Effect of P Reduction on phoD-Harboring Bacteria Community in Solar Greenhouse Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design and Soil Collection
2.2. Measurement of Morphological Traits, Yield and Nutrient Content of Cucumber Plants
2.3. Soil Physical and Chemical Properties Analyses
2.4. Extraction of Soil Phosphatase Activity and P Fractions
2.5. DNA Extraction, PCR Amplification and Illumina Miseq Sequencing of Soil phoD Community
2.6. Statistical Analyses
3. Results
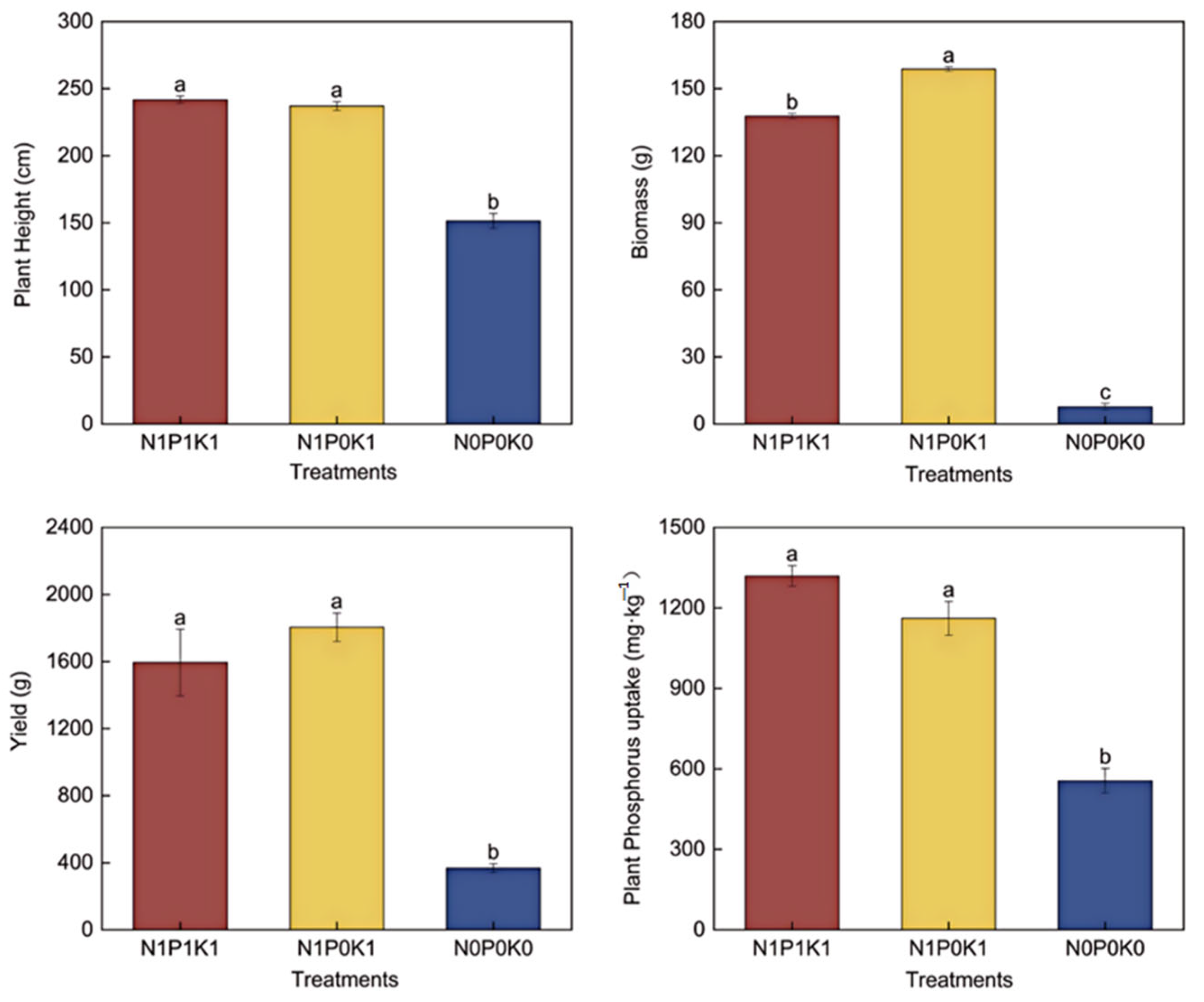
3.1. Effects of Reduced P on Morphological Characteristics, Yield and Nutrients of Cucumber Plants in Solar Greenhouse
3.2. Effects of P Fertilizer Reduction on Soil Chemical Properties of Cucumber in Solar Greenhouse
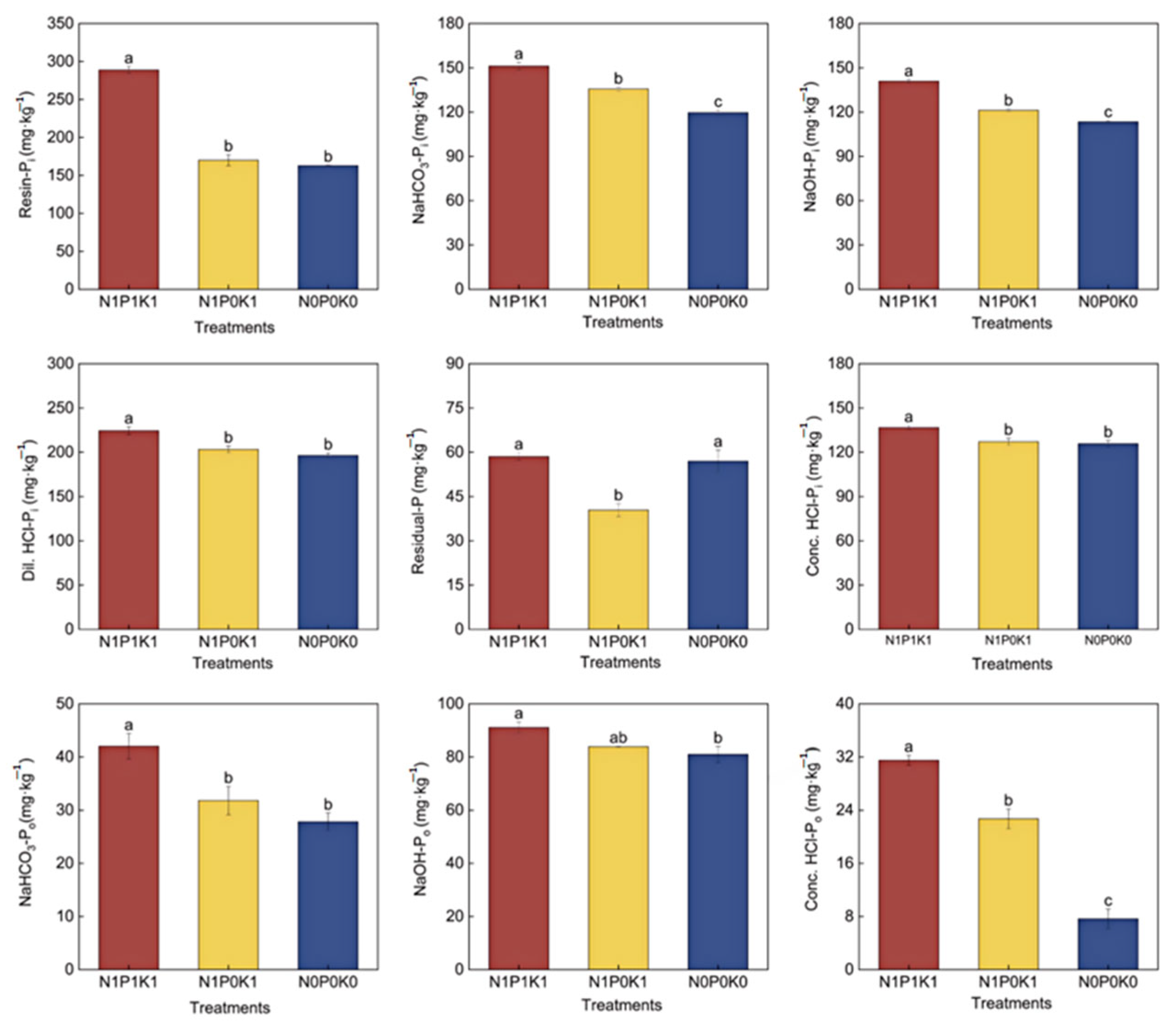
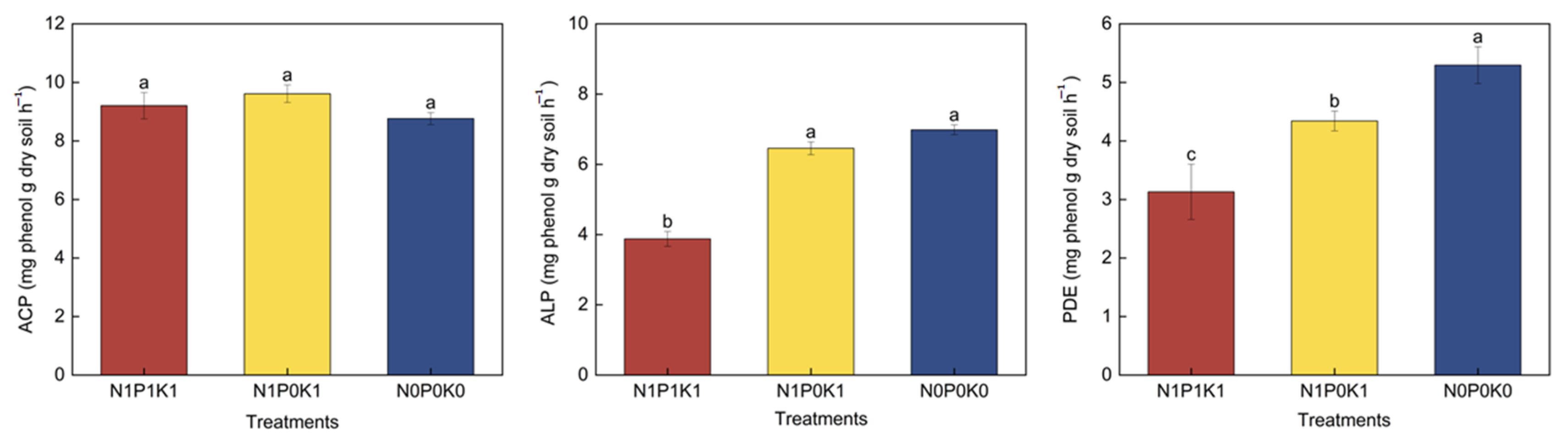
3.3. Effects of P Fertilizer Reduction on Soil P Fractions and Enzyme Activities of Cucumber in Solar Greenhouse
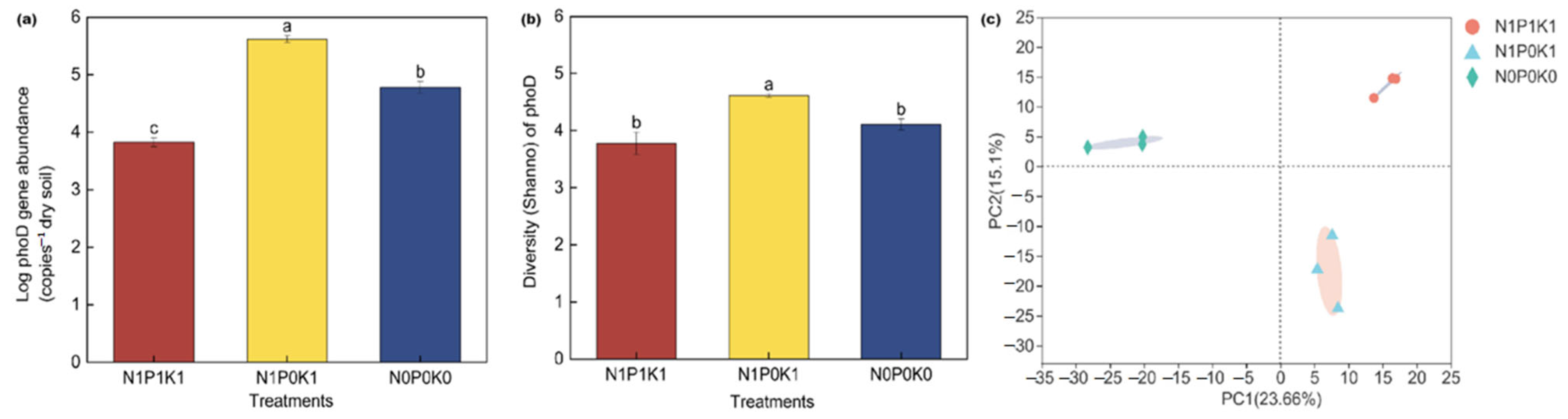
3.4. Effects of P Fertilizer Reduction on Soil phoD Gene Abundance and Community Diversity of Cucumber in Solar Greenhouse
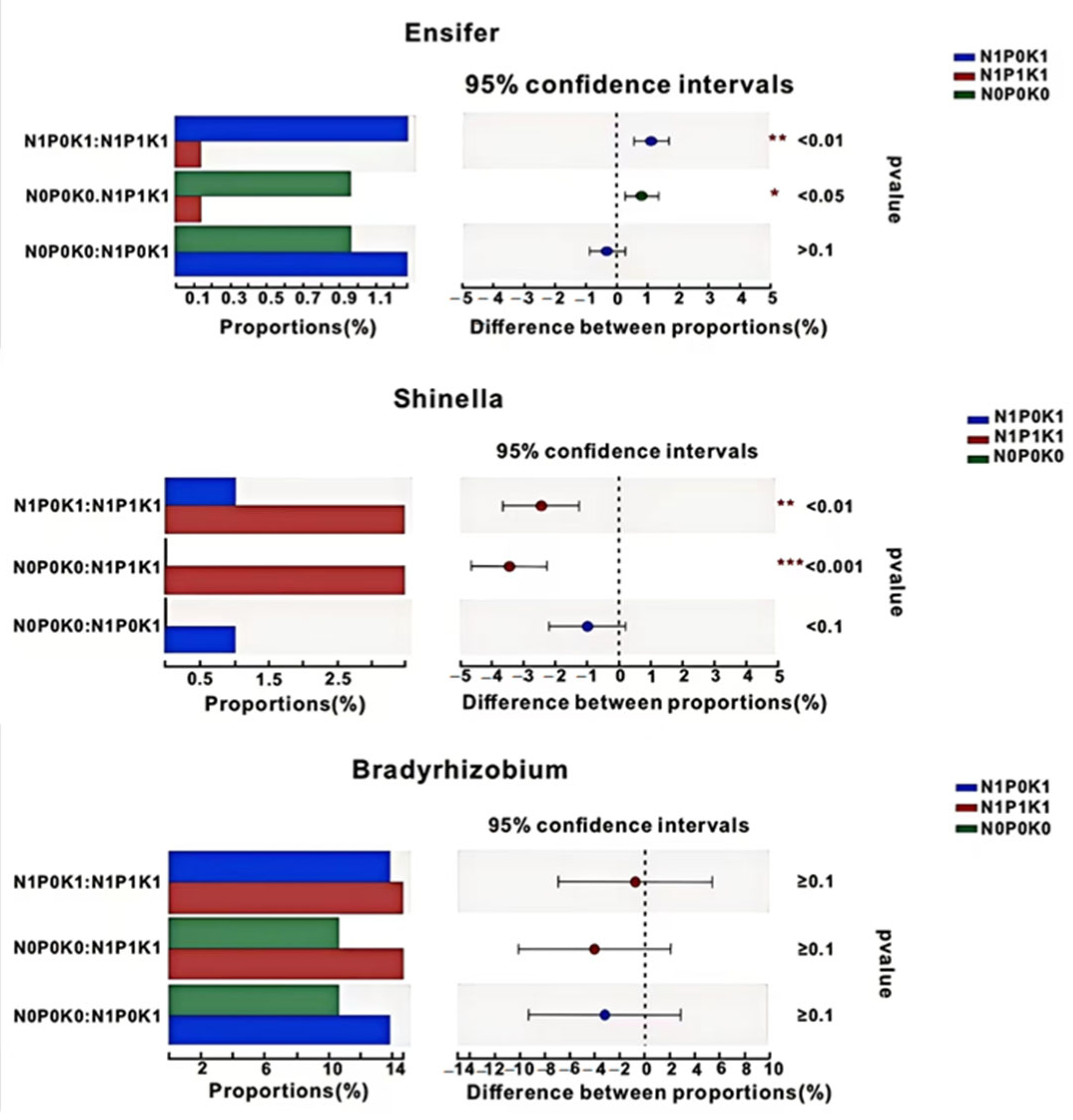
3.5. Effects of P Fertilizer Reduction on Soil phoD-Harboring Bacterial Community of Cucumber in Solar Greenhouse
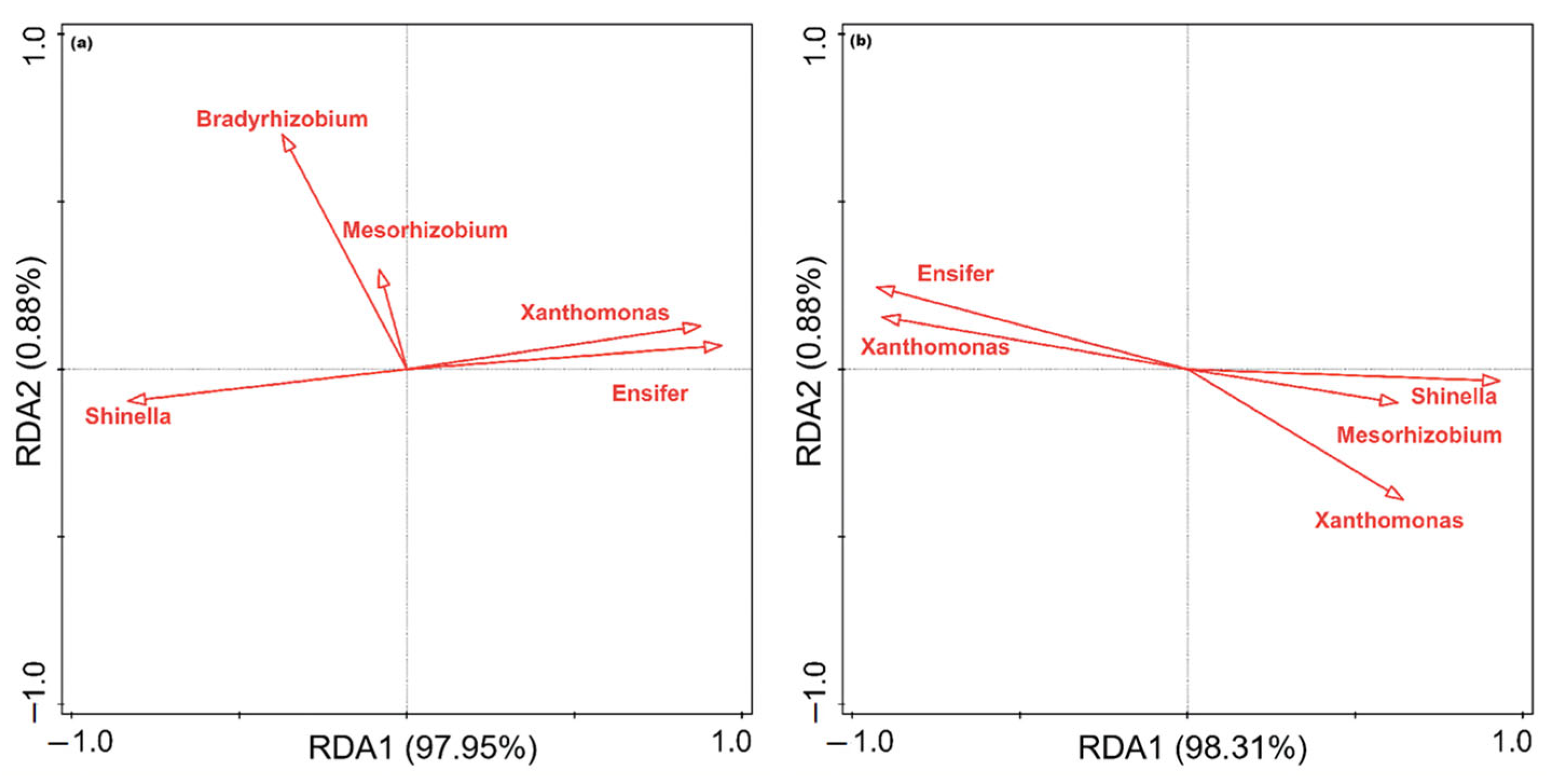
3.6. Effects of Reducing P Fertilizer on Soil Properties and the Relationship between P Fractions and the phoD-Harboring Bacterial Community of Cucumbers in Solar Greenhouses
4. Discussion
4.1. Changes in the Soil phoD-Harboring Bacterial Community and Its Relationship with Soil Physical and Chemical Properties
4.2. The Soil P Fraction and Its Relationship with the phoD-Harboring Community
4.3. Characteristics of the Variations in Cucumber Yield and P Uptake and the Relationship with Soil P Fractions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, X.; Liu, C.; Liang, C.; Wang, T.; Tian, J. The Phosphorus-Iron Nexus: Decoding the Nutrients Interaction in Soil and Plant. Int. J. Mol. Sci. 2024, 25, 6992. [Google Scholar] [CrossRef]
- Li, J.; Wan, X.; Liu, X.; Chen, Y.; Slaughter, L.C.; Weindorf, D.C.; Dong, Y. Changes in soil physical and chemical characteristics in intensively cultivated greenhouse vegetable fields in North China. Soil Tillage Res. 2019, 195, 104366. [Google Scholar] [CrossRef]
- Xu, M.-Z.; Wang, Y.-H.; Nie, C.-E.; Song, G.-P.; Xin, S.-N.; Lu, Y.-L.; Bai, Y.-L.; Zhang, Y.-J.; Wang, L. Identifying the critical phosphorus balance for optimizing phosphorus input and regulating soil phosphorus effectiveness in a typical winter wheat–summer maize rotation system in North China. J. Integr. Agric. 2023, 22, 3769–3782. [Google Scholar] [CrossRef]
- Lan, Z.M.; Lin, X.J.; Wang, F.; Zhang, H.; Chen, C.R. Phosphorus availability and rice grain yield in a paddy soil in response to long-term fertilization. Biol. Fertil. Soils 2012, 48, 579–588. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; An, Y.; Chen, X. Phosphorus fractionation related to environmental risks resulting from intensive vegetable cropping and fertilization in a subtropical region. Environ. Pollut. 2021, 269, 116098. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, X.; Guo, Z.; Jia, Z.; Wang, S.; Ding, K. Response of soil microbes to a reduction in phosphorus fertilizer in rice-wheat rotation paddy soils with varying soil P levels. Soil Tillage Res. 2018, 181, 127–135. [Google Scholar] [CrossRef]
- Bai, Y.; Zhu, J.; Deng, B.; Shi, H.; Wang, Z.; Han, D.; Duan, J.; Wang, D. Short-Term Effect of the Addition of Rice Husk Gasification Slag on the Movement and Transformation of Phosphorus in Different Soil Types. Sustainability 2020, 12, 2458. [Google Scholar] [CrossRef]
- Wei, K.; Chen, Z.; Jiang, N.; Zhang, Y.; Feng, J.; Tian, J.; Chen, X.; Lou, C.; Chen, L. Effects of mineral phosphorus fertilizer reduction and maize straw incorporation on soil phosphorus availability, acid phosphatase activity, and maize grain yield in northeast China. Arch. Agron. Soil Sci. 2020, 67, 66–78. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, W.; Luan, H.; Tang, J.; Li, R.; Li, M.; Zhang, H.; Huang, S. Long-Term organic substitution management affects soil phosphorus speciation and reduces leaching in greenhouse vegetable production. J. Clean. Prod. 2021, 327, 129464. [Google Scholar] [CrossRef]
- Wu, Q.; Huang, Z.; Zhu, P.; Wang, B.; Zhang, S.; Ao, J. Long-term change in phosphorus behavior and the degree of P saturation in typical cropland after different fertilization practices. Soil Sci. Plant Nutr. 2021, 68, 202–214. [Google Scholar] [CrossRef]
- Schaap, K.J.; Fuchslueger, L.; Hoosbeek, M.R.; Hofhansl, F.; Martins, N.P.; Valverde-Barrantes, O.J.; Hartley, I.P.; Lugli, L.F.; Quesada, C.A. Litter inputs and phosphatase activity affect the temporal variability of organic phosphorus in a tropical forest soil in the Central Amazon. Plant Soil 2021, 469, 423–441. [Google Scholar] [CrossRef]
- Nishigaki, T.; Tsujimoto, Y.; Rinasoa, S.; Rakotoson, T.; Andriamananjara, A.; Razafimbelo, T. Phosphorus uptake of rice plants is affected by phosphorus forms and physicochemical properties of tropical weathered soils. Plant Soil 2018, 435, 27–38. [Google Scholar] [CrossRef]
- Gatiboni, L.; Djalma, E.S.; Tiecher, T.; Murilo, G.V.; Danilo, R.D.S.; João, K.; Gustavo, B. Plant uptake of legacy phosphorus from soils without P fertilization. Nutr. Cycl. Agroecosystems 2021, 119, 139–151. [Google Scholar] [CrossRef]
- Bahramnejad, A.R.; Abad, H.H.S.; Madani, H. Effect of Irrigation Regimes and Phosphorus Fertilization on Water-use Efficiency, Phosphorus-agronomic Efficiency and Yield of Grass Pea (Lathyrus sativus L.) Ecotypes. Legume Res. Int. J. 2021, 44, 1038–1045. [Google Scholar] [CrossRef]
- Ye, L.; Zhao, X.; Bao, E.; Li, J.S.; Zhou, Z.R.; Cao, K. Bio-Organic fertilizer with reduced rates of chemical fertilization improves soil fertility and enhances tomato yield and quality. Sci. Rep. 2020, 10, 177. [Google Scholar] [CrossRef]
- Ragot, S.A.; Kertesz, M.A.; Bünemann, E.K. Diversity of the phoD alkaline phosphatase gene in soil. Appl. Environ. Microbiol. 2015, 81, 7281. [Google Scholar] [CrossRef]
- Shi, W.; Xing, Y.; Zhu, Y.; Gao, N.; Ying, Y. Diverse responses of pqqC- and phoD-harbouring bacterial communities to variation in soil properties of Moso bamboo forests. Microb. Biotechnol. 2022, 15, 2097–2111. [Google Scholar] [CrossRef]
- Luo, G.; Ling, N.; Nannipieri, P.; Chen, H.; Raza, W.; Wang, M.; Guo, S.; Shen, Q. Long-Term fertilisation regimes affect the composition of the alkaline phosphomonoesterase encoding microbial community of a vertisol and its derivative soil fractions. Biol. Fertil. Soils 2017, 53, 375–388. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, N.; Condron, L.M.; Dunfield, K.E.; Chen, Z.; Wang, J.; Chen, L. Impact of long-term phosphorus fertilizer inputs on bacterial phoD gene community in a maize field, Northeast China. Sci. Total Environ. 2019, 669, 1011–1018. [Google Scholar] [CrossRef]
- Gou, X.; Ren, Y.; Qin, X.; Wei, X.; Wang, J. Global patterns of soil phosphatase responses to nitrogen and phosphorus fertilization. Pedosphere 2024, 34, 200–210. [Google Scholar] [CrossRef]
- Bi, Q.F.; Li, K.J.; Zheng, B.X.; Liu, X.P.; Li, H.Z.; Jin, B.J.; Ding, K.; Yang, X.R.; Lin, X.Y.; Zhu, Y.G. Partial replacement of inorganic phosphorus (P) by organic manure reshapes phosphate mobilizing bacterial community and promotes P bioavailability in a paddy soil. Sci. Total Environ. 2020, 703, 134977. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Z.; Chen, Q.; Shi, W.C.; Sun, X.; Dong, J.J.; Li, J.; Wang, H.T.; Gao, J.G.; Liu, Z.G.; Zhang, M. Interactions between phosphorus availability and microbes in a wheat–maize double cropping system: A reduced fertilization scheme. J. Integr. Agric. 2022, 21, 840–854. [Google Scholar] [CrossRef]
- Roohi, M.; Arif, M.S.; Yasmeen, T.; Bragazza, L. Effects of cropping system and fertilization regime on soil phosphorous are mediated by rhizosphere-microbial processes in a semi-arid agroecosystem. J. Environ. Manag. 2020, 271, 111033. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Tong, D.; Wang, S.; Wang, D.; Xu, L.; Li, B.; Sun, W.; Wang, Y. Long-Term rice cultivation promoted microbial mineralization of organic P in a black soil. Soil Sci. Soc. Am. J. 2022, 86, 540–551. [Google Scholar] [CrossRef]
- Subramanyam, R.; Meng, X.; Chen, W.-W.; Wang, Y.-Y.; Huang, Z.-R.; Ye, X.; Chen, L.-S.; Yang, L.-T. Effects of phosphorus deficiency on the absorption of mineral nutrients, photosynthetic system performance and antioxidant metabolism in Citrus grandis. PLoS ONE 2021, 16, e0246944. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Agricultural Chemistry Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Tiessen, H.; Moir, J.O. Characterization of available P by sequential extraction. Soil Sampl. Methods Anal. 1993, 7, 5–229. [Google Scholar]
- Hedley, M.J.; Stewart, J.W.B.; Chauhan, B.S. Changes in Inorganic and Organic Soil Phosphorus Fractions Induced by Cultivation Practices and by Laboratory Incubations. Soil Sci. Soc. Am. J. 1982, 46, 970–976. [Google Scholar] [CrossRef]
- Tabatabai, M. Methods of soil analysis: Part 2—Microbiological and biochemical properties. Soil Enzym. 1994, 5, 775–833. [Google Scholar]
- Ragot, S.A.; Huguenin-Elie, O.; Kertesz, M.A.; Frossard, E.; Bünemann, E.K. Total and active microbial communities and phoD as affected by phosphate depletion and pH in soil. Plant Soil 2016, 408, 15–30. [Google Scholar] [CrossRef]
- Ragot, S.A. Development and Application of Molecular Tools to Investigate Microbial Alkaline Phosphatase Genes in Soil. Ph.D. Thesis, ETH Zurich, Zurich, Switzerland, 2016. [Google Scholar]
- Wang, Y.; Huang, R.; Xu, G.; Li, J.; Wang, Z.; Ci, E.; Gao, M. Soil alkaline phosphatase activity and bacterial phoD gene abundance and diversity under regimes of inorganic fertilizer reduction with straw. J. Soils Sediments 2020, 21, 388–402. [Google Scholar] [CrossRef]
- Fraser, T.D.; Lynch, D.H.; Bent, E.; Entz, M.H.; Dunfield, K.E. Soil bacterial phoD gene abundance and expression in response to applied phosphorus and long-term management. Soil Biol. Biochem. 2015, 88, 137–147. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, L.; Liu, X.; Ma, Q.; Liu, J. Impact of Maize Growth Stage on Soil Bacterial Communities and Functions in Dryland Under P Fertilization: A Comprehensive Analysis. J. Soil Sci. Plant Nutr. 2024, 24, 1172–1182. [Google Scholar] [CrossRef]
- Wang, M.; Wu, Y.; Zhao, J.; Liu, Y.; Chen, Z.; Tang, Z.; Tian, W.; Xi, Y.; Zhang, J. Long-term fertilization lowers the alkaline phosphatase activity by impacting the phoD-harboring bacterial community in rice-winter wheat rotation system. Sci. Total Environ. 2022, 821, 153406. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Xia, Y.; Sun, Q.; Liu, K.; Chen, X.; Ge, T.; Zhu, B.; Zhu, Z.; Zhang, Z.; Su, Y. Effects of long-term fertilization on phoD-harboring bacterial community in Karst soils. Sci. Total Environ. 2018, 628, 53–63. [Google Scholar] [CrossRef]
- Lang, M.; Zou, W.; Chen, X.; Zou, C.; Zhang, W.; Deng, Y.; Zhu, F.; Yu, P.; Chen, X. Soil Microbial Composition and phoD Gene Abundance Are Sensitive to Phosphorus Level in a Long-Term Wheat-Maize Crop System. Front. Microbiol. 2021, 11, 605955. [Google Scholar] [CrossRef]
- Long, X.-E.; Yao, H.; Huang, Y.; Wei, W.; Zhu, Y.-G. Phosphate levels influence the utilisation of rice rhizodeposition carbon and the phosphate-solubilising microbial community in a paddy soil. Soil Biol. Biochem. 2018, 118, 103–114. [Google Scholar] [CrossRef]
- Xin, Y.-Y.; Rahman, A.; Li, H.-X.; Xu, T.; Ding, G.-C.; Li, J. Modification of total and phosphorus mineralizing bacterial communities associated with Zea mays L. through plant development and fertilization regimes. J. Integr. Agric. 2021, 20, 3026–3038. [Google Scholar] [CrossRef]
- Khan, A.; Zhang, G.; Li, T.; He, B. Fertilization and cultivation management promotes soil phosphorus availability by enhancing soil P-cycling enzymes and the phosphatase encoding genes in bulk and rhizosphere soil of a maize crop in sloping cropland. Ecotoxicol. Environ. Saf. 2023, 264, 115441. [Google Scholar] [CrossRef]
- Zheng, M.M.; Wang, C.; Li, W.X.; Guo, L.; Cai, Z.J.; Wang, B.R.; Chen, J.; Shen, R.F. Changes of acid and alkaline phosphatase activities in long-term chemical fertilization are driven by the similar soil properties and associated microbial community composition in acidic soil. Eur. J. Soil Biol. 2021, 104, 103312. [Google Scholar] [CrossRef]
- Nunes, R.d.S.; de Sousa, D.M.G.; Goedert, W.J.; de Oliveira, L.E.Z.; Pavinato, P.S.; Pinheiro, T.D. Distribution of Soil Phosphorus Fractions as a Function of Long-Term Soil Tillage and Phosphate Fertilization Management. Front. Earth Sci. 2020, 8, 350. [Google Scholar] [CrossRef]
- Kumar, Y.; Naresh, R.K.; Dhaliwal, S.S.; Sharma, V.; Kumar, R.; Mandal, A. Impact of NPK Enriched Bio-Compost on Rice Yield and Sustainability of Nutrients in Sandy Loam Soils of India. Commun. Soil Sci. Plant Anal. 2022, 53, 2996–3007. [Google Scholar] [CrossRef]
- Sun, F.; Sun, N.; Ma, X.; Zhou, B.; Zhu, P.; Gao, H.; Xu, M. The Application of Fertilizer Phosphorus Affected Olsen P and the Phosphorus Fractions of Hedley Method in Black Soil. Agronomy 2022, 12, 3146. [Google Scholar] [CrossRef]
- Pavinato, P.S.; Gotz, L.F.; Teles, A.P.B.; Arruda, B.; Herrera, W.B.; Chadwick, D.R.; Jones, D.L.; Withers, P.J.A. Legacy soil phosphorus bioavailability in tropical and temperate soils: Implications for sustainable crop production. Soil Tillage Res. 2024, 244, 106228. [Google Scholar] [CrossRef]
- Bibi, S.; Irshad, M.; Mohiuddin, M.; Sher, S.; Tariq, M.A.U.R.; Ng, A.W.M. Distribution of Phosphorus Fractions in Orchard Soils in Relation to Soil Properties and Foliar P Contents. Sustainability 2022, 14, 3966. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, N.; Chen, Y.; Qin, Z.; Jin, Y.; Zhu, P.; Peng, C.; Colinet, G.; Zhang, S.; Liu, J. The Phosphorus Availability in Mollisol Is Determined by Inorganic Phosphorus Fraction under Long-Term Different Phosphorus Fertilization Regimes. Agronomy 2022, 12, 2364. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Z.; Zhang, L.; Chen, W.; Feng, G. Dynamics between soil fixation of fertilizer phosphorus and biological phosphorus mobilization determine the phosphorus budgets in agroecosystems. Agric. Ecosyst. Environ. 2024, 375, 109174. [Google Scholar] [CrossRef]
- Guppy, C. Is soil phosphorus fractionation as valuable as we think? Plant Soil 2021, 459, 19–21. [Google Scholar] [CrossRef]
- Mahmood, M.; Tian, Y.; Ma, Q.; Ahmed, W.; Mehmood, S.; Hui, X.; Wang, Z. Changes in Phosphorus Fractions and Its Availability Status in Relation to Long Term P Fertilization in Loess Plateau of China. Agronomy 2020, 10, 1818. [Google Scholar] [CrossRef]
- Huang, B.; Yan, D.; Ouyang, C.; Zhang, D.; Zhu, J.; Liu, J.; Li, Y.; Wang, Q.; Han, Q.; Cao, A. Chloropicrin fumigation alters the soil phosphorus and the composition of the encoding alkaline phosphatase PhoD gene microbial community. Sci. Total Environ. 2020, 711, 135080. [Google Scholar] [CrossRef]
- Zhuo, T.Y.; Ding, Y.; Wan, Q.; Li, S.M.; Chai, B.B.; Lei, X.H. Effects of hydrostatic pressure on phosphorus transformation at the water-sediment interface of a deep reservoir:novel insights into bacterial community and functional genes. J. Soils Sediments 2021, 21, 3367–3379. [Google Scholar] [CrossRef]
- Li, K.S.; Van Zeghbroeck, J.; Liu, Q.C.; Zhang, S.A. Isolating and Characterizing Phosphorus Solubilizing Bacteria from Rhizospheres of Native Plants Grown in Calcareous Soils. Front. Environ. Sci. 2021, 9, 802563. [Google Scholar] [CrossRef]
- Chen, W.; Zhan, Y.; Zhang, X.; Shi, X.; Wang, Z.; Xu, S.; Chang, Y.; Ding, G.; Li, J.; Wei, Y. Influence of carbon-to-phosphorus ratios on phosphorus fractions transformation and bacterial community succession in phosphorus-enriched composting. Bioresour. Technol. 2022, 362, 127786. [Google Scholar] [CrossRef] [PubMed]
- Maity, A.; Marathe, R.A.; Sarkar, A.; Basak, B.B. Phosphorus and potassium supplementing bio-mineral fertilizer augments soil fertility and improves fruit yield and quality of pomegranate. Sci. Hortic. 2022, 303, 111234. [Google Scholar] [CrossRef]
- Bai, S.; Tan, J.; Zhang, Z.; Wei, M.; Zhang, H.; Jiang, X. Phosphorus speciation and colloidal phosphorus responses to short-term cessation of fertilization in a lime concretion black soil. Pedosphere 2023, 33, 948–959. [Google Scholar] [CrossRef]
- Wang, Y.L.; Zhang, H.L.; Tang, J.W.; Xu, J.B.; Kou, T.J.; Huang, H.M. Accelerated phosphorus accumulation and acidification of soils under plastic greenhouse condition in four representative organic vegetable cultivation sites. Sci. Hortic. 2015, 195, 67–73. [Google Scholar] [CrossRef]
- Xiao, L.; Chen, G.L.; Wang, H.; Li, Y.X.; Li, C.; Cheng, L.; Wu, W.E.; Xiao, X.; Zhu, Y.Y. Improvement of P Use Efficiency and P Balance of Rice-Wheat Rotation System According to the Long-Term Field Experiments in the Taihu Lake Basin. Front. Environ. Sci. 2022, 10, 932833. [Google Scholar] [CrossRef]
- Nziguheba, G.; Merckx, R.; Palm, C.A. Soil phosphorus dynamics and maize response to different rates of phosphorus fertilizer applied to an Acrisol in western Kenya. Plant Soil 2002, 243, 1–10. [Google Scholar] [CrossRef]




| Materials | pH | TC | TN | TP | TK | SOM | AN | AP | AK |
|---|---|---|---|---|---|---|---|---|---|
| g·kg−1 | g·kg−1 | g·kg−1 | g·kg−1 | g·kg−1 | mg·kg−1 | mg·kg−1 | mg·kg−1 | ||
| Original soil | 7.30 | 21.36 | 2.09 | 0.91 | 23.72 | 36.82 | 40.30 | 156.30 | 231.30 |
| Resin-P | NaHCO3-Pi | NaOH-Pi | Dil. HCl-Pi | Conc. HCl-Pi | NaHCO3-Po | NaOH-Po | Conc. HCl-Po | Residual-P | |
| mg·kg−1 | mg·kg−1 | mg·kg−1 | mg·kg−1 | mg·kg−1 | mg·kg−1 | mg·kg−1 | mg·kg−1 | mg·kg−1 | |
| 185.10 | 142.10 | 130.70 | 208.70 | 130.10 | 29.20 | 81.30 | 17.80 | 57.20 |
| Treatment | N1P1K1 | N1P0K1 | N0P0K0 |
|---|---|---|---|
| pH | 7.15 ± 0.03 a | 7.26 ± 0.05 a | 7.27 ± 0.03 a |
| TC (g·kg−1) | 21.73 ± 0.05 ab | 20.51 ± 0.37 b | 22.43 ± 0.52 a |
| TN (g·kg−1) | 2.19 ± 0.04 a | 2.01 ± 0.05 b | 2.05 ± 0.02 b |
| TP (g·kg−1) | 1.05 ± 0.05 a | 0.83 ± 0.03 b | 0.77 ± 0.01 b |
| TK (g·kg−1) | 23.94 ± 0.35 a | 23.38 ± 1.64 a | 23.70 ± 0.69 a |
| SOM (g·kg−1) | 36.23 ± 3.18 a | 33.71 ± 1.59 a | 37.60 ± 0.61 a |
| AN (mg·kg−1) | 41.61 ± 1.08 ab | 43.36 ± 0.70 a | 38.5 ± 1.01 b |
| AP (mg·kg−1) | 233.37 ± 17.03 a | 121.92 ± 5.87 b | 113.45 ± 5.09 b |
| AK (mg·kg−1) | 677.92 ± 24.23 a | 587.32 ± 11.21 b | 129.59 ± 12.69 c |
| M3-Ca (mg·kg−1) | 1073.67 ± 12.49 c | 1110.67 ± 60.92 b | 1383.33 ± 62.81 a |
| M3-Al (mg·kg−1) | 262.67 ± 18.67 a | 286.67 ± 13.86 a | 309.67 ± 7.45 a |
| M3-Fe (mg·kg−1) | 100.67 ± 6.89 b | 113.67 ± 0.33 ab | 118.00 ± 3.06 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bian, T.; Wang, Z.; Wang, S.; Shan, X.; Wang, T.; Fu, H.; Sun, Z. Effect of P Reduction on phoD-Harboring Bacteria Community in Solar Greenhouse Soil. Agriculture 2024, 14, 1919. https://doi.org/10.3390/agriculture14111919
Bian T, Wang Z, Wang S, Shan X, Wang T, Fu H, Sun Z. Effect of P Reduction on phoD-Harboring Bacteria Community in Solar Greenhouse Soil. Agriculture. 2024; 14(11):1919. https://doi.org/10.3390/agriculture14111919
Chicago/Turabian StyleBian, Ting, Zhen Wang, Shuang Wang, Xuan Shan, Tianqi Wang, Hongdan Fu, and Zhouping Sun. 2024. "Effect of P Reduction on phoD-Harboring Bacteria Community in Solar Greenhouse Soil" Agriculture 14, no. 11: 1919. https://doi.org/10.3390/agriculture14111919
APA StyleBian, T., Wang, Z., Wang, S., Shan, X., Wang, T., Fu, H., & Sun, Z. (2024). Effect of P Reduction on phoD-Harboring Bacteria Community in Solar Greenhouse Soil. Agriculture, 14(11), 1919. https://doi.org/10.3390/agriculture14111919






