Abstract
Biochar derived from poultry manure increases nutrient availability and promotes plant growth. This study investigated the effect of biochar with mycorrhizal and/or plant growth-promoting rhizobacteria on soil fertility, chemical properties, oil, and seed yield of Black Cumin (Nigella sativa L.) plants. A split-plot design with three replicates was employed, with biochar derived from poultry litter (BC) applied at rates of 0, 5, and 10 t ha−1, with beneficial microbes such as arbuscular mycorrhizal fungi (AMF) and plant growth-promoting rhizobacteria (PGPR) affecting the growth of Black Cumin plants, and some soil properties, such as pH, electrical conductivity (EC), soil organic matter (SOM) and fertility index (FI), showing significant differences (p ≤ 0.05) among biochar and/or bio-fertilizer treatments. All biochar treatments with or without bio-fertilizers significantly increased pH, EC, OM and FI in comparison to the control treatment. The results demonstrated that applying biochar at the highest rate (10 t ha−1) increased fresh and dry capsule weights by 94.51% and 63.34%, respectively, compared to the control treatment (C). These values were significantly increased by 53.05 and 18.37%, compared to untreated plants when combined with AMF and PGPR. Furthermore, when biochar was applied in conjunction with both AMF and PGPR, fresh and dry capsule weights saw significant increases of 208.84% and 91.18%, respectively, compared to the untreated control treatment. The interaction between biochar, AMF, and PGPR significantly improved plant growth, yield, soil properties, and the fixed and volatile oil content of Black Cumin. These findings suggest that the combined application of biochar, AMF, and PGPR enhances nutrient availability and uptake, leading to improved growth and higher yields in Black Cumin plants, resulting in increased yield production.
1. Introduction
In arid and semi-arid regions, most of the soils contain less than 1% organic matter (OM), and the rate of decomposition and mineralization is high as a result of the high temperature. Poultry manure is produced by the decomposition of chicken droppings, which contain essential nutrients for plant growth due to their high organic matter content []. Moreover, the addition of manure improves both the chemical and physical properties of the soil, consequently leading to increased yield production [,]. Biochar made from animal-origin feedstocks, such as poultry litter, has a higher quality and nutrient status than that made from plant residues [,].
Biochar could improve soil fertility, enhance agricultural production, secure environmental sustainability, and convert organic matter into more stable materials. Poultry manure-derived biochar is regarded as a useful soil amendment and stable carbon; thus, it has high potential use as an organic fertilizer for soil improvement [,]. It is a carbon-rich substance formed through the pyrolysis process of biomass materials under either oxygen-free or oxygen-limited conditions []. Additionally, biochar contains many nutrients, improves soil quality and productivity, amplifies microorganisms’ colonies, and has great adsorption capacity [,]. Biochar could significantly increase root parameters by significantly increasing the number of root tips, the most active part of the root system that increases a plant’s ability to uptake soil nutrients [].
Arbuscular mycorrhizal fungi (AMF) are essential components of the microbial colony, which has a close symbiotic relationship with plant roots. It uses plants to effectively uptake soil nutrients that are useful for plant growth and development and increase yield production. This is achieved by using organic compounds that strengthen the interaction between AMF and plants. AMF have both positive and negative impacts on rhizosphere fungal structures [,,]. There is a significant improvement in the absorption of some macronutrients such as N, P, K Ca, and Mg by plants after inoculation with AMF [,].
The incorporation of biochar with arbuscular mycorrhizal fungi (AMF) enhances plant growth factors, such as root length, improves fungal colonization rate, and boosts nutrient uptake through the mycorrhizal pathway [,]. Moreover, biochar addition enhances soil bacterial populations by providing a porous structure for microbial habitats, improving nutrient retention, soil aeration, and water retention, while also buffering soil pH. These improved conditions create a more favorable environment for nitrogen-fixing microbes, which convert atmospheric nitrogen into ammonia, a form usable by plants. The presence of biochar promotes microbial diversity and fosters symbiotic relationships between nitrogen-fixing bacteria and plant roots, ultimately increasing biological nitrogen fixation and enhancing soil fertility. Bacteria involved in nitrogen-fixation (e.g., Rhizobium, Azospirillum, etc.) and nitrification (ammonia-oxidizing and nitrite-oxidizing bacteria) (e.g., Nitrospira, Nitrobacter) as well as methanotrophic bacteria (e.g., Methylobacterium) were reported to increase in soil amended with biochar []. Thus, biochar improves the biological nitrogen fixation processes by stimulating bacterial nitrification rates and increasing the nitrogen available for plant uptake.
Adding several microbial strains (bio-fertilizers) plays an important role in improving soil health, plant growth, and enhancing food quality [,,].
Plant growth-promoting rhizobacteria (PGPR) are advantageous microbes that can potentially improve soil environment and enhance plant growth by transferring nutrients from the soil to plants [,]. Soil fertility was improved by AMF with PGPR through direct and indirect mechanisms, such as nitrogen transformation and the solubilization process of phosphate and potassium [,].
Biochar and PGPR are used as amendments to increase nutrient availability and the diversity of bacterial communities within soil []. Bacillus species are among the most preponderant plant growth-promoting bacteria. Bacillus sp. inoculated with biochar application increased nutrient availability, growth parameters, nutrient uptake, and yield [,].
Black Cumin (Nigella sativa L.) is an annual plant of the species of the Ranunculaceae family. Black Cumin is native to the Eastern Mediterranean countries, especially to Egypt. It is considered one of the most vital medicinal plants, utilized for diverse purposes, particularly prized for oil production, and mainly cultivated for its seeds [,]. Black Cumin plants have been widely used as a condiment on bread and pickles, and it has important components such as metarbin, nigellin, glycosides, anthraquinones, saponines, melanthin, fixed oils, volatile oils, tannin, albuminous, proteins, glucose, and mucilage resins, which are known to promote human health [,]. Furthermore, it is considered in modern pharmaceutical and food industries as well as in the preparation of functional cosmetics and medicine [,]. Furthermore, the seeds of Black Cumin are mainly characterized by the spicy taste and smell. The seeds have also been found to contain minerals such as P, K, Ca, Mg, Cu, Zn, Na, and Fe [,].
This study hypothesizes that both organic and bio-fertilizers offer sustainable and eco-friendly solutions to improve low soil productivity, as they are cost-effective materials. The objectives of this study are to (i) evaluate the effects of co-inoculating arbuscular mycorrhizal fungi (AMF) and plant growth-promoting rhizobacteria (PGRP) with biochar on the availability and uptake of nitrogen (N), phosphorus (P), and potassium (K) by Black Cumin plants, and (ii) examine the impact of AMF and PGRP co-inoculation, along with biochar, on a soil’s chemical properties, as well as the oil and seed yield of Black Cumin plants.
2. Materials and Methods
2.1. Experimental Site and Design
This study was carried out at a private farm in Qena governorate, Upper Egypt (26°3′10.28″ N, and 32°5′13.95″ E) during the 2020/21 and 2021/22 winter seasons. The region dominates arid climate conditions, with winter temperatures ranging from 7 to 28 °C and summer temperatures from 27 to 49 °C. Annual rainfall is extremely low, averaging between 0.01 and 4 mm per year, while the daily evaporation rate is high at 8.97 mm day−1. Relative humidity stands at 36.62%, and wind speeds range from 2.3 to 3.8 m s−1 throughout the year. The soil texture of the studied area is the sandy loam class and has good drainage conditions. The soil’s physical and chemical properties are shown in Table 1.

Table 1.
Physical and chemical properties of the initial soil.
The tested bio-fertilizers consist of arbuscular mycorrhizal fungi (AMF), Azotobacter chroococcum, and Bacillus circulars (PGPR), and a mixture of both. The bio-fertilizers contain potassium-solubilizing bacteria obtained from the National Research Center, Giza, Egypt. The bio-fertilizers contain Bacillus circulars and this was confirmed by the 16S Ribosomal DNA Sequence Analysis (16S rDNA).
The bio-fertilizers used in the study were sourced from the National Research Center in Giza, Egypt. The spores were previously recovered from alkaline soil. The mycorrhizal fungi were colonized with Glomus mosseae (NRC31) and Glomus fasciculatum (NRC15) originally isolated from Egyptian soils and multiplied on sterilized 1:1:1 (v:v:v) peat:vermiculite:perlite []. Mycorrhizal inoculum material comprises 109 spores per gram, in addition to fragments of colonization roots. The bio-fertilization used was Azotobacter chroococcum + Bacillus circulars 8 × 107 and 8 × 106 CFU mL−1, respectively.
The Black Cumin (Nigella sativa L., local variety) seeds were acquired from the Al-Awamer Research Station, Agricultural Research Center, Assiut, Egypt, and were sown on the 9th and 11th November of the 2020 and 2021 winter growing seasons. The seeds underwent a 5-min surface sterilization process using 1% sodium hypochlorite. This was followed by a sterilized water wash with the addition of bacterial inoculants at a rate of 3 kg ha−1 of seed and with bacterial inoculants containing Arabic gum solution at a rate of 5 g L−1. This was added to the Black Cumin seeds, which were spread on a clean plastic sheet under shade for 30 min before sowing, followed immediately by irrigation, according to Gao et al. (2020) []. Each plot (3 × 3.5 m2) consisted of four ridges spaced at 60 cm; the hills were 30 cm apart, and 5–7 seeds were sown on one side of the ridge. In the end, every plot contained five ridges and 55 plants. The second inoculations of plants using 2 g of AMF and PGPR inoculant 2 cm below the soil surface were performed 3 weeks after cultivation. Weeds were controlled by hand hoeing when it was necessary. Biochar derived from poultry manure was added during soil preparation. Three levels of biochar, i.e., BC0 (without any addition), BC1 (at a rate of 5 t ha−1), and BC2 (at a rate of 10 t ha−1) were recorded as main plots, while the bio-fertilizers were recorded as sub-main plots, i.e., (C, AMF, PGPR, and AMF + PGPR) with four bio-fertilizers each (Figure 1).

Figure 1.
The design of field plots.
A split-plot experimental design was used with three replicates (R1, R2, and R3). The main plot factor was the application rate of biochar derived from poultry litter (BC), and the sub-plot factor was the microbial inoculation (AMF, PGPR, or a combination). The experiment consisted of the following two factors and treatment combinations:
- Biochar application (main plot factor):
- BC0 (Control): no biochar applied.
- BC1: biochar applied at 5 t ha−1.
- BC2: biochar applied at 10 t ha−1.
- Microbial inoculation (sub-plot factor):
- C (Control): no microbial inoculation.
- AMF: arbuscular mycorrhizal fungi (AMF) inoculation.
- PGPR: plant growth-promoting rhizobacteria (PGPR) inoculation.
- AMF + PGPR: a combination of AMF and PGPR inoculation.
- Treatment combinations:The combinations of these factors resulted in 12 treatment groups, as follows:
- BC0 + C (control: no biochar, no microbes),
- BC0 + AMF,
- BC0 + PGPR,
- BC0 + AMF + PGPR,
- BC1 + C,
- BC1 + AMF,
- BC1 + PGPR,
- BC1 + AMF + PGPR,
- BC2 + C,
- BC2 + AMF,
- BC2 + PGPR,
- BC2 + AMF + PGPR.
Each treatment was replicated three times, as shown in Figure 1. This experimental design allowed for the evaluation of individual and interactive effects of biochar and microbial inoculation on the soil fertility, chemical properties, and growth performance (including oil and seed yield) of Black Cumin (Nigella sativa L.) plants.
2.2. Biochar Preparation
For this experiment, the poultry manure was collected from a local private farm. The BC was made from poultry manure at 350 °C for 3 h under oxygen isolation, which refers to conditions where oxygen is either completely excluded or minimized. The physical and chemical properties of the obtained biochar were measured before handling (Table 2). The biochar was crushed and subsequently mixed into the soil. Plots where no biochar was applied were considered as the control treatment.

Table 2.
Chemical composition of the tested biochar.
2.3. Soil and Plant Analysis
During the harvest period (180 days after planting (DAP)), 36 surface soil samples (0–30 cm) were randomly collected from each plot for both seasons. The samples were air-dried, crushed up, and then passed through a 2 mm sieve. Soil texture was determined using the pipette method, as described by Sparks et al. (2020) []. Soil pH was determined in a soil:water solution of 1:2.5 with a digital pH meter. Soil salinity (EC) was measured in soil:water extract of 1:2.5 using an EC meter model AD310, brand Adwa (Szeged, Hungary). Soil Organic Carbon (SOC) content was determined according to the method of Walkley and Black (1934) []. Available Nitrogen (N) was determined following the Kjeldahl method [], which involves digesting the sample in sulfuric acid with a catalyst, followed by distillation and titration to determine ammonium content. Phosphorus (P) was estimated according to the Olsen and Sommers (1982) [] method, where a sodium bicarbonate solution at pH 8.5 is used, followed by shaking, filtration, and spectrophotometric measurement at 640 nm. Potassium (K) was extracted using 1 M of ammonium acetate (NH4OAc) at a pH of 7.0, with the solution shaken and filtered and potassium levels measured by flame photometry []. Biochar (2 g) was digested with H2O2 and H2SO4, and then, total N, P, and K concentrations were measured in the digested extract []. The dried ground plant material was digested using a mixture of sulfuric and perchloric acids in a 7:3 ratio to determine the nutritional contents in Black Cumin shoots. Total N, P and K were determined as described by Burt (2004) [].
2.4. Relative Water Content (RWC)
At the matured phase of Black Cumin plants, leaves were collected to determine the fresh weight (FW) of each treatment, the samples were immediately weighed and then soaked in a dark test tube for 24 h. After using filter paper to wipe them dry, their turgid weight (TW) was calculated by weighing them. To determine the dry weight (DW) of the leaves, they were oven-dried for 24 h at 70 °C. The leaf RWC was estimated according to Smart and Bingham (1974) [] using the following equation:
2.5. Fertility Index (FI)
The fertility index was estimated according to the formula of Abdellatif et al. (2021) [] as follows:
where , fertility index; , and , available nitrogen, phosphorus and potassium, respectively; , organic matter (%).
2.6. Fixed Oil Determination
The seeds (50 g) were powdered mechanically and extracted with petroleum ether (250 mL) at 40–60 °C over the 4 h period in the Soxhlet apparatus, and then, the solvent was removed under reduced pressure to estimate the percentage of the fixed oil [].
2.7. Volatile Oil Determination
The volatile oil % of seeds was determined by hydro-distillation in a Clevenger apparatus in five samples (100 g) for each replicate in both seasons, according to methods described by the Egyptian Pharmacopoeia (1984) [].
2.8. Plant Sampling and Analysis
In both seasons, the plants were harvested after 180 DAP. Harvest time was deemed when the plants started to turn yellow and at least 90% of the capsules were dry and yellow before the capsules opened and dropped seed. Ten plants were randomly selected from each experimental plot and transferred to the laboratory to record the data. Some plant growth parameters (branch number, plant height, capsule number and fresh and dry plant weights) were recorded. Threshing was performed manually by gently beating the pods with a wooden stick on a clean, dry surface. The seeds were then meticulously cleaned and further dried in a well-aired shady room. Chlorophyll in fresh plant leaves was measured by using a chlorophyll meter (Soil Plant Analysis Development (SPAD) 502 plus, Konica Minolta, Inc., Osaka, Japan). Black Cumin seeds from the whole plant were separated to calculate two important measurements: economic and biological yields.
2.9. Data Analysis
The Analysis of Variance (two-way ANOVA) and Duncan multiple range tests at 5% level of probability were performed to find the significant differences among the treatments. Statistical analyses were performed using CoStat 6.45 statistical software (CoHort; the cells in cultured bacterial software, Monterey, CA, USA).
3. Results
3.1. Biochar and Microbial Inoculants Effects on Soil Chemical Properties
At the time of harvest, the soil properties (pH, EC, OM and FI) showed significant differences (p ≤ 0.05) among biochar and/or bio-fertilizer treatments (Table 3 and Table 4). All biochar treatments with or without bio-fertilizers significantly increased pH, EC, OM and FI in comparison to the control treatment. Adding biochar at the high rate (BC2) slightly changed the soil pH values. In the same context, BC2 increased the EC values from 0.62 to 1.18 dS m−1, OM content increased from 4.25 to 11.24 g kg−1, and the fertility index increased from 6.57 to 11.70%, respectively, in the second season.

Table 3.
Impact of biochar and/or bio-fertilizer on some soil chemical properties after cultivating Black Cumin plants.

Table 4.
Analysis of variance of soil chemical properties.
The different bio-fertilizer treatments affected the soil pH, which increased by 0.12%, while EC, OM, and FI values increased by 29.87, 24.43 and 24.35%, respectively, in the second season when applying AMF + PGPR in comparison to the control treatment (C). Moreover, in the second season, soil pH, EC, OM and FI were significantly affected (p ≤ 0.05) by the interaction among biochar and bio-fertilizer treatments. The lowest decrease in pH (8.05) was observed in (BC0) with AMF + PGPR, whereas the highest increase in EC (1.29), OM (12.22) and FI (12.74) were found in the BC2 with AMF + PGPR.
3.2. Effects of Biochar and/or Microbial Inoculants on Nutrient Availability and Their Uptake
Nutrient availability and uptake (N, P, and K) were affected by both the biochar treatments and microbial inoculants (Table 5).

Table 5.
Impact of biochar and/or bio-fertilizer on soil nutrient availability after cultivating Black Cumin plants.
Concerning the biochar treatments, the addition of the high rate of biochar (BC) resulted in significant increases in available nutrients (N, P, and K) compared to the control treatment by 131.63, 77.92, and 63.28%, respectively. In the same trend, the nutrient uptake increased by 2.77, 2.84, and 4.62 times, respectively, in the second season over the control treatment (Figure 2a–c).
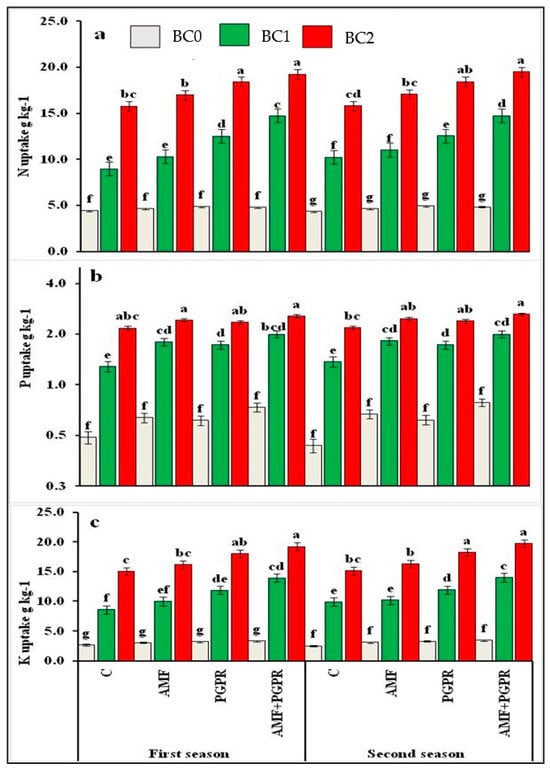
Figure 2.
Effect of biochar and/or bio-fertilizer on uptake nitrogen N of (a), (b) phosphorous P, and (c) potassium K of Black Cumin plants. BC0, BC1 and BC2, biochar at rates of 0, 5 and 10 t ha−1; AMF, arbuscular mycorrhizal fungi and PGPR, plant growth-promoting rhizobacteria. Means in each column followed by the same letters are not significantly different (p < 0.05) by Duncan’s multiple range test.
Compared to the control treatment in the second season, the PGPR + AMF treatment significantly increased the nutrient (N, P and K) availability by 70.17, 22.69, and 18.38%, respectively. Adding BC2 and AMF + PGPR resulted in a maximum increase in nutrient (N, P, and K) availability by 186.56, 97.06, and 81.00%, respectively, compared to the control treatment.
3.3. Effect of Biochar and/or Bio-Fertilizers on Black Cumin Growth Attributes
Data presented in (Table 6, Table 7 and Table 8) detail the impact of biochar and bio-fertilizer applications on Black Cumin plant growth parameters. The morphological growth parameters of a plant increased when BC + PGPR was with AMF.

Table 6.
Impact of biochar and or/bio-fertilizer on some growth parameters of Black Cumin plants.

Table 7.
Impact of biochar and/or bio-fertilizer on some growth parameters of Black Cumin plant capsules.

Table 8.
Analysis of variance of growth parameters.
In the second season, the application of a high rate of biochar (BC) increased the fresh weight, plant height, capsule number and capsule weight by 55.37, 78.41, 55.97, 94.51 and 63.34%, respectively, compared to the control treatment.
Meanwhile, adding bio-fertilizer (AMF + PGRP) enhanced the growth parameters of fresh weight, plant height, capsule number, capsule fresh and plant dry weight by 15.96, 47.64, 41.57, 53.05 and 18.37%, respectively, compared to the control treatment in the second season.
Concerning the combinations of biochar and bio-fertilizers, the added high rate of BC with PGPR + AMF increased fresh weight, plant height, capsule numbers, capsule fresh and plant dry weight by 71.79, 127.19, 108.28, 208.84, and 91.18%, respectively, compared to untreated plants.
3.4. Effect of Biochar and/or Bio-Fertilizers on Chlorophyll Contents of Black Cumin Plants
Biochar and bio-fertilizers significantly increased the total chlorophyll levels SPAD (p ≤ 0.05). Compared to the untreated plants, an increase of 40.40 and 40.78% in chlorophyll levels was noticed due to adding the high level of BC, respectively, in the first and second seasons (Table 9).

Table 9.
Impact of biochar and or/bio-fertilizer on chlorophyll content of Black Cumin leaves.
Regarding bio-fertilizer treatments, chlorophyll (SPAD) increased by 19.90 and 19.93%, respectively, in the first and second seasons due to AMF + PGPR treatments compared to the control treatment. However, adding PGPR and AMF alone or in combination increased the chlorophyll. Co-inoculation of AMF and PGPR along with BC1 and BC2 showed a drastic surge in the level of SPAD chlorophyll by 40.95 and 58.45%, respectively, in the second season compared to untreated plants.
3.5. Effects of Biochar and Microbial Inoculants on Black Cumin Yield
Seed yields were positively affected by all biochar and/or bio-fertilizer treatments compared to untreated plants (Table 10). The seed yield significantly increased as the applied biochar rate increased since it increased from 724 kg ha−1 without biochar to 1807 kg ha−1 at a high biochar rate in the second season.

Table 10.
Impact of biochar and or/bio-fertilizer on Black Cumin yield.
Moreover, bio-fertilizer application caused a noticeable increment in Black Cumin yield by 32.29% compared to untreated plants.
The interaction between biochar level and/or bio-fertilizers resulted in a significant positive effect on seed weight. The BC2 with AMF + PGPR treatment increased seed weight from 1606 to 2037 kg ha−1 in the second season.
3.6. Effects of Biochar and/or Microbial Inoculants on Black Cumin Oil Content
The content of Black Cumin oil was significantly impacted by biochar, bio-fertilizers, and their combination (Table 10 and Table 11). Overall, all treatments of biochar significantly increased the fixed and volatile oil compared to untreated plants during both seasons.

Table 11.
Analysis of variance of yield attributes.
Biochar application at a high rate resulted in significant effects on fixed and volatile oil since they increased by 16.97 and 60.52%, respectively, in the second season over the control treatment. In addition, adding bio-fertilizers increased the fixed and volatile oil by 26.65 and 36.95%, respectively, compared to the control treatment. Furthermore, BC added at high rate with PGPR + AMF increased fixed and volatile oil by 47.12 and 103.18%, respectively, compared to untreated plants.
4. Discussion
Adding biochar and bio-fertilizers individually or in combination enhances soil fertility through the superior positive impact on nutrient availability and growth parameters, consequently increasing yield production. The addition of biochar improved soil properties such as pH, electrical conductivity (EC), and soil organic matter (SOM), while enhancing the availability and uptake of nutrients such as nitrogen (N), phosphorus (P), and potassium (K). The soil pH increased slowly in the second season compared to the first season with the increasing rate of biochar application due to several factors, including the inherently high pH level of biochar, high alkaline mineral concentrations, the presence of functional groups such as hydroxyl and carboxyl associated with active soil reactions, as well as the presence of exchangeable basic cations within the biochar amendment [,,]. Moreover, biochar might provide a shelter for AMF and/or PGPR, causing it to be more helpful for microorganisms; thus, the combination of biochar and bio-fertilizer raises the soil pH [,,]. Wen et al. (2022) [] observed that the BC and AMF together increased soil pH. The tested sandy loam soil was alkaline in reaction with a safe EC threshold. The high concentration of soluble salts in biochar, which produces a higher EC value than the control treatment, may be the cause of the increase in soil EC [,]. Plots treated with BC levels alone or combined with bio-fertilizer generally had EC values that were greater than those of untreated plots.
The biochar decomposition is slowed down with bio-fertilizer application; therefore, increased soil organic matter content could be a plausible explanation for the high content of soil organic matter [,]. Moreover, this may be explained by the observation that, compared to soil labile carbon from chicken manure, the high carbon stability of soils treated with biochar increases soil persistence for organic material decomposition []. Bai et al. (2024) [] revealed that biochar amendment significantly mitigated the relative mineralization amount by 22.7% likely due to its potent adsorption potential, which reduced the available and dissolved resources []. Chen et al. (2023) [] revealed that biochar amendment significantly increased the condensed aromatic structures in DOC and the intensity of aromatic C:O functional groups in SOC—the magnitude of the increase correlated with the dosage of the biochar amendment. Our results revealed that the interaction between AMF alone or a combination with PGPR increased organic matter. These results are in agreement with Vahedi et al. (2022) [], who indicated that the soil organic matter content after AMF inoculation and biochar addition was 8.38% higher than after AMF inoculation alone and that microbial inoculation strengthened this effect. Ortas (2012) [] showed that when organic matter was incorporated into the soil, mycorrhiza consumed some of the organic matter to improve nutrient availability, while the remaining organic matter improved soil texture via interactions with hyphae, thereby contributing to the storage of organic matter in the soil. Accordingly, Ippolito et al. (2015) [] found that applying biochar increased the organic carbon content of the soil since biochar consists mainly of carbon. In addition, root exudates, such as phenolic compounds, stimulate microbial growth in the rhizosphere soil and can enhance soil organic matter and nutrients after decomposing in the soil. Dry conditions significantly slow or inhibit the decomposition of organic matter. Decomposition relies on the activity of microbes, fungi, and other organisms that require moisture to thrive. Without sufficient moisture, these organisms cannot function effectively, leading to a reduced breakdown of organic material. Additionally, in dry conditions, organic matter may remain exposed to the air, further limiting microbial activity and slowing the overall decomposition process. Moisture is essential because it allows for chemical reactions and nutrient cycling, which are crucial for breaking down organic materials [].
The increase in nutrient availability by increasing biochar addition with or without bio-fertilizer could be associated with various mechanisms and processes such as improved nutrient retention, increased organic material decomposition, increased nutrient solubility, and improved uptake, which in turn leads to better root growth and increased seed yield [,].
The addition of biochar and bio-fertilizer improved nitrogen availability and significantly increased its soil content. This could be attributed to biochar, which promotes spore germination and hyphal growth of arbuscular mycorrhizal fungi (AMF), consequently enhancing the colonization rate of plant roots. Moreover, AMF can also take up extra nitrogen from the soil to supply plants [,]. Moreover, the host plant was better able to quickly and widely absorb inorganic nitrogen from the soil because the hyphal network of AMF can extend more than 10 cm beyond the root surface [,,]. In addition, azotobacter bacteria might increase nitrogen availability [,]. Biochar with or without bio-fertilizer significantly increased phosphorus availability and led to an increase in P shoot content. Higher phosphorus content in soil and plant inoculation may be explained by the fact that plants produce organic acids, fungi, and side effects of azotobacter bacteria in the rhizosphere, which in turn stimulates P availability [,]. In sandy loam soil, Agbede and Oyewumi (2022) [] reported that the application of biochar increased pH, OC, and available P by 25.92, 143.93, and 31.94%, respectively, by increasing the biochar rate from 0 to 30.0 t/ha in sweet potato plants as an average value of both seasons. The application of biochar alone and/or bio-fertilizer significantly improved the soil chemical properties compared to the control treatment. The incorporation of biochar and AMF hyphae increased the surface area for absorbing phosphorus by altering the morphology of the roots to encourage phosphorus uptake and release organic acid, which dissolves and mineralizes phosphorus [,,]. Wang et al. (2015) [] showed that the poultry-derived biochar exhibited less than 10% phosphorus (P) release, further supporting the notion that biochar serves as an effective alternative for the slow release of phosphorus (P) from livestock manure. Biochar protects soil microorganisms, encourages the increase in microbial colonies and extra nutrients, and raises the P uptake by arbuscular mycorrhizal fungi [,].
Nevertheless, biochar itself contains some of the available phosphorus, and the incorporation of biochar with bio-fertilizers might have increased the nutrient availability and thus increased the Black Cumin seed yield. Mycorrhizal inoculation combined with biochar can function as an innovative and sustainable phosphorus (P) source for plants [,]. The interactions between biochar and bio-fertilizers were found to be beneficial for soil chemical properties, growth, seed yield, and oil % of Black Cumin, indicating the potential of bio-fertilizers to improve biochar use efficiency and enhance better use of nutrients in biochar. Vesicular arbuscular mycorrhizal fungus and bacteria interacting significantly enhanced the beneficial effects of bio-fertilizer on marigold plant growth, flowering, yield, and carotenoid content [,]. Wen et al. (2022) [] demonstrated that the combining of biochar with arbuscular mycorrhizal fungi boosted phosphorus availability. Biochar and microbes act as a powerful duo, promoting plant growth through enzymes and secondary metabolites they contribute []. Biochar with bio-fertilizer can act on various medicinal and aromatic plants to increase seed yield production. The addition of biochar both with or without bio-fertilizers significantly increased potassium availability and total potassium in plants. The reason behind this is that biochar can effectively augment soil potassium levels owing to its unique structure and properties []. The combination of BC and PGPR notably boosts available potassium in the soil and facilitates its uptake by plants [,]. Biochar enhances plant growth and nutrient absorption, even in the absence of microbial inoculation. Under different environmental conditions, biochar has gained great attention in recent years, which has changed the chemical properties of the soil and increased plant growth [,]. It has been demonstrated that inoculation with azotobacter provides appropriate conditions for plant root growth and biological functions.
The application of biochar derived from poultry manure promoted the growth and productivity of Black Cumin. Growth, seed yield and oil were significantly increased by the bio-fertilizer and biochar amendments and their application rate, with yield increments relative to the control treatment. These results were attributed largely to increased native soil N mineralization, available phosphorus, soil microbes, organic matter, soil productivity by bio-fertilizer, higher concentrations of micro- and macronutrients after the application of biochar with bio-fertilizer, and higher N supply from the biochar [,,]. The highest profitable net return in response to the application of a high rate from biochar with bio-fertilizer AMF + PGPR may be attributed to the increment of soil nutrients status, thereby the higher seed yields.
Chan et al. (2008) [] observed that biochar derived from poultry increased dry matter from 42% to 96% with increasing biochar from 10 to 50 t ha−1 in radish plants. In rice plants, Kimani et al. (2021) [] reported that poultry litter biochar significantly increased the grain yield and total biomass by 32.4 and 24.4%, respectively, compared to the control treatment. The grain and straw yields were significantly increased by 35.66 and 47.84%, respectively, for poultry manure-derived biochar compared to the control treatment in wheat plants []. Moradzadeh et al. (2021) [] found that the utilization of bio-fertilizers significantly enhanced growth parameters, seed production, and oil yields of Black Cumin.
5. Conclusions and Recommendations
The combined application of biochar and bio-fertilizers has shown significant benefits in enhancing soil fertility, improving nutrient availability, and ultimately increasing crop yields. The addition of biochar not only improved critical soil properties such as pH, electrical conductivity (EC), and soil organic matter (SOM) but also facilitated the availability and uptake of essential nutrients such as nitrogen (N), phosphorus (P), and potassium (K). The increase in soil pH, attributed to the alkaline nature of biochar and the presence of functional groups and basic cations, further enhances the soil’s capacity to support plant growth. Additionally, biochar’s role in providing a favorable environment for beneficial microorganisms like arbuscular mycorrhizal fungi (AMF) and plant growth-promoting rhizobacteria (PGPR) underscores its potential as a key amendment in sustainable agriculture. The observed improvements in soil organic matter content, nutrient retention, and nutrient solubility, particularly when biochar is used in conjunction with bio-fertilizers, highlight the synergistic effects of these amendments. Therefore, it is recommended to incorporate biochar and bio-fertilizers, either individually or in combination, into agricultural practices to enhance soil health, improve crop productivity, and promote sustainable farming systems. Future research should focus on optimizing application rates and exploring the long-term impacts of these amendments on different soil and crop types.
Author Contributions
Conceptualization, Y.A.S., A.M.A., M.F.I., M.E.F., A.S. and H.M.A.-S.; methodology, Y.A.S., A.M.A., M.F.I., M.E.F., C.C., M.D. and H.M.A.-S.; software, Y.A.S., A.M.A., M.F.I., M.E.F. and H.M.A.-S.; validation, Y.A.S., A.M.A., M.F.I., M.E.F., C.C., M.D. and H.M.A.-S.; formal analysis, Y.A.S., A.M.A., M.F.I., M.E.F. and H.M.A.-S.; investigation, Y.A.S., A.M.A., M.F.I., M.E.F. and H.M.A.-S.; resources, Y.A.S., A.M.A., M.F.I., M.E.F., C.C., M.D., A.S. and H.M.A.-S.; data curation, Y.A.S., A.M.A., M.F.I., M.E.F., C.C., M.D., A.S. and H.M.A.-S.; writing—original draft preparation, Y.A.S., A.M.A., M.F.I., M.E.F., C.C., M.D. and H.M.A.-S.; writing—review and editing, Y.A.S., A.M.A., M.F.I., M.E.F., C.C., M.D., A.S. and H.M.A.-S.; visualization, Y.A.S., A.M.A., M.F.I., M.E.F. and H.M.A.-S.; supervision, Y.A.S., A.M.A., M.F.I., M.E.F., A.S. and H.M.A.-S.; project administration, Y.A.S., A.M.A., M.F.I., M.E.F., C.C., M.D., A.S. and H.M.A.-S.; funding acquisition, Y.A.S., A.M.A., M.F.I., M.E.F., C.C., M.D., A.S. and H.M.A.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The manuscript presented is a scientific collaboration between scientific institutions in two countries (Egypt and Italy). The authors would like to thank Al-Azhar University, the National Authority for Remote Sensing and Space Science (NARSS), and Università Politecnica delle Marche and Università degli Studi della Basilicata for support with the field survey and data analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mohamed, W.S.; Hammam, A.A. Poultry manure-derived biochar as a soil amendment and fertilizer for sandy soils under arid conditions. Egypt. J. Soil Sci. 2019, 59, 1–14. [Google Scholar] [CrossRef]
- Agbede, T.M.; Oyewumi, A. Benefits of biochar, poultry manure and biochar–poultry manure for improvement of soil properties and sweet potato productivity in degraded tropical agricultural soils. Resour. Environ. Sustain. 2022, 7, 100051. [Google Scholar] [CrossRef]
- Sayed, Y.A.; Al-Sayed, H.M.; Ali, A.M. Impact of Different Fertilizers on Black Cumin (Nigella Sativa L.) Plants and Their Relation to Release Kinetics of Nitrogen and Phosphorus. Egypt. J. Soil Sci. 2024, 64, 911–925. [Google Scholar]
- Chan, K.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Using poultry litter biochars as soil amendments. Soil Res. 2008, 46, 437–444. [Google Scholar] [CrossRef]
- Sikder, S.; Joardar, J. Biochar production from poultry litter as management approach and effects on plant growth. Int. J. Recycl. Org. Waste Agric. 2019, 8, 47–58. [Google Scholar] [CrossRef]
- Agyarko-Mintah, E.; Cowie, A.; Singh, B.P.; Joseph, S.; Van Zwieten, L.; Cowie, A.; Harden, S.; Smillie, R. Biochar increases nitrogen retention and lowers greenhouse gas emissions when added to composting poultry litter. Waste Manag. 2017, 61, 138–149. [Google Scholar] [CrossRef]
- Pituello, C.; Francioso, O.; Simonetti, G.; Pisi, A.; Torreggiani, A.; Berti, A.; Morari, F. Characterization of chemical–physical, structural and morphological properties of biochars from biowastes produced at different temperatures. J. Soils Sediments 2015, 15, 792–804. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for environmental management: An introduction. In Biochar for Environmental Management; Routledge: Oxfordshire, UK, 2015; pp. 1–13. [Google Scholar]
- Dong, Y.; Wang, H.; Chang, E.; Zhao, Z.; Wang, R.; Xu, R.; Jiang, J. Alleviation of aluminum phytotoxicity by canola straw biochars varied with their cultivating soils through an investigation of wheat seedling root elongation. Chemosphere 2019, 218, 907–914. [Google Scholar] [CrossRef]
- Shi, Y.; Yu, Y.; Chang, E.; Wang, R.; Hong, Z.; Cui, J.; Zhang, F.; Jiang, J.; Xu, R. Effect of biochar incorporation on phosphorus supplementation and availability in soil: A review. J. Soils Sediments 2023, 23, 672–686. [Google Scholar] [CrossRef]
- Awad, M.; Moustafa-Farag, M.; Liu, Z.; El-Shazoly, R.M. Combined effect of biochar and salicylic acid in alleviating heavy metal stress, antioxidant enhancement, and Chinese mustard growth in a contaminated soil. J. Soil Sci. Plant Nutr. 2022, 22, 4194–4206. [Google Scholar] [CrossRef]
- Javeed, H.M.R.; Naeem, R.; Ali, M.; Qamar, R.; Sarwar, M.A.; Nawaz, F.; Shehzad, M.; Farooq, A.; Khalid, S.; Mubeen, K. Coupling biochar with microbial inoculants improves maize growth and nutrients acquisition under phosphorous-limited soil. Acta Physiol. Plant. 2022, 44, 110. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, W.; Feng, Z.; Feng, G.; Zhu, H.; Yao, Q. Arbuscular mycorrhizal fungus differentially regulates P mobilizing bacterial community and abundance in rhizosphere and hyphosphere. Appl. Soil Ecol. 2022, 170, 104294. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H. The application of arbuscular mycorrhizal fungi as microbial biostimulant, sustainable approaches in modern agriculture. Plants 2023, 12, 3101. [Google Scholar] [CrossRef] [PubMed]
- Hodge, A.; Storer, K. Arbuscular mycorrhiza and nitrogen: Implications for individual plants through to ecosystems. Plant Soil 2015, 386, 1–19. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, S.; Hu, W.; Xiao, L.; Tang, M. Arbuscular mycorrhizal fungus Rhizophagus irregularis increased potassium content and expression of genes encoding potassium channels in Lycium barbarum. Front. Plant Sci. 2017, 8, 440. [Google Scholar] [CrossRef]
- Hammer, E.; Balogh-Brunstad, Z.; Jakobsen, I.; Olsson, P.; Stipp, S.; Rillig, M. A mycorrhizal fungus grows on biochar and captures phosphorus from its surfaces. Soil Biol. Biochem. 2014, 77, 252–260. [Google Scholar] [CrossRef]
- Hammer, E.C.; Forstreuter, M.; Rillig, M.C.; Kohler, J. Biochar increases arbuscular mycorrhizal plant growth enhancement and ameliorates salinity stress. Appl. Soil Ecol. 2015, 96, 114–121. [Google Scholar] [CrossRef]
- Abujabhah, I.S.; Doyle, R.B.; Bound, S.A.; Bowman, J.P. Assessment of bacterial community composition, methanotrophic and nitrogen-cycling bacteria in three soils with different biochar application rates. J. Soils Sediments 2018, 18, 148–158. [Google Scholar] [CrossRef]
- Al-Sayed, H.M.; Khalafalla, M.Y.; Ali, A.M. Effects of compost and biofertilizer on carbon dioxide emission, yield, and quality of roselle (Hibiscus sabdariffa L.) plants grown on clay loam. J. Plant Nutr. 2023, 46, 2707–2723. [Google Scholar] [CrossRef]
- Ali, A.M.; Awad, M.Y.; Hegab, S.A.; Gawad, A.M.A.E.; Eissa, M.A. Effect of potassium solubilizing bacteria (Bacillus cereus) on growth and yield of potato. J. Plant Nutr. 2021, 44, 411–420. [Google Scholar] [CrossRef]
- Yousef, A.F.; Ali, A.M.; Azab, M.A.; Lamlom, S.F.; Al-Sayed, H.M. Improved plant yield of potato through exogenously applied potassium fertilizer sources and biofertilizer. AMB Express 2023, 13, 124. [Google Scholar] [CrossRef] [PubMed]
- Daryabeigi Zand, A.; Tabrizi, A.M.; Heir, A.V. The influence of association of plant growth-promoting rhizobacteria and zero-valent iron nanoparticles on removal of antimony from soil by Trifolium repens. Environ. Sci. Pollut. Res. 2020, 27, 42815–42829. [Google Scholar] [CrossRef] [PubMed]
- Mashabela, M.D.; Piater, L.A.; Dubery, I.A.; Tugizimana, F.; Mhlongo, M.I. Rhizosphere tripartite interactions and PGPR-mediated metabolic reprogramming towards ISR and plant priming: A metabolomics review. Biology 2022, 11, 346. [Google Scholar] [CrossRef]
- Zafar-ul-Hye, M.; Bhutta, T.S.; Shaaban, M.; Hussain, S.; Qayyum, M.F.; Aslam, U.; Zahir, Z.A. Influence of plant growth promoting rhizobacterial inoculation on wheat productivity under soil salinity stress. Phyton 2019, 88, 119. [Google Scholar] [CrossRef]
- Chen, D.; Saeed, M.; Ali, M.N.H.A.; Raheel, M.; Ashraf, W.; Hassan, Z.; Hassan, M.Z.; Farooq, U.; Hakim, M.F.; Rao, M.J. Plant growth promoting rhizobacteria (PGPR) and arbuscular mycorrhizal fungi combined application reveals enhanced soil fertility and rice production. Agronomy 2023, 13, 550. [Google Scholar] [CrossRef]
- Ren, H.; Li, Z.; Chen, H.; Zhou, J.; Lv, C. Effects of Biochar and Plant Growth-Promoting Rhizobacteria on Plant Performance and Soil Environmental Stability. Sustainability 2022, 14, 10922. [Google Scholar] [CrossRef]
- Hassan, S.E.-D. Plant growth-promoting activities for bacterial and fungal endophytes isolated from medicinal plant of Teucrium polium L. J. Adv. Res. 2017, 8, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, F.; Rafeeq, R.; Majeed, S.; Ismail, M.S.; Ahsan, M.; Ahmad, K.S.; Akram, A.; Haider, G. Biochar amendment in combination with endophytic bacteria stimulates photosynthetic activity and antioxidant enzymes to improve soybean yield under drought stress. J. Soil Sci. Plant Nutr. 2023, 23, 746–760. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Rezadoost, H.; Nadjafi, F.; Asareh, M.H. Comparative essential oil composition and fatty acid profiling of some Iranian black cumin landraces. Ind. Crops Prod. 2019, 140, 111628. [Google Scholar] [CrossRef]
- Randhawa, M.A.; Alghamdi, M.S. Anticancer activity of Nigella sativa (black seed)—A review. Am. J. Chin. Med. 2011, 39, 1075–1091. [Google Scholar] [CrossRef]
- Amin, B.; Hosseinzadeh, H. Black cumin (Nigella sativa) and its active constituent, thymoquinone: An overview on the analgesic and anti-inflammatory effects. Planta Medica 2016, 82, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Abdou, N.M.; Roby, M.H.; Al-Huqail, A.A.; Elkelish, A.; Sayed, A.A.; Alharbi, B.M.; Mahdy, H.A.; Abou-Sreea, A.I.B. Compost improving morphophysiological and biochemical traits, seed yield, and oil quality of Nigella sativa under drought stress. Agronomy 2023, 13, 1147. [Google Scholar] [CrossRef]
- Albakry, Z.; Karrar, E.; Ahmed, I.A.M.; Oz, E.; Proestos, C.; El Sheikha, A.F.; Oz, F.; Wu, G.; Wang, X. Nutritional composition and volatile compounds of black cumin (Nigella sativa L.) seed, fatty acid composition and tocopherols, polyphenols, and antioxidant activity of its essential oil. Horticulturae 2022, 8, 575. [Google Scholar] [CrossRef]
- Ozer, H.; Coban, F.; Sahin, U.; Ors, S. Response of black cumin (Nigella sativa L.) to deficit irrigation in a semi-arid region: Growth, yield, quality, and water productivity. Ind. Crops Prod. 2020, 144, 112048. [Google Scholar] [CrossRef]
- Ibrahim, M. The role of vermicompost and chitosan nanoparticles as foliar application to enhancing growth, yield and oil of black cumin (Nigella sativa L.) plants. Arch. Agric. Sci. J. 2020, 3, 205–223. [Google Scholar] [CrossRef]
- Ramadan, M.F. Nutritional value, functional properties and nutraceutical applications of black cumin (Nigella sativa L.): An overview. Int. J. Food Sci. Technol. 2007, 42, 1208–1218. [Google Scholar] [CrossRef]
- El-Din, S.; Attia, M.; Abo-Sedera, S. Evaluation of several substrates for mass multiplication of arbuscular mycorrhizal (AM) fungi grown on onion. Egypt. J. Microbiol. 1999, 34, 57–65. [Google Scholar]
- Gao, C.; El-Sawah, A.M.; Ali, D.F.I.; Alhaj Hamoud, Y.; Shaghaleh, H.; Sheteiwy, M.S. The integration of bio and organic fertilizers improve plant growth, grain yield, quality and metabolism of hybrid maize (Zea mays L.). Agronomy 2020, 10, 319. [Google Scholar] [CrossRef]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H. Methods of Soil Analysis, Part 3: Chemical Methods; John Wiley & Sons: Hoboken, NJ, USA, 2020; Volume 14. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Burt, R. Soil Survey Laboratory Methods Manual, Investigations Reports No 42, Version 4.0; Natural Resources Conservation Service; United States Department of Agriculture: Washington, DC, USA, 2004. [Google Scholar]
- Olsen, S.; Sommers, L. Methods of Soil Analysis: Chemical and Microbiological Properties Part 2, American Society of Agronomy; Soil Science Society of America: Madison, WI, USA, 1982. [Google Scholar]
- Smart, R.E.; Bingham, G.E. Rapid estimates of relative water content. Plant Physiol. 1974, 53, 258–260. [Google Scholar] [CrossRef]
- Abdellatif, M.A.; El Baroudy, A.A.; Arshad, M.; Mahmoud, E.K.; Saleh, A.M.; Moghanm, F.S.; Shaltout, K.H.; Eid, E.M.; Shokr, M.S. A GIS-based approach for the quantitative assessment of soil quality and sustainable agriculture. Sustainability 2021, 13, 13438. [Google Scholar] [CrossRef]
- Chemists, A.o.O.A.; Chemists, A.o.O.A. Official Methods of Analysis of the Association of Official Analytical Chemists; Association of Official Analytical Chemists: Rockville, MD, USA, 1931; Volume 3. [Google Scholar]
- Pharmacopoeia, E. General Organization for Governmental Printing Office; Ministry of Health: Cairo, Egypt, 1984; pp. 31–33.
- Alkharabsheh, H.M.; Seleiman, M.F.; Battaglia, M.L.; Shami, A.; Jalal, R.S.; Alhammad, B.A.; Almutairi, K.F.; Al-Saif, A.M. Biochar and its broad impacts in soil quality and fertility, nutrient leaching and crop productivity: A review. Agronomy 2021, 11, 993. [Google Scholar] [CrossRef]
- Khaledi, S.; Delbari, M.; Galavi, H.; Bagheri, H.; Chari, M.M. Effects of biochar particle size, biochar application rate, and moisture content on thermal properties of an unsaturated sandy loam soil. Soil Tillage Res. 2023, 226, 105579. [Google Scholar] [CrossRef]
- Vahedi, R.; Rasouli-Sadaghiani, M.H.; Barin, M.; Vetukuri, R.R. Effect of biochar and microbial inoculation on P, Fe, and Zn bioavailability in a calcareous soil. Processes 2022, 10, 343. [Google Scholar] [CrossRef]
- Jiang, N.; Guo, Q.; Yu, Y.; Guan, Y.; Yang, W. Soil sodicity affected the arbuscular mycorrhizal community and its interactions with bacteria in the Western Songnen Plain. Appl. Soil Ecol. 2022, 180, 104602. [Google Scholar] [CrossRef]
- Wen, Z.; Chen, Y.; Liu, Z.; Meng, J. Biochar and arbuscular mycorrhizal fungi stimulate rice root growth strategy and soil nutrient availability. Eur. J. Soil Biol. 2022, 113, 103448. [Google Scholar] [CrossRef]
- Al-Sayed, H.M.; Ali, A.M.; Mohamed, M.A.; Ibrahim, M.F. Combined effect of prickly pear waste biochar and Azolla on soil fertility, growth, and yield of Roselle (Hibiscus sabdariffa L.) plants. J. Soil Sci. Plant Nutr. 2022, 22, 3541–3552. [Google Scholar] [CrossRef]
- Phares, C.A.; Amoakwah, E.; Danquah, A.; Afrifa, A.; Beyaw, L.R.; Frimpong, K.A. Biochar and NPK fertilizer co-applied with plant growth promoting bacteria (PGPB) enhanced maize grain yield and nutrient use efficiency of inorganic fertilizer. J. Agric. Food Res. 2022, 10, 100434. [Google Scholar] [CrossRef]
- Bai, J.; Huang, Y.; Bai, Y.; Chen, D.; Haider, S.; Song, J.; Moreira, B.R.D.A.; Ren, G.; Yang, G.; Feng, Y. Impact of straw-biochar amendments on microbial activity and soil carbon dynamics in wheat-maize system. Soil Tillage Res. 2024, 244, 106284. [Google Scholar] [CrossRef]
- Lu, W.; Ding, W.; Zhang, J.; Li, Y.; Luo, J.; Bolan, N.; Xie, Z. Biochar suppressed the decomposition of organic carbon in a cultivated sandy loam soil: A negative priming effect. Soil Biol. Biochem. 2014, 76, 12–21. [Google Scholar] [CrossRef]
- Chen, Y.; Du, Z.; Weng, Z.; Sun, K.; Zhang, Y.; Liu, Q.; Yang, Y.; Li, Y.; Wang, Z.; Luo, Y. Formation of soil organic carbon pool is regulated by the structure of dissolved organic matter and microbial carbon pump efficacy: A decadal study comparing different carbon management strategies. Glob. Chang. Biol. 2023, 29, 5445–5459. [Google Scholar] [CrossRef] [PubMed]
- Ortas, I. The effect of mycorrhizal fungal inoculation on plant yield, nutrient uptake and inoculation effectiveness under long-term field conditions. Field Crops Res. 2012, 125, 35–48. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Spokas, K.A.; Novak, J.M.; Lentz, R.D.; Cantrell, K.B. Biochar elemental composition and factors influencing nutrient retention. In Biochar for Environmental Management; Routledge: Oxfordshire, UK, 2015; pp. 139–163. [Google Scholar]
- Rabbi, S.M.; Warren, C.R.; Swarbrick, B.; Minasny, B.; McBratney, A.B.; Young, I.M. Microbial decomposition of organic matter and wetting–drying promotes aggregation in artificial soil but porosity increases only in wet-dry condition. Geoderma 2024, 447, 116924. [Google Scholar] [CrossRef]
- Wei, M.; Liu, X.; He, Y.; Xu, X.; Wu, Z.; Yu, K.; Zheng, X. Biochar inoculated with Pseudomonas putida improves grape (Vitis vinifera L.) fruit quality and alters bacterial diversity. Rhizosphere 2020, 16, 100261. [Google Scholar] [CrossRef]
- Cavagnaro, T.R.; Bender, S.F.; Asghari, H.R.; van der Heijden, M.G. The role of arbuscular mycorrhizas in reducing soil nutrient loss. Trends Plant Sci. 2015, 20, 283–290. [Google Scholar] [CrossRef]
- Fellbaum, C.R.; Gachomo, E.W.; Beesetty, Y.; Choudhari, S.; Strahan, G.D.; Pfeffer, P.E.; Kiers, E.T.; Bücking, H. Carbon availability triggers fungal nitrogen uptake and transport in arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. USA 2012, 109, 2666–2671. [Google Scholar] [CrossRef]
- Al-Sayed, H.M.; Hegab, S.; Youssef, M.; Khalafalla, M.Y.; Eissa, M.A. Compost and non-symbiotic nitrogen fixers to reduce inorganic-N rates for roselle (Hibiscus sabdariffa L.). Commun. Soil Sci. Plant Anal. 2023, 54, 431–443. [Google Scholar] [CrossRef]
- Esitken, A.; Yildiz, H.E.; Ercisli, S.; Donmez, M.F.; Turan, M.; Gunes, A. Effects of plant growth promoting bacteria (PGPB) on yield, growth and nutrient contents of organically grown strawberry. Sci. Hortic. 2010, 124, 62–66. [Google Scholar] [CrossRef]
- Kim, K.; Neuberger, P.; Daly, E.J.; Gorzelak, M.; Hernandez-Ramirez, G. Arbuscular mycorrhizal fungi community linkages to soil nutrient availability across contrasting agroecosystems. Appl. Soil Ecol. 2022, 176, 104464. [Google Scholar] [CrossRef]
- Koide, R.; Kabir, Z. Extraradical hyphae of the mycorrhizal fungus Glomus intraradices can hydrolyse organic phosphate. New Phytol. 2000, 148, 511–517. [Google Scholar] [CrossRef]
- Mackay, J.E.; Cavagnaro, T.R.; Stöver, D.S.M.; Macdonald, L.M.; Grønlund, M.; Jakobsen, I. A key role for arbuscular mycorrhiza in plant acquisition of P from sewage sludge recycled to soil. Soil Biol. Biochem. 2017, 115, 11–20. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Y.; Chiu, P.C.; Imhoff, P.T.; Guo, M. Phosphorus release behaviors of poultry litter biochar as a soil amendment. Sci. Total Environ. 2015, 512, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Chintala, R.; Schumacher, T.E.; McDonald, L.M.; Clay, D.E.; Malo, D.D.; Papiernik, S.K.; Clay, S.A.; Julson, J.L. Phosphorus sorption and availability from biochars and soil/B iochar mixtures. CLEAN–Soil Air Water 2014, 42, 626–634. [Google Scholar] [CrossRef]
- Ali, S.; Rizwan, M.; Noureen, S.; Anwar, S.; Ali, B.; Naveed, M.; Abd_Allah, E.F.; Alqarawi, A.A.; Ahmad, P. Combined use of biochar and zinc oxide nanoparticle foliar spray improved the plant growth and decreased the cadmium accumulation in rice (Oryza sativa L.) plant. Environ. Sci. Pollut. Res. 2019, 26, 11288–11299. [Google Scholar] [CrossRef]
- Rafique, M.; Ortas, I.; Ahmed, I.A.; Rizwan, M.; Afridi, M.S.; Sultan, T.; Chaudhary, H.J. Potential impact of biochar types and microbial inoculants on growth of onion plant in differently textured and phosphorus limited soils. J. Environ. Manag. 2019, 247, 672–680. [Google Scholar] [CrossRef]
- Habib, A. Response of pearl millet to fertilization by mineral phosphorus, humic acid and mycorrhiza under calcareous soils conditions. Egypt. J. Soil Sci. 2021, 61, 399–411. [Google Scholar] [CrossRef]
- Nada, R.S.; Soliman, M.N.; Zarad, M.M.; Sheta, M.H.; Ullah, S.; Abdel-Gawad, A.I.; Ghoneim, A.H.; Elateeq, A.A. Effect of Organic Fertilizer and Plant Growth-Promoting Microbes on Growth, Flowering, and Oleanolic Acid Content in Calendula officinalis under Greenhouse Conditions. Egypt. J. Soil Sci. 2024, 64, 815–831. [Google Scholar] [CrossRef]
- Taha, N.; Kamel, S.; Elsakhawy, T.; Bayoumi, Y.; Omara, A.E.-D.; El-Ramady, H.R. Sustainable approaches of trichoderma under changing environments for vegetable production. Environ. Biodivers. Soil Secur. 2020, 4, 291–311. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota–a review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Alotaibi, M.O.; Alotibi, M.M.; Eissa, M.A.; Ghoneim, A.M. Compost and plant growth-promoting bacteria enhanced steviol glycoside synthesis in stevia (Stevia rebaudiana Bertoni) plants by improving soil quality and regulating nitrogen uptake. S. Afr. J. Bot. 2022, 151, 306–314. [Google Scholar] [CrossRef]
- Azhar, M.; ur Rehman, M.Z.; Ali, S.; Qayyum, M.F.; Naeem, A.; Ayub, M.A.; ul Haq, M.A.; Iqbal, A.; Rizwan, M. Comparative effectiveness of different biochars and conventional organic materials on growth, photosynthesis and cadmium accumulation in cereals. Chemosphere 2019, 227, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Sammama, H.; Mazri, M.A.; Ouahmane, L.; Sammama, A.; Hsissou, D.; El Kaoua, M.; Alfeddy, M.N. Microbial inoculation improves soil properties, nutrient uptake, and plant growth in soft wheat-faba bean intercropping. J. Soil Sci. Plant Nutr. 2022, 22, 5159–5173. [Google Scholar] [CrossRef]
- Ali, A.M.; Mahdy, A.Y.; Al-Sayed, H.M.; Bayomi, K.M. Phosphorus sources and sheep manure fertilization for soil properties enhancement and sugar beet yield. Gesunde Pflanz. 2023, 75, 2785–2795. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Hu, T.; Mahmoud, A.; Li, J.; Zhu, R.; Jiao, X.; Jing, P. A quantitative review of the effects of biochar application on rice yield and nitrogen use efficiency in paddy fields: A meta-analysis. Sci. Total Environ. 2022, 830, 154792. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, M.; Tian, Y.; Zhao, M.; Zeng, K.; Zhang, B.; Zhao, M.; Yin, B. Azolla biofertilizer for improving low nitrogen use efficiency in an intensive rice cropping system. Field Crops Res. 2018, 216, 158–164. [Google Scholar] [CrossRef]
- Kimani, S.M.; Bimantara, P.O.; Kautsar, V.; Tawaraya, K.; Cheng, W. Poultry litter biochar application in combination with chemical fertilizer and Azolla green manure improves rice grain yield and nitrogen use efficiency in paddy soil. Biochar 2021, 3, 591–602. [Google Scholar] [CrossRef]
- Moradzadeh, S.; Siavash Moghaddam, S.; Rahimi, A.; Pourakbar, L.; Sayyed, R. Combined bio-chemical fertilizers ameliorate agro-biochemical attributes of black cumin (Nigella sativa L.). Sci. Rep. 2021, 11, 11399. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).