.97 | 1.26 ± 0.02 |
| 10–20 cm | 1.61 ± 0.74 | 1.34 ± 0.63 | 1.51 ± 0.63 | 0.85 ± 0.12 | 0.58 ± 0.17 | 0.91 ± 0.04 | 0.73 ± 0.07 | 1.51 ± 0.87 | 0.82 ± 0.17 | |
| arbuscular mycorrhizal fungi (nmol/g) | 0–10 cm | 0.78 ± 0.33 | 0.23 ± 0.06 * | 0.24 ± 0.10 | 0.25 ± 0.01 | 0.24 ± 0.06 | 0.32 ± 0.11 | 0.42 ± 0.14 | 0.25 ± 0.11 | 0.35 ± 0.10 |
| 10–20 cm | 0.70 ± 0.46 | 0.29 ± 0.17 | 0.17 ± 0.02 | 0.23 ± 0.06 | 0.17 ± 0.08 | 0.36 ± 0.06 | 0.27 ± 0.04 | 0.18 ± 0.00 | 0.25 ± 0.04 * | |
| Actinomycetes (nmol/g) | 0–10 cm | 0.59 ± 0.04 | 0.26 ± 0.06 ** | 0.32 ± 0.04 | 0.42 ± 0.11 | 0.11 ± 0.03 | 0.32 ± 0.07 ** | 0.50 ± 0.15 * | 0.34 ± 0.08 | 0.53 ± 0.17 |
| 10–20 cm | 0.68 ± 0.16 | 0.27 ± 0.04 * | 0.21 ± 0.03 | 0.36 ± 0.11 | 0.09 ± 0.01 | 0.37 ± 0.14 * | 0.36 ± 0.01 ** | 0.22 ± 0.06 | 0.31 ± 0.04 |
References
- Shan, H.J.; Li, M.L.; Sun, Y.; Zhou, H. Recent Development of Turf Grass Industry China. Acta Agrestia Sin. 2013, 21, 222–229. [Google Scholar]
- Zhao, J.L.; Tang, F.L.; Liu, Y.J. Thoughts on development of China’s turf industry in the context of ecological civilization construction. Pratacultural Sci. 2021, 38, 2077–2086. [Google Scholar]
- Haydu, J.J.; Hodges, A.W.; Hall, C.R. Economic Impacts of the Turfgrass and Lawncare Industry in the United States. Edis 2006, 2006. [Google Scholar] [CrossRef]
- Hu, L.; Bian, X.J.; Yang, X.L. Turf Science and Management; China Agricultural University Press: Beijing, China, 2001. [Google Scholar]
- Griffith, S.; Bero, N.; Stier, J.; Obear, G.; Ruis, S.; Soldat, D. Biosolids as an Alternative Fertilizer for Kentucky Bluegrass Sod Production in Wisconsin. Crop Sci. 2017, 57, S227–S237. [Google Scholar] [CrossRef]
- Zhang, J.; Maleski, J.; Jespersen, D.; Waltz, F.C.; Rains, G.; Schwartz, B. Unmanned Aerial System-Based Weed Mapping in Sod Production Using a Convolutional Neural Network. Front. Plant Sci. 2021, 12, 702626. [Google Scholar] [CrossRef] [PubMed]
- Braun, R.C.; Watkins, E.; Hollman, A.B.; Mihelich, N.T.; Patton, A.J. Investigation of cool-season species, seeding rate, and nitrogen fertilization in sod production: II. Management and shelf-life. Agron. J. 2021, 113, 3460–3474. [Google Scholar] [CrossRef]
- Griffith, S.; Bero, N.; Stier, J.; Obear, G.; Ruis, S.J.; Soldat, D. Use of biosolids for sod production: Impact on the import/export of nutrients, heavy metals, and soil mineral matter. Agron. J. 2020, 112, 3371–3382. [Google Scholar] [CrossRef]
- Braun, R.C.; Watkins, E.; Hollman, A.B.; Mihelich, N.T.; Patton, A.J. Investigation of cool-season species, seeding rate, and nitrogen fertilization in sod production: I. Establishment and sod tensile strength. Agron. J. 2021, 113, 4176–4189. [Google Scholar] [CrossRef]
- Li, D.Y.; Fang, W.J.; Han, L.B. Nitrogen Fertilization Influences Shear Strength and Quality of Kentucky Bluegrass Sod Grown on Clay. Agron. J. 2011, 103, 751–755. [Google Scholar] [CrossRef]
- Cui, J.Y.; Mu, K.G.; Hu, L.; Zhang, F.S.; Xu, S.H. Studies on the effects of sod-production on soil quality in Beijing area. Pratacultural Sci. 2003, 20, 68–72. [Google Scholar]
- Lin, H.S.; Yu, Y.M.; Xie, X.H. Effect of Continuous Annual Carpet Sod Production on Soil Properties. North. Hortic. 2009, 6, 172–174. [Google Scholar]
- Tesfamariam, E.H.; Annandale, J.G.; Steyn, J.M.; Stirzaker, R.J. Exporting Large Volumes of Municipal Sewage Sludge through Turfgrass Sod Production. J. Environ. Qual. 2009, 38, 1320–1328. [Google Scholar] [CrossRef] [PubMed]
- Parlak, M.; Everest, T.; Ruis, S.J.; Blanco, H. Impact of urbanization on soil loss: A case study from sod production. Environ. Monit. Assess. 2020, 192, 588. [Google Scholar] [CrossRef]
- Chapin, F.S., III; McFarland, J.; McGuire, A.D.; Euskirchen, E.S.; Ruess, R.W.; Kielland, K. The changing global carbon cycle: Linking plant-soil carbon dynamics to global consequences. J. Ecol. 2009, 97, 840–850. [Google Scholar]
- Griffiths, B.S.; Philippot, L. Insights into the resistance and resilience of the soil microbial community. Fems Microbiol. Rev. 2013, 37, 112–129. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.B.; He, H.D.; Li, H.Q.; Yang, Y.S.; Wei, Y.X.; Luo, J.; Li, Y.N. Characteristics of Soil Bulk Density and Soil Water-Holding Capacity in Alpine Meadow Under Grazing Gradients. Res. Water Conserv. 2018, 25, 66–71. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Analysis in Agricultural Chemistry; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Bossio, D.A.; Scow, K.M.; Gunapala, N.; Graham, K.J. Determinants of soil microbial communities: Effects of agricultural management, season, and soil type on phospholipid fatty acid profiles. Microb. Ecol. 1998, 36, 1–12. [Google Scholar] [CrossRef]
- van Groenigen, K.J.; Osenberg, C.W.; Hungate, B.A. Increased soil emissions of potent greenhouse gases under increased atmospheric CO2. Nature 2011, 475, 214–216. [Google Scholar] [CrossRef]
- Liu, Q.F.; Li, M.D.; Duan, J.N.; Wu, H.Y. The Spatio-temporal Variation of Benefit of Cultivated Land Use Based on GIS Technology in Hunan Province. Econ. Geogr. 2013, 33, 142–147. [Google Scholar]
- Wang, Q.L.; Cao, Y.; Wang, B. Effects of sand covering on phycochemical properties of saline alkali soil and ryegrass growth. Land Dev. Eng. Res. 2018, 3, 53–57. [Google Scholar]
- Tao, J. Time-Scale Effect of Different Herb Plant Roots Distribution on Soil Physical and Chemical Properties in The Three Gorges Reservoir Area. Master’s Thesis, Southwest University, Chongqing, China, 2013. [Google Scholar]
- Zhang, H.H.; Wang, S.B.; Wang, J.R.; Wu, X.Y.; Ma, S.L.; Wu, Y.N.; Li, J.B.; Xu, N. Effects of different land use types on soil physicochemical properties and aggregate composition in Sanjiang Plain wetland. Chin. J. Ecol. 2019, 38, 1679–1687. [Google Scholar]
- Montemurro, F.; Maiorana, M.; Lacertosa, G. Plant and soil nitrogen indicators and performance of tomato grown at different nitrogen fertilization levels. J. Food Agric. Environ. 2007, 5, 143–148. [Google Scholar]
- Cheng, W.J.; Cui, J.Y.; Min, F.H.; Hu, L. Root distribution characteristics of three turfgrasses and their impact on soil nutrient content. Acta Prataculturae Sin. 2009, 18, 179–183. [Google Scholar]
- Robertson, G.P.; Gross, K.L.; Hamilton, S.K.; Landis, D.A.; Schmidt, T.M.; Snapp, S.S.; Swinton, S.M. Farming for Ecosystem Services: An Ecological Approach to Production Agriculture. Bioscience 2014, 64, 404–415. [Google Scholar] [CrossRef]
- Liu, X.; Herbert, S.J.; Hashemi, A.M.; Zhang, X.; Ding, G. Effects of agricultural management on soil organic matter and carbon transformation—A review. Plant Soil Environ. 2006, 52, 531–543. [Google Scholar] [CrossRef]
- Vesterdal, L.; Ritter, E.; Gundersen, P. Change in soil organic carbon following afforestation of former arable land. For. Ecol. Manag. 2002, 169, 137–147. [Google Scholar] [CrossRef]
- Zheng, L.W. Effect of Root System on Soil Properties. Master’s Thesis, Beijing Forestry University, Beijing, China, 2015. [Google Scholar]
- Qian, J. Study on Association Nitrogen Fixation with Azotobacter Chroococcum and Bermudagrass. Master’s Thesis, Yangzhou University, Yangzhou, China, 2020. [Google Scholar]
- Lu, S.F.; Wang, C.Y.; Du, Y.; Wu, Y.S.; Gao, Y.H.; Liu, S.X. Effects of rice planting years on physicochemical property and fungi community in soda saline-alkali soil. South China Agric. Univ. 2019, 40, 15–22. [Google Scholar]
- Wang, N.; Zang, J.; Guo, X.; Wang, H.; Huang, N.; Zhao, C.; Zhao, X.; Liu, J. Role of rice cultivation on fluorine distribution behavior in soda saline-alkali land. Sci. Total Environ. 2022, 835, 155543. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.P.; Tao, Y.X.; Wang, L.J.; Ji, J.H. The Effect of Different Cultivating on Nutrient and Crop Yield in Albic Soils. Heilongjiang August First Land Reclam. Univ. 2002, 9–11. [Google Scholar]
- Martin-Rueda, I.; Munoz-Guerra, L.M.; Yunta, F.; Esteban, E.; Tenorio, J.L.; Lucena, J.J. Tillage and crop rotation effects on barley yield and soil nutrients on a Calciortidic Haploxeralf. Soil Tillage Res. 2007, 92, 1–9. [Google Scholar] [CrossRef]
- Wang, Z.; Xiao, L.W.; Tan, Q.X.; Tian, L.L.; Zhu, B. Nitrogen and Phosphorus Absorption from Soil by the Dominant Herbaceous Species in the Water-Level-Fluctuation Zone of the Three Gorges Reservoir. Mt. Res. 2019, 37, 151–160. [Google Scholar]
- Garg, D.; Patel, N.; Rawat, A.; Rosado, A.S. Cutting edge tools in the field of soil microbiology. Curr. Res. Microb. Sci. 2024, 6, 100226. [Google Scholar] [CrossRef] [PubMed]
- Nan, L.L.; Shi, S.L.; Yu, J.h. Soil Microbial Properties in Alfalfa Field with Different Growing Years in Arid Desert Oasis. Acta Agrestia Sin. 2016, 24, 975–980. [Google Scholar]
- Liu, Y.J.; Wang, W.J.; Wang, H.G.; Wang, Q.; Hu, Q.G.; Chu, F.L. Effects of Crop Rotation on Soil Microbial Community in Sweet Potato Field. Crops 2021, 122–128. [Google Scholar]
- Li, R.; Liu, Y.; Chu, G.X. Effects of different cropping patterns on soil enzyme activities and soil microbial community diversity in oasis farmland. Chin. J. Appl. Ecol. 2015, 26, 490–496. [Google Scholar]
- Zhou, J.H.; Gao, R.R.; Wei, Q.; Yuan, Y.H.; Pu, H.Y. Effects of Different Land Use Patterns on Enzyme Activities and Microbial Diversity in Upland Red Soil. Soil Water Conserv. 2020, 34, 327–332. [Google Scholar]
- Wang, X.Y.; Zeng, M.; Wu, H.H. Analysis on the effect of microorganisms on soil rot transformation. South China Agric. 2018, 12, 190–193. [Google Scholar]
- Gu, H.F. Differences and Influencing Factors of PLFA Fingerprints under Different Land Use Patterns in Red Soil. Master’s Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2021. [Google Scholar]
- Cao, Z.P.; Li, D.P.; Han, X.M. The fungal to bacterial ratio in soil food webs, and its measurement. Acta Ecol. Sin. 2011, 31, 4741–4748. [Google Scholar]
- Du, Z.; Xie, Y.; Hu, L.; Hu, L.; Xu, S.; Li, D.; Wang, G.; Fu, J. Effects of Fertilization and Clipping on Carbon, Nitrogen Storage, and Soil Microbial Activity in a Natural Grassland in Southern China. PLoS ONE 2014, 9, e99385. [Google Scholar] [CrossRef]
- Galicia, L.; García-Oliva, F. The effects of C, N and P additions on soil microbial activity under two remnant tree species in a tropical seasonal pasture. Appl. Soil Ecol. 2004, 26, 31–39. [Google Scholar] [CrossRef]
- Zhu, K.; Wang, R.; Li, G.; Xiu, W.M.; Wang, J.; Li, B.; Wang, L.L.; Liu, H.F.; Zhao, J.N.; Yang, D.L. The response of microbial biomass carbon and metabolic characteristics of albic soil to land use change. Agro-Environ. Sci. 2018, 37, 2194–2201. [Google Scholar]
- Wang, Z.F. Characteristics of Soil Nutrients and Enzyme Activity Under Different Types of Land Use in Wetland of Sanjiang Plain. Res. Soil Water Conserv. 2019, 26, 43–48. [Google Scholar]
- Du, N.N.; Qiu, L.P.; Zhang, X.C.; Cheng, J.M. Effect of land use on mineralization of soil carbon and nitrogen in semi-arid grasslands. Agric. Res. Arid Areas 2017, 35, 73–78. [Google Scholar]
- Yan, P.C. Study on the nutrient and enzyme activity and microorganism property for the soil of three forests types in mountainous region from eastern Liaoning Province. Master’s Thesis, Shenyang Agricultural University, Shenyang, China, 2018. [Google Scholar]



| Sampling Site | Location | Climate Type | Annual Rainfall | Annual Mean Temperature | Maximum Summer Temperature | Minimum Winter Temperature | Annual Mean Insolation |
|---|---|---|---|---|---|---|---|
| Yongqing County, Langfang City, Hebei Province of China | 39°18′ N 116°24′ E | Warm temperate continental monsoon | 540 mm | 11.5 °C | 39 °C | −17 °C | 2740 h |
| Shunyi District, Beijing City of China | 40°7′ N 116°39′ E | Temperate continental semi-humid monsoon | 610 mm | 11.5 °C | 39 °C | −16 °C | 2746 h |
| Renze County Xingtai City, Hebei Province of China | 39°7′ N 114°43′ E | Temperate continental monsoon | 485 mm | 12.8 °C | 40 °C | −17 °C | 2440 h |
| Songjiang District, Shanghai City of China | 30°57′ N 121°18′ E | Subtropical monsoon | 1103 mm | 15.6 °C | 40 °C | −8 °C | 1817 h |
| Jinshan District, Shanghai City of China | 30°47′ N 121°8′ E | Subtropical monsoon | 1158 mm | 16 °C | 38 °C | −8 °C | 2021 h |
| Pinghu City, Jiaxing City, Zhejiang Province of China | 30°42′ N 121°1′ E | Subtropical humid | 1170 mm | 16 °C | 38 °C | −8 °C | 2017 h |
| Sampling Locations | Age of Sod Production (Year) | Planting Turfgrass | Cutting Frequency (per Year) | Mowing Frequency (per Cutting) | Fertilization Frequency (per Cutting) | Fertilization Dose (Compound Fertilizer Applied per Time, g/m2) | Irrigation (per Cutting) | Irrigation Duration (per Time/Min) | Other Tillage Practices | Control Plot | Abbreviation | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| North | Yongqing County, Langfang City, Hebei Province bright turf industry base | 3 | Tall fescue:Kentucky bluegrass (9:1) | 2 | Once a week | 3 | 30 | Water the seeds daily until emergence, then every other day | 30 | Deep plowing annually before turf planting | Corn and wheat | N-3 |
| Yongqing County, Langfang City, Hebei Province, Shunqing agricultural development turf industry base | 8 | Tall fescue (corn will be sown in June to September) | 3 (tow years) | When the turfgrass grows slowly, it is once a half month, and when it is fast, it is once every 10 days | 3 | 35 once or 22 twice | Water the seeds daily until emergence, then every other day | 30 | Deep plowing annually before turf planting | Forest | N-8 | |
| Beijing, Shunyi District, Beijing Green Garden turf planting base | 12 | Tall fescue:Kentucky bluegrass (15:8) | 1–2 | When the turfgrass grows slowly, it is once a half month, and when it is fast, it is once a week | 3 | 15 | Two days at a time | 30 | Deep plowing annually before turf planting | Forest | N-12 | |
| 6 | Tall fescue | N-6 | ||||||||||
| Xingtai City, Hebei Province, Renze County, Jingtai Garden family farm turf base | 10 | Tall fescue | 2–3 | When the turfgrass grows slowly, it is once a half month, and when it is fast, it is once a week | 3 | 30 once or 22 twice | Water every day until the sod is formed, and keep the ground moist after formation | 30–60 | Deep plowing annually before turf planting | Corns and wheat | N-10 | |
| 5 | Kentucky bluegrass | N-5 | ||||||||||
| 2.5 | Kentucky bluegrass | N-2.5 | ||||||||||
| South | Shanghai, Xuan, Yuan Lawn Company, Songjiang Ye Xie Unity Base | 14 | Bermudagrass (annual ryegrass will be sown in October) | 1–2 | Once or twice a week | 3 | 30 once or 25 twice | Once or twice a week | 30 | Sand mulching | Rice | S-14 |
| 11 | Creeping Bentgrass | 4 | First time at 10 and gradually increased to 25 | Water the seeds daily until germination, once a week after germination of 3–4 leaves | S-11 | |||||||
| Shanghai, Xuan, Yuan Lawn Company Shanghai, Jinshan, Langxia Zhonghua Base | 17 | Bermudagrass (annual ryegrass will be sown in October) | 1–2 | Once or twice a week | 3 | 15 | Two or three times a week | 30 | Sand mulching | Forest | S-17 | |
| Xuanjing Lawn, Yang Sheng Base, Yang Sheng Road, Pinghu City, Jiaxing City, Zhejiang Province | 10 | Bermudagrass (annual ryegrass will be sown in September) | 2 | Once or twice a week | 3 | 15 | Two or three times a week | 30 | Sand mulching | Forest | S-10 | |
| Jiaxing City, Zhejiang Province, Xincang Town, Pinghu City, Green Yi Lawn Professional Cooperative Lawn Base | 3 | Bermudagrass (annual ryegrass will be sown in October) | 2 | Once a half month | 3 | 22 | Once every 2–3 days in summer and once a week in winter | 30 | Sand mulching | Rice | S-3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, X.; Li, Y.; Wang, C.; Qiao, J.; Zhu, K.; Sun, Y.; Hu, Q. Effect of Sod Production on Physical, Chemical, and Biological Properties of Soils in North and South China. Agriculture 2024, 14, 1786. https://doi.org/10.3390/agriculture14101786
Qu X, Li Y, Wang C, Qiao J, Zhu K, Sun Y, Hu Q. Effect of Sod Production on Physical, Chemical, and Biological Properties of Soils in North and South China. Agriculture. 2024; 14(10):1786. https://doi.org/10.3390/agriculture14101786
Chicago/Turabian StyleQu, Xinyue, Yue Li, Chu Wang, Jiayue Qiao, Kai Zhu, Yan Sun, and Qiannan Hu. 2024. "Effect of Sod Production on Physical, Chemical, and Biological Properties of Soils in North and South China" Agriculture 14, no. 10: 1786. https://doi.org/10.3390/agriculture14101786
APA StyleQu, X., Li, Y., Wang, C., Qiao, J., Zhu, K., Sun, Y., & Hu, Q. (2024). Effect of Sod Production on Physical, Chemical, and Biological Properties of Soils in North and South China. Agriculture, 14(10), 1786. https://doi.org/10.3390/agriculture14101786






