Soil Properties and Rhizosphere Microbes Community Structure Reveal Nitrogen Uptake Preferences and Nitrogen Use Efficiency of Two Ecotypes of Paphiopedilum micranthum
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Sample Collection
2.2. Determination of Soil Physicochemical Properties and Nitrogen
2.3. DNA Extraction and Amplification with Polymerase Chain Reaction (PCR)
2.4. Statistical Analysis
3. Results
3.1. Soil Physiochemical Properties of P. micranthum in Different Habitats
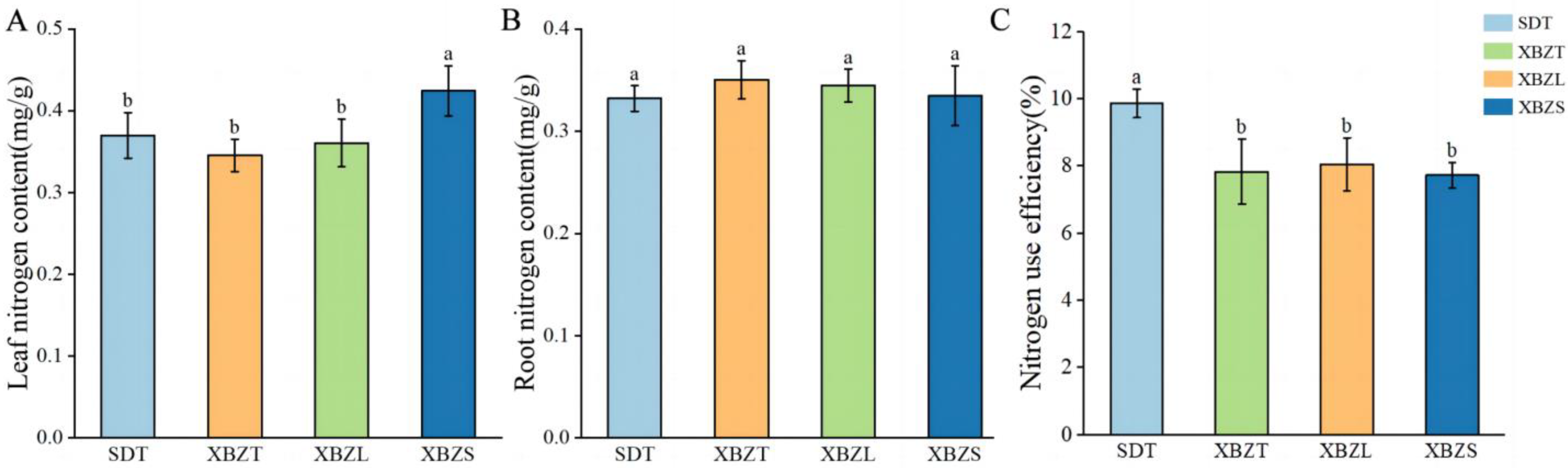
3.2. Nitrogen Content and Nitrogen-Use Efficiency of P. micranthum in Different Habitats
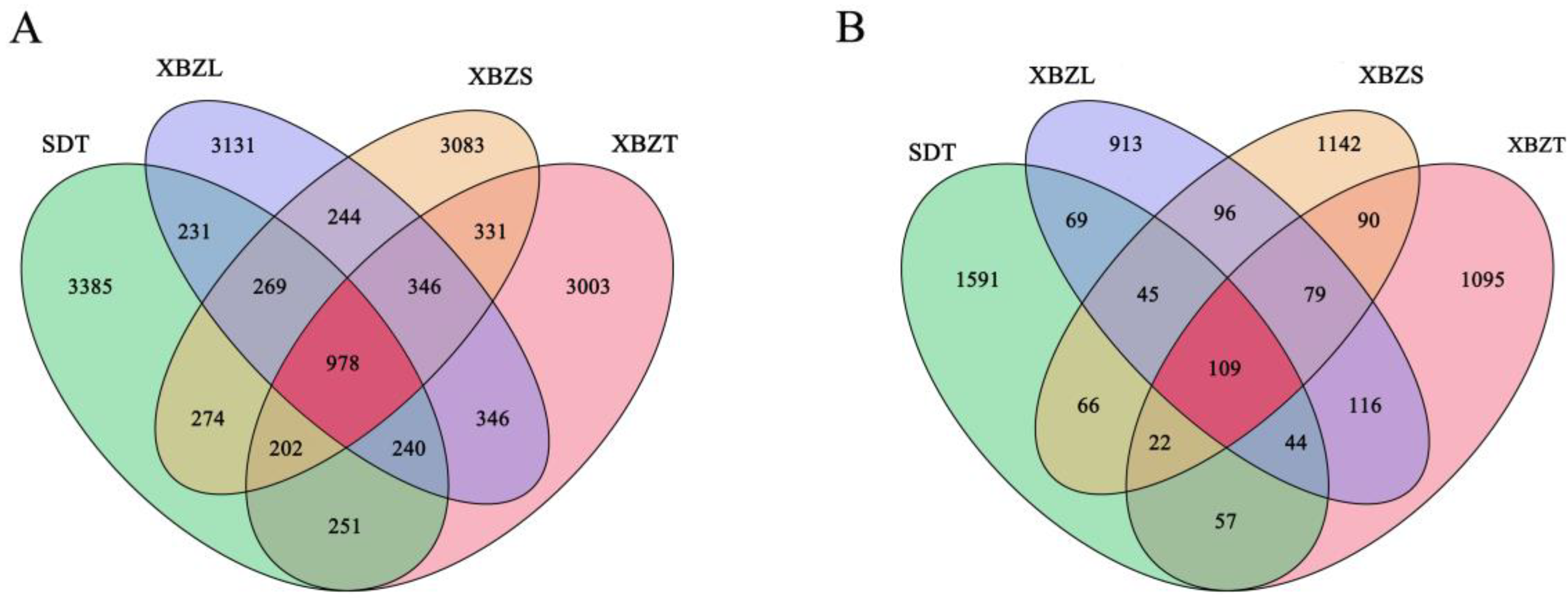
3.3. Analysis of the Illumina Sequencing Data
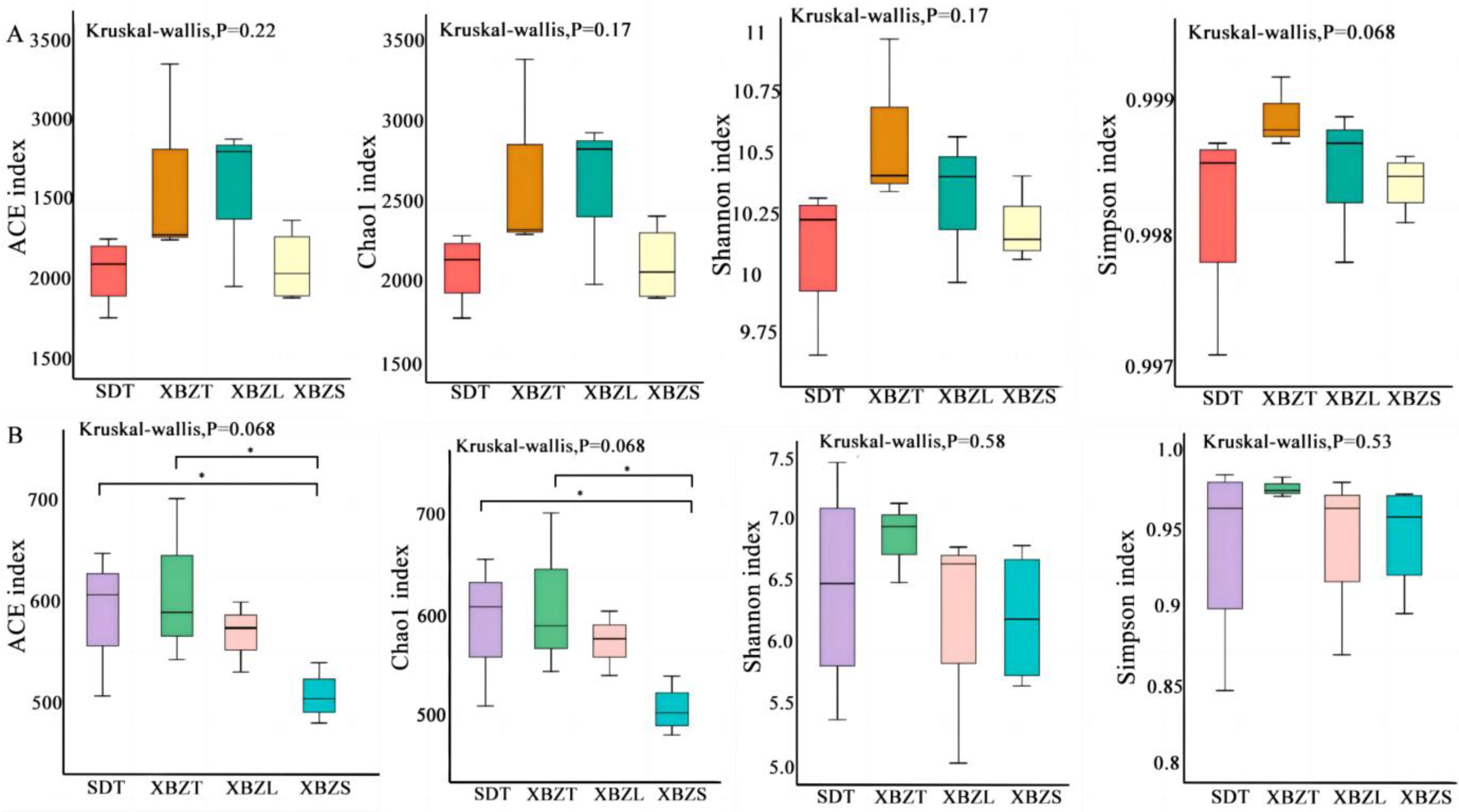
3.4. Diversity of Soil Bacterial and Fungal Communities in Different Habitats
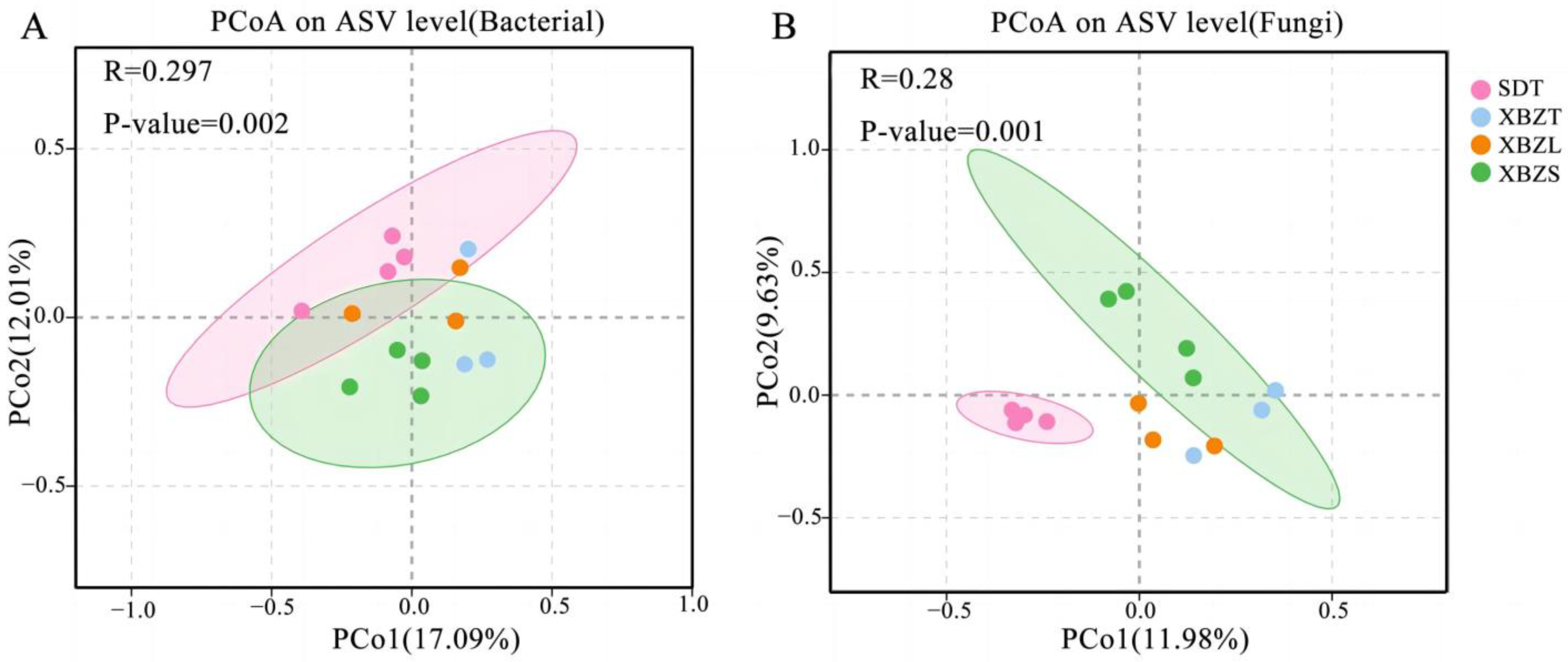
3.5. Rhizosphere Soil Microbial Community Structure of Different Ecotypes of P. micranthum
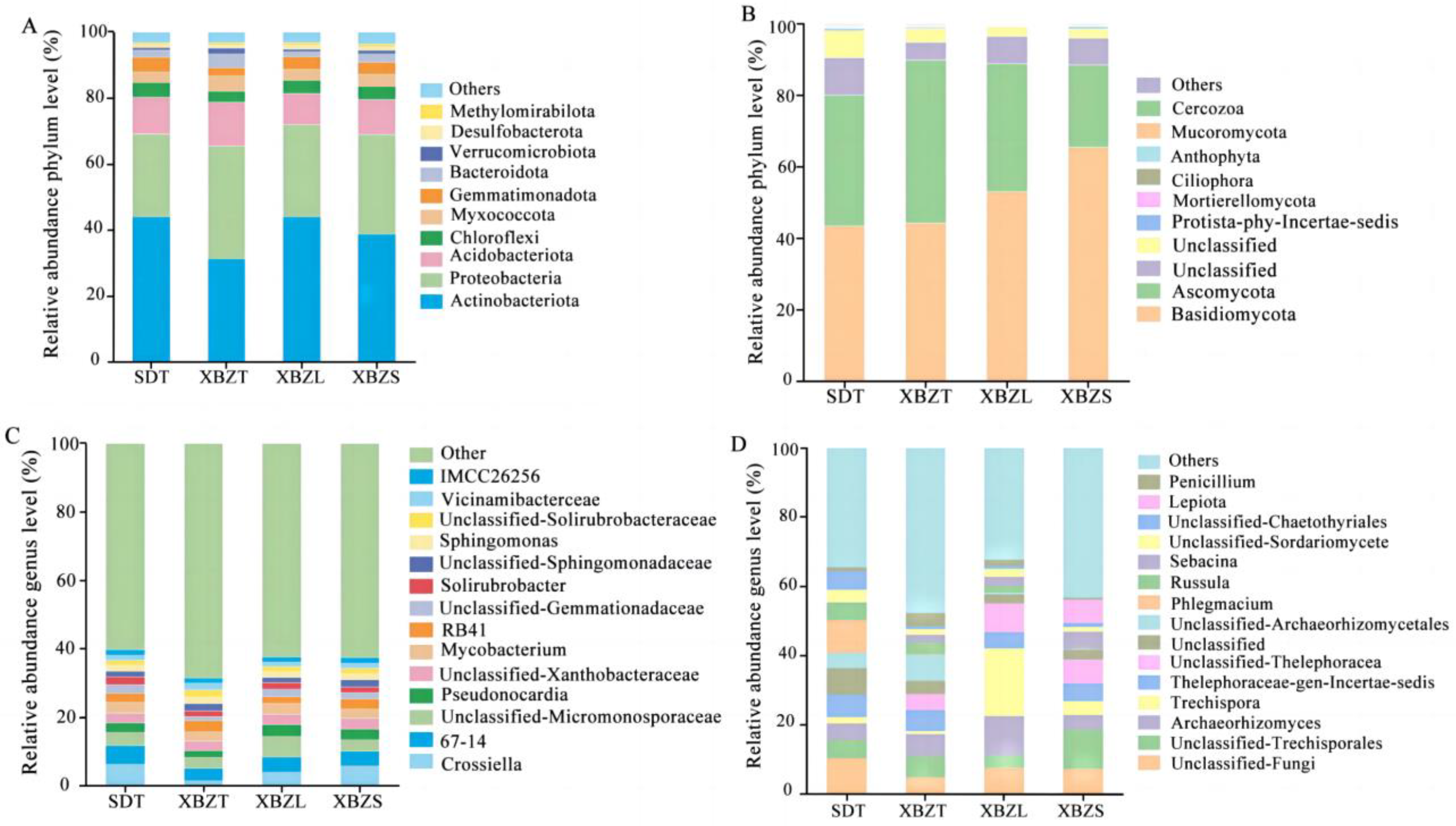
3.5.1. Soil Microbial Community Composition
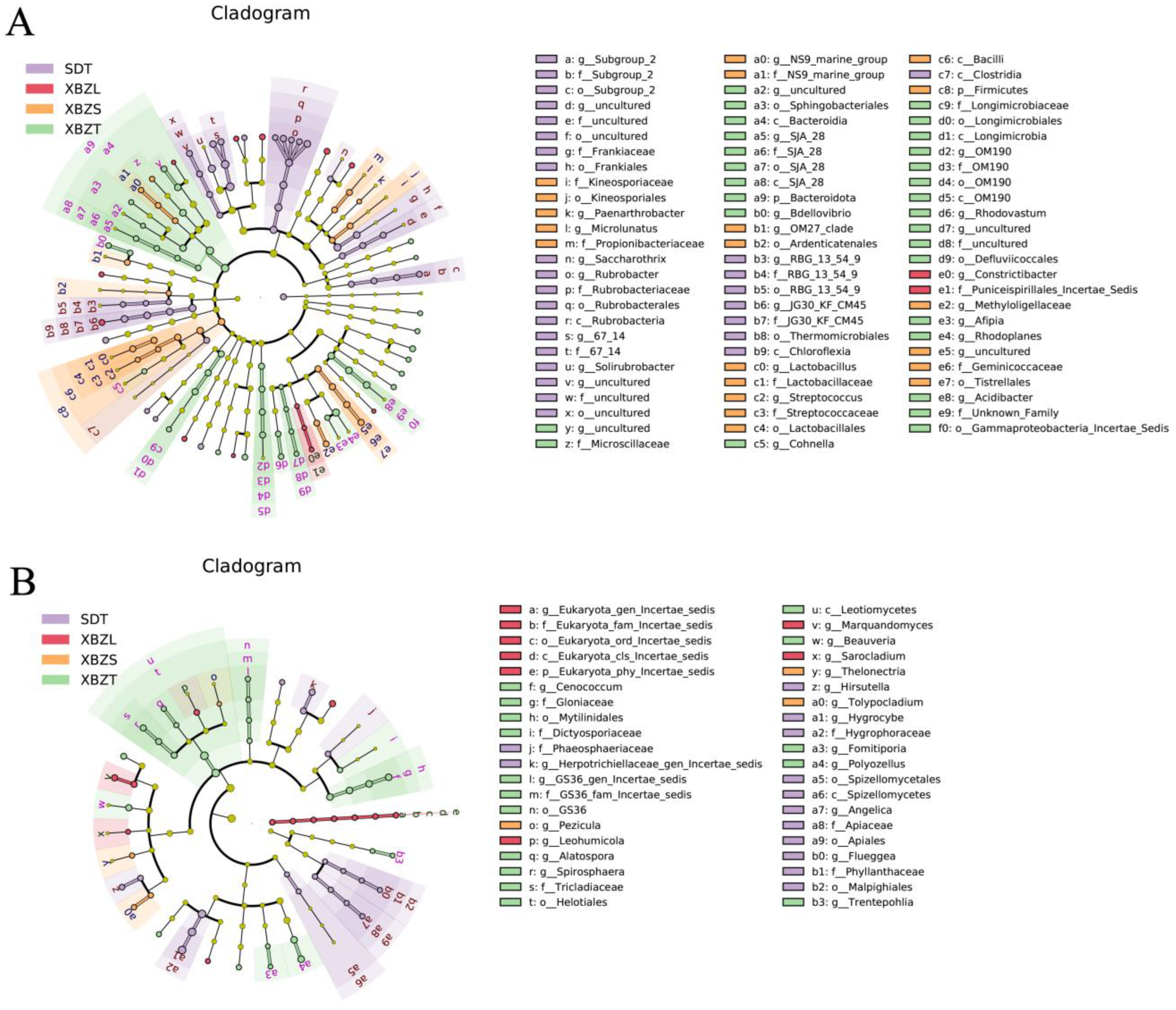
3.5.2. Differential Analysis of Microbial Community Composition
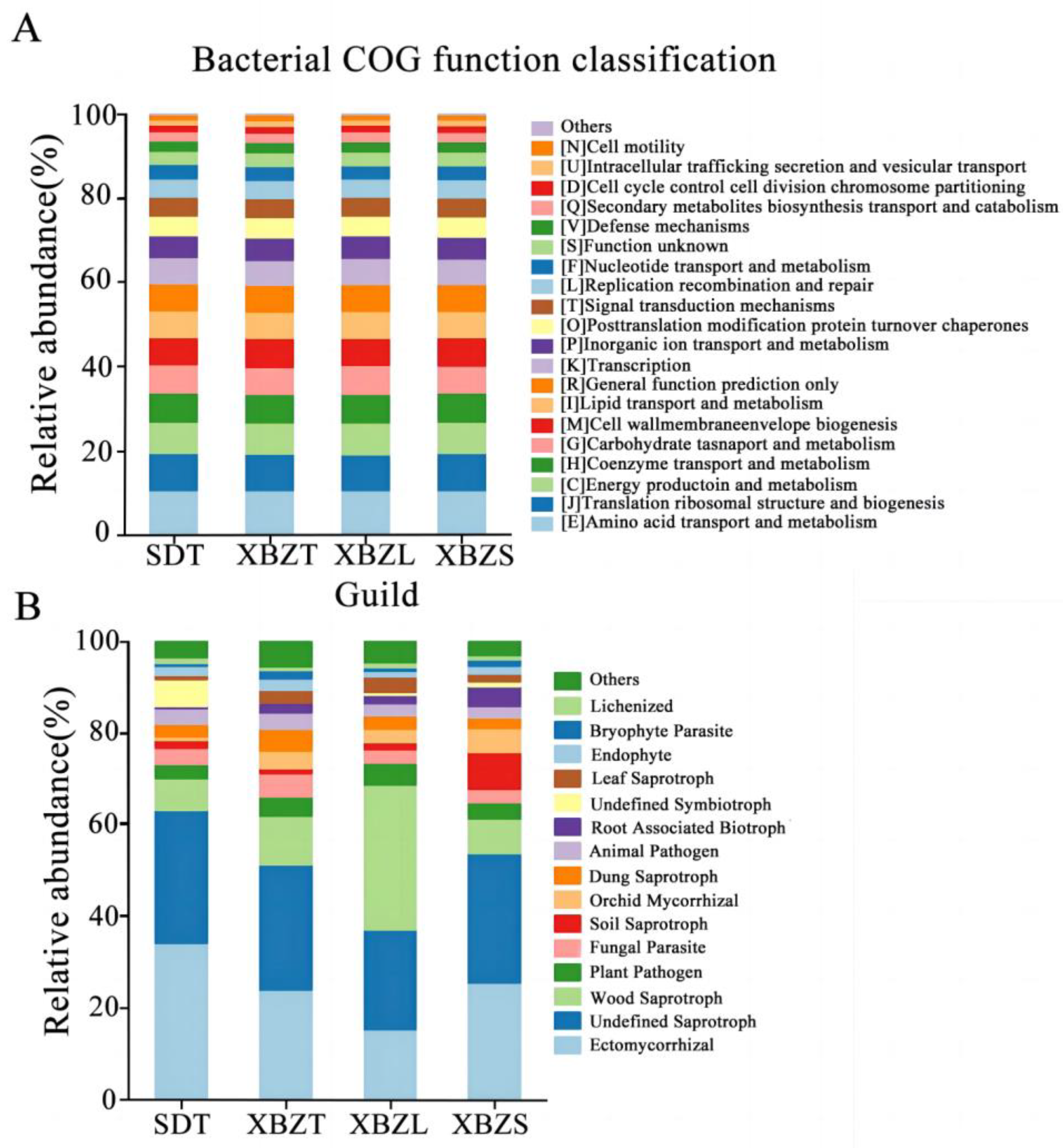
3.5.3. Soil Microbial Functional Prediction
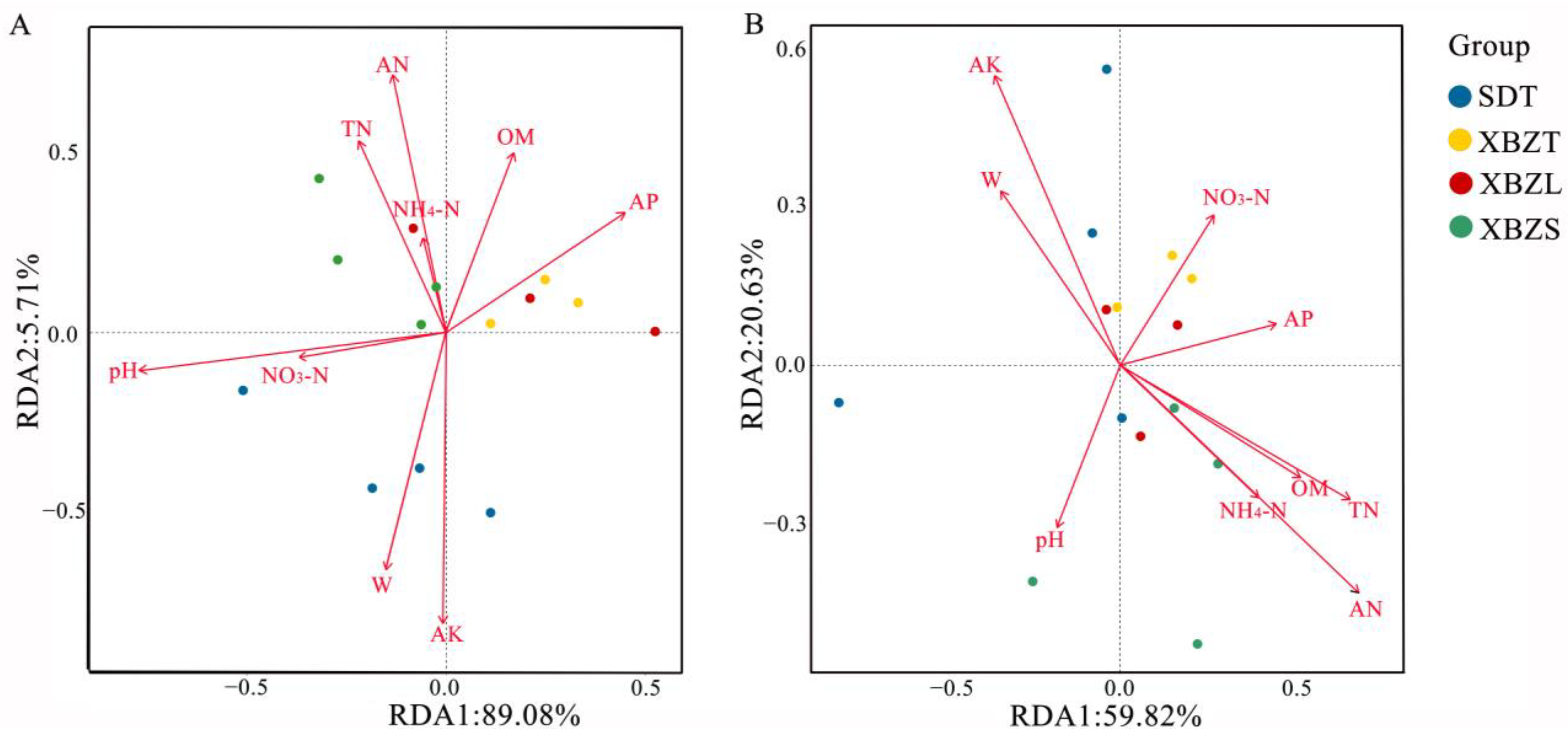
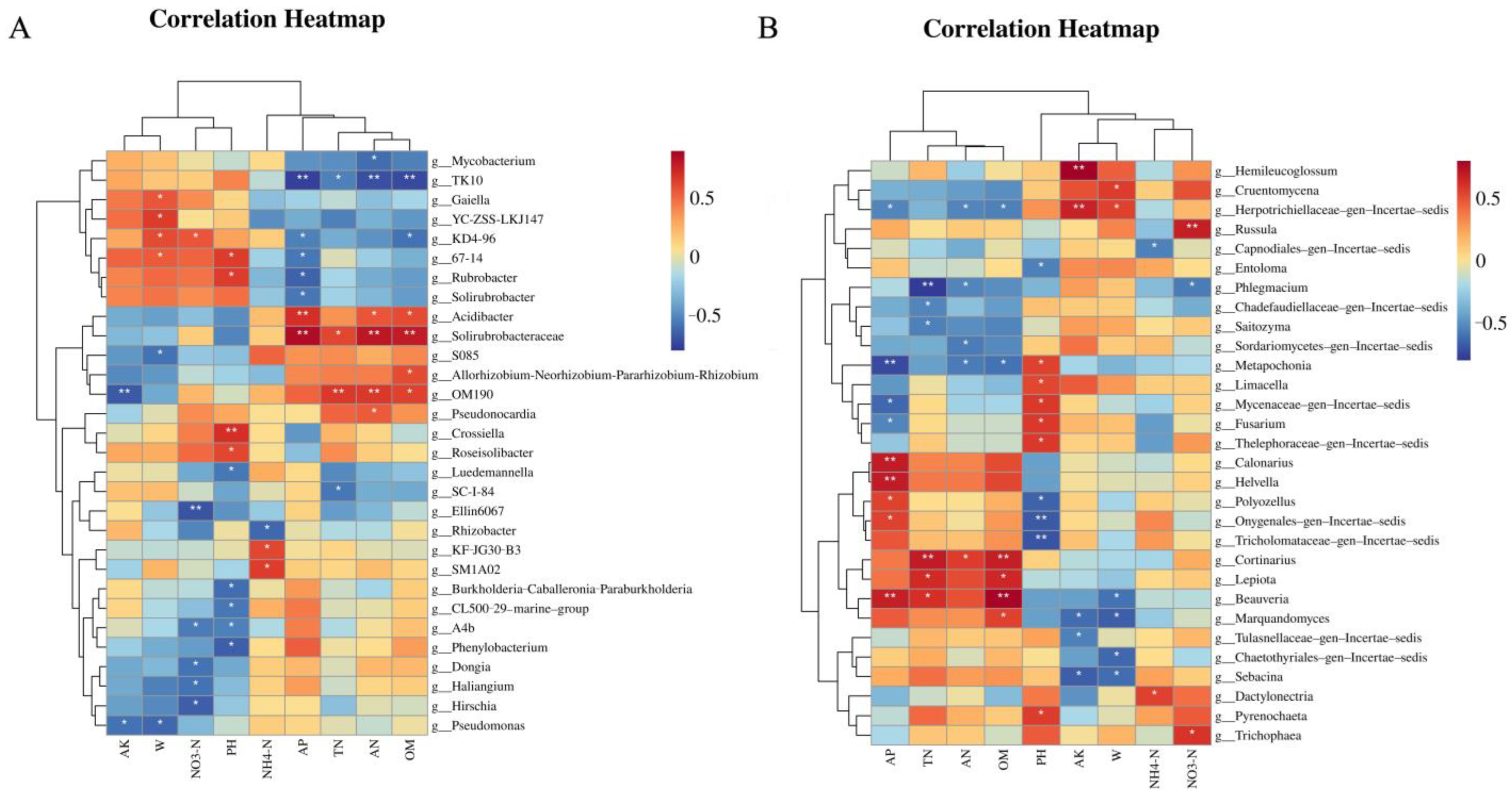
3.6. Relationship Between Soil Microbial Community Structure and Soil Properties
4. Discussion
4.1. Nitrogen Preference of P. micranthum in Different Habitats
4.2. Effects of Different Habitats on the Rhizosphere Microbial Communities of P. micranthum
4.3. Soil Adaptation of P. micranthum and the Function of Soil Microbial Communities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Z.J.; Chen, S.C.; Chen, L.J.; Lei, S.P. The Genus Paphiopedilum in China; Science Press: Beijing, China, 2009; pp. 4–12. [Google Scholar]
- CITES—Convention on International Trade in Endangered Species of Wild Fauna and Flora. Available online: https://cites.org/eng (accessed on 10 July 2023).
- Chen, S.C. Paphiopedilum. Flora Reipublicae Popularis Sinicae Tomus. Biodivers. Sci. 1999, 17, 52–72. [Google Scholar]
- Zhang, Y.; An, M.T.; Wu, J.Y.; Liu, F.; Wang, W. Geographical distribution pattern and dominant climatic factors of the Paphiopedilum Subgen. Brachypetalum in China. Chin. J. Plant Ecol. 2022, 46, 40. [Google Scholar]
- Wang, M.; Chen, H.; Zhang, W.; Wang, K. Influencing factors on soil nutrients at different scales in a karst area. Caten 2019, 175, 411–420. [Google Scholar] [CrossRef]
- Li, Z.Y.; Li, J.; Li, M.Y. Effect of human disturbance on genetic structure of rare and endangered Paphiopedilum micranthum implied the habitat status. Trop. Conserv. Sci. 2020, 13, 1940082920942012. [Google Scholar] [CrossRef]
- Zotz, G.; Armenia, L.; Einzmann, H.J. A new approach to an old problem: How to categorize the habit of ferns and lycophytes. Ann. Bot. 2023, 132, 513–522. [Google Scholar] [CrossRef]
- Averyanov, L.V.; Hiep, N.T.; Lộc, P.K.; Averyanova, A.L. Preliminary orchid checklist of Cao Bang province (Vietnam). Lindleyana 2000, 15, 130–164. [Google Scholar]
- Toure, D.; Ge, J.W.; Zhou, J.W. Interactions between soil characteristics, environmental factors, and plant species abundance: A case study in the Karst Mountains of Longhushan Nature Reserve, southwest China. J. Mt. Sci. 2015, 12, 943–960. [Google Scholar] [CrossRef]
- Geekiyanage, N.; Goodale, U.M.; Cao, K.; Kitajima, K. Plant ecology of tropical and subtropical karst ecosystems. Biotropica 2019, 51, 626–640. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, H.C.; Cheng, Z.; Wang, M.N.; LI, L.Q.; Long, C.L. Current Status of Wild Orchid Resources in China, Focusing on Their Conservation and Utilization. China Biotechnol. 2022, 42, 59–72. [Google Scholar]
- Gaskett, A.C.; Gallagher, R.V. Orchid diversity: Spatial and climatic patterns from herbarium records. Ecol. Evol. 2018, 8, 11235–11245. [Google Scholar] [CrossRef]
- Mikryukov, V.; Dulya, O.; Zizka, A.; Bahram, M.; Hagh-Doust, N.; Anslan, S.; Prylutskyi, O.; Delgado-Baquerizo, M.; Maestre, F.T.; Nilsson, H.; et al. Connecting the multiple dimensions of global soil fungal diversity. Sci. Adv. 2023, 9, eadj8016. [Google Scholar] [CrossRef] [PubMed]
- Eiserhardt, W.L.; Svenning, J.C.; Kissling, W.D.; Balslev, H. Geographical ecology of the palms (Arecaceae): Determinants of diversity and distributions across spatial scales. Ann. Bot. 2011, 108, 1391–1416. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Peng, W.X.; Song, T.Q.; Zeng, F.P.; Wang, K.L.; Song, M.; Zhang, H. Spatial pattern of woody plants and their environmental interpretation in the karst forest of southwest China. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2015, 149, 121–130. [Google Scholar] [CrossRef]
- Djordjević, V.; Tsiftsis, S.; Lakušić, D.; Jovanović, S.; Jakovljević, K.; Stevanović, V. Patterns of distribution, abundance and composition of forest terrestrial orchids. Biodivers Conserv. 2020, 29, 4111–4134. [Google Scholar] [CrossRef]
- Ye, P.; Wu, J.; An, M.; Chen, H.; Zhao, X.; Jin, X.; Si, Q. Geographical distribution and relationship with environmental factors of Paphiopedilum subgenus Brachypetalum Hallier (Orchidaceae) taxa in southwest China. Diversity 2021, 13, 634. [Google Scholar] [CrossRef]
- Kaur, J.; Phillips, C.; Sharma, J. Host population size is linked to orchid mycorrhizal fungal communities in roots and soil, which are shaped by microenvironment. Mycorrhiza 2021, 31, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wu, S.; Yang, W.; Selosse, M.A.; Gao, J. How mycorrhizal associations influence orchid distribution and population dynamics. Front. Plant Sci. 2021, 12, 647114. [Google Scholar] [CrossRef]
- Tian, L.; An, M.; Wu, M.; Liu, F.; Zhang, Y. Habitat ecological characteristics and soil fungal community structure of Paphiopedilum subgenus Brachypetalum Hallier (Orchidaceae) plants in Southwest China. Plant Signal. Behav. 2023, 18, 2227365. [Google Scholar] [CrossRef]
- Yuan, L.; Yang, Z.L.; Li, S.Y.; Hu, H.; Huang, J.L. Mycorrhizal specificity, preference, and plasticity of six slipper orchids from South Western China. Mycorrhiza 2010, 20, 559–568. [Google Scholar] [CrossRef]
- Karbarz, M.; Szlachcikowska, D.; Zapał, A.; Leśko, A. Unlocking the Genetic Identity of Endangered Paphiopedilum Orchids: A DNA Barcoding Approach. Genes 2024, 15, 689. [Google Scholar] [CrossRef]
- Deb, C.R.; Jakha, H.Y. Factors affecting asymbiotic immature seed culture and in vitro propagation of Paphiopedilum insigne (Wall. Ex. Lindl.) Pfitzer, a horticultural important vulnerable orchid. Plant Cell Biotechnol. Mol. Biol. 2020, 21, 129–141. [Google Scholar]
- McPherson, M.R.; Wang, P.; Marsh, E.L.; Mitchell, R.B.; Schachtman, D.P. Isolation and analysis of microbial communities in soil, rhizosphere, and roots in perennial grass experiments. JoVE 2018, 137, e57932. [Google Scholar]
- Wang, B.; Chen, H.; Qu, P.; Lin, R.; He, S.; Li, W.; Zhang, C.; Shi, X.; Liu, Y.; Du, H.; et al. Effect of Different Cultivation Patterns on Amomum villosum Yield and Quality Parameters, Rhizosphere Soil Properties, and Rhizosphere Soil Microbes. Horticulturae 2023, 9, 306. [Google Scholar] [CrossRef]
- Zhang, M.; Liang, G.; Ren, S.; Li, L.; Li, C.; Li, Y.; Yu, X.; Yin, Y.; Liu, T.; Liu, X. Responses of soil microbial community structure, potential ecological functions, and soil physicochemical properties to different cultivation patterns in cucumber. Geoderma 2023, 429, 116237. [Google Scholar] [CrossRef]
- Bao, S.D. Agrochemical Analysis of Soil; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Schinner, F.; Öhlinger, R.; Kandeler, E.; Margesin, R. Methods in Soil Biology; Springer Science and Business Media: New York, NY, USA, 2012. [Google Scholar]
- Zhang, M.; Wang, N.; Zhang, J.; Hu, Y.; Cai, D.; Guo, J.; Wu, D.; Sun, G. Soil physicochemical properties and the rhizosphere soil fungal community in a mulberry (Morus alba L.)/alfalfa (Medicago sativa L.) intercropping system. Forests 2019, 10, 167. [Google Scholar] [CrossRef]
- Huang, M.; Wang, Z.; Luo, L.; Wang, S.; Hui, X.; He, G.; Cao, H.; Ma, X.; Huang, T.; Zhao, Y.; et al. Soil testing at harvest to enhance productivity and reduce nitrate residues in dryland wheat production. Field Crops Res. 2017, 212, 153–164. [Google Scholar] [CrossRef]
- Lü, W.X.; Ge, Y.; Wu, J.Z.; Chang, J. Study on the method for the determination of nitric nitrogen, ammoniacal nitrogen and total nitrogen in plant. Guang Pu Xue Yu Guang Pu Fen Xi 2004, 24, 204–206. [Google Scholar]
- Lee, Y.J.; Sung, J.K.; Lee, S.B.; Lim, J.E.; Song, Y.S.; Lee, D.B.; Hong, S.Y. Plant analysis methods for evaluating mineral nutrient. Korean J. Soil Sci. Fertil. 2017, 50, 93–99. [Google Scholar] [CrossRef]
- Quan, Z.; Zhang, X.; Fang, Y.; Davidson, E.A. Different quantification approaches for nitrogen use efficiency lead to divergent estimates with varying advantages. Nat. Food 2021, 2, 241–245. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4516–4522. [Google Scholar] [CrossRef]
- De, L.C.; Biswas, S.S. Adaptational Mechanisms of Epiphytic Orchids: A Review. Int. J. Bio-Resour. Stress Manag. 2022, 13, 1312–1322. [Google Scholar] [CrossRef]
- Ashton, I.W.; Miller, A.E.; Bowman, W.D.; Suding, K.N. Niche complementarity due to plasticity in resource use: Plant partitioning of chemical N forms. Ecology 2010, 91, 3252–3260. [Google Scholar] [CrossRef]
- Warren, C.R. Does nitrogen concentration affect relative uptake rates of nitrate, ammonium, and glycine? J. Plant. Nutr. Soil. Sci. 2009, 172, 224–229. [Google Scholar] [CrossRef]
- Sun, S.; Chen, J.; Feng, W.; Zhang, C.; Huang, K.; Guan, M.; Feng, Y. Plant strategies for nitrogen acquisition and their effects on exotic plant invasions. Biodivers Sci. 2021, 29, 72. [Google Scholar]
- Liu, Q.; Wu, X.; Xing, H.; Chi, K.; Wang, W.; Song, L.; Xing, X. Orchid diversity and distribution pattern in karst forests in eastern Yunnan Province, China. For. Ecosyst. 2023, 10, 100117. [Google Scholar] [CrossRef]
- Li, S.X.; Wang, Z.H.; Stewart, B.A. Responses of crop plants to ammonium and nitrate N. Adv. Agron. 2013, 118, 205–397. [Google Scholar]
- Bartelheimer, M.; Poschlod, P. The response of grassland species to nitrate versus ammonium coincides with their pH optima. J. Veg. Sci. 2014, 25, 760–770. [Google Scholar] [CrossRef]
- Boczulak, S.A.; Hawkins, B.J.; Roy, R. Temperature effects on nitrogen form uptake by seedling roots of three contrasting conifers. Tree Physiol. 2014, 34, 513–523. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, H.; Xu, X. Root nitrogen acquisition strategy of trees and understory species in a subtropical pine plantation in southern China. Eur. J. Forest. Res. 2020, 139, 791–804. [Google Scholar] [CrossRef]
- Santillán, J.; López-Martínez, R.; Aguilar-Rangel, E.J.; Hernández-García, K.; Vásquez-Murrieta, M.S.; Cram, S.; Alcántara-Hernández, R.J. Microbial diversity and physicochemical characteristics of tropical karst soils in the northeastern Yucatan peninsula, Mexico. Appl. Soil Ecol. 2021, 165, 103969. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Shen, R.F.; Zhao, X.Q. Nitrogen source preference in maize at seedling stage is mainly dependent on growth medium pH. Agronomy 2022, 12, 2149. [Google Scholar] [CrossRef]
- Zhang, Z.L. Study on the Endangered Mechanism of Key Microorganisms in the Root-Endophytic and Rhizosphere of Four Extremely Small Populations of Paphiopedilum Plant Populations; Henan University of Science and Technology: Luoyang, China, 2023. [Google Scholar]
- Saikia, J.; Mazumdar, R.; Thakur, D. Phylogenetic afliation of endophytic actinobacteria associated with selected orchid species and their role in growth promotion and suppression of phytopathogens. Front. Plant Sci. 2022, 13, 1058867. [Google Scholar] [CrossRef] [PubMed]
- Tedsree, N.; Likhitwitayawuid, K.; Sritularak, B.; Tanasupawat, S. Diversity and Antimicrobial activity of plant growth promoting endophytic Actinomycetes isolated from Thai orchids. Environ. Nat. Resour. J. 2022, 20, 379–392. [Google Scholar] [CrossRef]
- Li, Y.Y.; Xu, T.T.; Ai, Z.; Wei, L.L.; Ma, F. Diversity and predictive functional of Caragana jubata bacterial community in rhizosphere and non-rhizosphere Soil at different altitudes. Environ. Sci. 2023, 44, 2304–2314. [Google Scholar]
- Yuan, R.W.; Liu, L.; Zhang, R.; Fan, S.Y. The interaction mechanism between plant rhizosphere secretion and soil microbe: A review. Chin. Agric. Sci. Bull. 2020, 36, 26–35. [Google Scholar]
- Liu, L.; Zhu, K.; Wurzburger, N.; Zhang, J. Relationships between plant diversity and soil microbial diversity vary across taxonomic groups and spatial scales. Ecosphere 2020, 11, e02999. [Google Scholar] [CrossRef]
- Aanderud, Z.T.; Bledsoe, C.S. Preferences for 15N-ammonium, 15N-nitrate, and 15N-glycine differ among dominant exotic and subordinate native grasses from a California Oak woodland. Environ. Exp Bot. 2009, 65, 205–209. [Google Scholar] [CrossRef]
- Wekesa, C.; Kiprotich, K.; Okoth, P.; Asudi, G.O.; Muoma, J.O.; Furch, A.C.U.; Oelmüller, R. Molecular characterization of indigenous rhizobia from Kenyan soils nodulating with common beans. Int. J. Mol. Sci. 2023, 24, 9509. [Google Scholar] [CrossRef]
- Xu, P.; Wang, E. Diversity and regulation of symbiotic nitrogen fixation in plants. Curr. Biol. 2023, 33, 543–559. [Google Scholar] [CrossRef]
- Yi, X.M.; Yuan, J.; Zhu, Y.H.; Yi, X.J.; Zhao, Q.; Fang, K.K.; Cao, L.K. Comparison of the abundance and community structure of N-cycling bacteria in paddy rhizosphere soil under different rice cultivation patterns. Int. J. Mol. Sci. 2018, 19, 3772. [Google Scholar] [CrossRef]
- Bai, B.; Liu, W.; Qiu, X.; Zhang, J.; Zhang, J.; Bai, Y. The root microbiome: Community assembly and its contributions to plant fitness. J. Integr. Plant Biol. 2022, 64, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.R.; Shi, R.H. The effect of soil pretreatment on the mineralization of nitrogen derived from different forms. J. Nanjing Agric. Univ. 1991, 14, 54–58. [Google Scholar]
- Fan, Q.S. Effect of soil microbial biomass on nitrogen conservation. Soils 1987, 19, 46–49. [Google Scholar]
- Finn, D.R.; Ziv-El, M.; Van Haren, J.; Park, J.G.; del Aguila-Pasquel, J.; Urquiza-Muñoz, J.D.; Cadillo-Quiroz, H. Methanogens and methanotrophs show nutrient-dependent community assemblage patterns across tropical peatlands of the Pastaza-Maranon Basin, Peruvian Amazonia. Front. Microbiol. 2020, 11, 746. [Google Scholar] [CrossRef]
- Kalam, S.; Basu, A.; Ahmad, I.; Sayyed, R.Z.; El-Enshasy, H.A.; Dailin, D.J.; Suriani, N.L. Recent understanding of soil acidobacteria and their ecological significance: A critical review. Front. Microbiol. 2020, 11, 580024. [Google Scholar] [CrossRef]
- Portillo, M.C.; Alloza, R.; Gonzalez, J.M. Three different phototrophic microbial communities colonizing a single natural shelter containing prehistoric paintings. Sci. Total Environ. 2009, 407, 4876–4881. [Google Scholar] [CrossRef]
- Rodriguez, V.; Moskwa, L.M.; Oses, R.; Kühn, P.; Riveras-Muñoz, N.; Seguel, O.; Scholten, T.; Wagner, D. Impact of climate and slope aspects on the composition of soil bacterial communities involved in pedogenetic processes along the Chilean coastal cordillera. Microorganisms 2022, 10, 847. [Google Scholar] [CrossRef] [PubMed]
- Filippini, G.; Bugnot, A.B.; Johnston, E.L.; Ruszczyk, J.; Potts, J.; Scanes, P.; Ferguson, A.; Ostrowski, M.; Varkey, D.; Dafforn, K.A. Sediment bacterial communities associated with environmental factors in intermittently closed and Open Lakes and lagoons (ICOLLs). Sci. Total Environ. 2019, 693, 133462. [Google Scholar] [CrossRef]
- Fu, Y.; Kumar, A.; Chen, L.; Jiang, Y.; Ling, N.; Wang, R.; Pan, Q.; Singh, B.P.; Redmile-Gordon, M.; Luan, L.; et al. Rhizosphere microbiome modulated effects of biochar on ryegrass 15N uptake and rhizodeposited 13C allocation in soil. Plant Soil 2021, 463, 359–377. [Google Scholar] [CrossRef]
- Wang, L.; Qiu, S.; Guo, J.; Ge, S. Light irradiation enables rapid start-up of nitritation through suppressing nxrB gene expression and stimulating ammonia-oxidizing bacteria. Environ. Sci. Technol. 2021, 55, 13297–13305. [Google Scholar]
- Li, L.; Dong, Y.; Qian, G.; Hu, X.; Ye, L. Performance and microbial community analysis of bio- electrocoagulation on simultaneous nitrification and denitrification in submerged membrane bioreactor at limited dissolved oxygen. Bioresour. Technol. 2018, 258, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.; Xie, T.X.; Liu, J.F.; Lan, S.R.; Peng, D.H.; Zhang, Q.H. Community structure and biological function of the root symbiotic fungi of Arundina graminifolia. Mycosystema 2019, 38, 1631–1642. [Google Scholar]
- Zhou, J.; Xie, T.X.; Liu, J.F.; Lan, L.Y.; Xu, Y.F.; Liu, Z.Y.; Ai, Y.; Zhang, Q.H. Community structure and biological function of the root symbiotic fungi of wild Cymbidium ensifolium. Acta Microbiol. Sin. 2021, 07, 2136–2153. [Google Scholar]
- Ying, Y.; Jiang, W.; He, X.; Fiaz, S.; Ahmad, S.; Lei, X.; Wang, X. A review of nitrogen translocation and nitrogen-use efficiency. J. Plant Nutr. 2019, 42, 2624–2641. [Google Scholar]
- Mou, Z.-m.; Yan, N.; Li, S.-y.; Hu, H. Nitrogen require ments for vegetative growth, flowering, seed production, and ramet growth of Paphiopedilum armeniacum (Orchid). HortScience 2012, 47, 585–588. [Google Scholar] [CrossRef]
- Shan, T.T.; Zhou, L.S.; Li, B.; Chen, X.M.; Guo, S.X.; Wang, A.R.; Tian, L.X.; Liu, J.T. The plant growth-promoting fungus MF23 (Mycena sp.) increases production of Dendrobium officinale (Orchidaceae) by affecting nitrogen uptake and NH4 + assimilation. Front. Plant Sci. 2021, 12, 693561. [Google Scholar] [CrossRef]
- Zahn, F.E.; Lee, Y.I.; Gebauer, G. Fungal association and root morphology shift stepwise during ontogenesis of orchid Cremastra appendiculata towards autotrophic nutrition. AoB Plants 2022, 14, plac021. [Google Scholar] [CrossRef]
- Hynson, N.A.; Madsen, T.P.; Selosse, M.A.; Adam, I.K.; Ogura-Tsujita, Y.; Roy, M.; Gebauer, G. The physiological ecology of mycoheterotrophy. In Mycoheterotrophy: The Biology of Plants Living on Fungi; Springer Science & Business Media: New York, NY, USA, 2013; pp. 297–342. [Google Scholar]

| Population | Paphiopedilum micranthum | |||
|---|---|---|---|---|
| Sample site | Wenshan County | Maguan County | ||
| Distribution altitude (m) | 1490–1510 | 1360–1370 | ||
| Ecotype | Lithophyte | Terrestrial | Terrestrial | |
| Slope aspect | North | North-West | North | |
| Distribution location | near mountaintop | Middle-up mountainside | near the top | |
| Vegetation type | Shrubwood | Evergreen and Deciduous broad-leaved forest | Evergreen and Deciduous broad-leaved forest | |
| Habitat | Stone wall, Stone crevice (XBZL) | Tree foot (XBZT) | Shrub foot (XBZS) | Tree foot (SDT) |
| Soil thickness (cm) | - | 10–20 | 10–15 | 10–15 |
| Number of populations | 3 | 3 | 4 | 4 |
| Number of samples | 15 | 18 | 24 | 16 |
| Sample | SDT | XBZT | XBZL | XBZS |
|---|---|---|---|---|
| pH | 7.00 ± 0.16 a | 6.73 ± 0.12 ab | 6.67 ± 0.31 b | 6.85 ± 0.21 ab |
| OM (g/kg) | 228.50 ± 19.60 b | 277.33 ± 40 ab | 285.00 ± 39.13 ab | 289.50 ± 38.27 a |
| W (%) | 30.20 ± 6.88 a | 18.61 ± 5.04 b | 22.33 ± 2.23 ab | 21.01 ± 4.28 b |
| TN (g/kg) | 7.81 ± 1.31 a | 9.08 ± 1.55 a | 8.79 ± 0.82 a | 9.73 ± 1.08 a |
| AN (mg/kg) | 561.50 ± 75.34 b | 656.67 ± 86.56 ab | 698.67 ± 31.34 a | 725.25 ± 86.50 a |
| AP (mg/kg) | 6.93 ± 1.17 b | 11.53 ± 3.06 ab | 10.97 ± 4.27 ab | 9.43 ± 1.24 a |
| AK (mg/kg) | 239.25 ± 11.62 a | 177.33 ± 14.01 b | 161.33 ± 46.29 b | 141.00 ± 42.31 b |
| NO3−-N (mg/kg) | 4.46 ± 2.32 a | 2.67 ± 1.92 a | 4.11 ± 1.97 a | 4.29 ± 1.86 a |
| NH4+-N (mg/kg) | 20.52 ± 5.52 a | 23.05 ± 9.42 a | 22.53 ± 4.70 a | 27.98 ± 5.82 a |
| NO3−/NH4+ | 0.22 ± 0.13 a | 0.14 ± 0.11 a | 0.20 ± 0.13 a | 0.15 ± 0.04 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Hu, J.; Ruan, Y.; Wu, Q.; Yue, Y.; Li, Z. Soil Properties and Rhizosphere Microbes Community Structure Reveal Nitrogen Uptake Preferences and Nitrogen Use Efficiency of Two Ecotypes of Paphiopedilum micranthum. Agriculture 2024, 14, 1909. https://doi.org/10.3390/agriculture14111909
Li Y, Hu J, Ruan Y, Wu Q, Yue Y, Li Z. Soil Properties and Rhizosphere Microbes Community Structure Reveal Nitrogen Uptake Preferences and Nitrogen Use Efficiency of Two Ecotypes of Paphiopedilum micranthum. Agriculture. 2024; 14(11):1909. https://doi.org/10.3390/agriculture14111909
Chicago/Turabian StyleLi, Yin, Jiaxue Hu, Yuehong Ruan, Qian Wu, Yan Yue, and Zongyan Li. 2024. "Soil Properties and Rhizosphere Microbes Community Structure Reveal Nitrogen Uptake Preferences and Nitrogen Use Efficiency of Two Ecotypes of Paphiopedilum micranthum" Agriculture 14, no. 11: 1909. https://doi.org/10.3390/agriculture14111909
APA StyleLi, Y., Hu, J., Ruan, Y., Wu, Q., Yue, Y., & Li, Z. (2024). Soil Properties and Rhizosphere Microbes Community Structure Reveal Nitrogen Uptake Preferences and Nitrogen Use Efficiency of Two Ecotypes of Paphiopedilum micranthum. Agriculture, 14(11), 1909. https://doi.org/10.3390/agriculture14111909





