Study of Pollen Traits, Production, and Artificial Pollination Methods in Zea mays L.
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. In Vitro Pollen Germination and Measurement of Pollen Diameter
2.3. Determination of Flavonoids and Phenolic Acids Content
2.4. Analysis of Tassel Morphology
2.5. “Smart” Pollination
2.6. Evaluation of Pollen Longevity in the Field
2.7. Informatic Tools
3. Results
3.1. Analysis of Pollen Germinability and Diameter, Flavonoid and Phenolic Acid Content
3.2. Analysis of Tassel Morphology
3.3. “Smart” Pollination Accuracy
3.4. Pollen Longevity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ertdman, G. Pollen Morphology and Plant Taxonomy, Angiosperms; Almqvist and Wiksell: Stockholm, Sweden, 1952. [Google Scholar] [CrossRef]
- Smith, E.G. Sampling and Identifying Allergenic Pollens and Molds; Blewstone Press: San Antonio, TX, USA, 1990; ISBN 0930961021/9780930961022. [Google Scholar]
- Percival, M.S. Pollen presentation and pollen collection. New Physiol. 1950, 49, 40–63. [Google Scholar] [CrossRef]
- Purseglov, J.W. Tropical Crops. Monocotyledons; Longiman Group: London, UK, 1972. [Google Scholar]
- Shull, G.H. The composition of a field of maize. J. Hered. 1908, 4, 296–301. [Google Scholar] [CrossRef]
- Shull, G.H. What is Heterosis? Genetics 1948, 33, 439–446. [Google Scholar] [CrossRef]
- Shull, G.H. Beginnings of the heterosis concept. In Heterosis; Gowen, J.W., Ed.; Iowa State College Press: Ames, IA, USA, 1952; pp. 14–48. [Google Scholar]
- East, E.M. Heterosis. Genetics 1936, 21, 375–397. [Google Scholar] [CrossRef]
- Rodgers, H.J.; Parkes, H.C. Transgenic plants and the environment. J. Exp. Bot. 1995, 46, 467–488. [Google Scholar]
- Louette, D.; Charrier, A.; Berthaud, J. In situ conservation of maize in Mexico: Genetic diversity and maize seed management in a traditional community. Econ. Bot. 1997, 51, 20–38. [Google Scholar] [CrossRef]
- Garcia, C.; Figueroa, M.J.; Gomez, L.; Towsend, R.; Schoper, J. Pollen control during transgenic hybrid maize development in Mexico. Crop Sci. 1998, 38, 1597–1602. [Google Scholar] [CrossRef]
- Oz, A.; Tugay, M.E. Variation on some agronomic characters in selfing generations in corn (Zea mays indentata Sturt). JAFAG 2003, 20, 123–132. [Google Scholar]
- Ogden, E.C.; Hayes, J.V.; Raynor, G.S. Diurnal patterns of pollen emission in Ambrosia, Phleum, Zea and Ricinus. Am. J. Bot. 1969, 56, 16–21. [Google Scholar] [CrossRef]
- Jarosz, N.; Loubet, B.; Durand, B.; Mc Cartney, H.A.; Foueillassar, X.; Huber, L. Field measurement of airborne concentration and deposition rate of maize pollen (Zea mays L.) downwind of an experimental field plot. Agric. For. Meteorol. 2003, 119, 37–51. [Google Scholar] [CrossRef]
- Kiesselbach, T.A. The Structure and Reproduction of Corn; Research Bulletin; University of Nebraska Press: Lincoln, NE, USA, 1949; p. 161. [Google Scholar]
- Saini, H. Effects of water stress on male gametophyte development in plants. Sex. Plant Reprod. 1997, 10, 67–73. [Google Scholar] [CrossRef]
- Barnabás, B. Effect of water loss on germination ability of maize (Zea mays L.) pollen. Ann. Bot. 1985, 55, 201–204. [Google Scholar] [CrossRef]
- Buitink, J.; Walters-Vertucci, C.; Hoekstra, F.A.; Leprince, O. Calorimetric properties of dehydrating pollen (analysis of a desiccation-tolerant and an intolerant species). Plant Physiol. 1996, 111, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Luna, S.; Figueroa, J.M.; Baltzar, M.; Gomez, L.; Towsend, R.; Schoper, J.B. Maize pollen longevity and distance isolation requirements for effective pollen control. Crop Sci. 2001, 41, 1551–1557. [Google Scholar] [CrossRef]
- Wang, S.; Xie, B.; Yin, L.; Duan, L.; Li, Z.; Eneji, A.E.; Tsuji, W.; Tsunekawa, A. Increased UV-B radiation affects the viability, reactive oxygen species accumulation and antioxidant enzyme activities in maize (Zea mays L.) pollen. Photochem. Photobiol. 2010, 86, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bruckmann, A.; Dresselhaus, T.; Begcy, K. Heat stress at the bicellular stage inhibits sperm cell development and transport into pollen tubes. Plant Physiol. 2024, 195, 2111–2128. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, X.; Sheng, D.; Hou, X.; Mandal, S.; Liu, X.; Zhang, P.; Shen, S.; Wang, P.; Krishna Jagadish, S.V.; et al. Heat-dependent postpollination limitations on maize pollen tube growth and kernel sterility. Plant Cell Environ. 2023, 46, 3822–3838. [Google Scholar] [CrossRef]
- Mo, Y.; Nagel, C.; Taylor, L.P. Biochemical complementation of chalcone synthase mutants defines a role for flavonols in functional pollen. Proc. Natl. Acad. Sci. USA 1992, 89, 7213–7217. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shirley, B.V. Flavonoids biosynthesis: “new” functions for an “old” pathway. Trends Plant Sci. 1996, 1, 11. [Google Scholar]
- Duvick, D.N.; Cassmann, K.G. Post-green revolution trends in yield potential of temperate maize in the North-Central United States. Crop Sci. 1999, 39, 16221630. [Google Scholar] [CrossRef]
- Postlethwait, S.N.; Nelson, O.E. Characterization of development in maize through the use of mutants. The polytypic (Pt) and ramosa-1 (Ra1) mutants. Am. J. Bot. 1964, 51, 238–243. [Google Scholar] [CrossRef]
- Fonseca, A.E.; Westgate, M.E.; Grass, L.; Dornbos, D.L. Tassel Morphology as an Indicator of Potential Pollen Production in Maize. Crop Manag. 2003, 2, 1–15. [Google Scholar] [CrossRef]
- Bódi, Z.; Pepó, P.; Kovács, A. Morphology of tassel components and their relationship to some quantitative features in maize. Cereal Res. Commun. 2008, 36, 353–360. [Google Scholar] [CrossRef]
- Ricci, B.; Monod, H.; Guérin, D.; Messéan, A.; Maton, C.; Balique, B.; Angevin, F. Predicting maize pollen production using tassel morphological characteristics. Field Crop Res. 2012, 136, 107–115. [Google Scholar] [CrossRef]
- Dari, S.; MacRobert, J.; Labuschagne, M. Effect of the few-branched-1 (Fbr1) tassel mutation on performance of maize inbred lines and hybrids evaluated under stress and optimum environments. Maydica 2016, 62. [Google Scholar]
- Gallavotti, A.; Long, J.A.; Stanfield, S.; Yang, X.; Jackson, D.; Vollbrecht, E.; Schmidt, R.J. The control of axillary meristem fate in the maize ramosa pathway. Development 2010, 137, 2849–2856. [Google Scholar] [CrossRef]
- Cassani, E.; Landoni, M.; Pilu, R. Characterization of the Ra1 mazie gene involved in inflorescence architecture. Sex. Plant Reprod. 2006, 19, 145–150. [Google Scholar] [CrossRef]
- Krieger, K.M.; Pollak, L.M.; Brumm, T.J.; White, P.J. Effects of pollination method and growing location on starch thermal properties of corn hybrids. Cereal Chem. 1998, 75, 656–659. [Google Scholar] [CrossRef]
- Letchworth, M.B.; Lambert, R.J. Pollen parent effects on oil, protein, and starch concentration in maize kernels. Crop Sci. 1998, 38, 363–367. [Google Scholar] [CrossRef]
- Sulewska, H.; Adamczzyk, H.; Gygert, J.; Rogacki, G.; Szymanska, K.; Smiatacz, K.; Panasiewicz, K.; Tomaszyk, K. A comparison of controlled self-pollination and open pollination results based on maize grain quality. Span. J. Agric. Res. 2014, 12, 492–500. [Google Scholar] [CrossRef]
- Kahriman, F.; Egesel, C.O.; Aydin, T.; Subasi, S. The role of artificial pollination and pollen effect on ear development and kernel structure of different maize genotypes. J. Pollinat. Ecol. 2015, 15, 6–14. [Google Scholar] [CrossRef]
- Kahriman, F.; Egesel, C.O.; Zorlu, E. Effects of open and self-pollination treatments on genetic estimations in maize diallel experiment. Span. J. Agric. Res. 2015, 13, e0704. [Google Scholar] [CrossRef]
- Broussard, M.A.; Coates, M.; Martinsen, P. Artificial Pollination Technologies: A Review. Agronomy 2023, 13, 1351. [Google Scholar] [CrossRef]
- Lago, C.; Landoni, M.; Cassani, E.; Cantaluppi, E.; Doria, E.; Nielsen, E.; Giorgi, A.; Pilu, R. Study and characterization of an ancient European flint white maize rich in anthocyanins: Millo Corvo from Galicia. PLoS ONE 2015, 10, e0126521. [Google Scholar]
- Giupponi, L.; Leoni, V.; Colombo, F.; Cassani, E.; Hejna, M.; Rossi, L.; Pilu, R. Characterization of “Mais delle Fiorine” (Zea mays L.) and nutritional, morphometric and genetic comparison with other maize landraces of Lombardy region (Northern Italy). Genet. Resour. Crop Evol. 2021, 68, 2075–2091. [Google Scholar] [CrossRef]
- Sangiorgio, S.; Colombo, F.; Ghidoli, M.; Giupponi, L.; Ferro, G.; Ferro, C.G.; Cassani, E.; Landoni, M.; Pilu, R. The ancient varieties of mountain maize: The inheritance of the pointed character and its effect on the natural drying process. Agronomy 2021, 11, 2295. [Google Scholar] [CrossRef]
- Lago, C.; Landoni, M.; Cassani, E.; Atanassiu, S.; Cantaluppi, E.; Pilu, R. Development and characterization of a coloured sweet corn line as a new functional food. Maydica 2014, 59, 191–200. [Google Scholar]
- Landoni, M.; Cassani, E.; Ghidoli, M.; Colombo, F.; Sangiorgio, S.; Papa, G.; Adani, F.; Pilu, R. Brachytic2 mutation is able to counteract the main pleiotropic effects of brown midrib3 mutant in maize. Sci. Rep. 2022, 12, 2446. [Google Scholar] [CrossRef]
- Chen, Z.J.; Cong, Y.; Tang, D.G.; Zhang, L.; Zhang, L.; Qu, J.T.; Jian, L. Dissection of the genetic architecture for tassel branch number by QTL analysis in two related populations in maize. J. Integr. Agric. 2017, 16, 1432–1442. [Google Scholar] [CrossRef]
- Aylor, D.E. Rate of dehydration of corn (Zea mays L.) pollen in the air. J. Exp. Bot. 2003, 54, 2307–2312. [Google Scholar] [CrossRef][Green Version]
- Torabinejad, J.; Caldwell, M.M.; Flint, S.D.; Durham, S. Susceptibility of pollen to UV-B radiation: An assay of 34 taxa. Am. J. Bot. 1998, 85, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Youmbi, E.; The, C.; Tedjacno, A. Conservation of the germination capacity of pollen grains in three varieties of maize (Zea mays L.). Grana 2005, 44, 152–159. [Google Scholar] [CrossRef]
- Kaefer, K.A.C.; Chiapetti, R.; FogaÃ, L.; Muller, A.L.; Calixto, G.B.; Dallâ, E.I.; Chaves, Ã. Viability of maize pollen grains in vitro collected at different times of the day. Afr. J. Agric. Res. 2016, 11, 1040–1047. [Google Scholar]
- Lephatsi, M.; Nephali, L.; Meyer, V.; Piater, L.A.; Buthelezi, N.; Dubery, I.A.; Opperman, H.; Brand, M.; Huyser, J.; Tugizimana, F. Molecular mechanisms associated with microbial biostimulant-mediated growth enhancement, priming and drought stress tolerance in maize plants. Sci. Rep. 2022, 21, 12–10450. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions and biotechnological applocations. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef]
- Westgate, M.E.; Lizaso, J.; Batchelor, W. Quantitative relationships between pollen shed density and grain yield in maize. Crop Sci. 2003, 43, 934–942. [Google Scholar] [CrossRef]
- Ruidong, S.; Shijin, H.; Yuwei, Q.; Yimeng, L.; Xiaohang, Z.; Ying, L.; Xihang, L.; Mingyang, D.; Xiangling, L.; Fenghai, L. Identification of QTLs and their candidate genes for the number of maize tassel branches in F2 from two higher generation sister lines using QTL mapping and RNA-seq analysis. Front. Plant Sci. 2023, 14, 1202755. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bortiri, E.; Chuck, G.; Vollbrecht, E.; Rocheford, T.; Martienssen, R.; Hake, S. ramosa2 encodes a LATERAL ORGAN BOUNDARY domain protein that determines the fate of stem cells in branch meristems of maize. Plant Cell 2006, 18, 574–585. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Satoh-Nagasawa, N.; Nagasawa, N.; Malcomber, S.; Sakai, H.; Jackson, D. A trehalose metabolic enzyme controls inflorescence architecture in maize. Nature 2006, 441, 227–230. [Google Scholar] [CrossRef]
- Landoni, M.; Cassani, E.; Pilu, R. Arabidopsis thaliana plants overexpressing Ramosa 1 maize gene show an increase in organ size due to cell expansion’. Sex. Plant Reprod. 2007, 20, 191–198. [Google Scholar] [CrossRef]
- Wang, Y.; Bao, J.; Wei, X.; Wu, S.; Fang, C.; Li, Z.; Qi, Y.; Gao, Y.; Dong, Z.; Wan, X. Genetic Structure and Molecular Mechanisms Underlying the Formation of Tassel, Anther, and Pollen in the Male Inflorescence of Maize (Zea mays L.). Cells 2022, 11, 1753. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, S.; Jiang, Y.; Yan, T.; Fang, C.; Hou, Q.; Wu, S.; Xie, K.; An, X.; Wan, X. Use of CRISPR/Cas9-Based Gene Editing to Simultaneously Mutate Multiple Homologous Genes Required for Pollen Development and Male Fertility in Maize. Cells 2022, 11, 439. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Zhang, S.; Jiang, Y.; Liu, X.; Fang, C.; Wang, J.; Zhao, L.; Hou, Q.; Zhang, J.; Xiangyuan Wan, X.; et al. CRISPR/Cas9-based genome editing of 14 lipid metabolic genes reveals a sporopollenin metabolon ZmPKSB-ZmTKPR1-1/-2 required for pollen exine formation in maize. Plant Biotechnol. J. 2024, 22, 216–232. [Google Scholar] [CrossRef]
- Errum, A.; Rehman, N.; Uzair, M.; Inam, S.; Ali, G.M.; Khan, M.R. CRISPR/Cas9 editing of wheat Ppd-1 gene homoeologs alters spike architecture and grain morphometric traits. Funct. Integr. Genom. 2023, 23, 66. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Lindsay, P.L.; Jackson, D. Next generation cereal crop yield enhancement: From knowledge of inflorescence development to practical engineering by genome editing. Int. J. Mol. Sci. 2021, 22, 5167. [Google Scholar] [CrossRef] [PubMed]
- Van Eck, J. Genome editing of tomatoes and other Solanaceae. In Genome Editing for Precision Crop Breeding; Burleigh Dodds Science Publishing: Cambridge, UK, 2021; pp. 421–440. [Google Scholar]
- Rodríguez-Leal, D.; Lemmon, Z.H.; Man, J.; Bartlett, M.E.; Lippman, Z.B. Engineering quantitative trait variation for crop improvement by genome editing. Cell 2017, 171, 470–480. [Google Scholar] [CrossRef]
- Kwon, C.T.; Heo, J.; Lemmon, Z.H.; Capua, Y.; Hutton, S.F.; Van Eck, J.; Park, S.J.; Lippman, Z.B. Rapid customization of Solanaceae fruit crops for urban agriculture. Nat. Biotechnol. 2020, 38, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Sriboon, S.; Li, H.; Guo, C.; Senkhamwong, T.; Dai, C.; Liu, K. Knock-out of TERMINAL FLOWER 1 genes altered flowering time and plant architecture in Brassica napus. BMC Genet. 2020, 21, 52. [Google Scholar] [CrossRef]
- Farinati, S.; Draga, S.; Betto, A.; Palumbo, F.; Vannozzi, A.; Lucchin, M.; Barcaccia, G. Current insights and advances into plant male sterility: New precision breeding technology based on genome editing applications. Front. Plant Sci. 2023, 14, 1223861. [Google Scholar] [CrossRef]
- Della Porta, G.; Ederle, D.; Bucchini, L.; Prandi, M.; Verderio, A.; Pozzi, C. Maize pollen mediated gene flow in the Po valley (Italy): Source–recipient distance and effect of flowering time. Eur. J. Agron. 2008, 28, 255–265. [Google Scholar] [CrossRef]
- Devos, Y.; Reheul, D.; de Schrijver, A. ‘The co-existence between transgenic and non-transgenic maize in the European Union: A focus on pollen flow and cross-fertilization’. Environ. Biosafety Res. 2005, 4, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Hollick, J.B.; Patterson, G.I.; Asmundsson, I.M.; Chandler, V.L. ‘Paramutation alters regulatory control of the maize pl locus’. Genetics 2000, 154, 1827–1838. [Google Scholar] [CrossRef] [PubMed]
- Pilu, R. Paramutation: Just a Curiosity or Fine Tuning of Gene Expression in the next generation? Curr. Genom. 2011, 12, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Pilu, R. Paramutation phenomena in plants. Semin. Cell. Biol. 2015, 44, 2–10. [Google Scholar] [CrossRef]
- Ceccarelli, S.; Grando, S. Decentralized-participatory plant breeding: An example of demand driven research. Euphytica 2007, 155, 349–360. [Google Scholar] [CrossRef]


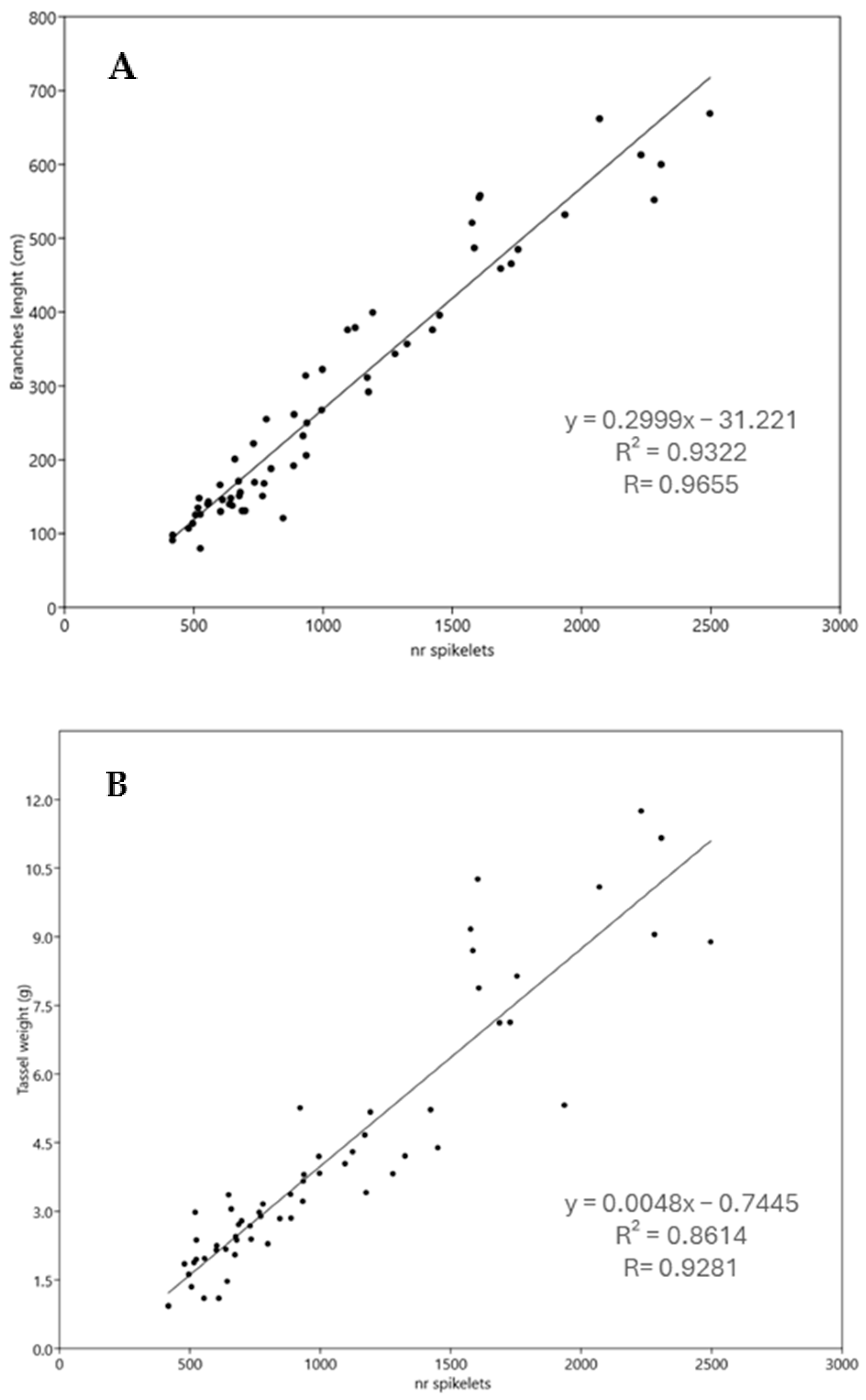
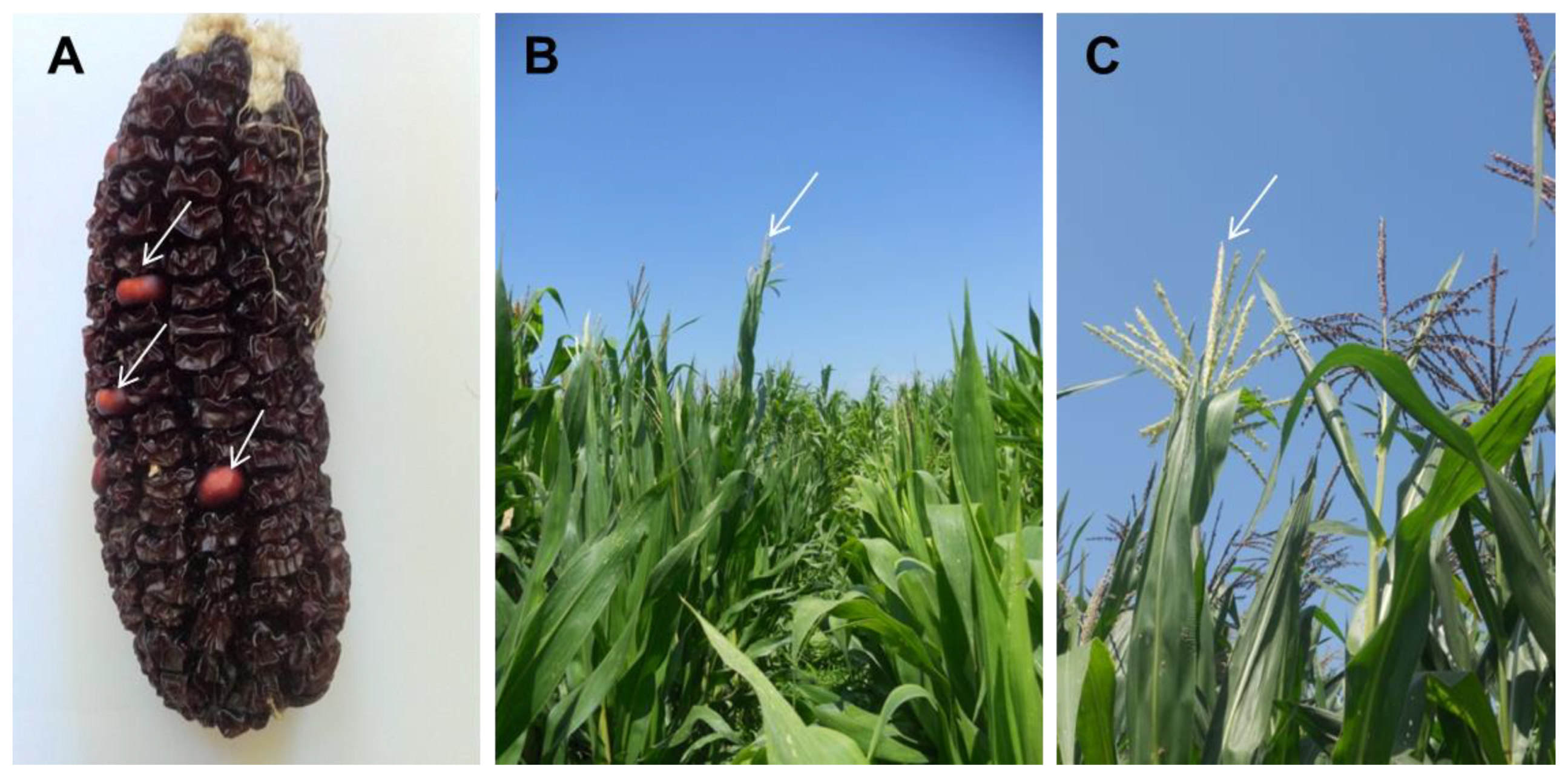

| Line ID | % of Pollen Germinability | Pollen Diameter (µm) | Flavonoids Content (mg/100 g) | Phenolic Acids Content (mg/100 g) |
|---|---|---|---|---|
| R1 | 48.51 ± 5.71 bc | 80.80 ± 1.63 b | 661.57 ± 45.71 b | 4.27 ± 0.48 bcd |
| R2 | 50.37 ± 1.87 bc | 82.33 ± 2.12 b | 832.55 ± 43.82 c | 5.58 ± 0.68 d |
| R3 | n.d. | 82.11 ± 1.64 b | 578.43 ± 111.44 b | 3.67 ± 0.75 bc |
| R4 | 39.32 ± 9.50 ab | 80.67 ± 0.73 b | 219.26 ± 1.96 a | 1.64 ± 0.14 a |
| R5 | 61.48 ± 4.24 c | 61.00 ± 2.24 a | 552.85 ± 48.54 b | 3.39 ± 0.22 b |
| R6 | 79.82 ± 5.08 d | 79.67 ± 0.82 b | 643.11 ± 3.57 b | 4.63 ± 0.18 bcd |
| R7 | 47.77 ± 7.23 bc | 89.56 ± 4.45 c | 531.32 ± 25.98 b | 3.63 ± 0.32 bc |
| R8 | 61.33 ± 5.72 c | 79.25 ± 2.12 b | 530.07 ± 21.5 b | 3.33 ± 0.26 b |
| R9 | n.d. | 80.00 ± 1.41 b | 1208.87 ± 99.24 d | 9.13 ± 0.65 e |
| R10 | 36.54 ± 5.69 ab | 80.00 ± 2.19 b | 652.17 ± 54.5 b | 4.83 ± 0.22 cd |
| R11 | 60.05 ± 14.04 c | 80.00 ± 4.24 b | 654.05 ± 52.6 b | 5.60 ± 0.61 d |
| ID | Genotype | N° of Tassel Branches | Total Length of Tassel Branches (cm) | Tassel Weight (g) | Counted Spikelets Number |
|---|---|---|---|---|---|
| R 6708 | B73 | 12 ± 3.4 ab | 135 ± 21.56 a | 1.67 ± 0.43 a | 531 ± 47.40 a |
| R 6706 | Mo17 | 11 ± 3.6 a | 242 ± 63.77 ac | 3.10 ± 0.44 abc | 783 ± 179.22 ab |
| R 6603 | F1 B73/Mo17 | 8 ± 2.1 a | 128 ± 30.73 a | 1.36 ± 0.61 a | 554 ± 126.55 a |
| R 6732 | F2 B73/Mo17 | 8 ± 1.1 a | 169 ± 30.42 a | 3.09 ± 0.58 abc | 776 ± 134.72 ab |
| R 6600 | Hybrid PI 817 Pioneer FAO 700 | 7 ± 2.3 a | 186 ± 62.99 a | 2.35 ± 0.09 abc | 798 ± 223.37 ab |
| R 6601 | Hybrid LG 32.85 Limagrain FAO 200 | 8 ± 2.1 a | 161 ± 54.27 a | 2.28 ± 0.87 ab | 637 ± 180.69 a |
| R 6602 | Hybrid PR33A46 Pioneer FAO 500 | 6 ± 1.5 a | 123 ± 26.29 a | 2.74 ± 0.23 abc | 704 ± 118.42 ab |
| R 6571 | Nero Spinoso | 33 ± 4.3 d | 597 ± 44.82 d | 9.99 ± 1.57 e | 2099.13 ± 289.84 c |
| R 6573 | Rostrato Rosso di Rovetta | 25 ± 2.5 c | 503 ± 35.40 d | 8.68 ± 1.17 e | 1649.31 ± 84.75 c |
| R 6637 | Spinato di Gandino | 27 ± 6.9 cd | 479 ± 127.28 d | 6.15 ± 1.86 d | 1773 ± 469.18 c |
| R 6639 | Mais delle Fiorine | 25 ± 5.0 c | 334 ± 80.88 c | 3.96 ± 0.86 bc | 1000 ± 213.43 b |
| R 6640 | Millo Corvo | 20 ± 4.2 bc | 310 ± 63.90 cd | 4.47 ± 0.54 cd | 1163 ± 213.38 b |
| Canonical Pollination | Smart Pollination | |||
|---|---|---|---|---|
| Genotype and cross type | su1/su1 ⊗ | su1/su1 ⊗ | br2/br2 ⊗ | P11/Pl1 × pl1/pl1 |
| Total number of individuals | 14,557 | 10,696 | 266 | 1122 |
| Expected genotype | su1/su1 | su1/su1 | br2/br2 | Pl1/pl1 |
| N° of off-type phenotypes scored | 10 | 65 | 1 | 20 |
| Off-type genotype | su1/+ | su1/+ | br2/+ | pl1/pl1 |
| % of pollen contamination | 0.07 ± 0.25 a | 0.6 ±1.48 a | 0.38 ± 0.74 a | 1.78 ± 0.77 b |
| Pollen Storage Time | % of Seed Set (Mean ± SD) | Category (Mean Data) |
|---|---|---|
| T0 (10 AM) | 92 ± 7.58 a | 4 |
| +1 h | 93 ± 9.83 a | 4 |
| +2 h | 99 ± 2.04 a | 4 |
| +3 h | 90 ± 17.61 a | 4 |
| +24 h | 99 ± 2.04 a | 4 |
| +48 h | 84 ± 6.65 b | 4 |
| +72 h | 40 ± 17.79 c | 2 |
| +5 gg | 0.33 ± 0.52 d | 1 |
| +6 gg | 0 ± 0 d | 1 |
| +7 gg | 3 ± 7.56 d | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landoni, M.; Sangiorgio, S.; Ghidoli, M.; Cassani, E.; Pilu, R. Study of Pollen Traits, Production, and Artificial Pollination Methods in Zea mays L. Agriculture 2024, 14, 1791. https://doi.org/10.3390/agriculture14101791
Landoni M, Sangiorgio S, Ghidoli M, Cassani E, Pilu R. Study of Pollen Traits, Production, and Artificial Pollination Methods in Zea mays L. Agriculture. 2024; 14(10):1791. https://doi.org/10.3390/agriculture14101791
Chicago/Turabian StyleLandoni, Michela, Stefano Sangiorgio, Martina Ghidoli, Elena Cassani, and Roberto Pilu. 2024. "Study of Pollen Traits, Production, and Artificial Pollination Methods in Zea mays L." Agriculture 14, no. 10: 1791. https://doi.org/10.3390/agriculture14101791
APA StyleLandoni, M., Sangiorgio, S., Ghidoli, M., Cassani, E., & Pilu, R. (2024). Study of Pollen Traits, Production, and Artificial Pollination Methods in Zea mays L. Agriculture, 14(10), 1791. https://doi.org/10.3390/agriculture14101791









